Rapamycin Mitigates Corneal Damage in a Mouse Model of Alkali Burn Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics Statement
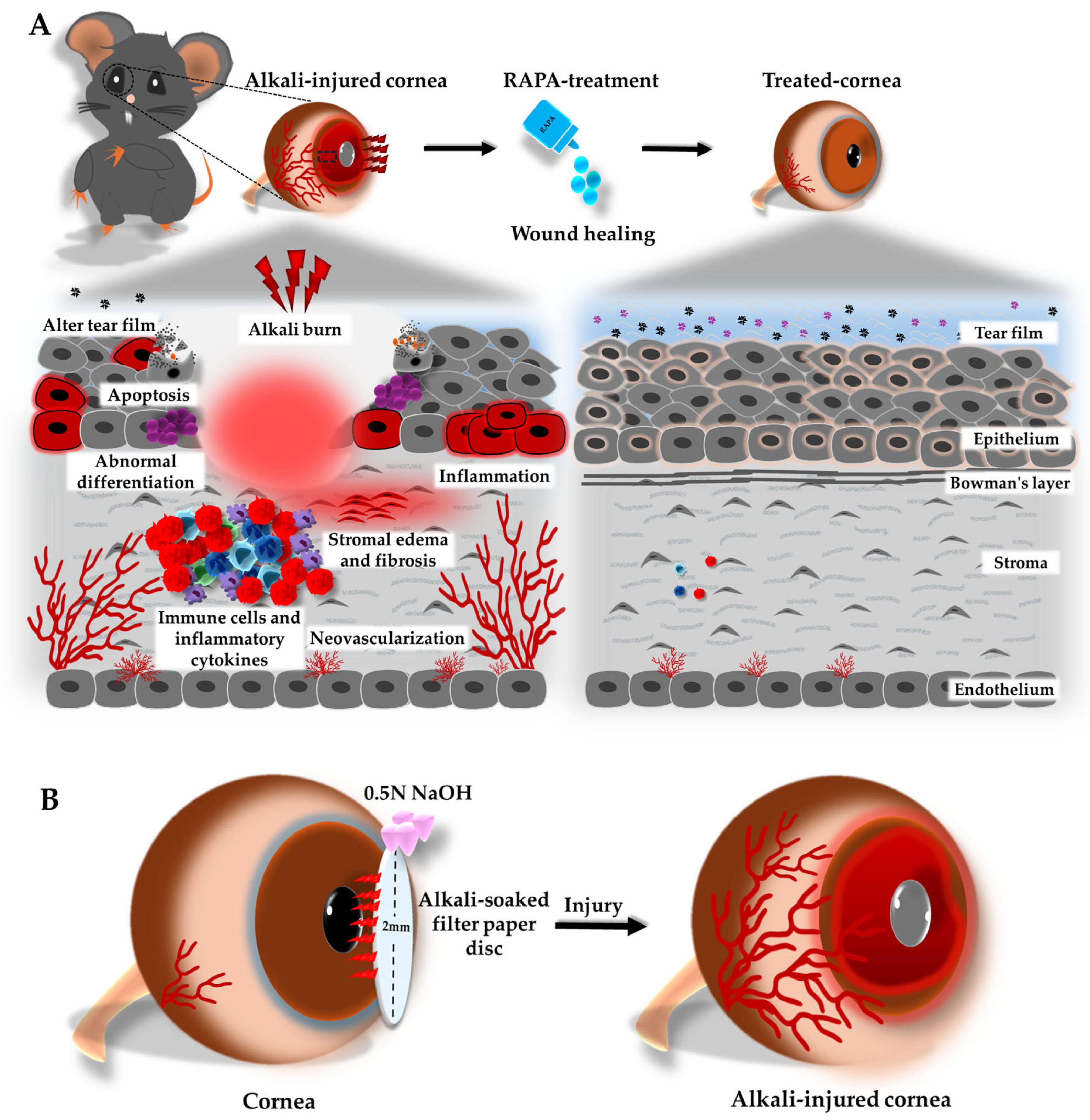
2.3. Mouse Model of Corneal Alkali Burn Injury and Experimental Groups
2.4. Evaluation of Corneal Clinical Score
2.5. Tear Secretion Measurements
2.6. Corneal Fluorescein Staining and Scoring
2.7. Corneal Neovascularization Assessment
2.8. Histological Analysis
2.9. Immunofluorescence Staining
2.10. Western Blot
2.11. TUNEL Assay
2.12. Statistical Analysis
3. Results
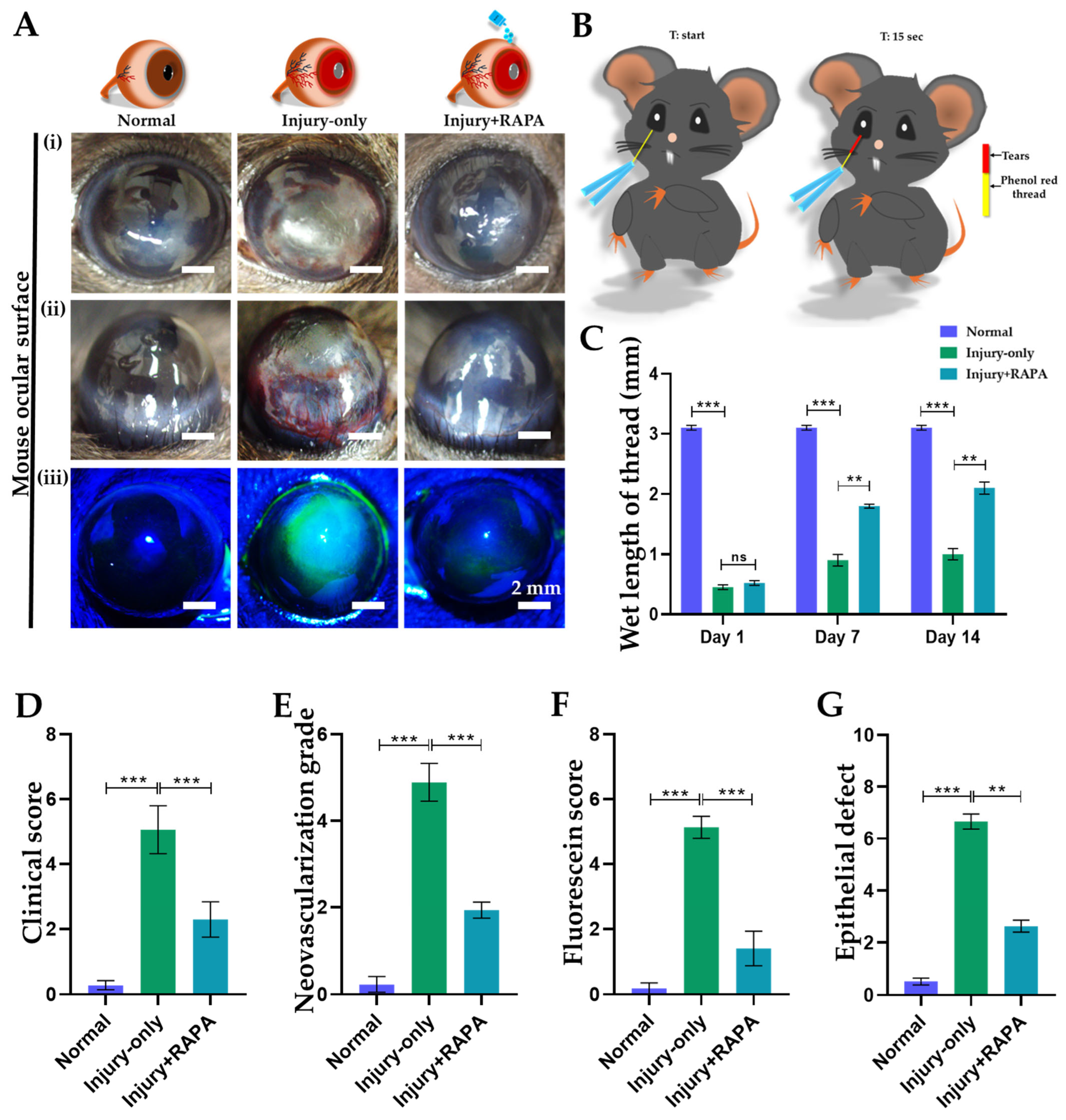
3.1. RAPA Preserves Corneal Integrity in a Mouse Model of Corneal Alkali Burn Injury
3.2. RAPA Reduces Corneal Clinical Scores in a Mouse Model of Corneal Alkali Burn Injury
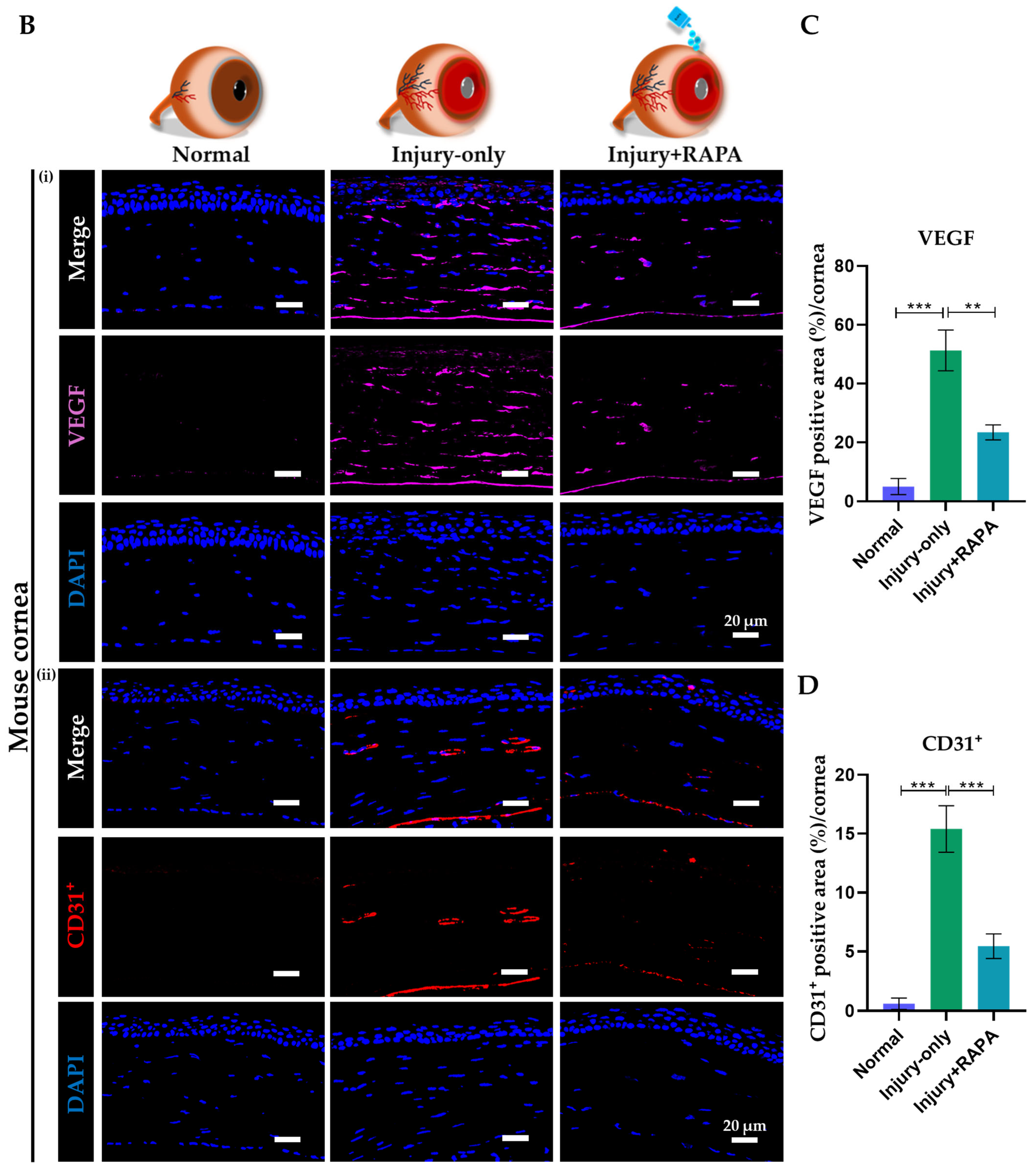
3.3. RAPA Attenuates Corneal Neovascularization Grade in a Mouse Model of Corneal Alkali Burn Injury
3.4. RAPA Enhances Tear Production in a Mouse Model of Corneal Alkali Burn Injury
3.5. RAPA Restores Corneal Epithelial Barrier Function by Maintaining ZO-1 Expression in a Mouse Model of Corneal Alkali Burn Injury
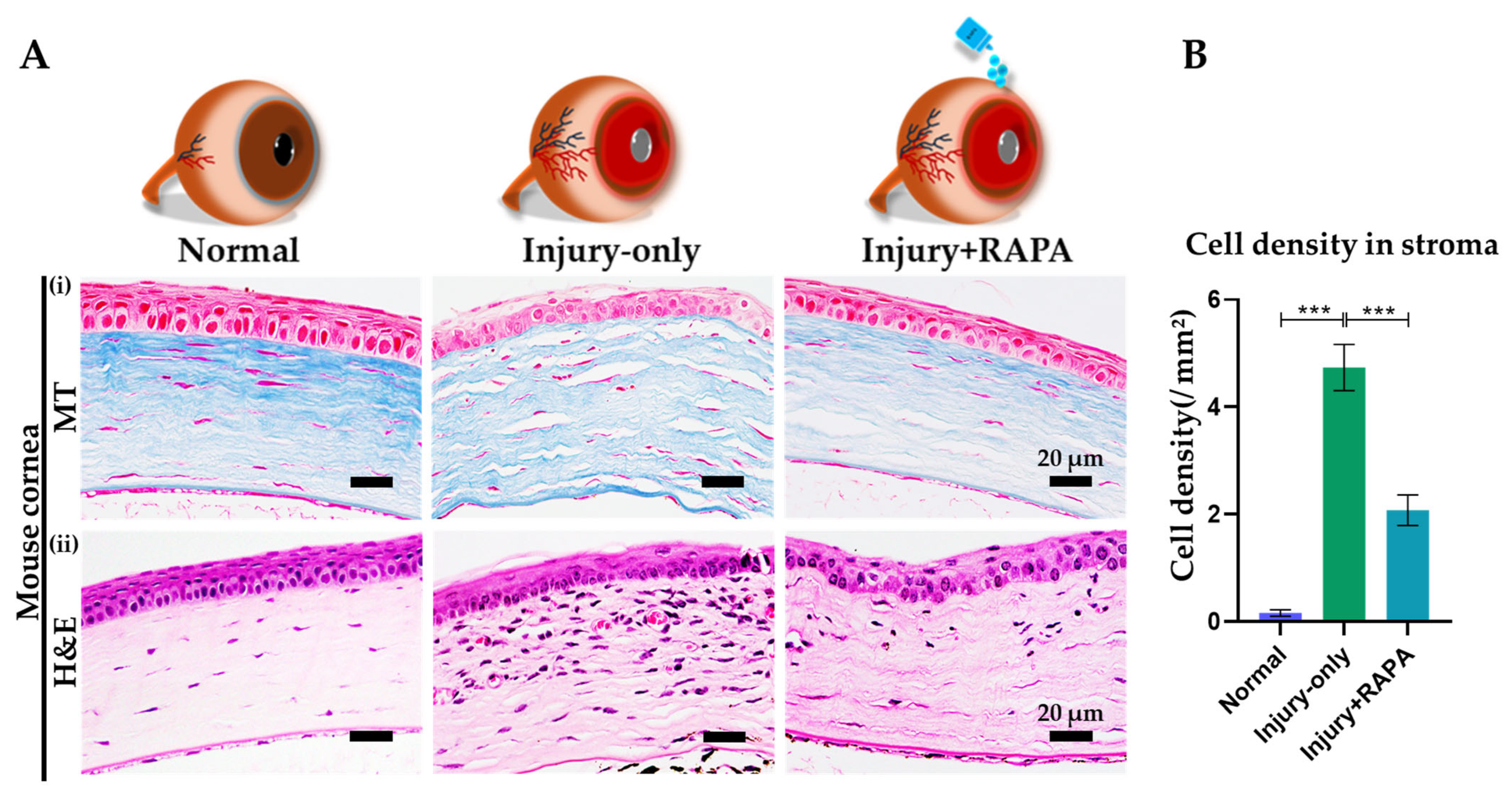
3.6. RAPA Reduces Fibrotic Tissue Deposition and Inflammatory Cell Infiltration in the Corneal Stroma of a Mouse Model of Corneal Alkali Burn Injury
3.7. RAPA Diminishes Macrophage and Pan-Leukocyte Infiltration in the Cornea of a Mouse Model of Corneal Alkali Burn Injury
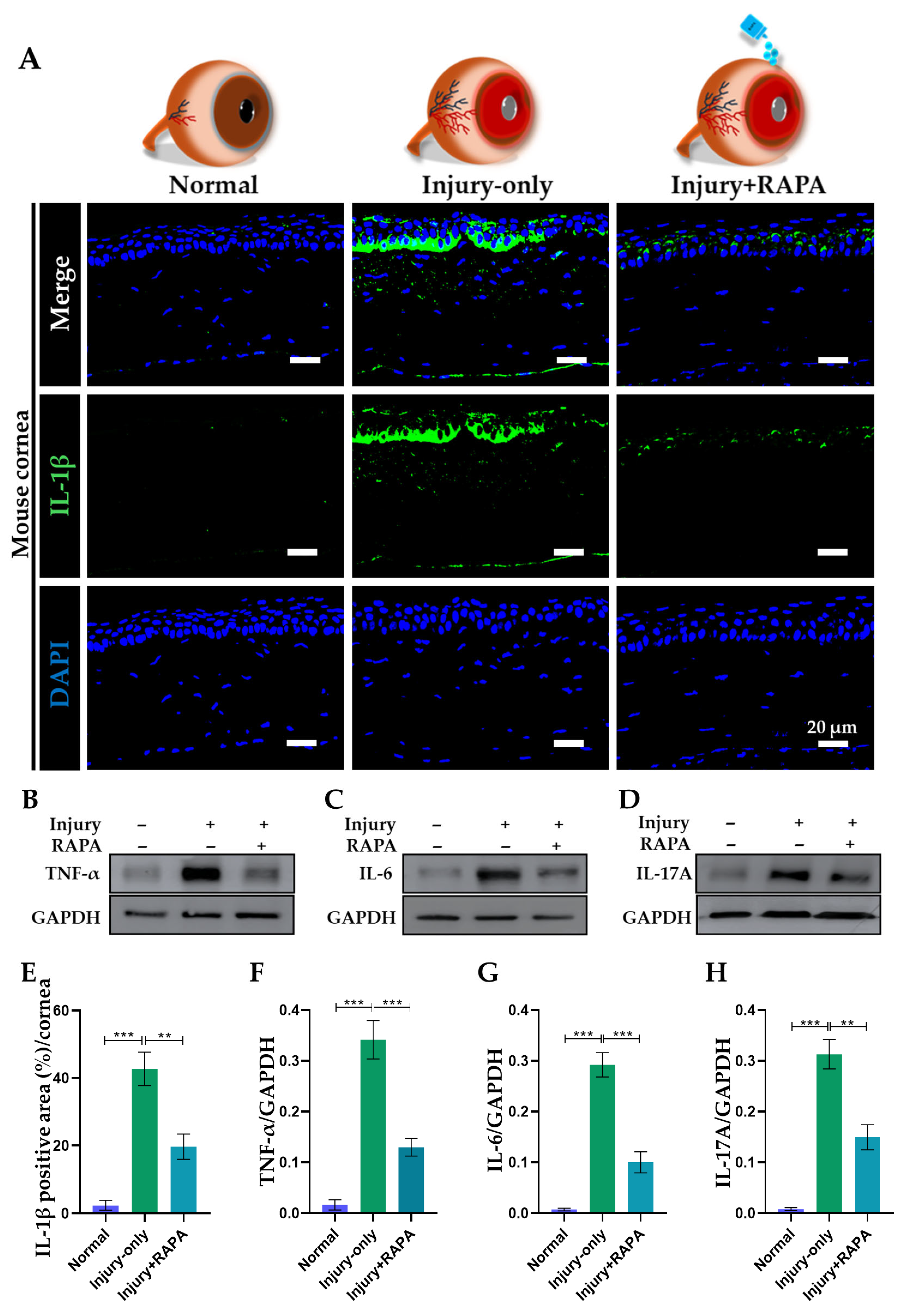
3.8. RAPA Downregulates Pro-Inflammatory Cytokines in the Cornea of a Mouse Model of Corneal Alkali Burn Injury
3.9. RAPA Represses Angiogenic Mediator and Endothelial Marker in the Cornea of a Mouse Model of Corneal Alkali Burn Injury
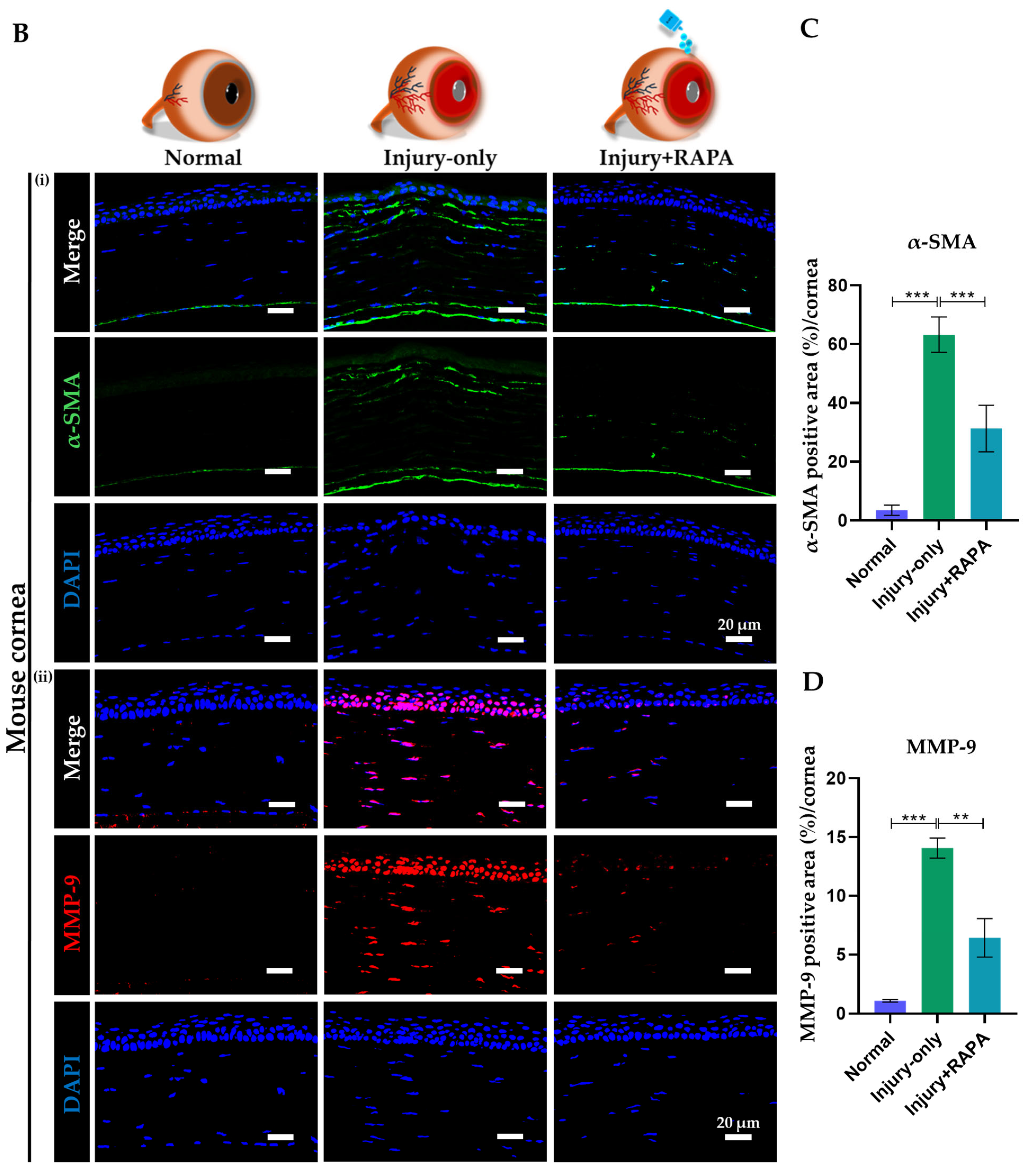
3.10. RAPA Suppresses α-SMA–Positive Myofibroblast-Associated Fibrosis and MMP-9–Mediated Pathological Matrix Remodeling in the Cornea of a Mouse Model of Corneal Alkali Burn Injury
3.11. RAPA Inhibits Cellular Apoptosis in the Cornea of a Mouse Model of Corneal Alkali Burn Injury
3.12. RAPA Limits Uncontrolled Cell Proliferation in the Cornea of a Mouse Model of Corneal Alkali Burn Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RAPA | Rapamycin |
| ECM | Extracellular Matrix |
| VEGF | Vascular Endothelial Growth Factor |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| mTOR | Mammalian Target of Rapamycin |
| MMP-2 | Matrix Metalloproteinase-2 |
| bFGF | Basic Fibroblast Growth Factor |
| IL-1β | Interleukin 1-beta |
| TNF-α | Tumor Necrosis Factor-Alpha |
| IL-6 | Interleukin 6 |
| IL-17B | Interleukin-17B |
| CD31 | Cluster of Differentiation 31 |
| CD45 | Cluster of Differentiation 45 |
| MMP-9 | Matrix Metalloproteinase-9 |
| ZO-1 | Zonula Occludens-1 |
| α-SMA | Alpha-Smooth Muscle Actin |
| TUNEL | Terminal Deoxynucleotidyl Transferase UTP nick end labeling |
| NaOH | Sodium Hydroxide |
| TIMPs | Tissue Inhibitors of Metalloproteinases |
| TLR4 | Toll-Like Receptor 4 |
| NLRP3 | NOD-Like Receptor Family Pyrin Domain Containing 3 |
| H&E | Hematoxylin and Eosin |
| MT | Masson’s Trichrome |
References
- Hakami, N.Y.; Dusting, G.J.; Chan, E.C.; Shah, M.H.; Peshavariya, H.M. Wound healing after alkali burn injury of the cornea involves Nox4-type NADPH oxidase. Invest. Ophthalmol. Vis. Sci. 2020, 61, 20. [Google Scholar] [CrossRef] [PubMed]
- Saccu, G.; Menchise, V.; Giordano, C.; Delli Castelli, D.; Dastrù, W.; Pellicano, R.; Tolosano, E.; Van Pham, P.; Altruda, F.; Fagoonee, S. Regenerative approaches and future trends for the treatment of corneal burn injuries. J. Clin. Med. 2021, 10, 317. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Yang, Y.; Qiu, J.; Yang, M.; Wu, C.; Lai, Z.; Tang, M.; Ge, J.; Yu, K. Tetramethylpyrazine (TMP) ameliorates corneal neovascularization via regulating cell infiltration into cornea after alkali burn. Biomed. Pharmacother. 2019, 109, 1041–1051. [Google Scholar] [CrossRef]
- Lu, P.; Li, L.; Wu, Y.; Mukaida, N.; Zhang, X. Essential contribution of CCL3 to alkali-induced corneal neovascularization by regulating vascular endothelial growth factor production by macrophages. Mol. Vis. 2008, 14, 1614. [Google Scholar]
- Zhao, Z.; Wen, Y.; Peng, Y.; Wang, W.; Ma, H. Aloin alleviates corneal injury in alkali burn via inhibiting neutrophil extracellular traps and promoting Nrf2. Immunopharmacol. Immunotoxicol. 2024, 46, 773–784. [Google Scholar] [CrossRef]
- Yuan, K.; Zheng, J.; Huang, X.; Zhang, Y.; Han, Y.; Hu, R.; Jin, X. Neutrophil extracellular traps promote corneal neovascularization-induced by alkali burn. Int. Immunopharmacol. 2020, 88, 106902. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cheng, Y.; Li, W.; Yu, H.; Li, Z.; Li, J.; Li, M.; Huang, Q.; Liu, Y.; Ling, S. Irradiated umbilical cord mesenchymal stem cell-coated high oxygen-permeable hydrogel lenses inhibit corneal inflammation and neovascularization after corneal alkali burns. Sci. Rep. 2025, 15, 10401. [Google Scholar] [CrossRef]
- Yu, J.; Shen, Y.; Luo, J.; Jin, J.; Li, P.; Feng, P.; Guan, H. Upadacitinib inhibits corneal inflammation and neovascularization by suppressing M1 macrophage infiltration in the corneal alkali burn model. Int. Immunopharmacol. 2023, 116, 109680. [Google Scholar] [CrossRef]
- Goto, H.; Arima, T.; Takahashi, A.; Tobita, Y.; Nakano, Y.; Toda, E.; Shimizu, A.; Okamoto, F. Trimebutine prevents corneal inflammation in a rat alkali burn model. Sci. Rep. 2024, 14, 12111. [Google Scholar] [CrossRef]
- Barry, Z.; Park, B.; Corson, T.W. Pharmacological potential of small molecules for treating corneal neovascularization. Molecules 2020, 25, 3468. [Google Scholar] [CrossRef] [PubMed]
- Joung, C.; Noh, H.; Jung, J.; Song, H.Y.; Bae, H.; Pahk, K.; Kim, W.-K. A novel CD147 inhibitor, SP-8356, attenuates pathological fibrosis in alkali-burned rat cornea. Int. J. Mol. Sci. 2020, 21, 2990. [Google Scholar] [CrossRef]
- Jin, H.; Yoon, H.-J.; Jiang, E.; Liu, J.; Yoon, H.S.; Choi, J.S.; Moon, J.; Qi, H.; Yoon, K.C. Therapeutic Effects of Human Placental Extracts Eye Drops in Experimental Dry Eye and Alkali Burn. J. Ocul. Pharmacol. Ther. 2025, 41, 141–149. [Google Scholar] [CrossRef]
- Tuft, S.; Shortt, A. Surgical rehabilitation following severe ocular burns. Eye 2009, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, X.; Guo, L.; Zheng, F.; Meng, C.; Zheng, Y.; Liu, G. Daphnetin inhibits corneal inflammation and neovascularization on a mouse model of corneal alkali burn. Int. Immunopharmacol. 2022, 103, 108434. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.L.; Basu, S.K.; Stiles, M.A.; Prislovsky, A.; Grambergs, R.C.; Nicholas, S.E.; Karamichos, D.; Allegood, J.C.; Proia, R.L.; Mandal, N. Ablation of Sphingosine Kinase 1 Protects Cornea from Neovascularization in a Mouse Corneal Injury Model. Cells 2022, 11, 2914. [Google Scholar] [CrossRef]
- Drzyzga, Ł.; Śpiewak, D.; Dorecka, M.; Wyględowska-Promieńska, D. Available therapeutic options for corneal neovascularization: A review. Int. J. Mol. Sci. 2024, 25, 5479. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Fan Gaskin, J.; Chan, E.C. Corneal Neovascularisation and Anti-VEGF Therapy. Targets 2025, 3, 9. [Google Scholar] [CrossRef]
- Soleimani, M.; Naderan, M. Management strategies of ocular chemical burns: Current perspectives. Clin. Ophthalmol. 2020, 14, 2687–2699. [Google Scholar] [CrossRef]
- Bakunowicz-Łazarczyk, A.; Urban, B. Assessment of therapeutic options for reducing alkali burn-induced corneal neovascularization and inflammation. Adv. Med. Sci. 2016, 61, 101–112. [Google Scholar] [CrossRef]
- Hobby, G.; Clark, R.; Woywodt, A. A treasure from a barren island: The discovery of rapamycin. Clin. Kidney J. 2022, 15, 1971–1972. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Hong, H.S.; Kim, J.C.; Shin, J.S.; Son, Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2005, 46, 454–460. [Google Scholar] [CrossRef]
- Shah, M.; Edman, M.C.; Janga, S.R.; Yarber, F.; Meng, Z.; Klinngam, W.; Bushman, J.; Ma, T.; Liu, S.; Louie, S. Rapamycin eye drops suppress lacrimal gland inflammation in a murine model of Sjögren’s syndrome. Invest. Ophthalmol. Vis. Sci. 2017, 58, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ko, J.H.; Kim, B.H.; Ryu, J.S.; Kim, H.J.; Kim, M.K.; Oh, J.Y. Inhibition of mTOR by rapamycin aggravates corneal epithelial stem cell deficiency by upregulating inflammatory response. Stem Cells 2019, 37, 1212–1222. [Google Scholar] [CrossRef]
- Li, J.; Han, J.; Shi, Y.; Liu, M. Rapamycin inhibits corneal inflammatory response and neovascularization in a mouse model of corneal alkali burn. Exp. Eye Res. 2023, 233, 109539. [Google Scholar] [CrossRef]
- Li, J.; Du, S.; Shi, Y.; Han, J.; Niu, Z.; Wei, L.; Yang, P.; Chen, L.; Tian, H.; Gao, L. Rapamycin ameliorates corneal injury after alkali burn through methylation modification in mouse TSC1 and mTOR genes. Exp. Eye Res. 2021, 203, 108399. [Google Scholar] [CrossRef]
- Li, X.; Chen, K.; Wang, Z.; Li, J.; Wang, X.; Xie, C.; Tong, J.; Shen, Y. The mTOR signalling in corneal diseases: A recent update. Biochem. Pharmacol. 2023, 213, 115620. [Google Scholar] [CrossRef]
- Saunders, R.N.; Metcalfe, M.S.; Nicholson, M.L. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001, 59, 3–16. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, L.; Fei, S.; Gao, X.; Chen, H.; Han, Z.; Tao, J.; Ju, X.; Wang, Z.; Tan, R. Long-term outcomes in rapamycin on renal allograft function: A 30-year follow-up from a single-center experience. BMC Nephrol. 2024, 25, 311. [Google Scholar] [CrossRef]
- Shin, Y.J.; Hyon, J.Y.; Choi, W.S.; Yi, K.; Chung, E.-S.; Chung, T.-Y.; Wee, W.R. Chemical injury-induced corneal opacity and neovascularization reduced by rapamycin via TGF-β1/ERK pathways regulation. Invest. Ophthalmol. Vis. Sci. 2013, 54, 4452–4458. [Google Scholar] [PubMed]
- Lee, K.-J.; Lee, J.-Y.; Lee, S.H.; Choi, T.H. Accelerating repaired basement membrane after bevacizumab treatment on alkali-burned mouse cornea. BMB Rep. 2013, 46, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, L.; Zhong, Y.; Zhang, Y.; Yin, Q.; Li, S.; Zhang, X.; Han, H.; Yao, K. Ferrostatin-1-loaded liposome for treatment of corneal alkali burn via targeting ferroptosis. Bioeng. Transl. Med. 2022, 7, e10276. [Google Scholar]
- Lee, K.E.; Oh, S.; Bhujel, B.; Kim, C.M.; Lee, H.; Park, J.H.; Kim, J.Y. Effect of topical programmed death-ligand1 on corneal epithelium in dry eye mouse. Biomolecules 2024, 14, 68. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Liu, X.; Wang, N.; Song, Z.; Wu, K. A comparison of the effects of benzalkonium chloride on ocular surfaces between C57BL/6 and BALB/c mice. Int. J. Mol. Sci. 2017, 18, 509. [Google Scholar] [CrossRef]
- Veettil, R.A.; Li, W.; Pflugfelder, S.C.; Koch, D.D. A Mouse Model for corneal neovascularization by alkali Burn. J. Vis. Exp. (JoVE) 2023, 196, e65289. [Google Scholar]
- Shadmani, A.; Dhowre, H.S.; Ercal, O.; Meng, X.Q.; Wu, A.Y. Corneal and limbal alkali injury induction using a punch-trephine technique in a mouse model. JoVE (J. Vis. Exp.) 2023, 198, e65609. [Google Scholar] [CrossRef]
- Lan, C.; Liu, G.; Huang, L.; Wang, X.; Tan, J.; Wang, Y.; Fan, N.; Zhu, Y.; Yu, M.; Liu, X. Forkhead domain inhibitor-6 suppresses corneal neovascularization and subsequent fibrosis after alkali burn in rats. Invest. Ophthalmol. Vis. Sci. 2022, 63, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Im, S.-T.; Yoon, J.Y.; Kim, S.; Kim, M.K.; Chung, M.-H.; Park, C.-K. Comparison of therapeutic effects between topical 8-oxo-2’-deoxyguanosine and corticosteroid in ocular alkali burn model. Sci. Rep. 2021, 11, 6909. [Google Scholar]
- Huang, Y.; Lin, L.; Yang, Y.; Duan, F.; Yuan, M.; Lou, B.; Lin, X. Effect of tauroursodeoxycholic acid on inflammation after ocular alkali burn. Int. J. Mol. Sci. 2022, 23, 11717. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, W.; Zhang, M.; Huo, Y.; Li, F.; Song, L.; Wu, S.; Yang, Q.; Li, X.; Zhang, J. Minocycline-loaded nHAP/PLGA microspheres for prevention of injury-related corneal angiogenesis. J. Nanobiotechnol. 2024, 22, 134. [Google Scholar]
- Ferrari, G.; Bignami, F.; Giacomini, C.; Franchini, S.; Rama, P. Safety and efficacy of topical infliximab in a mouse model of ocular surface scarring. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1680–1688. [Google Scholar]
- Yao, Q.; Wu, H.; Ren, H.; Cao, J.; Shao, Y.; Liu, G.; Lu, P. Inhibition of Experimental Corneal Neovascularization by the Tight Junction Protein ZO-1. J. Ocul. Pharmacol. Ther. 2024, 40, 379–388. [Google Scholar] [CrossRef]
- Gong, D.; Wu, N.; Chen, H.; Zhang, W.; Yan, C.; Zhang, C.; Fu, Y.; Sun, H. Phytic acid-loaded polyvinyl alcohol hydrogel promotes wound healing of injured corneal epithelium through inhibiting ferroptosis. Redox Biol. 2024, 76, 103354. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Z.; Jin, C.; Chen, Q.; Fang, Y.; Jin, J.; Chen, J.; Lu, L.; Tian, H.; Xu, J. Human amniotic epithelial cell-derived extracellular vesicles provide an extracellular matrix-based microenvironment for corneal injury repair. J. Tissue Eng. 2022, 13, 20417314221122123. [Google Scholar] [PubMed]
- Özkaya, D.; Karaca, U.; Usta Sofu, G.; Savran, M.; Özgöçmen, M.; Ertuğrul, A. Effect of adalimumab on experimental corneal neovascularization model. Int. Ophthalmol. 2023, 43, 2119–2128. [Google Scholar] [CrossRef]
- Uchiyama, M.; Shimizu, A.; Masuda, Y.; Nagasaka, S.; Fukuda, Y.; Takahashi, H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013, 19, 2135. [Google Scholar]
- Wang, H.; Guo, Z.; Liu, P.; Yang, X.; Li, Y.; Lin, Y.; Zhao, X.; Liu, Y. Luteolin ameliorates cornea stromal collagen degradation and inflammatory damage in rats with corneal alkali burn. Exp. Eye Res. 2023, 231, 109466. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Huang, Y.; Pan, Y.; Yu, Y.; Zhou, Y.; Wan, S.-S.; Yang, Y.-N. The potential protective effects of miR-497 on corneal neovascularization are mediated via macrophage through the IL-6/STAT3/VEGF signaling pathway. Int. Immunopharmacol. 2021, 96, 107745. [Google Scholar] [PubMed]
- Horwitz, V.; Dachir, S.; Cohen, M.; Gutman, H.; Cohen, L.; Gez, R.; Buch, H.; Kadar, T.; Gore, A. Differential expression of corneal and limbal cytokines and chemokines throughout the clinical course of sulfur mustard induced ocular injury in the rabbit model. Exp. Eye Res. 2018, 177, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.-W.; Jiang, D.; Qin, P.; Zhang, Y.; Qiu, B.; Chanda, S.; Tergaonkar, V.; Li, Q.; Wong, I.Y.; Yu, Z. Inhibition of NUCKS facilitates corneal recovery following alkali burn. Sci. Rep. 2017, 7, 41224. [Google Scholar] [CrossRef]
- Gong, Y.; Gao, J.; Li, M.; Zhang, X.-L.; Liao, Y.-H.; Bao, Y.-B. URP20 improves corneal injury caused by alkali burns combined with pathogenic bacterial infection in rats. Exp. Eye Res. 2024, 238, 109739. [Google Scholar]
- Kim, D.R.; Park, S.-K.; Kim, E.J.; Kim, D.-K.; Yoon, Y.C.; Myung, D.; Lee, H.J.; Na, K.-S. Dexamethasone acetate loaded poly (ε-caprolactone) nanofibers for rat corneal chemical burn treatment. Sci. Rep. 2024, 14, 21806. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shao, Y.; Lin, Z.; Qu, Y.-L.; Wang, H.; Zhou, Y.; Chen, W.; Chen, Y.; Chen, W.-L.; Hu, F.-R. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1285–1295. [Google Scholar] [CrossRef]
- Luisi, J.; Lin, J.L.; Karediya, N.; Kraft, E.R.; Sharifi, A.; Schmitz-Brown, M.E.; Zhang, W.; Ameredes, B.T.; Merkley, K.H.; Motamedi, M. Concentration-associated pathology of alkali burn in a mouse model using anterior segment optical coherence tomography with angiography. Exp. Eye Res. 2022, 223, 109210. [Google Scholar] [CrossRef] [PubMed]
- Sprogyte, L.; Park, M.; Di Girolamo, N. Pathogenesis of alkali injury-induced limbal stem cell deficiency: A literature survey of animal models. Cells 2023, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019. [Google Scholar] [CrossRef]
- Kwon, J.; Kang, C.; Moghtader, A.; Shahjahan, S.; Bibak Bejandi, Z.; Alzein, A.; Djalilian, A.R. Emerging Treatments for Persistent Corneal Epithelial Defects. Vision 2025, 9, 26. [Google Scholar] [CrossRef]
- Chang, C.-H.; Peng, I.-C.; Huang, Y.-H. Recombinant Thrombomodulin Domain 1 Modulates Macrophage Polarization and Enhances Healing in Corneal Alkali Burns. Invest. Ophthalmol. Vis. Sci. 2025, 66, 21. [Google Scholar]
- Liang, W.; Zhang, Y.; Zhou, L.; Lu, X.; Finn, M.E.; Wang, W.; Shao, H.; Dean, D.C.; Zhang, L.; Liu, Y. Zeb1 regulation of wound-healing-induced inflammation in alkali-damaged corneas. Iscience 2022, 25, 4. [Google Scholar]
- Gu, X.-J.; Liu, X.; Chen, Y.-Y.; Zhao, Y.; Xu, M.; Han, X.-J.; Liu, Q.-P.; Yi, J.-L.; Li, J.-M. Involvement of NADPH oxidases in alkali burn-induced corneal injury. Int. J. Mol. Med. 2016, 38, 75–82. [Google Scholar] [CrossRef]
- Shimizu, H.; Sakimoto, T.; Yamagami, S. Pro-inflammatory role of NLRP3 inflammasome in experimental sterile corneal inflammation. Sci. Rep. 2019, 9, 9596. [Google Scholar] [CrossRef]
- Do, K.K.; Wang, F.; Sun, X.; Zhang, Y.; Liang, W.; Liu, J.Y.; Jiang, D.Y.; Lu, X.; Wang, W.; Zhang, L. Conditional deletion of Zeb1 in Csf1r+ cells reduces inflammatory response of the cornea to alkali burn. Iscience 2024, 27, 5. [Google Scholar] [CrossRef]
- Bian, F.; Xiao, Y.; Zaheer, M.; Volpe, E.A.; Pflugfelder, S.C.; Li, D.-Q.; De Paiva, C.S. Inhibition of NLRP3 inflammasome pathway by butyrate improves corneal wound healing in corneal alkali burn. Int. J. Mol. Sci. 2017, 18, 562. [Google Scholar] [CrossRef]
- Song, M.-S.; Ku, Y.A.; Kim, S.; Chung, M.H.; Kim, Y.H.; Kim, D.H. Comparison of corneal epithelial wound healing between topical RCI001, solcoseryl, and polydeoxyribonucleotide in the murine ocular alkali burn model. Korean J. Ophthalmol. KJO 2023, 37, 236. [Google Scholar] [CrossRef]
- Chang, J.-H.; Garg, N.K.; Lunde, E.; Han, K.-Y.; Jain, S.; Azar, D.T. Corneal neovascularization: An anti-VEGF therapy review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef]
- Brodovsky, S.C.; McCarty, C.A.; Snibson, G.; Loughnan, M.; Sullivan, L.; Daniell, M.; Taylor, H.R. Management of alkali burns: An 11-year retrospective review. Ophthalmology 2000, 107, 1829–1835. [Google Scholar] [CrossRef]
- Anderson, C.; Zhou, Q.; Wang, S. An alkali-burn injury model of corneal neovascularization in the mouse. J. Vis. Exp. JoVE 2014, 86, 51159. [Google Scholar]
- Stevenson, W.; Cheng, S.-F.; Dastjerdi, M.H.; Ferrari, G.; Dana, R. Corneal neovascularization and the utility of topical VEGF inhibition: Ranibizumab (Lucentis) vs. bevacizumab (Avastin). Ocul. Surf. 2012, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Liu, L.; Ma, X.; Zou, J. Topical vitamin C promotes the recovery of corneal alkali burns in mice. J. Ophthalmol. 2021, 2021, 2406646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, J.; Wang, Y.; Jin, J.; Zhou, H.; Chen, J.; Su, S.B. Activation of Toll-like receptor 3 promotes pathological corneal neovascularization by enhancement of SDF-1-mediated endothelial progenitor cell recruitment. Exp. Eye Res. 2019, 178, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Gong, Y.; Yang, L. Effect of nintedanib nanothermoreversible hydrogel on neovascularization in an ocular alkali burn rat model. Curr. Eye Res. 2022, 47, 1578–1589. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, S.; Ye, X.; Wang, Y.; Wang, Q.; Chen, Z.; Wang, Z.; Zhang, J.; Wang, Q.; Chen, L. Genome editing VEGFA prevents corneal neovascularization in vivo. Adv. Sci. 2024, 11, 2401710. [Google Scholar] [CrossRef]
- Liu, G.; Wu, H.; Lu, P.; Zhang, X. Interleukin (IL)-17A promotes angiogenesis in an experimental corneal neovascularization model. Curr. Eye Res. 2017, 42, 368–379. [Google Scholar] [CrossRef]
- Lu, P.; Li, L.; Liu, G.; Baba, T.; Ishida, Y.; Nosaka, M.; Kondo, T.; Zhang, X.; Mukaida, N. Critical role of TNF-α-induced macrophage VEGF and iNOS production in the experimental corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3516–3526. [Google Scholar] [CrossRef]
- Zhong, Y.-Y.; Zhang, H.-F.; Lu, X.-H. Rapamycin eye drops inhibit rat corneal neovascularization: Role of vascular endothelial growth factor and inflammatory factor. Chin. J. Tissue Eng. Res. 2010, 14, 2709. [Google Scholar]
- Kim, J.W.; Jeong, H.; Yang, M.-S.; Lim, C.W.; Kim, B. Therapeutic effects of zerumbone in an alkali-burned corneal wound healing model. Int. Immunopharmacol. 2017, 48, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.-J.; Guo, X.-X.; Li, J.; Pu, Q.; Li, X.-Y. Cyclopamine inhibits corneal neovascularization and fibrosis by alleviating inflammatory macrophage recruitment and endothelial cell activation. Int. Immunopharmacol. 2025, 147, 114025. [Google Scholar] [CrossRef] [PubMed]
- Sosne, G.; Christopherson, P.L.; Barrett, R.P.; Fridman, R. Thymosin-β4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2388–2395. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Wang, Y.; Meng, F.; Ying, M.; Han, R.; Hao, P.; Wang, L.; Li, X. Blockade of extracellular high-mobility group box 1 attenuates inflammation-mediated damage and haze grade in mice with corneal wounds. Int. Immunopharmacol. 2020, 83, 106468. [Google Scholar] [CrossRef]
- Yan, Y.; Xing, S.; Liu, J.; Yan, X.; Guan, Y.; Jiang, Z.; Zhang, W.; Li, X. Lycium barbarum glycopeptide reduces inflammation and fibrosis in corneal injury by modulating the NF-κB/NLRP3/IL-1 β signaling pathway and microRNA-21a-5p/SMAD7. Exp. Eye Res. 2025, 257, 110438. [Google Scholar] [CrossRef]
- Mayangsari, D.; Agustini, L.; Fatmariyanti, S.; Ridholia, R.; Lestari, P. Expression of Matrix Metalloproteinase-9, Transforming Growth Factor Beta and Fibroblast in The Simblefaron Due to Alkali Burn: Literature Review. Pharmacogn. J. 2024, 16, 687–690. [Google Scholar] [CrossRef]
- Kiristioglu, M.O.; Baykara, M.; Yavas, O.; Kupeli, Z.A.; Ozyigit, M.O. The effect of platelet-rich plasma and sodium alginate hydrogel on corneal wound healing after corneal alkali burns in rats with computer-assisted anterior segment optical coherence tomography image analysis. Exp. Eye Res. 2024, 247, 110044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Nan, Y.; Yang, Y.; Li, X.; Jiang, W.; Jiao, T.; Li, J.; Jia, X.; Ye, M.; Niu, Y. Exploring the role of Lycium barbarum polysaccharide in corneal injury repair and investigating the relevant mechanisms through in vivo and in vitro experiments. Molecules 2023, 29, 49. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Sang, W.; Liu, F.; Yu, H.; Zhou, R.; Belin, M.W.; Zloty, P.; Chen, Y. High MMP-9 Expression May Contribute to Retroprosthetic Membrane Formation after KPro Implantation in Rabbit Corneal Alkali Burn Model. J. Ophthalmol. 2016, 2016, 1094279. [Google Scholar] [CrossRef]
- Emu, M.; Soleimani, M.; Baharnoori, M.; Djalilian, A.; Hatami-Marbini, H. In vivo Removal of KS GAGs from murine cornea by intrastromal injection of keratanase II enzyme. Exp. Eye Res. 2025, 259, 110518. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Emu, M. The role of KS GAGs in the microstructure of CXL-treated corneal stroma; a transmission electron microscopy study. Exp. Eye Res. 2023, 231, 109476. [Google Scholar] [CrossRef]
- Cho, B.-J.; Hwang, J.S.; Shin, Y.J.; Kim, J.W.; Chung, T.-Y.; Hyon, J.Y. Rapamycin rescues endoplasmic reticulum stress–induced dry eye syndrome in mice. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1254–1264. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Kutlehria, S.; Hernandez, H.; de Souza, R.G.; Lee, A.; Yu, Z.; Pflugfelder, S.C.; Singh, M.; de Paiva, C.S. Rapamycin eyedrops increased CD4+ Foxp3+ cells and prevented goblet cell loss in the aged ocular surface. Int. J. Mol. Sci. 2020, 21, 8890. [Google Scholar] [CrossRef]
- Bhujel, B.; Oh, S.-H.; Kim, C.-M.; Yoon, Y.-J.; Chung, H.-S.; Ye, E.-A.; Lee, H.; Kim, J.-Y. Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review. Bioengineering 2023, 11, 39. [Google Scholar] [CrossRef]
- Park, G.W.; Heo, J.; Kang, J.Y.; Yang, J.W.; Kim, J.S.; Kwon, K.D.; Yu, B.C.; Lee, S.J. Topical cell-free conditioned media harvested from adipose tissue-derived stem cells promote recovery from corneal epithelial defects caused by chemical burns. Sci. Rep. 2020, 10, 12448. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Zhang, X.; Yang, S.; Peng, G.; Wu, T.; Zhou, Y.; Huang, C.; Reinach, P.S.; Li, W. MK2 inhibitor reduces alkali burn-induced inflammation in rat cornea. Sci. Rep. 2016, 6, 28145. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, J.; Li, S.; Li, W.; Wang, B.; Deng, Y.; Yuan, J. The long-term effect of tacrolimus on alkali burn-induced corneal neovascularization and inflammation surpasses that of anti-vascular endothelial growth factor. Drug Des. Dev. Ther. 2018, 12, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, M.; Liu, Y.; Hu, D.; Gu, L.; Fei, X.; Zhang, J. Effect of rapamycin microspheres in Sjögren syndrome dry eye: Preparation and outcomes. Ocul. Immunol. Inflamm. 2019, 27, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Gidfar, S.; Milani, F.Y.; Milani, B.Y.; Shen, X.; Eslani, M.; Putra, I.; Huvard, M.J.; Sagha, H.; Djalilian, A.R. Rapamycin prolongs the survival of corneal epithelial cells in culture. Sci. Rep. 2017, 7, 40308. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhujel, B.; Hur, W.; Lee, S.; Lee, H.; Chung, H.S.; Kim, J.Y. Rapamycin Mitigates Corneal Damage in a Mouse Model of Alkali Burn Injury. Bioengineering 2025, 12, 998. https://doi.org/10.3390/bioengineering12090998
Bhujel B, Hur W, Lee S, Lee H, Chung HS, Kim JY. Rapamycin Mitigates Corneal Damage in a Mouse Model of Alkali Burn Injury. Bioengineering. 2025; 12(9):998. https://doi.org/10.3390/bioengineering12090998
Chicago/Turabian StyleBhujel, Basanta, Woojune Hur, Seorin Lee, Hun Lee, Ho Seok Chung, and Jae Yong Kim. 2025. "Rapamycin Mitigates Corneal Damage in a Mouse Model of Alkali Burn Injury" Bioengineering 12, no. 9: 998. https://doi.org/10.3390/bioengineering12090998
APA StyleBhujel, B., Hur, W., Lee, S., Lee, H., Chung, H. S., & Kim, J. Y. (2025). Rapamycin Mitigates Corneal Damage in a Mouse Model of Alkali Burn Injury. Bioengineering, 12(9), 998. https://doi.org/10.3390/bioengineering12090998







