Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations
Abstract
1. The Need for Mechanical Circulatory Support
1.1. Bridge Devices
1.2. Destination Therapy
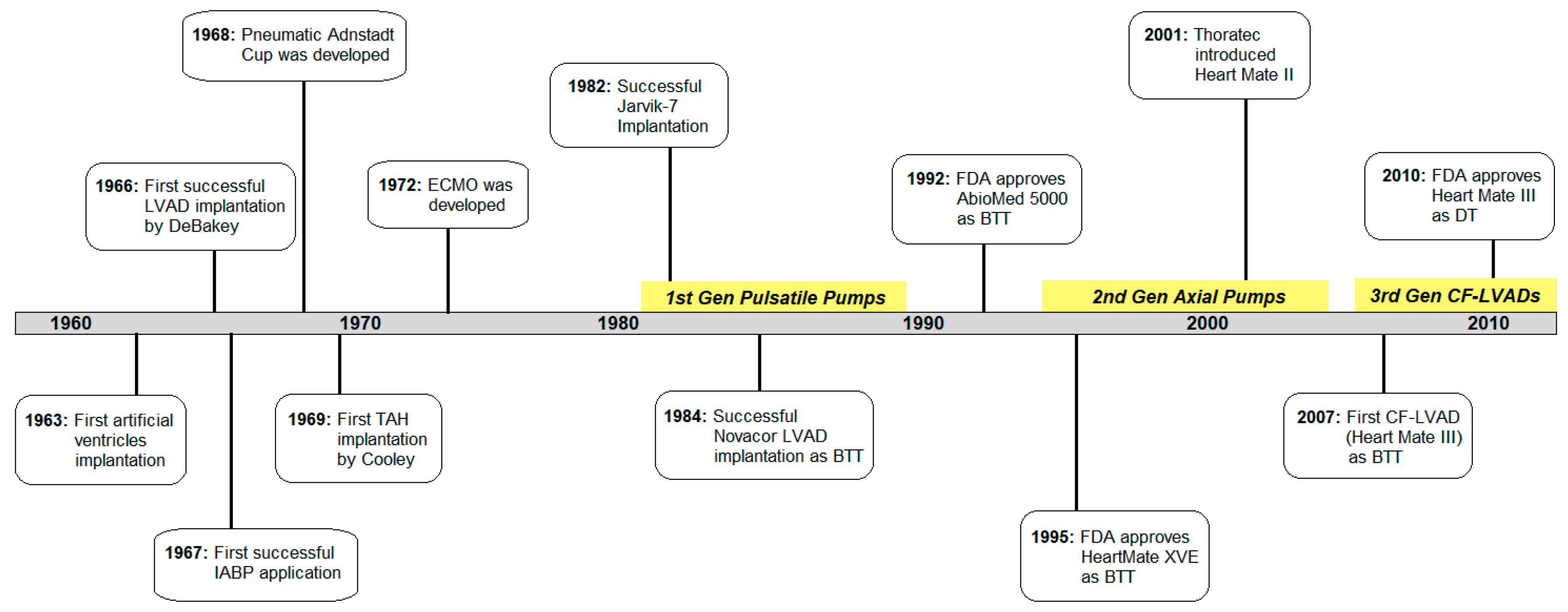
2. The History of Cardiac Assist Devices
2.1. The Beginning
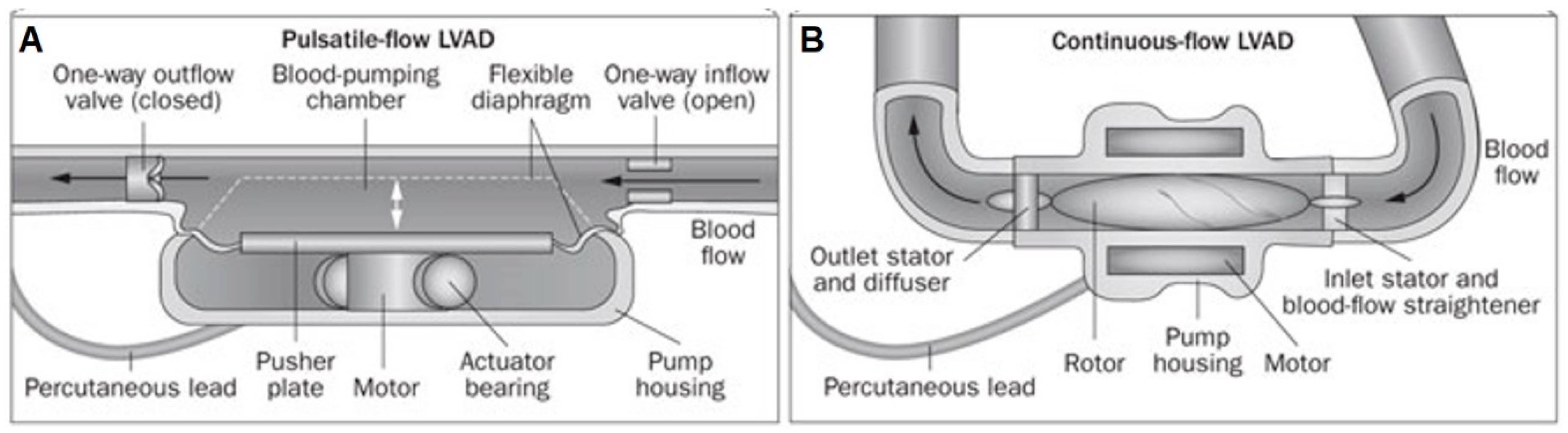
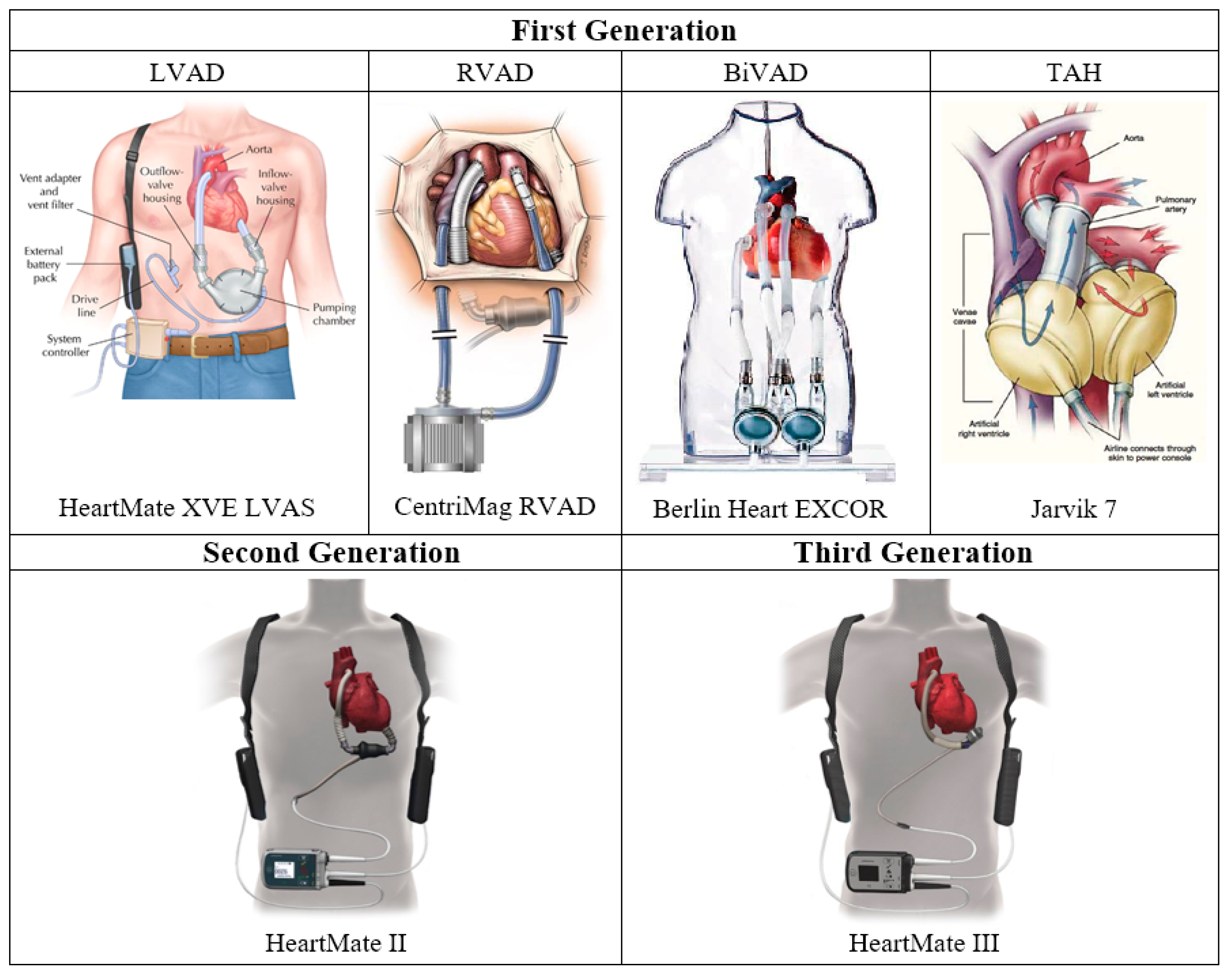
2.2. First Generation: Pulsatile Pumps
2.2.1. LVAD
2.2.2. RVAD
2.2.3. BiVAD
2.2.4. Total Artificial Heart
2.3. Second Generation: Continuous Axial Flow Pumps
2.4. Third Generation: Continuous Centrifugal Pumps
3. Current State of The Art
3.1. CADs in Clinical Settings Today
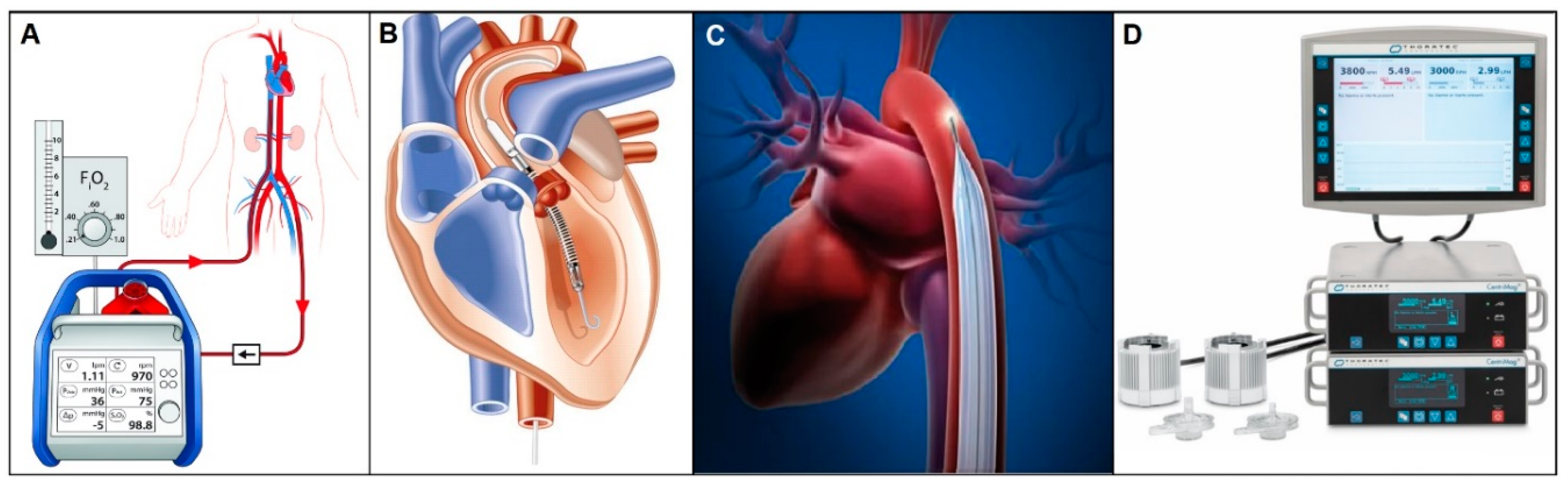
3.1.1. Short-term Circulatory Support
3.1.2. CADs for Extended Use
3.1.3. Pediatric Pumps
3.2. Clinical Complications of Current VADs
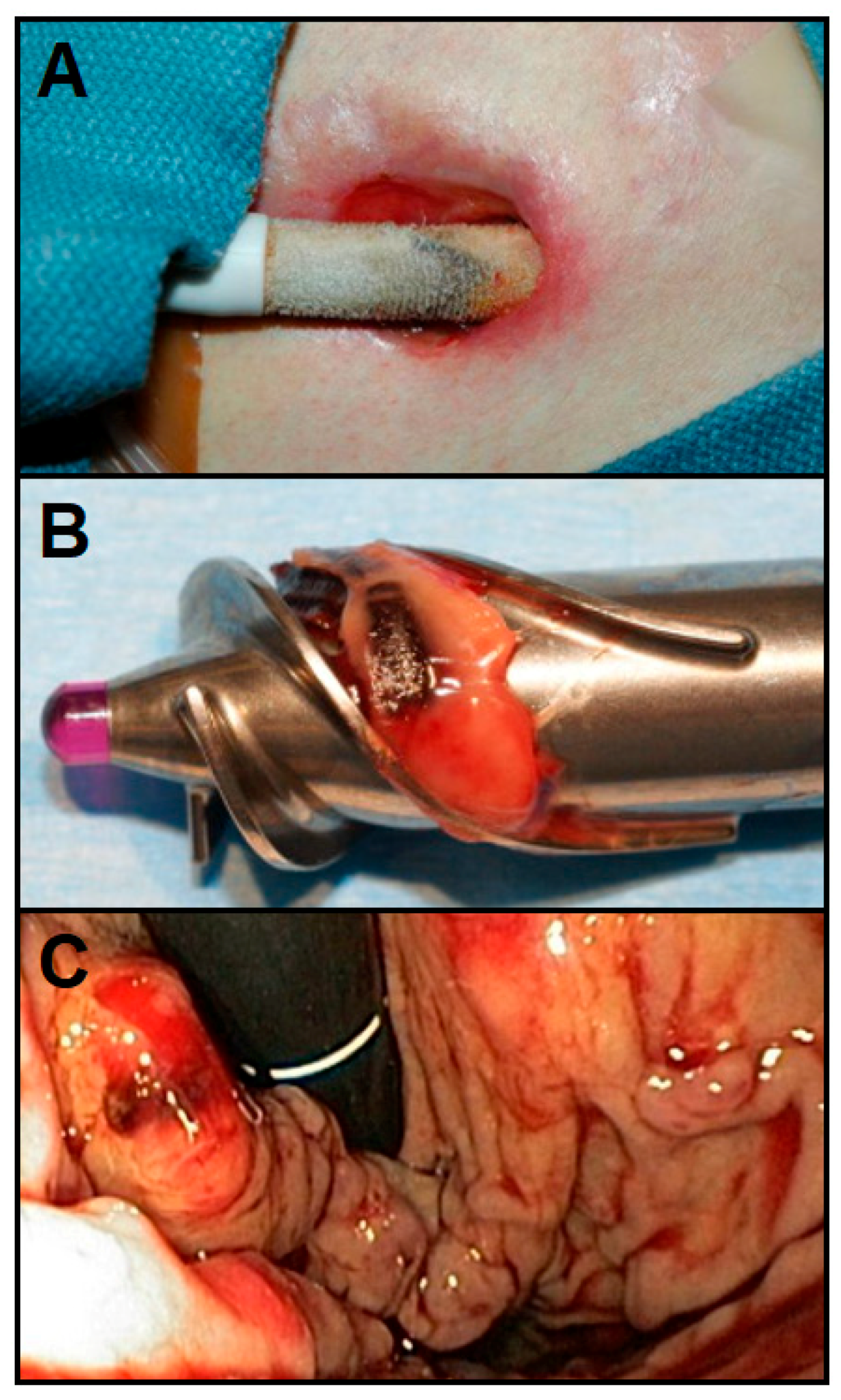
3.2.1. Driveline Infections
3.2.2. Pump Thrombosis
3.2.3. Gastrointestinal Bleeding
4. Innovations for Effective Long-Term Cardiac Support
4.1. Alternative Powering Methods for Untethered Cardiac Support
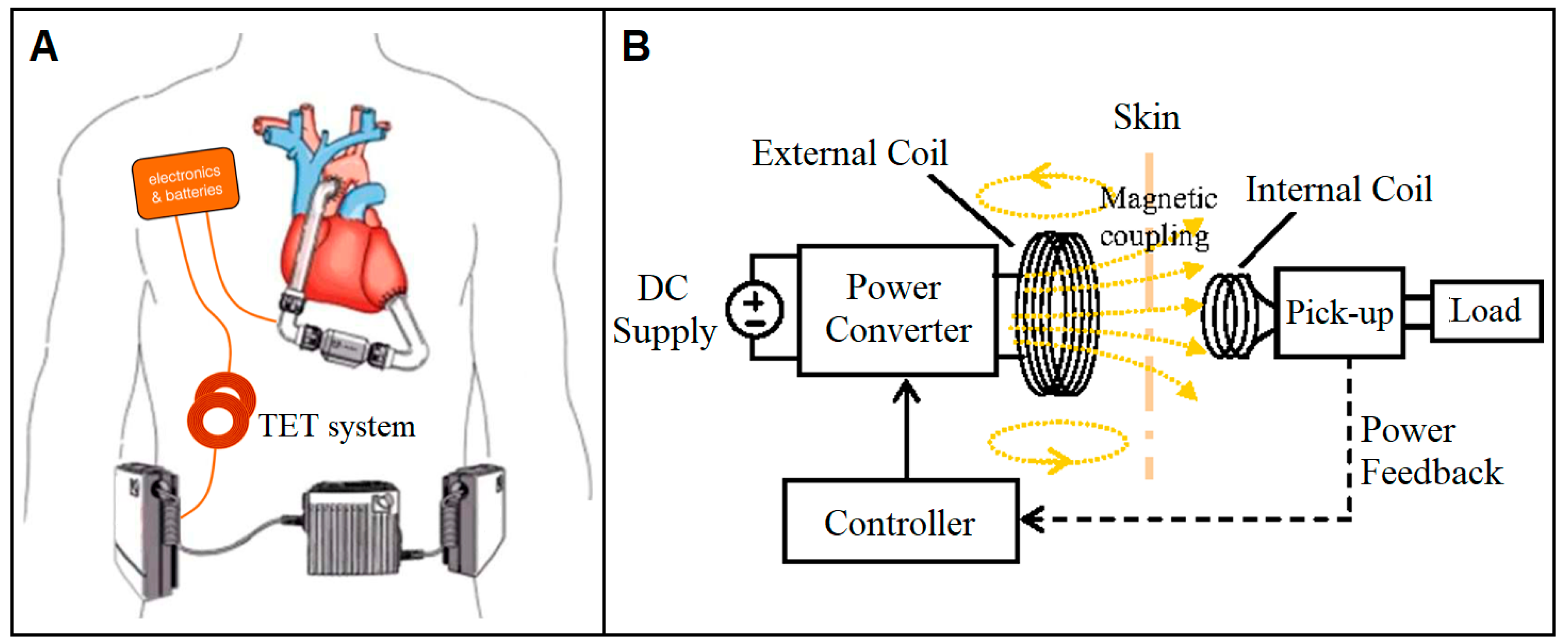
4.1.1. Transcutaneous Energy Transfer System
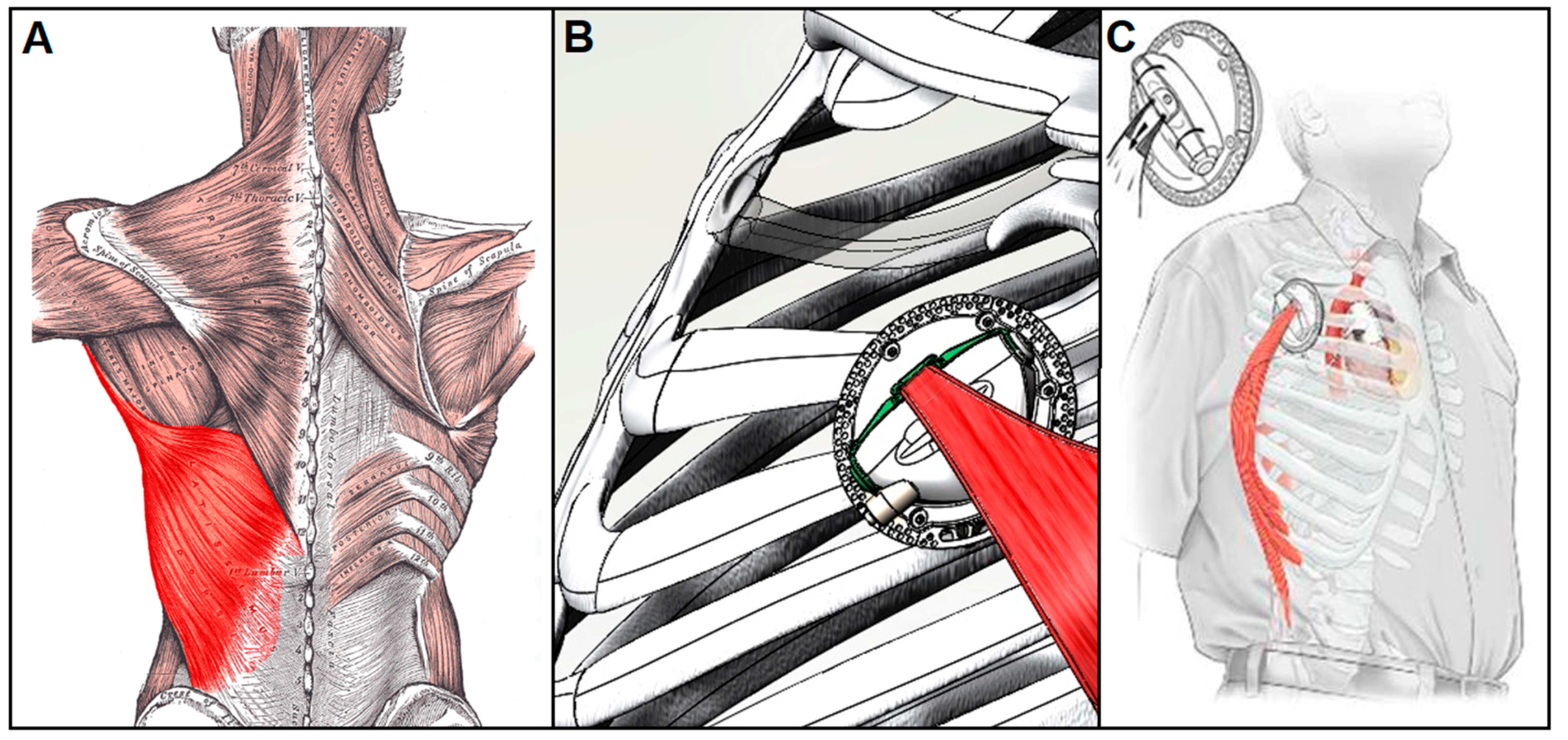
4.1.2. Muscle-powered VADs
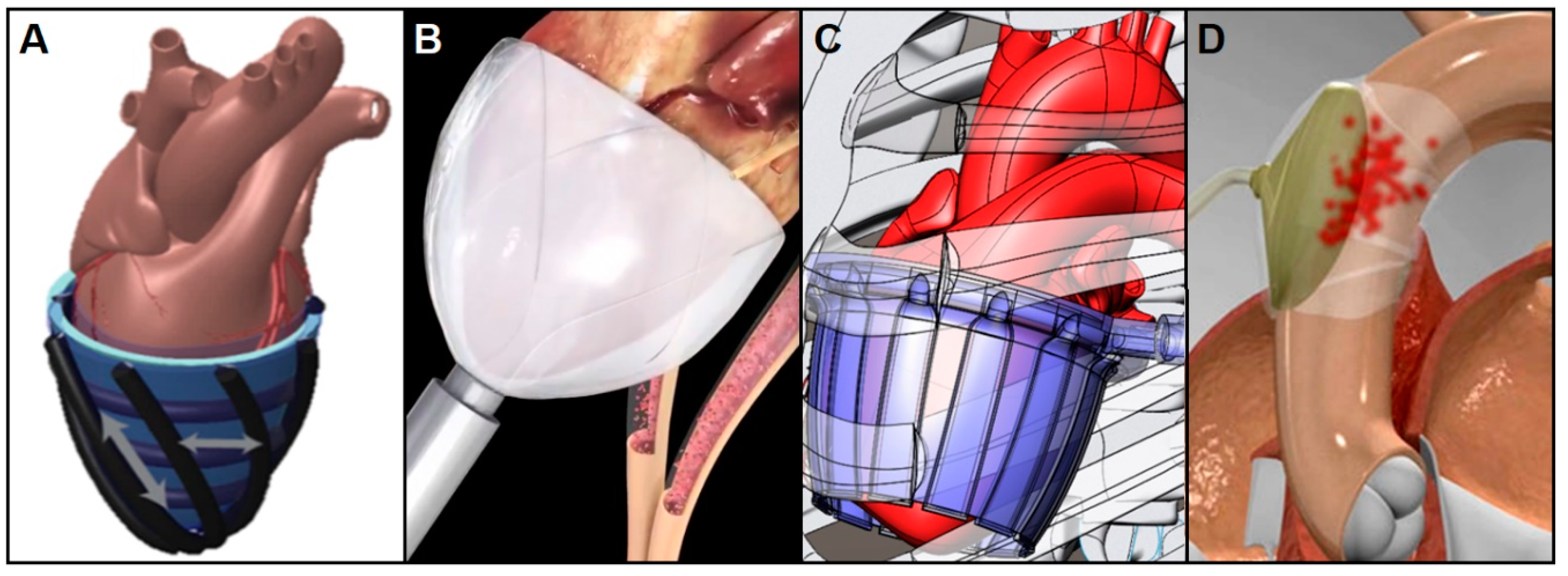
4.2. Non-Blood-Contacting Cardiac Assist Devices
4.2.1. Copulsation Direct Cardiac Compression Sleeve
4.2.2. Counterpulsation Extra-Aortic Balloon Pump
4.2.3. Passive Periventricular Restraint
4.3. CADs in Summary
4.4. Patient Management for Long-Term Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heart Failure | National Heart, Lung, and Blood Institute (NHLBI). Available online: https://www.nhlbi.nih.gov/health-topics/heart-failure (accessed on 13 November 2018).
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Mancini, D.; Colombo, P.C. Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. J. Am. Coll. Cardiol. 2015, 65, 2542–2555. [Google Scholar] [CrossRef]
- Mandras, S.A.; Crespo, J.; Patel, H.M. Innovative Application of Immunologic Principles in Heart Transplantation. Ochsner J. 2010, 10, 231–235. [Google Scholar]
- Lahpor, J.R. State of the art: Implantable ventricular assist devices. Curr. Opin. Organ Transplant. 2009, 14, 554–559. [Google Scholar] [CrossRef]
- Boyle, A. Current status of cardiac transplantation and mechanical circulatory support. Curr. Heart Fail. Rep. 2009, 6, 28–33. [Google Scholar] [CrossRef]
- Birks, E.J. A Changing Trend Toward Destination Therapy. Tex. Heart Inst. J. 2011, 38, 552–554. [Google Scholar]
- Susen, S.; Rauch, A.; Van Belle, E.; Vincentelli, A.; Lenting, P.J. Circulatory support devices: Fundamental aspects and clinical management of bleeding and thrombosis. J. Thromb. Haemost. 2015, 13, 1757–1767. [Google Scholar] [CrossRef]
- Xie, A.; Phan, K.; Yan, T.D. Durability of continuous-flow left ventricular assist devices: A systematic review. Ann. Cardiothorac. Surg. 2014, 3, 547–556. [Google Scholar]
- Kilic, A.; Emani, S.; Sai-Sudhakar, C.B.; Higgins, R.S.D.; Whitson, B.A. Donor selection in heart transplantation. J. Thorac. Dis. 2014, 6, 1097–1104. [Google Scholar]
- Holley, C.T.; Harvey, L.; John, R. Left ventricular assist devices as a bridge to cardiac transplantation. J. Thorac. Dis. 2014, 6, 1110–1119. [Google Scholar]
- Melvin, D.B.; Schima, H.; Losert, U.M.; Wolner, E. Long-Term Ventricular Wall Actuation: Can and Should It Be Systematically Explored? Artif. Organs 1996, 20, 63–68. [Google Scholar] [CrossRef]
- Trumble, D.R.; McGregor, W.E.; Kerckhoffs, R.C.P.; Waldman, L.K. Cardiac assist with a twist: Apical torsion as a means to improve failing heart function. J. Biomech. Eng. 2011, 133, 101003. [Google Scholar] [CrossRef]
- Stewart, G.C.; Givertz, M.M. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation 2012, 125, 1304–1315. [Google Scholar] [CrossRef]
- Anstadt, G.L.; Schiff, P.; Baue, A.E. Prolonged circulatory support by direct mechanical ventricular assistance. Trans. Am. Soc. Artif. Intern. Organs 1966, 12, 72–79. [Google Scholar]
- Whitson, B.A. Surgical implant techniques of left ventricular assist devices: An overview of acute and durable devices. J. Thorac. Dis. 2015, 7, 2097–2101. [Google Scholar]
- Kilic, A. The future of left ventricular assist devices. J. Thorac. Dis. 2015, 7, 2188–2193. [Google Scholar]
- Prinzing, A.; Herold, U.; Berkefeld, A.; Krane, M.; Lange, R.; Voss, B. Left ventricular assist devices—Current state and perspectives. J. Thorac. Dis. 2016, 8, E660–E666. [Google Scholar] [CrossRef]
- Cook, J.A.; Shah, K.B.; Quader, M.A.; Cooke, R.H.; Kasirajan, V.; Rago, K.K.; Smallfield, M.C.; Tchoukina, I.; Tang, D.G. The total artificial heart. J. Thorac. Dis. 2015, 7, 2172–2180. [Google Scholar]
- Englert, J.A.R.; Davis, J.A.; Krim, S.R. Mechanical Circulatory Support for the Failing Heart: Continuous-Flow Left Ventricular Assist Devices. Ochsner J. 2016, 16, 263–269. [Google Scholar]
- Shah, S.P.; Mehra, M.R. Durable left ventricular assist device therapy in advanced heart failure: Patient selection and clinical outcomes. Indian Heart J. 2016, 68, S45–S51. [Google Scholar] [CrossRef]
- Lee, L.S.; Ghanta, R.K.; Mokashi, S.A.; Coelho-Filho, O.; Kwong, R.Y.; Bolman, R.M.; Chen, F.Y. Ventricular restraint therapy for heart failure: The right ventricle is different from the left ventricle. J. Thorac. Cardiovasc. Surg. 2010, 139, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Schmack, B.; Weymann, A.; Popov, A.F.; Patil, N.P.; Sabashnikov, A.; Kremer, J.; Farag, M.; Brcic, A.; Lichtenstern, C.; Karck, M.; et al. Concurrent Left Ventricular Assist Device (LVAD) Implantation and Percutaneous Temporary RVAD Support via CardiacAssist Protek-Duo TandemHeart to Preempt Right Heart Failure. Med. Sci. Monit. Basic Res. 2016, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Chair, S.Y.; Yu, D.; Ng, M.T.; Wang, Q.; Cheng, H.Y.; Wong, E.; Sit, J. Evolvement of left ventricular assist device: The implications on heart failure management. J. Geriatr. Cardiol. JGC 2016, 13, 425–430. [Google Scholar] [PubMed]
- Vetter, H.O.; Kaulbach, H.G.; Schmitz, C.; Forst, A.; Uberfuhr, P.; Kreuzer, E.; Pfeiffer, M.; Brenner, P.; Dewald, O.; Reichart, B. Experience with the Novacor left ventricular assist system as a bridge to cardiac transplantation, including the new wearable system. J. Thorac. Cardiovasc. Surg. 1995, 109, 74–80. [Google Scholar] [CrossRef]
- Rodriguez, L.E.; Suarez, E.E.; Loebe, M.; Bruckner, B.A. Ventricular Assist Devices (VAD) Therapy: New Technology, New Hope? Methodist DeBakey Cardiovasc. J. 2013, 9, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.D.; Timms, D.; Gaddum, N.; Mason, D.G.; Fraser, J.F. Biventricular assist devices: A technical review. Ann. Biomed. Eng. 2011, 39, 2313–2328. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.S.; Grandin, E.W.; Brinkley, M.; Kapur, N.K.; Pham, D.T.; Ruthazer, R.; Rame, J.E.; Atluri, P.; Birati, E.Y.; Oliveira, G.H.; et al. Early Right Ventricular Assist Device Use in Patients Undergoing Continuous-Flow Left Ventricular Assist Device Implantation: Incidence and Risk Factors from the Interagency Registry for Mechanically Assisted Circulatory Support. Circ. Heart Fail. 2017, 10, e003863. [Google Scholar] [CrossRef]
- Haneya, A.; Philipp, A.; Puehler, T.; Rupprecht, L.; Kobuch, R.; Hilker, M.; Schmid, C.; Hirt, S.W. Temporary percutaneous right ventricular support using a centrifugal pump in patients with postoperative acute refractory right ventricular failure after left ventricular assist device implantation. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012, 41, 219–223. [Google Scholar] [CrossRef]
- Rigatelli, G.; Santini, F.; Faggian, G. Past and present of cardiocirculatory assist devices: A comprehensive critical review. J. Geriatr. Cardiol. JGC 2012, 9, 389–400. [Google Scholar]
- Gerosa, G.; Scuri, S.; Iop, L.; Torregrossa, G. Present and future perspectives on total artificial hearts. Ann. Cardiothorac. Surg. 2014, 3, 595–602. [Google Scholar]
- HeartMate II Left Ventricular Assist Device (LVAD) Fact Sheet. Available online: https://www.thoratec.com/downloads/heartmate-II-fact-sheet-b100-0812-final.pdf (accessed on 14 December 2018).
- HeartMate IITM LVAD—Used in Over 25,000 Patients. Available online: https://www.heartmate.com/healthcare-provider/heartmate-ii-lvad (accessed on 14 December 2018).
- Garbade, J.; Bittner, H.B.; Barten, M.J.; Mohr, F.-W. Current trends in implantable left ventricular assist devices. Cardiol. Res. Pract. 2011, 2011, 290561. [Google Scholar] [CrossRef]
- Recently-Approved Devices—HeartWareTM HVADTM—P100047/S090. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm581473.htm (accessed on 14 December 2018).
- HeartMate 3 Left Ventricular Assist System (LVAS)—P160054/S008. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm624155.htm (accessed on 14 December 2018).
- Thoratec CentriMag Blood Pump Fact Sheet. Available online: CentriMag_Product_Fact_Sheet111109.pdf (accessed on 5 December 2018).
- Timeline: First in VADS and Mechanical Assist Devices | Columbia University Department of Surgery. Available online: http://columbiasurgery.org/lvad/timeline-first-vads-and-mechanical-assist-devices (accessed on 5 December 2018).
- Part II.9—The Translational Pathway for Mechanical Circulatory Support | ISCTR. Available online: https://isctr.org/chapter-ii-9/ (accessed on 5 December 2018).
- Doty, D. Ventricular Assist Device and Destination Therapy Candidates from Preoperative Selection Through End of Hospitalization. Crit. Care Nurs. Clin. 2015, 27, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Griffith, K.; Jenkins, E.; Stulak, J.; Paugh, T.; Pagani, F. Long-term use of the CentriMag® Ventricular Assist System as a right ventricular assist device: A case report. Perfusion 2012, 27, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Maymi, M.A.; Gupta, D.; Barras, W.E.; Philip, J.; Fricker, F.J.; Bleiweis, M.S.; Udassi, J.P. Pediatric Mechanical Circulatory Support. JPCC 2014, 1, 186–192. [Google Scholar]
- Heart Views. Official Journal of Gulf Heart Association. Available online: http://www.heartviews.org/viewimage.asp?img=HeartViews_2007_8_2_70_63742_f2.jpg (accessed on 5 December 2018).
- Agnetti, G.; Piepoli, M.F.; Siniscalchi, G.; Nicolini, F. New Insights in the Diagnosis and Treatment of Heart Failure. BioMed Res. Int. 2015, 2015, 265260. [Google Scholar] [CrossRef]
- Park, W.H.; Seo, Y.G.; Sung, J.D. Exercise Therapy for an Older Patient with Left Ventricular Assist Device. Ann. Rehabil. Med. 2014, 38, 396–400. [Google Scholar] [CrossRef] [PubMed]
- HeartMate 3 Heart Pump Approved for Patients Not Eligible for Transplant. Available online: https://www.medgadget.com/2018/10/heartmate-3-heart-pump-approved-for-patients-not-eligible-for-transplant.html (accessed on 14 December 2018).
- Miller, L.W.; Guglin, M. Patient selection for ventricular assist devices: A moving target. J. Am. Coll. Cardiol. 2013, 61, 1209–1221. [Google Scholar] [CrossRef]
- UW Health. Ventricular Assist Device (VAD) Patient Selection Criteria. Available online: https://www.uwhealth.org/heart-cardiovascular/ventricular-assist-device-vad-patient-selection-criteria/39748 (accessed on 13 November 2018).
- Trinsey, A. Extracorporeal membrane oxygenation: A review. Nurs. Crit. Care 2017, 12, 16–23. [Google Scholar] [CrossRef]
- Extracorporeal Membrane Oxygenation (ECMO)| Boston Children’s Hospital. Available online: http://www.childrenshospital.org/centers-and-services/programs/a-_-e/extracoporeal-membrane-oxygenation-ecmo-program/frequently-asked-questions-for-parents (accessed on 13 November 2018).
- CDRH Transparency—Reclassification. Available online: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/ucm378724.htm (accessed on 14 December 2018).
- Mosier, J.M.; Kelsey, M.; Raz, Y.; Gunnerson, K.J.; Meyer, R.; Hypes, C.D.; Malo, J.; Whitmore, S.P.; Spaite, D.W. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: History, current applications, and future directions. Crit. Care 2015, 19, 431. [Google Scholar] [CrossRef]
- Rossiter-Thornton, M.; Arun, V.; Forrest, A.P.; Bayfield, M.S.; Wilson, M.K. Left Ventricular Support with the Impella® LP 5.0 for Cardiogenic Shock Following Cardiac Surgery. Heart Lung Circ. 2008, 17, 243–245. [Google Scholar] [CrossRef]
- Naidu, S.S. Novel Percutaneous Cardiac Assist Devices: The Science of and Indications for Hemodynamic Support. Circulation 2011, 123, 533–543. [Google Scholar] [CrossRef] [PubMed]
- ABIOMED, Inc. Abiomed Receives Approval for Expanded FDA Indication for Cardiomyopathy with Cardiogenic Shock. Available online: http://investors.abiomed.com/news-releases/news-release-details/abiomed-receives-approval-expanded-fda-indication-cardiomyopathy (accessed on 14 December 2018).
- ABIOMED, Inc. Abiomed Receives Approval for Expanded FDA Indication for High Risk Percutaneous Coronary Intervention (PCI) Procedures. Available online: http://investors.abiomed.com/news-releases/news-release-details/abiomed-receives-approval-expanded-fda-indication-high-risk-0 (accessed on 14 December 2018).
- U.S. Food and Drug Administration. Recently-Approved Devices—Impella Ventricular Support Systems—P140003/S018. Available online: https://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm598801.htm (accessed on 14 December 2018).
- ABIOMED. Impella Ventricular Support Systems for Use During Cardiogenic Shock and High-Risk PIC. Available online: http://abiomed-private.s3.amazonaws.com/assets/files/154202963952329325f54536fc43bdfb5343fbf029.pdf (accessed on 14 December 2018).
- Parissis, H.; Graham, V.; Lampridis, S.; Lau, M.; Hooks, G.; Mhandu, P.C. IABP: History-evolution-pathophysiology-indications: What we need to know. J. Cardiothorac. Surg. 2016, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K790241 (accessed on 5 December 2018).
- Counterpulsation Applied. An Introduction to Intra-Aortic Balloon Pumping. TELEFLEX. Available online: MC-000363_Counterpulsation-Manual.pdf (accessed on 5 December 2018).
- Thoratec Corporate Fact Sheet. Available online: GL-THO-03150196-Thoratec Corporate Fact Sheet-FINAL.pdf (accessed on 5 December 2018).
- 2nd Generation CentriMag Primary Console Operating Manual. Thoratec. Available online: PL-0047_Rev_08_2nd_Generation_CentriMag_System_Operating_Manual_(US).pdf (accessed on 5 December 2018).
- ECMO. The Dept of Anaesthesia and Intensive Care. Available online: https://www.aic.cuhk.edu.hk/web8/ecmo.htm (accessed on 5 December 2018).
- Samuels, L.E.; Casanova-Ghosh, E.; Droogan, C. Cardiogenic shock associated with loco-regional anesthesia rescued with left ventricular assist device implantation. J. Cardiothorac. Surg. 2010, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Intra-aortic Balloon Pump (IABP) Market 2017—Maquet (Getinge Group), Teleflex Incorporated—openPR. Available online: https://www.openpr.com/news/706293/Intra-aortic-Balloon-Pump-IABP-Market-2017-Maquet-Getinge-Group-Teleflex-Incorporated.html (accessed on 18 December 2018).
- Kazui, T.; Tran, P.L.; Echeverria, A.; Jerman, C.F.; Iwanski, J.; Kim, S.S.; Smith, R.G.; Khalpev, Z.I. Minimally invasive approach for percutaneous CentriMag right ventricular assist device support using a single PROTEKDuo Cannula. J. Cardiothorac. Surg. 2016, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Thoratec Coporation. HeartMate 3 Left Ventricular Assist System Instructions for Use. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160054C.pdf (accessed on 14 December 2018).
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.; Milano, C.A.; Patel, C.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- MOMENTUM 3 Data Shows Improved Survival for HeartMate 3 Heart Pump. Cardiac Rhythm News, 13 March 2018.
- HeartWare Ventricular Assist System Instructions for Use. Available online: ifu00001_rev_15.pdf (accessed on 14 December 2018).
- Villa, C.R.; Morales, D.L.S. The Total Artificial Heart in End-Stage Congenital Heart Disease. Front. Physiol. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- SynCardia. Total Artificial Heart Etiologies; Outcomes. 2018. Available online: https://syncardia.com/clinicians/clinical-resources/patients-outcomes-experience/ (accessed on 5 December 2018).
- Bartfay, S.; Dellgren, G.; Dahlberg, P.; Hallhagen, S.; Redfors, B.; Karason, K. Berlin Heart Excor as Bridge to Heart Transplantation. A Versatile Option in Patients Not Suitable for a Continuous Flow LVAD. J. Heart Lung Transpl. 2018, 37, S271. [Google Scholar] [CrossRef]
- EXCOR Pediatric VAD Instructions for Use. Available online: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM537702.pdf (accessed on 5 December 2018).
- Bockeria, L.A.; Bockeria, O.L.; Le, T.G.; Satyukova, A.S.; Glushko, L.A.; Shvartz, V.A. Miniature Rotary Blood Pumps for Use in Pediatric Cardiac Surgery. Biomed. Eng. 2017, 50, 291–295. [Google Scholar] [CrossRef]
- Lima, B.; Bansal, A.; Abraham, J.; Rich, J.D.; Lee, S.S.; Soleimani, B.; Katz, J.N.; Kilic, A.; Young, J.S.; Patel, C.B.; et al. Controversies and Challenges of Ventricular Assist Device Therapy. Am. J. Cardiol. 2018, 121, 1219–1224. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Nienaber, J.J.C.; Kusne, S.; Riaz, T.; Walker, R.C.; Baddour, L.M.; Wright, A.J.; Park, S.J.; Vikram, H.R.; Keating, M.R.; Arabia, F.A.; et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Leuck, A.-M. Left ventricular assist device driveline infections: Recent advances and future goals. J. Thorac. Dis. 2015, 7, 2151–2157. [Google Scholar] [PubMed]
- Harvey, L.; Holley, C.T.; John, R. Gastrointestinal bleed after left ventricular assist device implantation: Incidence, management, and prevention. Ann. Cardiothorac. Surg. 2014, 3, 475–479. [Google Scholar] [PubMed]
- Cushing, K.; Kushnir, V. Gastrointestinal Bleeding Following LVAD Placement from Top to Bottom. Dig. Dis. Sci. 2016, 61, 1440–1447. [Google Scholar] [CrossRef]
- Ventricular Assist Device Exit Site Care—PPT Video Online Download. Available online: https://slideplayer.com/slide/1442089/ (accessed on 18 December 2018).
- Uriel, N.; Morrison, K.A.; Garan, A.R.; Kato, T.S.; Yuzefpolskaya, M.; Latif, F.; Restaino, S.W.; Mancini, D.M.; Flannery, M.; Takayama, H.; et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: The Columbia ramp study. J. Am. Coll. Cardiol. 2012, 60, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Lünse, S.; Höhn, J.; Simon, P.; Heidecke, C.-D.; Glitsch, A. Dieulafoy-Like Lesion at the Brim of a Gastric Diverticulum: A Very Rare Cause of Gastrointestinal Bleeding. J. Gastrointest. Dig. Syst. 2018, 8, 572. [Google Scholar] [CrossRef]
- Magovern, G.J.; Park, S.B.; Magovern, G.J.; Christlieb, I.Y.; Kao, R.L. Mechanical Circulatory Assist Devices. Tex. Heart Inst. J. 1987, 14, 276–283. [Google Scholar] [PubMed]
- Tchantchaleishvili, V.; Bush, B.S.; Swartz, M.F.; Day, S.W.; Massey, H.T. Plutonium-238: An ideal power source for intracorporeal ventricular assist devices? ASAIO J. 2012, 58, 550–553. [Google Scholar] [CrossRef] [PubMed]
- LaForge, D.; Portner, P. An efficient pulsed-solenoid powered cardiac assist system. IEEE Trans. Magn. 1982, 18, 1481–1483. [Google Scholar] [CrossRef]
- Farrar, D.J.; Buck, K.E.; Coulter, J.H.; Kupa, E.J. Portable pneumatic biventricular driver for the Thoratec ventricular assist device. ASAIO J. 1997, 43, M631–M634. [Google Scholar] [CrossRef]
- Najjar, E.; Kristensen, A.H.; Thorvaldsen, T.; Hubbert, L.; Svenarud, P.; Dalen, M.; Broberg, A.M.; Lund, L.H. Controller and battery changes due to technical problems related to the HVAD® left ventricular assist device—A single center experience. J. Cardiothorac. Surg. 2018, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Strand, J.J.; Wordingham, S.E.; Stulak, J.M.; Luckhardt, A.J.; Swetz, K.M. Dying with a Left Ventricular Assist Device as Destination Therapy. Circ. Heart Fail. 2016, 9, e003096. [Google Scholar] [CrossRef] [PubMed]
- Bocan, K.N.; Sejdić, E. Adaptive Transcutaneous Power Transfer to Implantable Devices: A State of the Art Review. Sensors 2016, 16, 393. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Smith, J.R.; Bonde, P. Energy Transmission and Power Sources for Mechanical Circulatory Support Devices to Achieve Total Implantability. Ann. Thorac. Surg. 2014, 97, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Basaeri, H.; Christensen, D.B.; Roundy, S. A review of acoustic power transfer for bio-medical implants. Smart Mater. Struct. 2016, 25, 123001. [Google Scholar] [CrossRef]
- Denisov, A.; Yeatman, E.M. Ultrasonic vs. Inductive Power Delivery for Miniature Biomedical Implants. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 84–89. [Google Scholar] [CrossRef]
- Trumble, D.R.; Webster, J.G. Artificial Hearts and Cardiac Assist Devices: Performance, Control, and Power Delivery. In Wiley Encyclopedia of Electrical and Electronics Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- What are the Limitations of the AbioCor TAH for the Treatment of Heart Failure? Available online: https://www.medscape.com/answers/163062-86476/what-are-the-limitations-of-the-abiocor-tah-for-the-treatment-of-heart-failure (accessed on 5 December 2018).
- Lionheart Recipient at Penn State Hershey Medical Center First US Patient to Head Home with Device. EurekAlert! Available online: http://www.eurekalert.org/pub_releases/2003-06/ps-lra062403.php (accessed on 5 December 2018).
- Puers, R.; Vandevoorde, G. Recent progress on transcutaneous energy transfer for total artificial heart systems. Artif. Organs 2001, 25, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Enssle, A. Transcutaneous Energy Transfer for Left Ventricular Assist Devices: Create the Future Design Contest. Available online: http://contest.techbriefs.com/2016/entries/medical/7069 (accessed on 5 December 2018).
- Dissanayake, T.; Budgett, D.; Hu, A.P.; Malpas, S.; Bennet, L. Transcutaneous Energy Transfer System for Powering Implantable Biomedical Devices. In 13th International Conference on Biomedical Engineering; Lim, C.T., Goh, J.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 235–239. [Google Scholar]
- Trumble, D.R.; Magovern, J.A. A muscle-powered energy delivery system and means for chronic in vivo testing. J. Appl. Physiol. (1985) 1999, 86, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Trumble, D.R. A Muscle-Powered Counterpulsation Device for Tether-Free Cardiac Support: Form and Function. J. Med. Devices 2016, 10, 20903. [Google Scholar] [CrossRef]
- Trumble, D.; Magovern, J.A. Linear Muscle Power for Cardiac Support: A Progress Report. Basic Appl. Myol. BAM 1999, 9, 175–186. [Google Scholar]
- Salmons, S.; Jarvis, J. The Working Capacity of Skeletal Muscle Transformed for Use in a Cardiac Assist Role; Futura Publishing Co.: Mount Kisco, NY, USA, 1990; pp. 89–104. [Google Scholar]
- Muscles connecting the upper extremity to the vertebral column. In Gray’s Anatomy of the Human Body, 20th U.S. ed.; Lea & Febiger Publishing: Philadelphia, PA, USA, 1918.
- Han, J.; Kubala, M.; Trumble, D.R. Design of a Muscle-Powered Soft Robotic Bi-VAD for Long-Term Circulatory Support; American Society of Mechanical Engineers: New York, NY, USA, 2018; p. V001T01A003. [Google Scholar] [CrossRef]
- Yozu, R.; Ueda, T.; Inoue, Y.; Omoto, T.; Yoshito, H.; Kanda, K.; Tsutsui, N.; Kawada, S.; Mitsumaru, A. Experimental Study of Combination of Extraaortic Balloon Counterpulsation (EABC) and Ventricular Assist Cup (VAC) to Acute Heart Failure. ASAIO J. 1996, 42, 36. [Google Scholar] [CrossRef]
- Lowe, J.E.; Anstadt, M.P.; Van, T.P.; Smith, P.K.; Hendry, P.J.; Plunkett, M.D.; Anstadt, G.L. First successful bridge to cardiac transplantation using direct mechanical ventricular actuation. Ann. Thorac. Surg. 1991, 52, 1237–1243. [Google Scholar] [CrossRef]
- Oz, M.C.; Artrip, J.H.; Burkhoff, D. Direct cardiac compression devices. J. Heart Lung Transplant. 2002, 21, 1049–1055. [Google Scholar] [CrossRef]
- Conte, J.V. Surgical Ventricular Restoration: Technique and Outcomes. Congest. Heart Fail. 2004, 10, 248–251. [Google Scholar] [CrossRef] [PubMed]
- BBC NEWS | Artificial Heart Muscle Tested. Available online: http://news.bbc.co.uk/2/hi/health/3151891.stm (accessed on 30 May 2017).
- Roche, E.T.; Horvath, M.A.; Wamala, I.; Alazmani, A.; Song, S.E.; Whyte, W.; Machaidze, Z.; Payne, C.J.; Weaver, J.C.; Fishbein, G.; et al. Soft robotic sleeve supports heart function. Sci. Transl. Med. 2017, 9, eaaf3925. [Google Scholar] [CrossRef] [PubMed]
- Corinnova. Preclinical Results. Available online: https://www.corinnova.com/preclin (accessed on 5 December 2018).
- Reesink, K.D.; Dekker, A.L.; Ommen, V.; Soemers, C.; Geskes, G.G.; Van Der Veen, F.H.; Maessen, J.G. Miniature intracardiac assist device provides more effective cardiac unloading and circulatory support during severe left heart failure than intraaortic balloon pumping. Chest 2004, 126, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Mitsumaru, A.; Yozu, R.; Tanaka, S.; Yoshito, H.; Kanda, K.; Tsutsui, N.; Kawada, S. Experimental study of combination of extraaortic balloon counterpulsation and ventricular assist cup to acute heart failure in dogs. ASAIO J. 1997, 43, 187–192. [Google Scholar] [CrossRef] [PubMed]
- C-Pulse Extra-Aortic Heart Pump Wins Approvals in Europe (2012). Available online: https://www.medgadget.com/2012/07/c-pulse-extra-aortic-heart-pump-wins-approvals-in-europe.html (accessed on 30 April 2017).
- Kwon, M.H.; Cevasco, M.; Schmitto, J.D.; Chen, F.Y. Ventricular restraint therapy for heart failure: A review, summary of state of the art, and future directions. J. Thorac. Cardiovasc. Surg. 2012, 144, 771–777.e1. [Google Scholar] [CrossRef]
- Klodell, C.T.; Aranda, J.M.; McGiffin, D.C.; Ravburn, B.K.; Sun, B.; Abraham, W.T.; Pae, W.E.; Boehmer, J.P.; Klein, H.; Huth, C. Worldwide surgical experience with the Paracor HeartNet cardiac restraint device. J. Thorac. Cardiovasc. Surg. 2008, 135, 188–195. [Google Scholar] [CrossRef]
- Long, J.W.; Kfoury, A.G.; Slaughter, M.S.; Silver, M.; Milano, C.; Rogers, J.; Delgado, R.; Frazier, O.H. Long-term destination therapy with the HeartMate XVE left ventricular assist device: Improved outcomes since the REMATCH study. Congest. Heart Fail. 2005, 11, 133–138. [Google Scholar] [CrossRef]
- Jaquiss, R.D.B.; Humpl, T.; Canter, C.E.; Morales, D.L.; Rosenthal, D.N.; Fraser, C.D. Postapproval Outcomes: The Berlin Heart EXCOR Pediatric in North America. ASAIO J. 2017, 63, 193. [Google Scholar] [CrossRef]
- Pennington, D.G.; Lohmann, D.P. Novacor LVAS Implantation Technique. Oper. Tech. Thorac. Cardiovasc. Surg. 1999, 4, 318–329. [Google Scholar] [CrossRef]
- Thoratec PVAD & Thoratec IVAD Fact Sheet. Available online: https://www.thoratec.com/downloads/PVAD%20IVAD%20Fact%20Sheet-B100-0412-FINAL.pdf (accessed on 5 December 2018).
- Lad, V.; Elhenawy, A.; Harwood, S.; Maclver, J.; Badiwala, M.; Vallelonga, M.; Yau, T.M.; Cusimano, R.J.; Delgado, D.H.; Ross, H.J.; et al. Mechanical circulatory support with the ABIOMED BVS 5000: The Toronto General Hospital experience. Can. J. Cardiol. 2010, 26, 467–470. [Google Scholar] [CrossRef]
- Levinson, M.M.; Smith, R.G.; Cork, R.C.; Gallo, J.; Emery, R.W.; Icenogle, T.B.; Ott, R.A.; Burns, G.L.; Copeland, J.G. Thromboembolic complications of the Jarvik-7 total artificial heart: Case report. Artif. Organs 1986, 10, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Jarvik Heart Inc. Robert Jarvik, MD on the Jarvik-7. 2016. Available online: https://www.jarvikheart.com/history/robert-jarvik-on-the-jarvik-7/ (accessed on 5 December 2018).
- Gilotra, N.A.; Stevens, G.R. Temporary Mechanical Circulatory Support: A Review of the Options, Indications, and Outcomes. Clin. Med. Insights Cardiol. 2015, 8, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Class 2 Device Recall Jarvik 2000 Ventricular Assist System. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?ID=169598 (accessed on 5 December 2018).
- Schroder, J.N.; Milano, C.A. A tale of two centrifugal left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2017, 154, 850–852. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Recently-Approved Devices—HeartMate 3 Left Ventricular Assist System (LVAS) - P160054. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm581474.htm (accessed on 14 December 2018).
- Katz, J.N.; Waters, S.B.; Hollis, I.B.; Chang, P.P. Advanced Therapies for End-Stage Heart Failure. Curr. Cardiol. Rev. 2015, 11, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Melvin, D.B. Device-Induced Ventricular Geometric Remodeling: Appraisal of Critical Issues. J. Card. Surg. 2001, 16, 34–39. [Google Scholar] [CrossRef]
- Milano, C.A.; Rogers, J.G.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Mokadam, N.A.; Mahr, C.; Miller, J.S.; Markham, D.W.; et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018, 6, 792–802. [Google Scholar] [CrossRef]
- IOM; National Academy of Engineering. Healthcare System Complexities, Impediments, and Failures; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
| Category | Product | Type of Support | Duration of Support | Advantages | Limitations |
|---|---|---|---|---|---|
| Early Methods of Cardiac Support | ECMO | BiVAD | Short-term | Extracorporeal artificial heart-lung bypass for acute support | Upper body hypoxia, LV dilatation, thrombosis |
| IABP | Descending Aorta | Short-term | Increases myocardial oxygen perfusion and cardiac output | Thrombosis, aortic rupture, arterial flow obstruction | |
| 1st Generation—Pulsatile Flow | HeartMate XVE | LVAD | Long-term | Improved enough to receive FDA approval for DT in 2003 and CE mark in 2004 | Bulky and Heavy |
| Berlin Heart EXCOR | BiVAD | BTT | Pediatric uses with various pump sizes | Not completely implanted | |
| Novacor LVAS | LVAD | BTT | Longer durability and higher reliability at the time | Still large and bulky with three extracorporeal hardware | |
| HeartMate I | LVAD | BTT/BTR | Introduced textured blood contacting surface to reduce thrombosis | Large size and complications like bleeding and driveline infection | |
| Thoratec PVAD | Uni or BiVAD | Short-term | Weeks to months support for patient’s home discharge post-cardiotomy | Common side effects from pneumatic driveline | |
| ABioMed BVS 5000 | Uni or BiVAD | Short-term | Resuscitate critically ill patients for acute stabilization | Risks of bleeding, coagulopathy, and end-organ damage | |
| Jarvik 7 | TAH | Long-term | World’s first permanent total artificial heart; more used as a BTT now | Thrombotic deposition and cerebral embolic events | |
| AbioCor TAH | TAH | Long-term | Uses TET technology without aid of wires | Discomfort with TET system, bulkiness, clotting at device surfaces | |
| ABioMed Impella RP | IVC-to-PA | Short-term | First and only FDA approved percutaneous heart pump for RV support | Thrombotic vascular complications and hemolysis | |
| Tandem Heart | LA-to-FA | Short-term | Significantly reduces preload and augments cardiac output | Risks of cannula migration, thromboembolism, and cardiac tamponade | |
| 2nd Generation—Continuous Axial Flow | HeartMate II | LVAD | Long-term | FDA approval for DT, Improved survival rate and patient quality of life, Most commonly installed LVAD in 2000s | Bleeding, cardiac arrhythmia, infection, sepsis |
| Heart Assist 5 | LVAD | Long-term | Small size and weight, CE mark approved remote monitoring system in 2012 | Bleeding, thrombosis, infections | |
| Jarvik 2000 | LVAD | Long-term | Pediatric uses, FDA approval for trial using as a DT in 2012 | Class 2 device recall for a potential external cable damage in 2018 | |
| ABioMed Impella | FA-to-LV | Short-term | Minimally invasive, Varying sizes | Hemolysis, aortic valve injury, infection | |
| 3rd Generation—Continuous Centrifugal Flow | HeartWare HVAD | LVAD | Long-term | Small size, magnetically levitated rotor, FDA approval for DT in 2017 | Risks of infection, bleeding, arrhythmia, stroke |
| HeartMate III | LVAD | Long-term | Magnetically levitated rotor, FDA approval for DT in 2018 | Risks of infection, bleeding, arrhythmia, stroke | |
| DuraHeart | LVAD | Long-term | Favorable clinical outcomes as BTT in Japan and Europe | Hemolysis, thromboembolism, bleeding | |
| HeartWare MVAD | LVAD | Long-term | Miniature size for pediatric uses | Risks of infection, bleeding, and thrombosis | |
| CentriMag | Uni-VAD | Short-term | Magnetically suspended rotor for acute therapy, Minimal shear force on RBCs and hemolysis | Bleeding, infection, respiratory failure, hemolysis, neurologic dysfunction | |
| Non-blood-contacting VADs | CorInnova | Ventricular Epicardium | Potentially Long-term | Minimally invasive, Non-blood-contacting, soft material | Studies done on large animals only |
| Biomimetic DCCS | Ventricular Epicardium | Potentially Long-term | Soft material, Non-blood-contacting, compression and torsion applications | Still under development | |
| Muscled-powered DCCS | Ventricular Epicardium | Potentially Long-term | Tether-free, Non-blood-contacting, Biocompatible soft material | Still under development | |
| C-pulse Device | Ascending Aorta | Short-term | Non-blood-contacting | No longer commercially available |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Trumble, D.R. Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations. Bioengineering 2019, 6, 18. https://doi.org/10.3390/bioengineering6010018
Han J, Trumble DR. Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations. Bioengineering. 2019; 6(1):18. https://doi.org/10.3390/bioengineering6010018
Chicago/Turabian StyleHan, Jooli, and Dennis R. Trumble. 2019. "Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations" Bioengineering 6, no. 1: 18. https://doi.org/10.3390/bioengineering6010018
APA StyleHan, J., & Trumble, D. R. (2019). Cardiac Assist Devices: Early Concepts, Current Technologies, and Future Innovations. Bioengineering, 6(1), 18. https://doi.org/10.3390/bioengineering6010018





