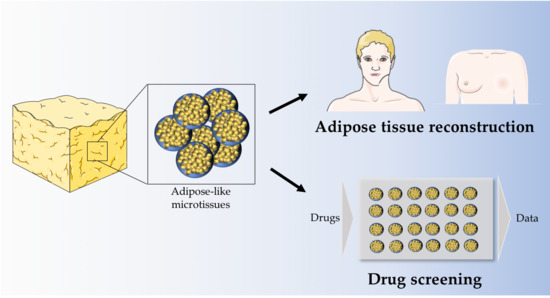
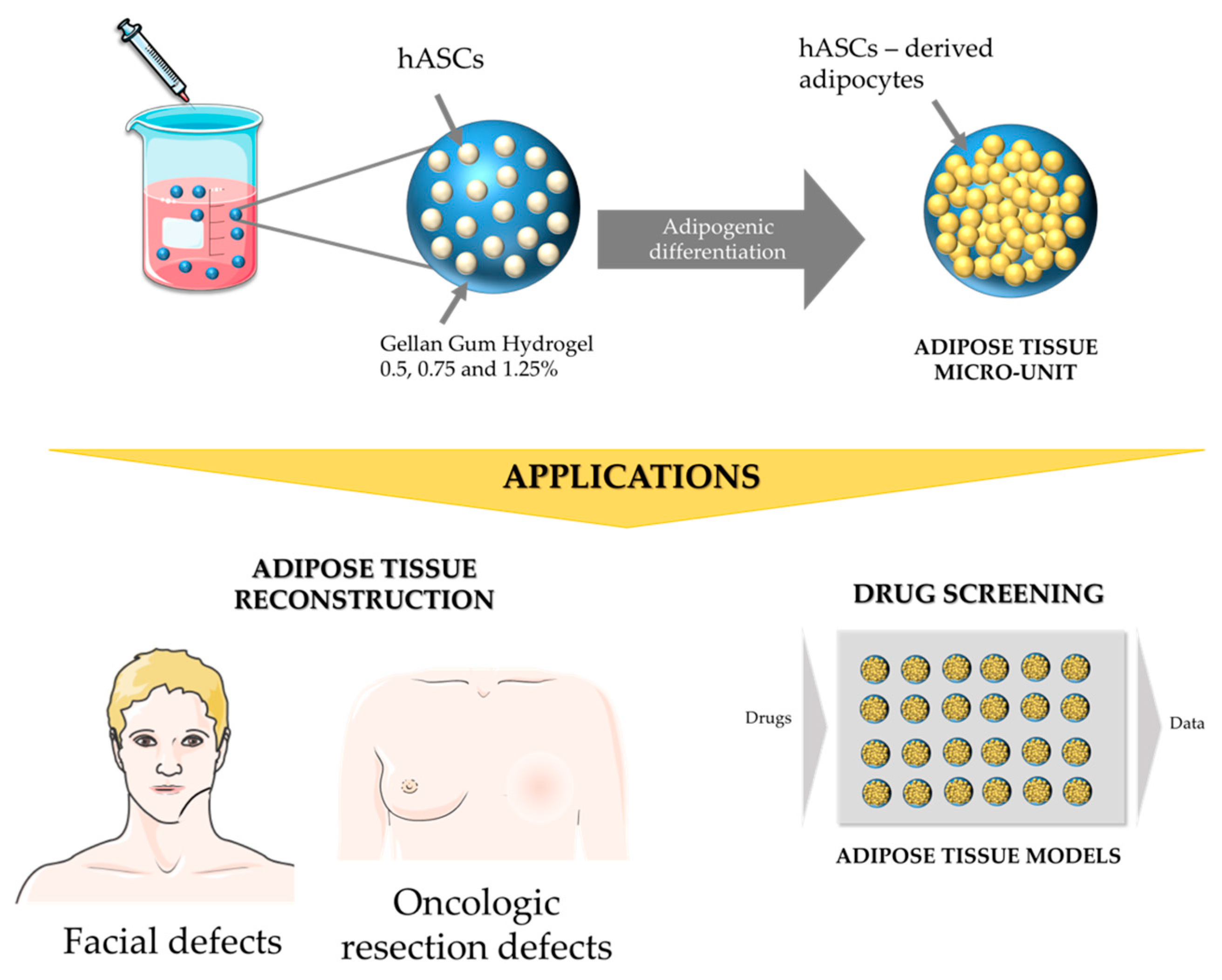
Generation of Gellan Gum-Based Adipose-Like Microtissues
Abstract
1. Introduction
2. Materials and Methods
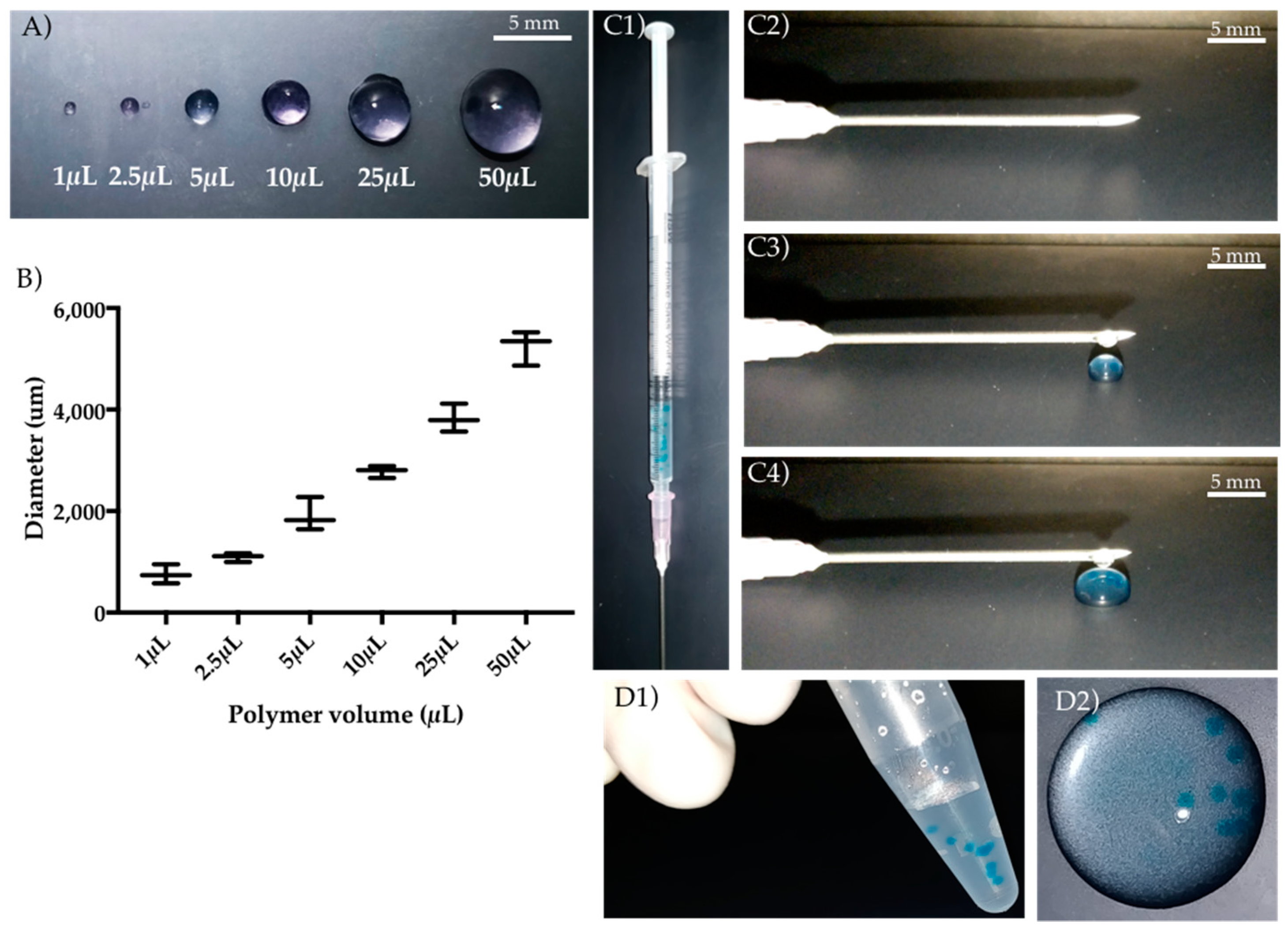
2.1. Gellan Gum Hydrogel Particles Preparation
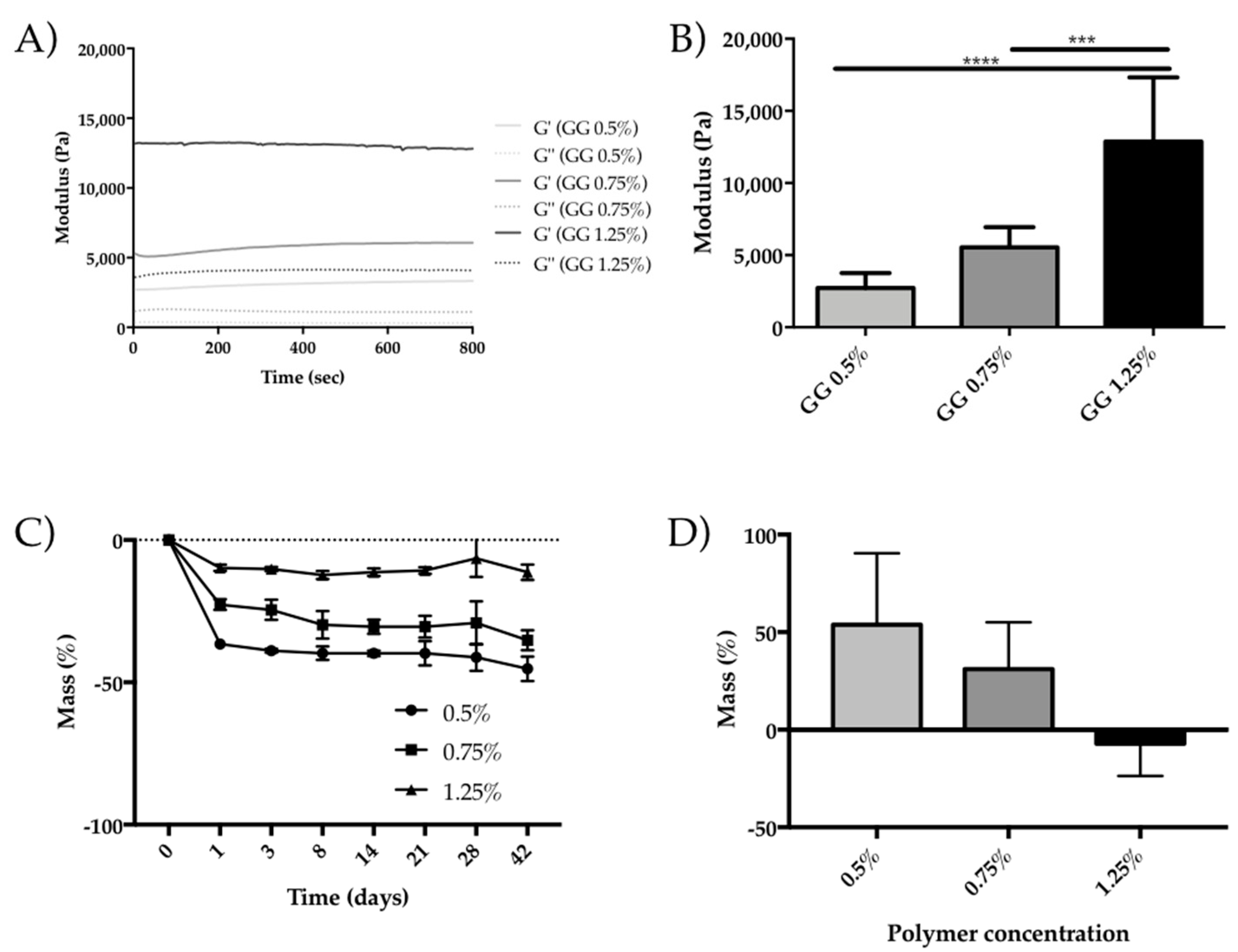
2.2. Oscillatory Rheology
2.3. Degradation
2.4. Isolation and Characterization of Human Adipose Stem Cells
2.5. Cell Encapsulation within Gellan Gum Hydrogels Particles
2.6. Adipogenic Differentiation within Gellan Gum Hydrogels Particles
2.7. Incubation with TNF Alpha and ROCK Inhibitor
2.8. Quantitative Real Time Polymerase Chain Reaction (RT-PCR)
2.9. Western Blot
2.10. Immunocytochemistry
2.11. Nile Red Staining and Quantification
2.12. Statistical Analysis
3. Results
3.1. GG Hydrogel Particles Properties
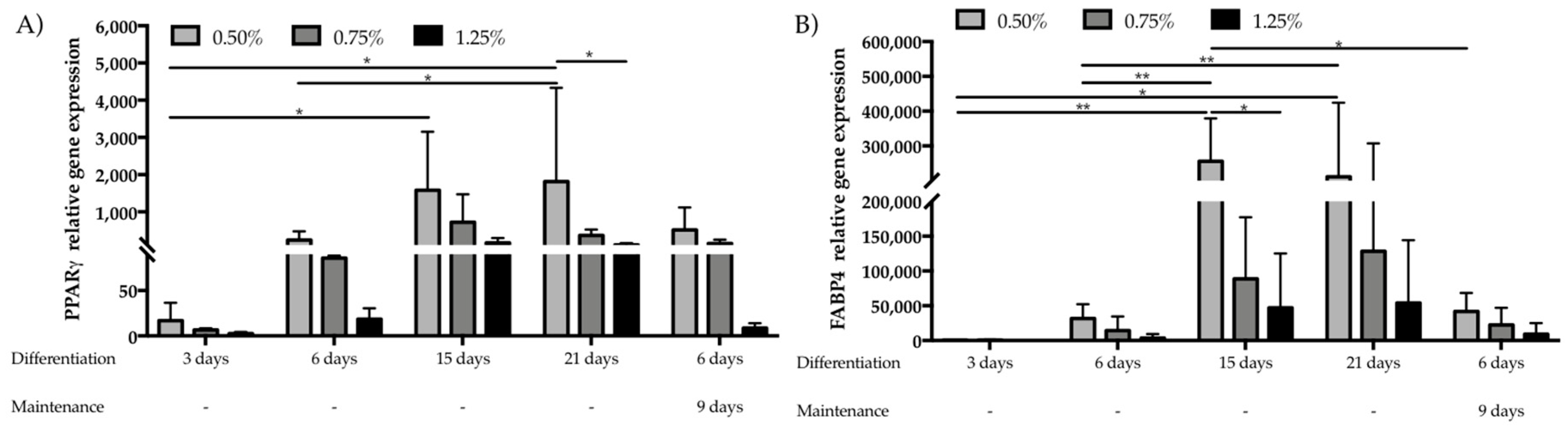
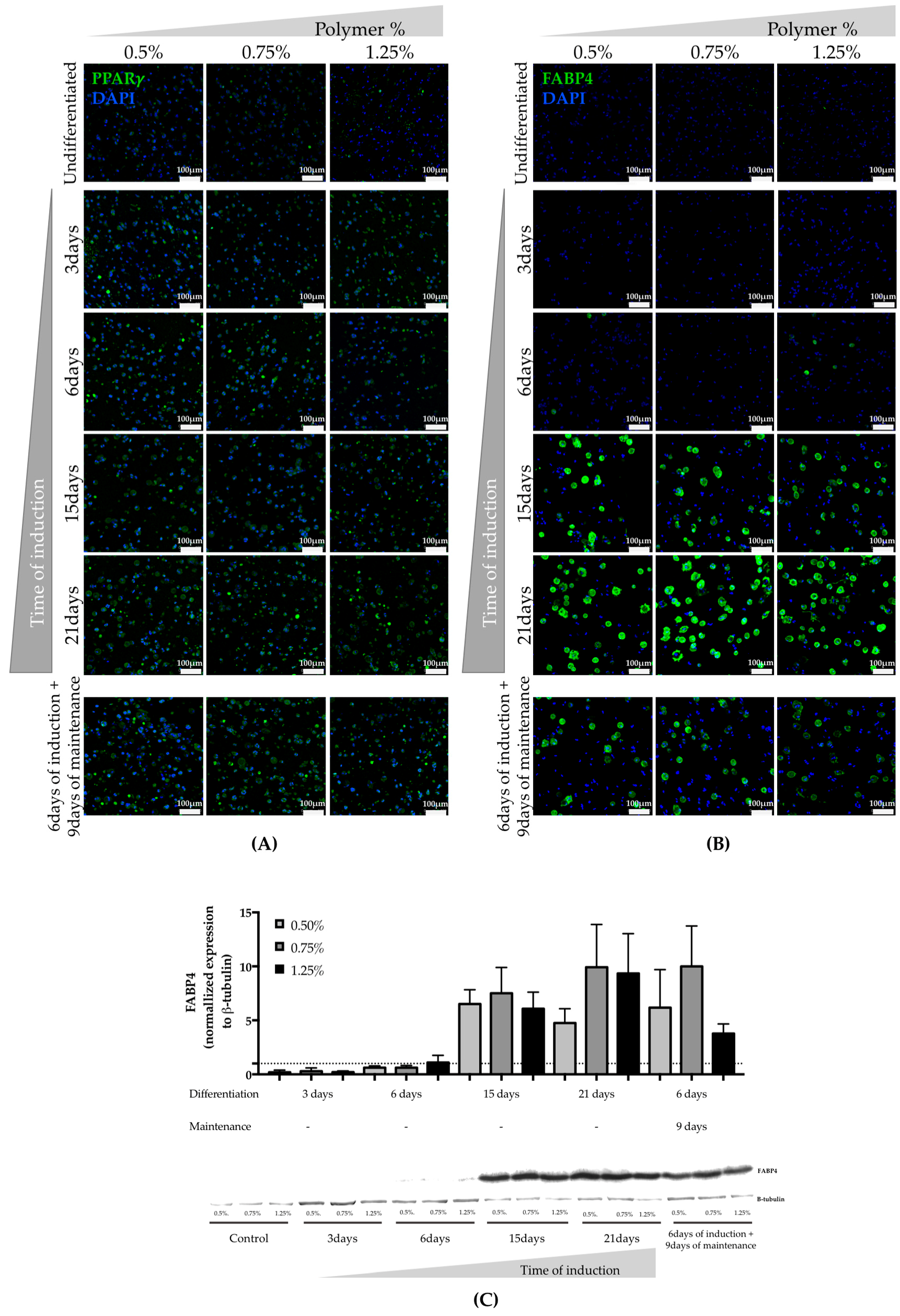
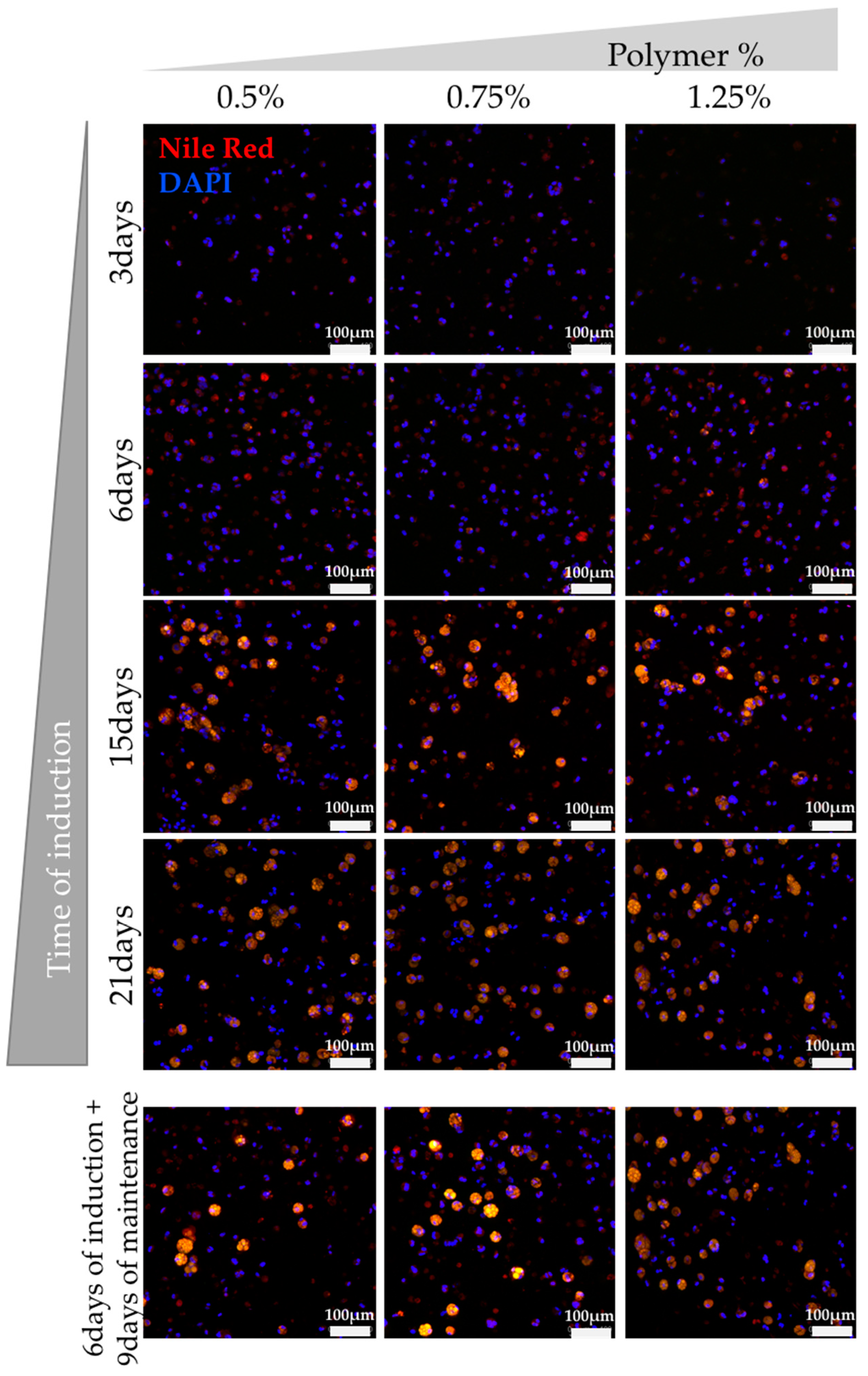
3.2. Assessment of hASCs Differentiation within GG Hydrogel Particles
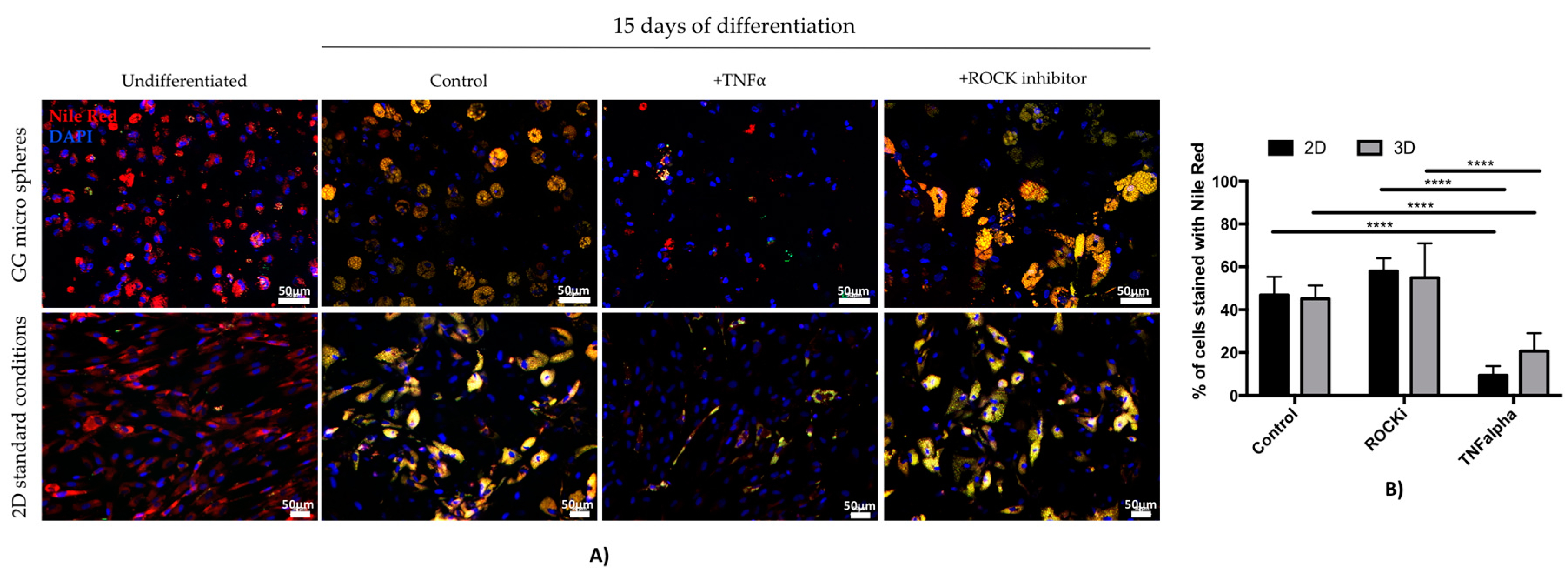
3.3. Assessment of TNF Alpha and ROCK Inhibitor Effect on Adipogenic Differentiation
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pope, B.D.; Warren, C.R.; Parker, K.K.; Cowan, C.A. Microenvironmental control of adipocyte fate and function. Trends Cell Biol. 2016, 26, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.W. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 2001, 263, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Chen, Y.Y.; Hsueh, Y.S.; Tai, H.C.; Lin, F.H. Modifying alginate with early embryonic extracellular matrix, laminin and hyaluronic acid for adipose tissue engineering. J. Biomed. Mater. Res. Part A 2015. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.S.; Chen, Y.S.; Tai, H.C.; Mestak, O.; Chao, S.C.; Chen, Y.Y.; Shih, Y.; Lin, J.F.; Shieh, M.J.; Lin, F.H. Laminin-alginate beads as preadipocyte carriers to enhance adipogenesis in vitro and in vivo. Tissue Eng. Part A 2016. [Google Scholar] [CrossRef]
- Rossi, E.; Gerges, I.; Tocchio, A.; Tamplenizza, M.; Aprile, P.; Recordati, C.; Martello, F.; Martin, I.; Milani, P.; Lenardi, C. Biologically and mechanically driven design of an RGD-mimetic macroporous foam for adipose tissue engineering applications. Biomaterials 2016, 104, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Zhang, R.; Lin, F.; Luan, J. Injectable cell/hydrogel microspheres induce the formation of fat lobule-like microtissues and vascularized adipose tissue regeneration. Biofabrication 2012, 4, 045003. [Google Scholar] [CrossRef] [PubMed]
- Girandon, L.; Kregar-Velikonja, N.; Bozikov, K.; Barlic, A. In vitro models for adipose tissue engineering with adipose-derived stem cells using different scaffolds of natural origin. Folia Biol. (Praha) 2011, 57, 47–56. [Google Scholar] [PubMed]
- Korurer, E.; Kenar, H.; Doger, E.; Karaoz, E. Production of a composite hyaluronic acid/gelatin blood plasma gel for hydrogel-based adipose tissue engineering applications. J. Biomed. Mater. Res. Part A 2014, 102, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Bellas, E.; Marra, K.G.; Kaplan, D.L. Sustainable three-dimensional tissue model of human adipose tissue. Tissue Eng. Part C Methods 2013, 19, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, T.N.; Hinman, C.R.; Ashley Rubin, R.K.; Smither, K.; Burke, D.J.; Hawker, C.J.; Messina, D.; Van Epps, D.; Clegg, D.O. Vitronectin-based, biomimetic encapsulating hydrogel scaffolds support adipogenesis of adipose stem cells. Tissue Eng. Part A 2016, 22, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.D.; Wang, R.Y.; Reagan, M.R.; Chen, Y.; Borowsky, F.E.; Zieba, A.; Marra, K.G.; Rubin, J.P.; Ghobrial, I.M.; Kaplan, D.L. The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv. Healthc. Mater. 2016, 5, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; in het Panhuis, M. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.; Cencetti, C.; Ria, R.; Alhaique, F.; Coviello, T. Preparation and characterization of novel Gellan gum hydrogels suitable for modified drug release. Molecules 2009, 14, 3376–3391. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Gardel, L.S.; Rada, T.; Martins, L.; Gomes, M.E.; Reis, R.L. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J. Orthop. Res. 2010, 28, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Salgado, A.J.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Reis, R.L. Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue Eng. Part C Methods 2011, 17, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Cooke, M.J.; Tam, R.Y.; Sousa, N.; Salgado, A.J.; Reis, R.L.; Shoichet, M.S. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomaterials 2012, 33, 6345–6354. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, Y.; Lin, Y.; Shen, J.; Wang, D.A. A novel gellan gel-based microcarrier for anchorage-dependent cell delivery. Acta Biomater. 2008, 4, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Cerqueira, M.T.; Sousa, R.A.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta Biomater. 2014, 10, 4787–4797. [Google Scholar] [CrossRef] [PubMed]
- Pereira Da Silva, L.; Teixeira Cerqueira, M.; Romero Amandi De Sousa, R.P.; Pinto Marques, A.M.; Correlo Da Silva, V.M.; Goncalves Dos Reis, R.L. Gellan Gum Spongy-Like Hydrogel, its Preparation and Biomedical Applications Thereof. U.S. Patent 14782812, 9 April 2014. [Google Scholar]
- Cerqueira, M.T.; Pirraco, R.P.; Santos, T.C.; Rodrigues, D.B.; Frias, A.M.; Martins, A.R.; Reis, R.L.; Marques, A.P. Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules 2013, 14, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Floyd, Z.E.; Wu, X.; Hebert, T.; Halvorsen, Y.D.C.; Buehrer, B.M.; Gimble, J.M. Adipogenic differentiation of adipose-derived stem cells. In Adipose-Derived Stem Cells: Methods and Protocols; Springer: Berlin, Germany, 2011; pp. 193–200. [Google Scholar]
- Samani, A.; Bishop, J.; Luginbuhl, C.; Plewes, D.B. Measuring the elastic modulus of ex vivo small tissue samples. Phys. Med. Biol. 2003, 48, 2183–2198. [Google Scholar] [CrossRef] [PubMed]
- Comley, K.; Fleck, N.A. A micromechanical model for the Young’s modulus of adipose tissue. Int. J. Solids Struct. 2010, 47, 2982–2990. [Google Scholar] [CrossRef]
- Samani, A.; Zubovits, J.; Plewes, D. Elastic moduli of normal and pathological human breast tissues: An inversion-technique-based investigation of 169 samples. Phys. Med. Biol. 2007, 52, 1565. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, N.; Mansfield, J.; Green, E.; Bell, J.; Knight, B.; Liversedge, N.; Tham, J.C.; Welbourn, R.; Shore, A.C.; Kos, K.; et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1427–E1435. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Bianco, J.; Brown, C.; Fuetterer, L.; Watkins, J.F.; Samani, A.; Flynn, L.E. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013, 34, 3290–3302. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.P.; Hedrick, M.H.; Chang, J.; Mehrara, B.J.; Longaker, M.T. The impact of biomolecular medicine and tissue engineering on plastic surgery in the 21st century. Plast. Reconstr. Surg. 2000, 105, 2467–2481. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, M.C.; Farè, S. Adipose tissue engineering: State of the art, recent advances and innovative approaches. Expert Rev. Med. Devices 2009, 6, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Stosich, M.S.; Mao, J.J. Adipose tissue engineering from human adult stem cells: Clinical implications in plastic and reconstructive surgery. Plast. Reconstr. Surg. 2007, 119, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Hosoda, K.; Fujikura, J.; Fujimoto, M.; Iwakura, H.; Tomita, T.; Ishii, T.; Arai, N.; Hirata, M.; Ebihara, K.; et al. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J. Biol. Chem. 2007, 282, 29574–29583. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Heyd, F.; Hegyi, K.; Sethi, J.K. Tumour necrosis factor-α inhibits adipogenesis via a β-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007, 14, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sethi, J.K.; Hotamisligil, G.S. Transmembrane tumor necrosis factor (TNF)-α inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 1999, 274, 26287–26295. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Forward (5′–3′) | Primer Reverse (5′–3′) | Tm (°C) 1 |
|---|---|---|---|
| PPARγ | TGGGTGAAACTCTGGGAGAT (20) | TGGCATCTCTGTGTCAACCA (20) | 57.3 |
| FABP4 | AAACTGGTGGTGGAATGCGT (20) | GCGAACTTCAGTCCAGGTCA (20) | 58.4 |
| GAPDH | AGCCTCAAGATCATCAGCAA (20) | GTCATGAGTCCTTCCACGAT (20) | 56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lago, M.E.L.; Da Silva, L.P.; Henriques, C.; Carvalho, A.F.; Reis, R.L.; Marques, A.P. Generation of Gellan Gum-Based Adipose-Like Microtissues. Bioengineering 2018, 5, 52. https://doi.org/10.3390/bioengineering5030052
Lago MEL, Da Silva LP, Henriques C, Carvalho AF, Reis RL, Marques AP. Generation of Gellan Gum-Based Adipose-Like Microtissues. Bioengineering. 2018; 5(3):52. https://doi.org/10.3390/bioengineering5030052
Chicago/Turabian StyleLago, Manuela E. L., Lucília P. Da Silva, Catarina Henriques, Andreia F. Carvalho, Rui L. Reis, and Alexandra P. Marques. 2018. "Generation of Gellan Gum-Based Adipose-Like Microtissues" Bioengineering 5, no. 3: 52. https://doi.org/10.3390/bioengineering5030052
APA StyleLago, M. E. L., Da Silva, L. P., Henriques, C., Carvalho, A. F., Reis, R. L., & Marques, A. P. (2018). Generation of Gellan Gum-Based Adipose-Like Microtissues. Bioengineering, 5(3), 52. https://doi.org/10.3390/bioengineering5030052