Sources of Collagen for Biomaterials in Skin Wound Healing
Abstract
1. Introduction
1.1. The Molecular Structure of Collagen
1.2. Collagen Stability
1.3. Collagen in Skin and Wound Healing
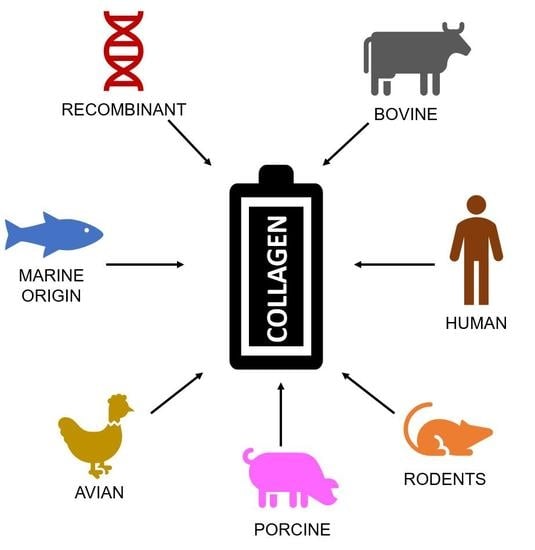
2. Sources of Collagen
2.1. Bovine Sources
2.2. Rodent Sources
2.3. Fish, Mollusc, and Marine Invertebrate Sources
2.4. Recombinant Expression Systems
2.4.1. Prokaryotic Expression Systems
E. Coli
2.4.2. Eukaryotic Expression Systems
Yeast
Plant
Insect
Mammals and Cultured Human Cells
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Gelse, K. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Carrique, L.; Goff, S.V.-L.; Mariano, N.; Georges, R.-N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Hongo, C.; Fukushima, R.; Wu, G.; Narita, H.; Noguchi, K.; Tanaka, Y.; Nishino, N. Crystal structures of collagen model peptides with Pro-Hyp-Gly repeating sequence at 1.26 Å resolution: Implications for proline ring puckering. Biopolymers 2004, 76, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.W.; Raines, R.T. The n→π∗ Interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Hojima, Y.; Prockops, D.J. Assembly of collagen fibrils de novo by cleavage of the type 1 pC-collagen with procollagen C-proteinase. J. Biol. Chem. 1987, 262, 15696–15701. [Google Scholar]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 16, 1–11. [Google Scholar] [CrossRef]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Boudreau, N.J.; Jones, P.L. Extracellular matrix and integrin signalling: the shape of things to come. Biochem. J. 1999, 339, 481–488. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Sharma, V.; Kang, N.V.; García-Gareta, E. A Universal Classification System of Skin Substitutes Inspired by Factorial Design. Tissue Eng. Part B: Rev. 2018, 24, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Ariffin, F.; Huda, N. An alternative source of type I collagen based on by-product with higher thermal stability. Food Hydrocoll. 2017, 63, 372–382. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Saito, T. Biocompatibility of Novel Type I Collagen Purified from Tilapia Fish Scale: An In Vitro Comparative Study. BioMed Res. Int. 2015, 2015, 139476. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Ohtaki, A.; Mizuno, M.; Tonozuka, T.; Sakano, Y.; Kamitori, S.; Dadu, E.; Nykvist, P.; Kapyla, J.; White, D.J.; et al. Integrin-mediated Cell Adhesion to Type I Collagen Fibrils. J. Boil. Chem. 2004, 279, 31956–31963. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef] [PubMed]
- Bretaud, S.; Nauroy, P.; Malbouyres, M.; Ruggiero, F. Fishing for collagen function: About development, regeneration and disease. Semin. Cell Dev. Biol. 2018, 89, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Cooperman, L.; Michaeli, D. The immunogenicity of injectable collagen. I. A 1-year prospective study. J. Am. Acad. Dermatol. 1984, 10, 638–646. [Google Scholar] [CrossRef]
- Cooperman, L.; Michaeli, D. The immunogenicity of injectable collagen. II. A retrospective review of seventy-two tested and treated patients. J. Am. Acad. Dermatol. 1984, 10, 647–651. [Google Scholar] [CrossRef]
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, H.M. Plant-Derived Human Collagen Scaffolds for Skin Tissue Engineering. Tissue Eng. Part A 2013, 19, 1507–1518. [Google Scholar] [CrossRef]
- Shoseyov, O.; Posen, Y.; Grynspan, F. Human collagen produced in plants: More than just another molecule. Bioengineered 2014, 5, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Li, B.; Lareu, R.R.; Chan, C.K.; Raghunath, M.; Zeugolis, D. Collagen solubility testing, a quality assurance step for reproducible electro-spun nano-fibre fabrication. A technical note. J. Biomater. Sci. Polym. Ed. 2008, 19, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Naing, M.W. Production of recombinant collagen: state of the art and challenges. Eng. Boil. 2017, 1, 18–23. [Google Scholar] [CrossRef]
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. Int. J. Biol. Macromol. 2017, 97, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Henrickson, R. Chemical, Biochemical, Functional, and Nutritional Characteristics of Collagen in Food Systems. Adv. Food Res. 1982, 28, 231–372. [Google Scholar] [PubMed]
- Timpl, R.; Glanville, R.W.; Nowack, H.; Wiedemann, H.; Fietzek, P.P.; Kuhn, K. Isolation, Chemical and Electron Microscopical Characterization of Neutral-Salt-Soluble Type III Collagen and Procollagen from Fetal Bovine Skin. Hoppe-Seyler´s Zeitschrift für physiologische Chemie 1975, 356, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Raspanti, M.; Viola, M.; Sonaggere, M.; Tira, M.E.; Tenni, R. Collagen Fibril Structure Is Affected by Collagen Concentration and Decorin. Biomacromolecules 2007, 8, 2087–2091. [Google Scholar] [CrossRef]
- Snowden, J.M.K.; Swann, D.A. Effects of glycosaminoglycans and proteoglycan on the in vitro assembly and thermal stability of collagen fibrils. Biopolymers 1980, 19, 767–780. [Google Scholar] [CrossRef]
- Kalamajski, S.; Oldberg, A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Boil. 2010, 29, 248–253. [Google Scholar] [CrossRef]
- E Scott, J.; Orford, C.R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem. J. 1981, 197, 213–216. [Google Scholar] [CrossRef]
- Vogel, K.G.; Paulsson, M.; Heinegård, D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 1984, 223, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian-Schwarz, A.; Held, M.; Knoeller, T.; Stachon, S.; Schmidt, T.; Schaller, H.E.; Just, L. In vivo biocompatibility and biodegradation of a novel thin and mechanically stable collagen scaffold. J. Biomed. Mater. Res. Part A. 2014, 102, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Eikenberry, E.F. Characterization of fibrous forms of collagen. Methods Enzymol. 1982, 82, 127–174. [Google Scholar] [PubMed]
- Haut, R.C. Age-Dependent Influence of Strain Rate on the Tensile Failure of Rat-Tail Tendon. J. Biomech. Eng. 1983, 105, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Friess, W. Collagen--biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T. Isolation of collagen from fish waste material — skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-K.; Jin, Y.-G.; Rha, S.-J.; Kim, S.-J.; Hwang, J.-H. Biochemical characteristics of four marine fish skins in Korea. Food Chem. 2014, 159, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process. Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- Rastian, Z.; Pütz, S.; Wang, Y.; Kumar, S.; Fleissner, F.; Weidner, T.; Parekh, S.H. Type I Collagen from Jellyfish Catostylus mosaicus for Biomaterial Applications. ACS Biomater. Sci. Eng. 2018, 4, 2115–2125. [Google Scholar] [CrossRef]
- Wichuda, J.; Sunthorn, C.; Busarakum, P. Comparison of the properties of collagen extracted from dried jellyfish and dried squid. Afr. J. Biotechnol. 2016, 15, 642–648. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLOS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Sun, D. Graded/gradient porous biomaterials. Materials 2010, 3, 26–47. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, S.; Nagai, N.; Suzuki, T.; Munekata, M. Novel biomaterial from reinforced salmon collagen gel prepared by fibril formation and cross-linking. J. Biosci. Bioeng. 2004, 98, 40–47. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Zhao, Y.; Wang, H.; Zhang, H. Effect of heat treatment on the enzymatic stability of grass carp skin collagen and its ability to form fibrils in vitro. J. Sci. Food Agric. 2015, 95, 329. [Google Scholar] [CrossRef] [PubMed]
- Burjanadze, T.V. New analysis of the phylogenetic change of collagen thermostability. Biopolymers 2000, 53, 523–536. [Google Scholar] [CrossRef]
- Krishnan, S.; Perumal, P. Preparation and biomedical characterization of jellyfish (Chrysaora Quinquecirrha) collagen from southeast coast of India. Int. J. Pharm. Pharm. Sci. 2013, 5, 698–701. [Google Scholar]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Tan, C.C.; Karim, A.A.; Latiff, A.A.; Gan, C.Y.; Ghazali, F.C. Extraction and characterization of pepsin-solubilized collagen from the body wall of crown-of-thorns Starfish (Acanthaster planci). Int. Food Res. J. 2013, 20, 3013–3020. [Google Scholar]
- Bama, P.; Vijayalakshimi, M.; Jayasimman, R.; Kalaichelvan, P.T.; Deccaraman, M. Extraction of collagen from cat fish (Tachysurus maculatus) by pepsin digestion and preparation and characterization of collagen chitosan sheet. Int. J. Pharm. Pharm. Sci. 2010, 2, 133–137. [Google Scholar]
- Sugiura, H.; Yunoki, S.; Kondo, E.; Ikoma, T.; Tanaka, J.; Yasuda, K. In Vivo Biological Responses and Bioresorption of Tilapia Scale Collagen as a Potential Biomaterial. J. Biomater. Sci. Polym. Ed. 2009, 20, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and properties of acid- and pepsin-soluble collagen from the skin of blacktip shark (Carcharhinus limbatus). Eur. Food Res. Technol. 2010, 230, 475. [Google Scholar] [CrossRef]
- A Werkmeister, J.; Ramshaw, J.A.M. Recombinant protein scaffolds for tissue engineering. Biomed. Mater. 2012, 7, 12002. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, C.; Baumann, S.; Cabalzar, J.; Luther, K.B.; Hennet, T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, D.; Shang, L.; Ma, X.; Luo, Y.; Xue, W.; Gao, P. Optimization of Fermentation Process for Human-like Collagen Production of Recombinant Escherichia coli Using Response Surface Methodology. Chin. J. Chem. Eng. 2010, 18, 137–142. [Google Scholar] [CrossRef]
- Hua, X.; Fan, D.; Luo, Y.; Zhang, X.; Shi, H.; Mi, Y.; Ma, X.; Shang, L.; Zhao, G. Kinetics of high cell density fed-batch culture. Chin. J. Chem. Eng. 2006, 14, 242–247. [Google Scholar] [CrossRef]
- Ghosh, N.; McKillop, T.J.; Jowitt, T.A.; Howard, M.; Davies, H.; Holmes, D.F.; Roberts, I.S.; Bella, J. Collagen-Like Proteins in Pathogenic E. coli Strains. PLOS ONE 2012, 7, e37872. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, J.; Harsch, M.; Jiménez, S. Hydroxyproline content determines the denaturation temperature of chick tendon collagen. Arch. Biochem. Biophys. 1973, 158, 478–484. [Google Scholar] [CrossRef]
- Perret, S.; Merle, C.; Bernocco, S.; Berland, P.; Garrone, R.; Hulmes, D.J.S.; Theisen, M.; Ruggiero, F. Unhydroxylated Triple Helical Collagen I Produced in Transgenic Plants Provides New Clues on the Role of Hydroxyproline in Collagen Folding and Fibril Formation. J. Boil. Chem. 2001, 276, 43693–43698. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Ramshaw, J.A.M. Bioengineered collagens. Subcell Biochem. 2017, 82, 601–629. [Google Scholar] [PubMed]
- Konitsiotis, A.D.; Raynal, N.; Bihan, D.; Hohenester, E.; Farndale, R.W.; Leitinger, B. Characterization of High Affinity Binding Motifs for the Discoidin Domain Receptor DDR2 in Collagen. J. Boil. Chem. 2008, 283, 6861–6868. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; An, B.; Ramshaw, J.A.; Brodsky, B. Bacterial collagen-like proteins that form triple-helical structures. J. Struct. Boil. 2014, 186, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Luo, Y. The selective roles of chaperone systems on over-expression of human-like collagen in recombinant Escherichia coli. J. Ind. Microbiol. Biotechnol. 2014, 41, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Hitzeman, R.A.; Hagie, F.E.; Levine, H.L.; Goeddel, D.V.; Ammerer, G.; Hall, B.D. Expression of a human gene for interferon in yeast. Nature 1981, 293, 717–722. [Google Scholar] [CrossRef]
- Ferrer-Miralles, N.; Domingo-Espín, J.; Corchero, J.L.; Vázquez, E.; Villaverde, A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Factories 2009, 8, 17. [Google Scholar] [CrossRef]
- Myllyharju, J.; Nokelainen, M.; Vuorela, A.; Kivirikko, K.I. Expression of recombinant human type I-III collagens in the yeastPichia pastoris. Biochem. Soc. Trans. 2000, 28, 353–357. [Google Scholar] [CrossRef]
- Vaughan, P.R.; Galanis, M.; Richards, K.M.; Tebb, T.A.; Ramshaw, J.A.; Werkmeister, J.A. Production of Recombinant Hydroxylated Human Type III Collagen Fragment in Saccharomyces cerevisiae. DNA Cell Boil. 1998, 17, 511–518. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, X.; Zhang, F.; Li, L.; Deng, J.; Xue, W.; Zhu, C.; Fan, D. New strategy for expression of recombinant hydroxylated human collagen α1(III) chains in Pichia pastoris GS115. Biotechnol. Appl. Biochem. 2015, 62, 293–299. [Google Scholar] [CrossRef]
- Yang, C.; Hillas, P.; Tang, J.; Balan, J.; Notbohm, H.; Polarek, J. Development of a recombinant human collagen-type III based hemostat. J. Biomed. Mater. Res. 2004, 69, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hait, M.R.; Robb, C.A.; Baxter, C.R.; Borgmann, A.; Tippett, L. Comparative evaluation of avitene microcrystalline collagen hemostat in experimental animal wounds. Am. J. Surg. 1973, 125, 284–287. [Google Scholar] [CrossRef]
- Davol Inc. Avitene - Microfibrillar Collagen Hemostat. Available online: https://www.crbard.com/CRBard/media/ProductAssets/DavolInc/PF10462/en-US/emmjq1t27osvjz36giw1wo86gqnxnu43.pdf (accessed on 10 November 2018).
- Liu, Y.; Griffith, M.; Watsky, M.A.; Forrester, J.V.; Kuffova, L.; Grant, D.; Merrett, K.; Carlsson, D.J.; Watsky, M. Properties of Porcine and Recombinant Human Collagen Matrices for Optically Clear Tissue Engineering Applications. Biomacromolecules 2006, 7, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, T.; Takada, H.; Wu, S.-H.; Kanayama, K.; Feng, J.; Tashiro, K.; Asahi, R.; Sunaga, A.; Hoshi, K.; Kurisaki, A.; et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.; Perret, S.; Lacour, T.; Jonval, V.; Hudaverdian, S.; Garrone, R.; Ruggiero, F.; Theisen, M. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens -mediated transient expression and in transgenic tobacco plant. FEBS Lett. 2002, 515, 114–118. [Google Scholar] [CrossRef]
- Setina, C.M.; Haase, J.P.; Glatz, C.E. Process integration for recovery of recombinant collagen type I α1 from corn seed. Biotechnol. Prog. 2016, 32, 98–107. [Google Scholar] [CrossRef]
- Xu, X.; Gan, Q.; Clough, R.C.; Pappu, K.M.; A Howard, J.; A Baez, J.; Wang, K. Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase. BMC Biotechnol. 2011, 11, 69. [Google Scholar] [CrossRef]
- Stein, H.; Wilensky, M.; Tsafrir, Y.; Rosenthal, M.; Amir, R.; Avraham, T.; Ofir, K.; Dgany, O.; Yayon, A.; Shoseyov, O. Production of Bioactive, Post-Translationally Modified, Heterotrimeric, Human Recombinant Type-I Collagen in Transgenic Tobacco†. Biomacromolecules 2009, 10, 2640–2645. [Google Scholar] [CrossRef]
- Yan, J.; Hu, K.; Xiao, Y.; Zhang, F.; Han, L.; Pan, S.; Li, L.; Wei, Y.; Cui, F. Preparation of recombinant human-like collagen/fibroin scaffold and its promoting effect on vascular cells biocompatibility. J. Bioact. Compat. Polym. 2018, 33, 416–425. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.-P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Shilo, S.; Roth, S.; Amzel, T.; Harel-Adar, T.; Tamir, E.; Grynspan, F.; Shoseyov, O. Cutaneous Wound Healing After Treatment with Plant-Derived Human Recombinant Collagen Flowable Gel. Tissue Eng. Part A 2013, 19, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Farkash, U.; Avisar, E.; Volk, I.; Slevin, O.; Shohat, N.; El Haj, M.; Dolev, E.; Ashraf, E.; Luria, S. First clinical experience with a new injectable recombinant human collagen scaffold combined with autologous platelet-rich plasma for the treatment of lateral epicondylar tendinopathy (tennis elbow). J. Shoulder Elb. Surg. 2018, 28, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Strauß, C.; Stoiber, S.; Kasper, C.; Charwat, V. Impact of Source and Manufacturing of Collagen Matrices on Fibroblast Cell Growth and Platelet Aggregation. Materials 2017, 10, 1086. [Google Scholar]
- Tomital, M.; Munetsuna, H.; Sato, T.; Adachi, T.; Hino, R.; Hayashi, M.; Shimizu, K.; Nakamura, N.; Tamura, T.; Yoshizato, K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 2003, 21, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Lv, Q.; Cui, F.Z.; Feng, Q.L.; Kong, X.D.; Wang, H.L.; Huang, L.Y.; Li, T. Biocompatible Fibroin Blended Films with Recombinant Human-like Collagen for Hepatic Tissue Engineering. J. Bioact. Compat. Polym. 2006, 21, 23–37. [Google Scholar] [CrossRef]
- High FiveTM Cells in Express FiveTM Medium. Available online: https://www.thermofisher.com/order/catalog/product/B85502 (accessed on 3 December 2018).
- Lamberg, A.; Helaakoski, T.; Myllyharju, J.; Peltonen, S.; Notbohm, H.; Pihlajaniemi, T.; I Kivirikko, K. Characterization of human type III collagen expressed in a baculovirus system. Production of a protein with a stable triple helix requires coexpression with the two types of recombinant prolyl 4-hydroxylase subunit. J. Boil. Chem. 1996, 271, 11988–11995. [Google Scholar] [CrossRef]
- David, T.P.; Pieper, F.; Sakai, N.; Karatzas, C.; Platenburg, E.; De Wit, I.; Samuel, C.; Dekker, A.; Daniels, G.A.; Berg, R.A.; et al. Production of recombinant human type I procollagen homotrimer in the mammary gland of transgenic mice. Transgenic Res. 1999, 8, 415–427. [Google Scholar]
- Hou, Y.; Guey, L.T.; Wu, T.; Gao, R.; Cogan, J.; Wang, X.; Hong, E.; Ning, W.V.; Keene, D.; Liu, N.; et al. Intravenously Administered Recombinant Human Type VII Collagen Derived from Chinese Hamster Ovary Cells Reverses the Disease Phenotype in Recessive Dystrophic Epidermolysis Bullosa Mice. J. Investig. Dermatol. 2015, 135, 3060–3067. [Google Scholar] [CrossRef]
- Woodley, D.T.; Keene, D.R.; Atha, T.; Huang, Y.; Lipman, K.; Li, W.; Chen, M. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat. Med. 2004, 10, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Costa, F.K.; Lindvay, C.R.; Han, Y.P.; Woodley, D.T. The recombinant expression of full-length type VII collagen and characterization of molecular mechanisms underlying dystrophic epidermolysis bullosa. J. Biol. Chem. 2002, 277, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Geddis, A.E.; Prockop, D.J. Expression of Human COL1A1 Gene in Stably Transfected HT1080 Cells: The Production of a Thermostable Homotrimer of Type I Collagen in a Recombinant System. Matrix 1993, 13, 399–405. [Google Scholar] [CrossRef]
- Fichard, A.; Tillet, E.; Delacoux, F.; Garrone, R.; Ruggiero, F. Human recombinant α1(V) collagen chain. Homotrimeric assembly and subsequent processing. J. Boil. Chem. 1997, 272, 30083–30087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, F.Z.; Hu, K.; Zhu, X.D.; Fan, D.D. Bone regeneration by using scaffold based on mineralized recombinant collagen. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2008, 86, 29–35. [Google Scholar] [CrossRef]
- Nokelainen, M.; Tu, H.; Vuorela, A.; Notbohm, H.; Kivirikko, K.I.; Myllyharju, J. High-level production of human type I collagen in the yeastPichia pastoris. Yeast 2001, 18, 797–806. [Google Scholar] [CrossRef]
- Chan, S.W.P.; Hung, S.-P.; Raman, S.K.; Hatfield, G.W.; Lathrop, R.H.; Da Silva, N.A.; Wang, S.-W. Recombinant Human Collagen and Biomimetic Variants Using a De Novo Gene Optimized for Modular Assembly. Biomacromolecules 2010, 11, 1460–1469. [Google Scholar] [CrossRef]
- Que, R.; Mohraz, A.; Da Silva, N.A.; Wang, S.-W. Expanding Functionality of Recombinant Human Collagen Through Engineered Non-Native Cysteines. Biomacromolecules 2014, 15, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
| Source | Expressed Collagen | Notes | Reference | |
|---|---|---|---|---|
| Marine Invertebrates | Jelly blubber (Catostylus mosaicus) | Type I | Low denaturation temperature; reduced viscosity and proline content compared to RASC | [43] |
| Flame jellyfish (Rhopilema esculentum) | Type I | Collagen sponge used as hemostat; effective due to physical properties, no noted superiority to traditional protein-based hemostatic agents | [45] | |
| Atlantic sea nettle (Chrysaora quinquecirrha) | Type I | High thermal denaturation temperature (37 °C); large variance in amino acid content compared to RASC; significant amount of hydroxyproline | [51] | |
| Barrel jellyfish (Rhizostoma pulmo) | Type I | Heparin inhibited cellular adhesion to jellyfish-derived collagen by 55%; fibrillar morphology similar to mammalian collagen | [37] | |
| Squid (Doryteuthis singhalensis) | Type I | High thermal denaturation temperature (35 °C), indicating potential for commercial use | [52] | |
| Bigfin reef squid (Sepioteuthis lessoniana) | Type I | Variance in amino acid composition compared to RASC; high solubility at narrow acidic pH range 4–5 | [44] | |
| Crown-of-thorns starfish (Acanthaster planci) | Type I | Denaturation temperature of 33 °C, comparable to mammalian collagen; proline content similar to mammalian collagen | [53] | |
| Teleost Fish | Atlantic salmon (Salmo salar) | Type I | Fish skin collagen less resistant to high temperatures, with lower denaturation and thermal decomposition temperatures being observed in fish skin collagen compared to bovine-derived collagen | [13] |
| Pacific cod (Gadus macrocephalus) | Type I | Proline and hydroxyproline content lower than bovine- and porcine-derived collagen; extremely low thermal denaturation temperature (14.5 °C), likely not useful for biomaterials without significant crosslinking | [42] | |
| Olive flounder (Paralichthys olivaceus) | Type I | Significant collagen extraction yield from skin | [41] | |
| Catfish (Tachysurus maculatus) | Type I | Type I collagen extracted from the swim bladder and used to form chitosan scaffold; crosslinking with glutaraldehyde yielded a scaffold with high tensile strength, low antigenicity, and high thermal stability | [54] | |
| Nile tilapia (Oreochromis niloticus) | Type I | Tilapia-derived collagen sponges rarely elicited an inflammatory response in vivo, statistically similar to those elicited by bovine-derived collagen | [55] | |
| Chum salmon (Oncorhynchus keta) | Type I | Very low denaturation temperature (18.6 °C), indicating a necessity to crosslink if used in biomaterials | [48] | |
| Elasmobranch Fish | Blacktip shark (Carcharhinus limbatus) | Type I | Denaturation temperature (34 °C) similar to that of mammalian-derived collagen | [56] |
| Expression System | Transduced Gene | Expressed Collagen | Notes | Reference | |
|---|---|---|---|---|---|
| Prokaryote | Escherichia coli | COL1A1 | Type I | Different amino acid expression when compared to natural collagen | [82,97] |
| Escherichia coli | COL3A1; L230, L593 (APMV) | Type III | Expression collagen III and mimivirus propyl and lysyl hydroxylases yielded hydroxylation levels similar to those expressed in humans | [58,59] | |
| Yeast | Pichia pastoris | COL1A1, PH4A/B | Type I | - | [60,76,98] |
| Pichia pastoris | COL3A1, PH4A/B | Type III | Recombinant hydroxylated collagen III exhibited hemostatic properties in vivo | [70,72,73] | |
| Saccharomyces cerevisiae | COL3A1, PH4A/B | Type III | Computational algorithm determined optimal oligonucleotide sequence | [99] | |
| Addition of non-native cysteine residues created crosslinking and anchoring sites; increased melting point compared to other RHC | [100] | ||||
| Plant | Nicotiana tabacum | COL1A1/2, P4HA/B, LH3 | Type I | Expressed triple helix similar to native collagen; supported growth and proliferation of vascular endothelial cells | [81] |
| Zea mays seed | COL1A1, P4HA/B | Type 1 | High yield collagen I produced by recombinant corn seed; hydroxylation of collagen led to enhanced thermostability. | [79,80] | |
| Human cell lines | HT1080 fibrosarcoma cells | COL1A1 | Type I | BP loss during initial propagation in E. coli necessitated reconstruction via PCR; recombinant expression produced over-modified pro 1(I) chains | [85] |
| HEK 293 kidney epithelial cells | COL5A1 | Type V | Addition of ascorbate to medium resulted in correctly folded, stable triple helix | [96] | |
| HEK 293 kidney epithelial cells | COL7A1 | Type VII | Anchoring type VII collagen used to treat dystrophic epidermolysis bullosa, by establishing dermal-epidermal adherence | [92,93,94] | |
| Mammal | Mus Musculus (Mammary gland) | COL1A1 | Type I | Soluble (1)3 (I) procollagen with post-translational proline and lysine hydroxylation secreted in milk | [91] |
| Insect | Spodoptera frugiperda (Sf9 cells) | COL3A1 | Type III | Hydroxylated triple helix molecules expressed intracellularly | [90] |
| Trichoplusia ni (High Five, Invitrogen) | COL3A1 | Type III | Hydroxylysine residue content slightly lower than non-recombinant expression | [90] | |
| Bombyx mori | COL1A1 | Gly-X-Y collage-like homodimer | Amino acid sequence and contents varied from natural collagen | [88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. https://doi.org/10.3390/bioengineering6030056
Davison-Kotler E, Marshall WS, García-Gareta E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering. 2019; 6(3):56. https://doi.org/10.3390/bioengineering6030056
Chicago/Turabian StyleDavison-Kotler, Evan, William S. Marshall, and Elena García-Gareta. 2019. "Sources of Collagen for Biomaterials in Skin Wound Healing" Bioengineering 6, no. 3: 56. https://doi.org/10.3390/bioengineering6030056
APA StyleDavison-Kotler, E., Marshall, W. S., & García-Gareta, E. (2019). Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering, 6(3), 56. https://doi.org/10.3390/bioengineering6030056