Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration
Abstract
:1. Introduction
2. Noble Metal NPs, Hydrogel, and NP–Hydrogel Composite
2.1. Noble Metal NPs
2.2. Hydrogel
2.3. NP–Hydrogel Composite
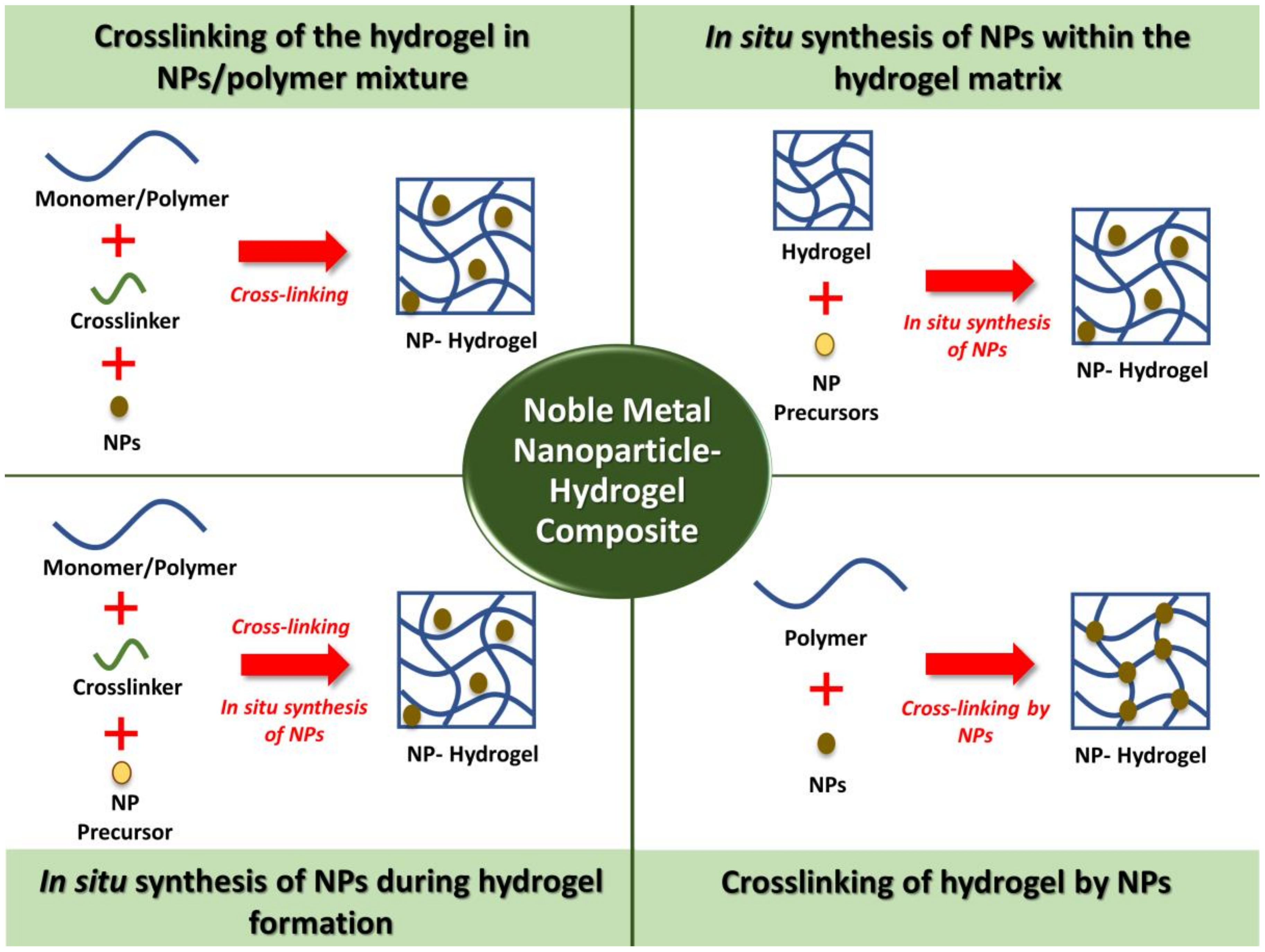
3. Synthesis Methods of Noble Metal NPs–Hydrogels Composites
3.1. Crosslinking of the Hydrogel in NPs/Polymer Mixture
3.2. In Situ Synthesis of NPs within the Hydrogel Matrix
3.3. In Situ Synthesis of NPs during Hydrogel Formation
3.4. Crosslinking of Hydrogels by NPs
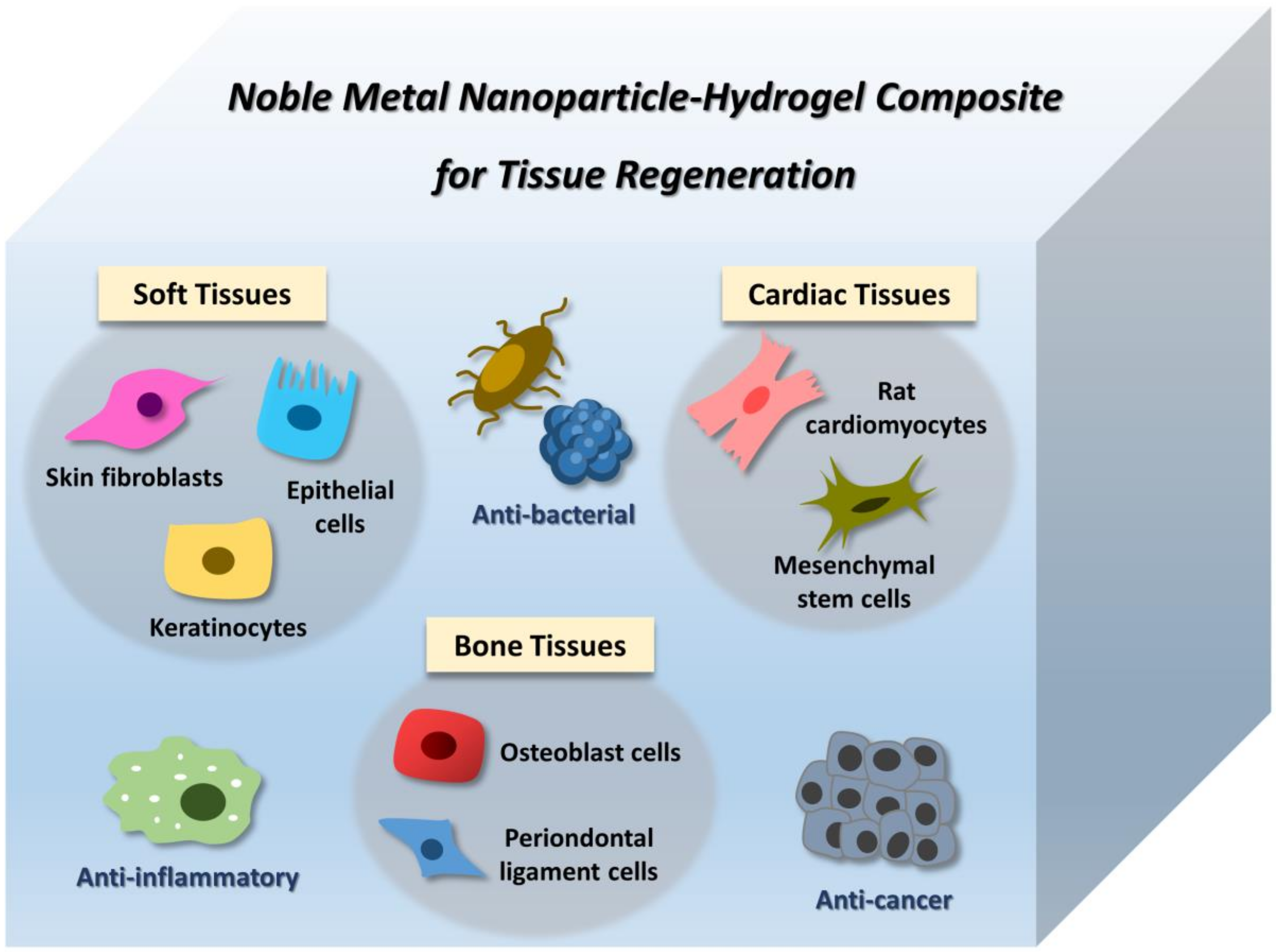
4. Application of Noble Metal NP–Hydrogel Composites in Tissue Engineering
4.1. Soft Tissues
4.2. Bone Tissues
4.3. Cardiac Tissues
5. Limitations and Challenges
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Network. Available online: https://optn.transplant.hrsa.gov/ (accessed on 20 December 2018).
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Zohora, F.T.; Azim, A.Y.M.A. Biomaterials as porous scaffolds for tissue engineering applications: A review. Eur. Sci. J. 2014, 10. [Google Scholar] [CrossRef]
- Brahatheeswaran Dhandayuthapani, Y.Y.; Maekawa, T.; Sakthi Kumar, D. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Farrell, H.; Higginbotham, C.L. Compressive Strength and Bioactivity Properties of Photopolymerizable Hybrid Composite Hydrogels for Bone Tissue Engineering. Int. J. Polym. Mater. Po. 2014, 63, 641–650. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef]
- Teow, S.Y.; Wong, M.M.; Yap, H.Y.; Peh, S.C.; Shameli, K. Bactericidal Properties of Plants-Derived Metal and Metal Oxide Nanoparticles (NPs). Molecules (Basel, Switzerland) 2018, 23, 1366. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules (Basel, Switzerland) 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ahn, S.; Kang, J.P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and applications of noble metal nanoparticles: A review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Conde, J.; Doria, G.; Baptista, P. Noble Metal Nanoparticles Applications in Cancer. J. Drug Deliv. 2012, 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine AU—Chugh, Heerak. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomedicine 2017, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yang, D.; Zhao, C.; Song, Z.; Liu, P.; Wang, Y.; Chen, Z.; Shen, J. Protein–Gold Hybrid Nanocubes for Cell Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Borzenkov, M.; Moros, M.; Tortiglione, C.; Bertoldi, S.; Contessi, N.; Fare, S.; Taglietti, A.; D’Agostino, A.; Pallavicini, P.; Collini, M.; et al. Fabrication of photothermally active poly(vinyl alcohol) films with gold nanostars for antibacterial applications. Beilstein J. Nanotechnol. 2018, 9, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Malki, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold Nanorod-Based Engineered Cardiac Patch for Suture-Free Engraftment by Near IR. Nano. Lett. 2018, 18, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Lee, H.R.; Lee, S.J.; Lee, D.; Um, S.H.; Lee, J.H.; Woo, Y.-H.; Zhang, L.G.; Lee, D.-W.; et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016, 469, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/beta-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Vial, S.; Maia, F.R.; Carvalho, M.; Reis, R.L.; Granja, P.L.; Oliveira, J.M. Gellan gum-coated gold nanorods: an intracellular nanosystem for bone tissue engineering. RSC Adv. 2015, 5, 77996–78005. [Google Scholar] [CrossRef]
- Li, J.J.; Kawazoe, N.; Chen, G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials 2015, 54, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver nanoparticles: synthesis methods, properties and applications. Int. J. Mater. Sci. Appl 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, J.H.; Kim, H.J.; Ji, Y.H.; Kim, J.H.; Son, S.W. Silver nanoparticle-induced hMSC proliferation is associated with HIF-1alpha-mediated upregulation of IL-8 expression. J. Invest. Dermatol. 2014, 134, 3003–3007. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.; Sohaebuddin, S.; Poudyal, N.; Liu, J.P.; Tang, L. Magnetic Nanoparticles to Enhance Cell Seeding and Distribution in Tissue Engineering Scaffolds. In Proceedings of the 2008 8th IEEE Conference on Nanotechnology, Arlington, TX, USA, 18–21 August 2008; pp. 646–649. [Google Scholar]
- Eid, K.; Eldesouky, A.; Fahmy, A.; Shahat, A.; AbdElaal, R. Calcium phosphate scaffold loaded with platinum nanoparticles for bone allograft. Am. J. Biomed. Sci. 2013, 5, 242–249. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; Wani, W.A.; Manickam, P.; Nair, M. Nanocomposite Hydrogels: Advances in Nanofillers Used for Nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert. Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, F.; Tsang, W.P.; Wan, C.; Wu, C. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 2017, 120, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Wang, H.-J.; Kim, T.-H.; Choi, J.S.; Kulkarni, G.; Jackson, J.D.; Atala, A.; Yoo, J.J. In Situ Tissue Regeneration of Renal Tissue Induced by Collagen Hydrogel Injection. Stem. Cells Transl. Med. 2018, 7, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Pena, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C. Fibrin Hydrogels for Endothelialized Liver Tissue Engineering with a Predesigned Vascular Network. Polymers 2018, 10, 1048. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, D.; Li, Y.; Xie, W.; Wang, X.; Tao, L.; Wei, Y.; Wang, X.; Zhao, L. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci. China Life Sci. 2018, 61, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater. Res. 2017, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible Hydrogel Nanocomposite with Covalently Embedded Silver Nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.A.J.; Franchi, L.P.; Rosa, L.R.; da Veiga, M.A.M.S.; Takahashi, C.S. Cytotoxicity and genotoxicity of silver nanoparticles of different sizes in CHO-K1 and CHO-XRS5 cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 795, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.X.; Liu, M.C.; Kempson, I.M.; Fa, Y.C.; Huang, K.Y. Light-triggered methylcellulose gold nanoparticle hydrogels for leptin release to inhibit fat stores in adipocytes. Int. J. Nanomed. 2017, 12, 7603–7611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials (Basel, Switzerland) 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.H.H.; Amir, N.; Yahya, N.; Saaid, I.M. Characterization and Colloidal Stability of Surface Modified Zinc Oxide Nanoparticle. J. Phys. Conf. Ser. 2018, 11. [Google Scholar] [CrossRef]
- Varaprasad, K.; Mohan, Y.M.; Vimala, K.; Mohana Raju, K. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Kumari, M. A green approach to prepare silver nanoparticles loaded gum acacia/poly(acrylate) hydrogels. Int. J. Biol. Macromol. 2015, 80, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Deen, G.; Chua, V. Synthesis and Properties of New “Stimuli” Responsive Nanocomposite Hydrogels Containing Silver Nanoparticles. Gels 2015, 1, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, A.; de Jesus Oliveira, A.C.; Quelemes, P.V.; Placido, A.; da Silva, F.V.; Oliveira, I.S.; de Almeida, M.P.; Amorim, A.; Delerue-Matos, C.; de Oliveira, R.C.M.; et al. In Situ Synthesis of Silver Nanoparticles in a Hydrogel of Carboxymethyl Cellulose with Phthalated-Cashew Gum as a Promising Antibacterial and Healing Agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Nadeau, B.; An, X.; Cheng, D.; Long, Z.; Ni, Y. Silver nanoparticles-containing dual-function hydrogels based on a guar gum-sodium borohydride system. Sci. Rep. 2016, 6, 36497. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Khampieng, T.; Brikshavana, P.; Supaphol, P. Silver nanoparticle-embedded poly(vinyl pyrrolidone) hydrogel dressing: gamma-ray synthesis and biological evaluation. J. Biomater. Sci. Polym. Ed. 2014, 25, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Deekonda, K.; Muniyandy, S.; Lim, Y.Y.; Janarthanan, P. Electron beam radiation mediated green synthesis of silver nanoparticles using carboxymethyl sago pulp obtained from sago waste. Polymer 2016, 86, 147–156. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Mallaiah, S.H. Swelling and mechanical properties of radiation crosslinked Au/PVA hydrogel nanocomposites. Radiat. Eff. Defect. S. 2016, 171, 869–878. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Wang, L.; Xu, L.; Zhai, M.; Wei, S. Radiation synthesis and characterization of nanosilver/gelatin/carboxymethyl chitosan hydrogel. Radiat. Phys. Chem. 2012, 81, 553–560. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically Crosslinked Gold Nanoparticle—Hyaluronan Hydrogels. Adv. Mater. 2010, 22, 4736–4740. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An Injectable Self-Assembling Collagen–Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Kato, T.; Tanaka, T.; Maruyama, T. A DNA–gold nanoparticle hybrid hydrogel network prepared by enzymatic reaction. Chem. Commun. 2017, 53, 5802–5805. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Hernández, R.; López, D. Crosslinking of poly(vinyl alcohol) using functionalized gold nanoparticles. Eur. Polym. J. 2010, 46, 2099–2104. [Google Scholar] [CrossRef]
- Schuetz, T.; Richmond, N.; Harmon, M.E.; Schuetz, J.; Castaneda, L.; Slowinska, K. The microstructure of collagen type I gel cross-linked with gold nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, J.; Zhu, Y.; Zhao, W.; Pan, C.; Ma, H.; Zhang, L. A poly(hydroxyethyl methacrylate)-Ag nanoparticle porous hydrogel for simultaneous in vivo prevention of the foreign-body reaction and bacterial infection. Nanotechnology 2018, 29, 395101. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.W.; El-Hotaby, W.; Hemdan, B.; Abdel-Fattah, W.I. Thermosensitive chitosan/phosphate hydrogel-composites fortified with Ag versus Ag@Pd for biomedical applications. Life Sci. 2018, 194, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, F.H.; Hussain, F.S.J.; Zeyohannes, S.S.; Rasad, M.S.B.A.; Yusuff, M.M. A facile synthesis method of hydroxyethyl cellulose-silver nanoparticle scaffolds for skin tissue engineering applications. Mater. Sci. Eng. C. 2017, 79, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Udekwu, K.I.; Noel, C.W.; Gagnon, L.B.; Taylor, P.K.; Vulesevic, B.; Simpson, M.J.; Gkotzis, S.; Islam, M.M.; Lee, C.J.; et al. Safety and efficacy of composite collagen-silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale 2015, 7, 18789–18798. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Desagani, D.; Chandran, G.; Ghosh, N.N.; Karthikeyan, G.; Waigaonkar, S.; Ganguly, A. Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.A.; Zhu, J.; Gootee, J.; Snider, C.L.; Bellrichard, M.; Grant, D.A. Gold Nanoparticle-Collagen Gels for Soft Tissue Augmentation. Tissue Eng. Part A 2018, 24, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, W.; Yu, Y.; Zhang, Y.; Zhang, J.; Yuan, Z. The study of angiogenesis stimulated by multivalent peptide ligand-modified alginate. Colloids Surf. B Biointerfaces 2017, 154, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, G.; Xu, K.; Wang, L.; Yu, L.; Xing, M.M.Q.; Qiu, X. Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 379–386. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.I.; Perni, S.; Tommasi, G.; Morris, N.G.; Hawkins, K.; López-Cabarcos, E.; Prokopovich, P. Silver nanoparticle based antibacterial methacrylate hydrogels potential for bone graft applications. Mater. Sci. Eng. C. 2015, 50, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Tentor, F.R.; de Oliveira, J.H.; Scariot, D.B.; Lazarin-Bidoia, D.; Bonafe, E.G.; Nakamura, C.V.; Venter, S.A.S.; Monteiro, J.P.; Muniz, E.C.; Martins, A.F. Scaffolds based on chitosan/pectin thermosensitive hydrogels containing gold nanoparticles. Int. J. Biol. Macromol. 2017, 102, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Kumar, P.T.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Antibacterial and bioactive alpha- and beta-chitin hydrogel/nanobioactive glass ceramic/nano silver composite scaffolds for periodontal regeneration. J. Biomed. Nanotechnol. 2013, 9, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.M.; Kim, B.C.; Park, J.H.; Kwon, I.K.; Mantalaris, A.; Hwang, Y.S. Stem cells in bone tissue engineering. Biomed. Mater. 2010, 5, 062001. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Lee, D.; Heo, D.N.; Nah, H.R.; Lee, S.J.; Ko, W.-K.; Lee, J.S.; Moon, H.-J.; Bang, J.B.; Hwang, Y.-S.; Reis, R.L.; et al. Injectable hydrogel composite containing modified gold nanoparticles: implication in bone tissue regeneration. Int. J. Nanomed. 2018, 13, 7019–7031. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yuan, K.; Ma, R.; Gao, L.; Jiang, W.; Hu, X.; Lin, W.; Zhang, X.; Huang, Z. Gold nanoparticles promote osteogenic differentiation of human periodontal ligament stem cells via the p38 MAPK signaling pathway. Mol. Med. Rep. 2017, 16, 4879–4886. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Qiu, X. Conductive biomaterials in cardiac tissue engineering. Biotarget 2017, 8. [Google Scholar] [CrossRef]
- Shevach, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano. Lett. 2014, 14, 5792–5796. [Google Scholar] [CrossRef] [PubMed]
- Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol 2016, 6. [Google Scholar] [CrossRef]
- You, J.-O.; Rafat, M.; Ye, G.J.C.; Auguste, D.T. Nanoengineering the Heart: Conductive Scaffolds Enhance Connexin 43 Expression. Nano. Lett. 2011, 11, 3643–3648. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, K.; Ahumada, M.; McTiernan, C.D.; Bejjani, J.; Variola, F.; Ruel, M.; Xu, B.; Liang, W.; Suuronen, E.J.; Alarcon, E.I. Multi-functional thermo-crosslinkable collagen-metal nanoparticle composites for tissue regeneration: nanosilver vs. nanogold. RSC Adv. 2017, 7, 47704–47708. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Moore, N.; Sullivan, R.T.; Truong, D.; Migrino, R.Q.; Nikkhah, M. Electrically conductive hydrogel-based micro-topographies for the development of organized cardiac tissues. RSC Adv. 2017, 7, 3302–3312. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.-C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef] [PubMed]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Travan, A.; Donati, I.; Di Luca, A.; Benincasa, M.; Crosera, M.; Paoletti, S. Biological response of hydrogels embedding gold nanoparticles. Colloids Surf. B Biointerfaces 2011, 83, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Cuttle, L. Cytotoxicity testing of silver-containing burn treatments using primary and immortal skin cells. Burns 2014, 40, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and Gold Nanoparticles Exposure to In Vitro Cultured Retina—Studies on Nanoparticle Internalization, Apoptosis, Oxidative Stress, Glial- and Microglial Activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef] [PubMed]
- Kostić, D.D.; Malagurski, I.S.; Obradović, B.M. Transport of silver nanoparticles from nanocomposite Ag/alginate hydrogels under conditions mimicking tissue implantation. Hemijska Industrija 2017, 71, 12. [Google Scholar] [CrossRef]
| Tissue Regeneration | Nanoparticles | Scaffolds | Synthesis Method | Cell Line/Animal Tested | Effect of NPs Addition on the Physical Property of Material | Effect of NPs Addition on the Biological Property of Material | Reference |
|---|---|---|---|---|---|---|---|
| Soft Tissues | Collagen-coated Ag NPs | Collagen | Crosslinking of the hydrogel in NPs/polymer mixture | Primary human epidermal keratinocytes; Dermal fibroblasts; Mice | Hydrogel containing 0.2 µM Ag NPs has similar Young’s modulus as human skin | Biocompatibility, anti-inflammatory, and anti-bacterial activities | [71] |
| Ag NPs | Poly(hydroxyethyl methacrylate) | In situ synthesis of NPs during hydrogel formation | Mouse embryo fibroblasts (NIH-3T3); BALB/c female mice | Increased amounts of Ag NPs loading slightly enhanced the compressive modulus of hydrogel | Biocompatibility, anti-bacterial, and in vivo resistance to foreign-body reactions | [68] | |
| Ag NPs | Hydroxyethyl cellulose | Crosslinking of the hydrogel in NPs/polymer mixture | Human fibroblasts | Glass transition temperature of scaffold increases as concentration of AgNO3 increases | Biocompatibility | [70] | |
| Ag NPs & Ag-Palladium NPs | Chitosan/Hydroxyapatite & Chitosan/Beta-tricalcium phosphate | Crosslinking of hydrogel in NPs/polymer mixture | Normal skin fibroblasts (BJ1); Hepatocellular carcinoma cells (HEPG2); Breast cancer cells (MCF7); | N/A | Biocompatibility and anti-bacterial activity | [69] | |
| Chitosan-coated Ag NPs | Agarose | Crosslinking of the hydrogel in NPs/polymer mixture | Human cervical carcinoma cells (HeLa); Human pancreatic epithelial carcinoma cells (MiaPaCa2); Human embryonic kidney cells (HEK); | Mechanical strength (five to eight Mpa) falls within range for soft tissue engineering | Biocompatibility, anti-bacterial activity, and hemocompatibility | [72] | |
| Au NPs | Alginate | Crosslinking of the hydrogel in NPs/polymer mixture | Human umbilical vein endothelial cells (HUVECs) | N/A | Enhanced HUVECs adhesion rate and cell spreading | [74] | |
| Au NPs | Collagen | Conjugation of Au NPs to collagen fibrils | Swine | Enhanced longevity of the material | Biocompatibility and low irritation | [73] | |
| Bone Tissues | Ag NPs | α-chitin and β-chitin/Bioactive glass ceramic NPs | Crosslinking of hydrogel in NPs/polymer mixture | Human periodontal ligament cells (hPDL); Human primary osteoblasts (POB) | Composite scaffold has decreased porosity and enhanced compressive strength. | Anti-bacterial activity, differentiation, and mineralization of POB in the absence of osteogenic supplements | [78] |
| Ag NPs | Poly (ethylene glycol) | In situ synthesis of NPs within the hydrogel matrix | Osteoblast cells (MC3T3-E1); Sprague–Dawley rats | N/A | Anti-bacterial activity, promoted osteogenesis in vitro and in vivo | [75] | |
| Ag NPs | Methacrylate | Crosslinking of hydrogel in NPs/polymer mixture; diffusion reaction; adsorption of NPs | Osteoblast cells (MC-3T3) | No effect on mechanical properties (absorption method) | Biocompatibility and anti-bacterial activity (absorption method) | [76] | |
| Au NPs | Chitosan/Pectin | Crosslinking of the hydrogel in NPs/polymer mixture; diffusion reaction; adsorption of NPs | Normal kidney epithelial cells (VERO); Epithelial colorectal adenocarcinoma cells (HT-29); HPV-16 positive human cervical tumor cells (SiHa); Kidney epithelial cells (LLCMK2); Murine macrophage cells (J774A1 cells); Mouse preosteoblastic cells (MC3T3-E1) | Gelation temperature decreases with decrease in pectin concentration and increase in Au NPs levels | Biocompatibility and promoted growth of MC3T3-E1 cells | [77] | |
| Au NPs | Gelatin | Crosslinking of the hydrogel in NPs/polymer mixture | Human adipose-derived stem cells (ADSCs); New Zealand Rabbit | N/A | Biocompatibility, promoted differentiation toward osteoblast cells, and improved bone regeneration in vivo | [80] | |
| N-acetyl cysteine-Au NPs | Gelatin-tyramine | Crosslinking of hydrogel in NPs/polymer mixture | Human adipose derived-stem cells (hASCs) | N/A | Biocompatibility and promoted osteodifferentiation | [81] | |
| Ag and Au NPs | Silk fibroin/Nanohydroxyapatite | In situ synthesis of NPs within the hydrogel matrix | Osteoblast-like cells (MG63) | Hydrogels containing Ag and Au NPs have enhanced mechanical stiffness | Biocompatibility and anti-bacterial activity | [16] | |
| Cardiac Tissues | Peptide-modified Ag and Au NPs | Collagen | Crosslinking of the hydrogel in NPs/polymer mixture | Neonatal rat ventricular cardiomyocytes and cardiac fibroblasts | Enhanced mechanical and electrical properties of the material | Promoted reparative macrophage migration | [87] |
| Au NPs | Decellularized omental matrices | Evaporation of Au for deposition | Neonatal rat ventricular cardiomyocytes, Cardiac fibroblasts | Au NPs patches have enhanced conductivity and similar longitudinal elastic modulus as pristine patches | Aligned cardiac cells with organized connexin 43 and attenuation of fibroblast proliferation | [84] | |
| Au NPs | Thiol 2-hydroxyethyl methacrylate (HEMA)/HEMA | In situ synthesis of NPs within the hydrogel matrix | Neonatal rat ventricular cardiomyocytes | Conductive hydrogel has tunable conductive and mechanical property, with Young’s modulus similar to myocardium | Increased expression of connexin 43 | [86] | |
| Chitosan-modified Au NPs | Chitosan | Crosslinking of the hydrogel in NPs/polymer mixture | Mesenchymal stem cells | Tunable electrical conductivity of the hydrogel by different concentration of Au NPs | Biocompatibility, enhanced differentiation into cardiac lineages | [91] | |
| Au nanorods | Gelatin methacrylate | Crosslinking of the hydrogel in NPs/polymer mixture | Neonatal rat ventricular cardiomyocytes | Enhanced mechanical and electrical properties of the material | Enhanced formation of cardiac tissues | [88,89] | |
| Au nanorods | Gelatin methacryloyl | Crosslinking of the hydrogel in NPs/polymer mixture (3D bioprinting) | Neonatal rat ventricular cardiomyocytes and cardiac fibroblasts | Nanocomposite bioink has increased shear-thinning effect and enhanced printability | Enhanced cell adhesion and organization, electrical propagation, and synchronized contraction | [90] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.-L.; Teow, S.-Y.; Pushpamalar, J. Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration. Bioengineering 2019, 6, 17. https://doi.org/10.3390/bioengineering6010017
Tan H-L, Teow S-Y, Pushpamalar J. Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration. Bioengineering. 2019; 6(1):17. https://doi.org/10.3390/bioengineering6010017
Chicago/Turabian StyleTan, Hui-Li, Sin-Yeang Teow, and Janarthanan Pushpamalar. 2019. "Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration" Bioengineering 6, no. 1: 17. https://doi.org/10.3390/bioengineering6010017
APA StyleTan, H.-L., Teow, S.-Y., & Pushpamalar, J. (2019). Application of Metal Nanoparticle–Hydrogel Composites in Tissue Regeneration. Bioengineering, 6(1), 17. https://doi.org/10.3390/bioengineering6010017





