Development of the Direct Deuteration Method for Amino Acids and Characterization of Deuterated Tryptophan
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Configuration of a Simplified Deuteration Apparatus Used for Deuteration
2.3. Procedure for Deuteration of Amino Acids
2.4. Analytical Methods Using NMR to Determine the Deuteration Level of Amino Acids
2.5. Measurement of Optical Rotation
2.6. Liquid Chromatography Setup for Enantiomeric Separation
2.7. Fluorescence Measurement Method for Evaluating UV-Induced Degradation and Acid-Induced Changes
2.8. Crystallization
3. Results and Discussion
3.1. Deuteration of 20 Proteinogenic Amino Acids
3.2. Chiral Separation of Deuterated Tryptophan
3.3. Physicochemical Characterization of Deuterated Tryptophan
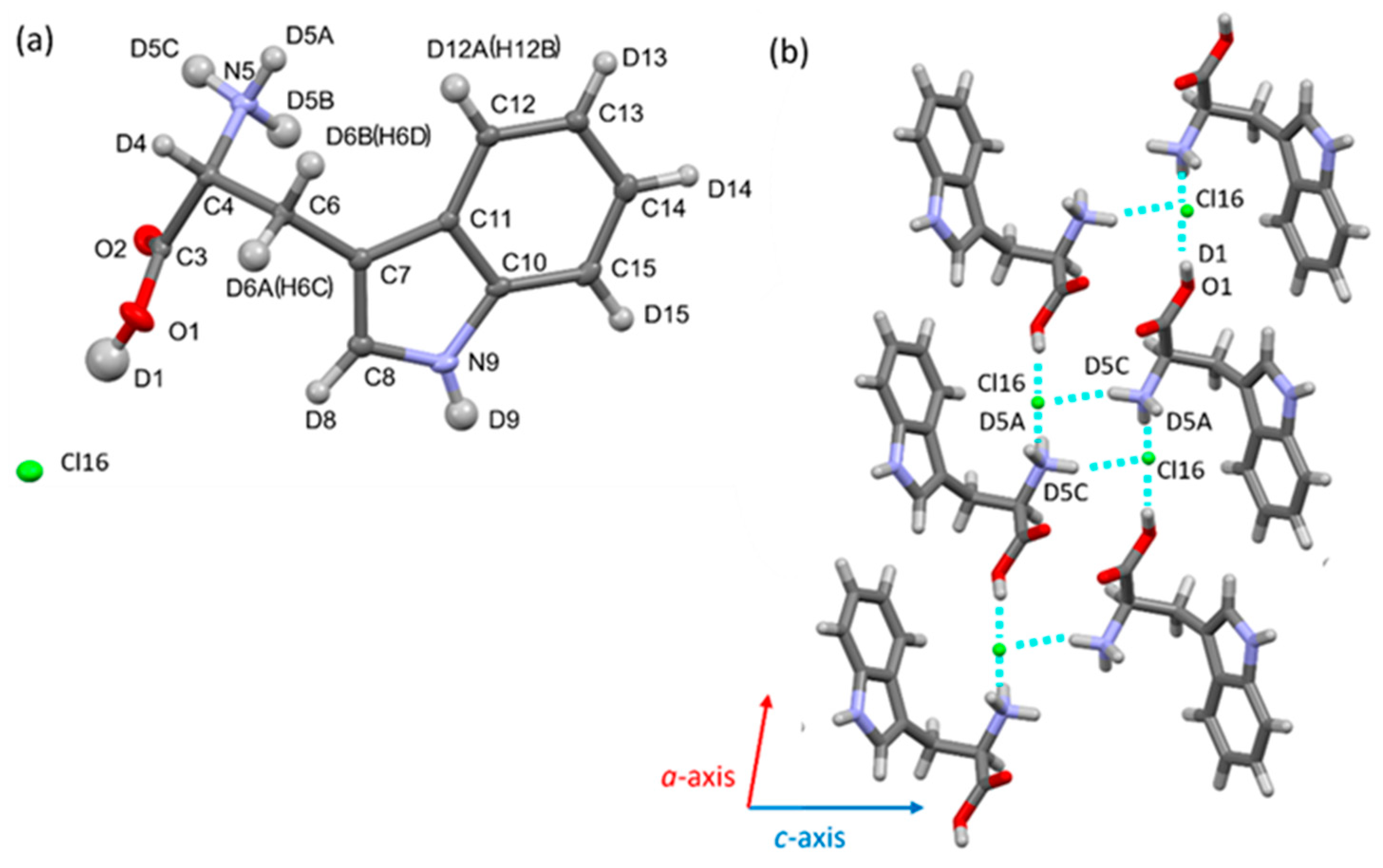
3.4. Structural Comparison Between Protonated and Deuterated Tryptophan Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L-Trp | The optically active L-form of tryptophan |
| D-Trp | The optically active D-form of tryptophan |
| h-Trp | Protiated tryptophan |
| d-Trp | Deuterated tryptophan |
| d-L-Trp | Deuterated L-form of tryptophan |
| d-D-Trp | Deuterated D-form of tryptophan |
References
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Matsuda, Y.; Mine, A.; Shikida, N.; Takahashi, K.; Miyairi, K.; Shimbo, K.; Kikuchi, Y.; Konishi, A. Single-chain tandem macrocyclic peptides as a scaffold for growth factor and cytokine mimetics. Commun. Biol. 2022, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Schousboe, A.; Bak, L.K.; Waagepetersen, H.S. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front. Endocrinol. 2013, 4, 102. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Wang, D.; Kim, A.K.; Lau, E.; Lin, A.J.; Liem, D.A.; Zhang, J.; Zong, N.C.; Lam, M.P.Y.; Ping, P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol. Cell. Proteom. 2012, 11, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Harbeson, S.L.; Tung, R.D. Deuterium medicinal chemistry: A new approach to drug discovery and development. MedChem News 2014, 24, 8–22. [Google Scholar]
- Russak, E.M.; Bednarczyk, E.M. Impact of deuterium substitution on the pharmacokinetics of pharmaceuticals. Ann. Pharmacother. 2019, 53, 211–216. [Google Scholar] [CrossRef]
- Oksanen, E.; Chen, J.C.-H.; Fisher, S.Z. Neutron crystallography for the study of hydrogen bonds in macromolecules. Molecules 2017, 22, 596. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, C.; Shimizu, R.; Kagotani, Y.; Ostermann, A.; Schrader, T.E.; Adachi, M. Direct observation of the protonation states in the mutant green fluorescent protein. J. Phys. Chem. Lett. 2020, 11, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Krueger, S. Small-angle neutron scattering contrast variation studies of biological complexes: Challenges and triumphs. Curr. Opin. Struct. Biol. 2022, 74, 102375. [Google Scholar] [CrossRef]
- Bradshaw, J.P. Orientation of the headgroup of phosphatidylinositol in a model biomembrane as determined by neutron diffraction. Biochemistry 1999, 38, 8393–8401. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, A.T.; Cedergren, R.J.; Hiscock, B.F.; Harpp, D.N. Synthesis of Highly Deuterated Amino Acids. Proc. Natl. Acad. Sci. USA 1966, 55, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Zhang, S.; Ma, L.; Zeng, Z.; Zhou, Z.; Gandon, V.; Xu, H.; Yi, W.; Wang, S. Access to N-α-deuterated amino acids and DNA conjugates via Ca(II)-HFIP-mediated reductive deutero-amination of α-oxo-carbonyl compounds. Nat. Commun. 2025, 16, 1816. [Google Scholar] [CrossRef] [PubMed]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological Production of Amino Acids and Derivatives: Current Status and Prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mosin, O.V.; Shvets, V.I.; Skladnev, D.A.; Ignatov, I. Microbial synthesis of 2H-labelled L-phenylalanine with different levels of isotopic enrichment by a facultative methylotrophic bacterium Brevibacterium methylicum with RuMP assimilation of carbon. Biochem. Moscow Suppl. Ser. B 2013, 7, 247–258. [Google Scholar] [CrossRef]
- Chun, S.W.; Narayan, A.R.H. Biocatalytic, Stereoselective Deuteration of α-Amino Acids and Methyl Esters. ACS Catal. 2020, 10, 7413–7418. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, C.; Hai, Y. Stereoselective Biocatalytic α-Deuteration of L-Amino Acids by a Pyridoxal 5′-Phosphate-Dependent Mannich Cyclase. ChemBioChem 2023, 24, e202300561. [Google Scholar] [CrossRef] [PubMed]
- Doyon, T.J.; Buller, A.R. Site-Selective Deuteration of Amino Acids through Dual-Protein Catalysis. J. Am. Chem. Soc. 2022, 144, 7327–7336. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Krishnakumar, V.; Gunanathan, C. Selective α-Deuteration of Amines and Amino Acids Using D2O. Org. Lett. 2016, 18, 5892–5895. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, A.; Rodrigues, F.; Roche, M. Development and Scale-Up of Stereoretentive α-Deuteration of Amines. Org. Process Res. Dev. 2017, 21, 1741–1744. [Google Scholar] [CrossRef]
- Moozeh, K.; So, S.M.; Chin, J. Catalytic Stereoinversion of L-Alanine to Deuterated D-Alanine. Angew. Chem. Int. Ed. 2015, 127, 9513–9517. [Google Scholar] [CrossRef]
- Sawama, Y.; Matsuda, T.; Moriyama, S.; Ban, K.; Fujioka, H.; Kamiya, M.; Shou, J.; Ozeki, Y.; Akai, S.; Sajiki, H. Unprecedented regioselective deuterium-incorporation of alkyltrimethylammonium chlorides and Raman analysis. Asian J. Org. Chem. 2023, 12, e202200710. [Google Scholar] [CrossRef]
- Sawama, Y.; Park, K.; Yamada, T.; Sajiki, H. New gateways to the platinum group metal-catalyzed direct deuterium-labeling method utilizing hydrogen as a catalyst activator. Chem. Pharm. Bull. 2018, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Ohtaki, R.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Novel Pd/C-catalyzed redox reactions between aliphatic secondary alcohols and ketones under hydrogenation conditions: Application to H–D exchange reaction and the mechanistic study. J. Org. Chem. 2007, 72, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Ihara, T.; Saito, T.; Kato, H.; Kinugasa, S. Investigation of Analytical Conditions for Accurate Quantitative NMR Measurements. Bunseki Kagaku 2012, 61, 963–967. [Google Scholar] [CrossRef]
- Sawama, Y.; Mori, M.; Yamada, T.; Monguchi, Y.; Sajiki, H. Hydrogen Self-Sufficient Arene Reduction to Cyclohexane Derivatives Using a Combination of Platinum on Carbon and 2-Propanol. Adv. Synth. Catal. 2015, 357, 3667–3670. [Google Scholar] [CrossRef]
- Akutsu-Suyama, K.; Ueda, M.; Shibazaki, C.; Fisher, Z. Development of a Simple and Easy Chemical Deuteration System. CROSS Rep. 2024, 2, 1–5. [Google Scholar]
- Shibazaki, C.; Suzuki, H.; Ikegami, T.; Yoshida, K.; Oku, T.; Adachi, M.; Akutsu-Suyama, K. Recycling of Used Heavy Water and Its Application to Amino Acid Deuteration. JSP Conf. Proc. 2024. preprint. [Google Scholar]
- Munz, D.; Webster-Gardiner, M.; Fu, R.; Strassner, T.; Goddard, W.A., III; Gunnoe, T.B. Proton or metal? The H/D exchange of arenes in acidic solvents. ACS Catal. 2015, 5, 769–775. [Google Scholar] [CrossRef]
- Patel, M.; Saunthwal, R.K.; Verma, A.K. Base-mediated deuteration of organic molecules: A mechanistic insight. ACS Omega 2018, 3, 10612–10623. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Aoki, F.; Mizumoto, T.; Maejima, T.; Esaki, H.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Facile and convenient method of deuterium gas generation using a Pd/C-catalyzed H2-D2 exchange reaction and its application to synthesis of deuterium-labeled compounds. Chem. Eur. J. 2008, 14, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, G.J.; Sak, M.H.; Jacobsen, E.N. Catalysis of an SN2 pathway by geometric preorganization. Nature 2024, 632, 1052. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E. Effects of L-tryptophan on sleepiness and on sleep. J. Psychiatr. Res. 1982, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatr. Neurosci. 2007, 32, 394–399. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Onoue, S.; Tsuda, Y. Photoreactivity of Amino Acids: Tryptophan-Induced Photochemical Events via Reactive Oxygen Species Generation. Anal. Sci. 2007, 23, 829–833. [Google Scholar] [CrossRef]
- Friedman, M.; Finley, J.W. Methods of tryptophan analysis. J. Agric. Food Chem. 1971, 19, 626–631. [Google Scholar] [CrossRef]
- Friedman, M.; Cuq, J.-L. Chemistry, analysis, nutritional value, and toxicology of tryptophan in food. A review. J. Agric. Food Chem. 1988, 36, 1079–1093. [Google Scholar] [CrossRef]
- Ohta, T. The Decomposition of Tryptophan in Acid Solutions: Specific Effect of Hydrochloric Acid. Chem. Pharm. Bull. 1981, 29, 1767–1771. [Google Scholar] [CrossRef]
- Gotoh, Y.; Shibata, T. Heat Decomposition of Amino Acids with Autoclave (Part 1). Jpn. J. Home Econ. 1970, 21, 428–431. [Google Scholar]
- Schöneich, C. Novel Chemical Degradation Pathways of Proteins Mediated by Tryptophan Oxidation: Tryptophan Side Chain Fragmentation. J. Pharm. Pharmacol. 2018, 70, 655–665. [Google Scholar] [CrossRef]
- Zhang, K.; Fei, W.; Ji, J.; Yang, Y. Degradation of Tryptophan by UV Irradiation: Influencing Parameters and Mechanisms. Water 2021, 13, 2368. [Google Scholar] [CrossRef]
- Fujii, N.; Uchida, H.; Saito, T. The Damaging Effect of UV-C Irradiation on Lens α-Crystallin. Mol. Vis. 2004, 10, 814–820. [Google Scholar] [PubMed]
- Kimura, Y.; Kanematsu, Y.; Sakagami, H.; Rivera Rocabado, D.S.; Shimazaki, T.; Tachikawa, M.; Ishimoto, T. Hydrogen/Deuterium Transfer from Anisole to Methoxy Radicals: A Theoretical Study of a Deuterium-Labeled Drug Model. J. Phys. Chem. A 2022, 126, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Xue, B.; Rehak, P.; Jacoby, G.; Ji, W.; Shimon, L.J.W.; Beck, R.; Král, P.; Cao, Y.; Gazit, E. Self-Assembly of Aromatic Amino Acid Enantiomers into Supramolecular Materials of High Rigidity. ACS Nano 2020, 14, 1694–1706. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Messerschmidt, M.; Luger, P. Crystal Structure of DL-Tryptophan at 173 K. Cryst. Res. Technol. 2004, 39, 274–278. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, Y. Solid Tryptophan as a Pseudoracemate: Physicochemical and Crystallographic Characterization. Chirality 2015, 27, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Ashida, T.; Sasada, Y.; Kakudo, M. The Crystal Structures of L-Tryptophan Hydrochloride and Hydrobromide. Bull. Chem. Soc. Jpn. 1966, 39, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhang, L.; Xu, M.; Liu, B.; Sun, X. Effect of Deuterium Content on the Structural, Optical, and Thermal Properties of DKDP Crystals: A Systematic Analysis. RSC Adv. 2024, 14, 26115–26122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hanson, B.L.; Langan, P.; Viola, R.E. The Effect of Deuteration on Protein Structure: A High-Resolution Comparison of Hydrogenous and Perdeuterated Haloalkane Dehalogenase. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 1000–1008. [Google Scholar] [CrossRef] [PubMed]


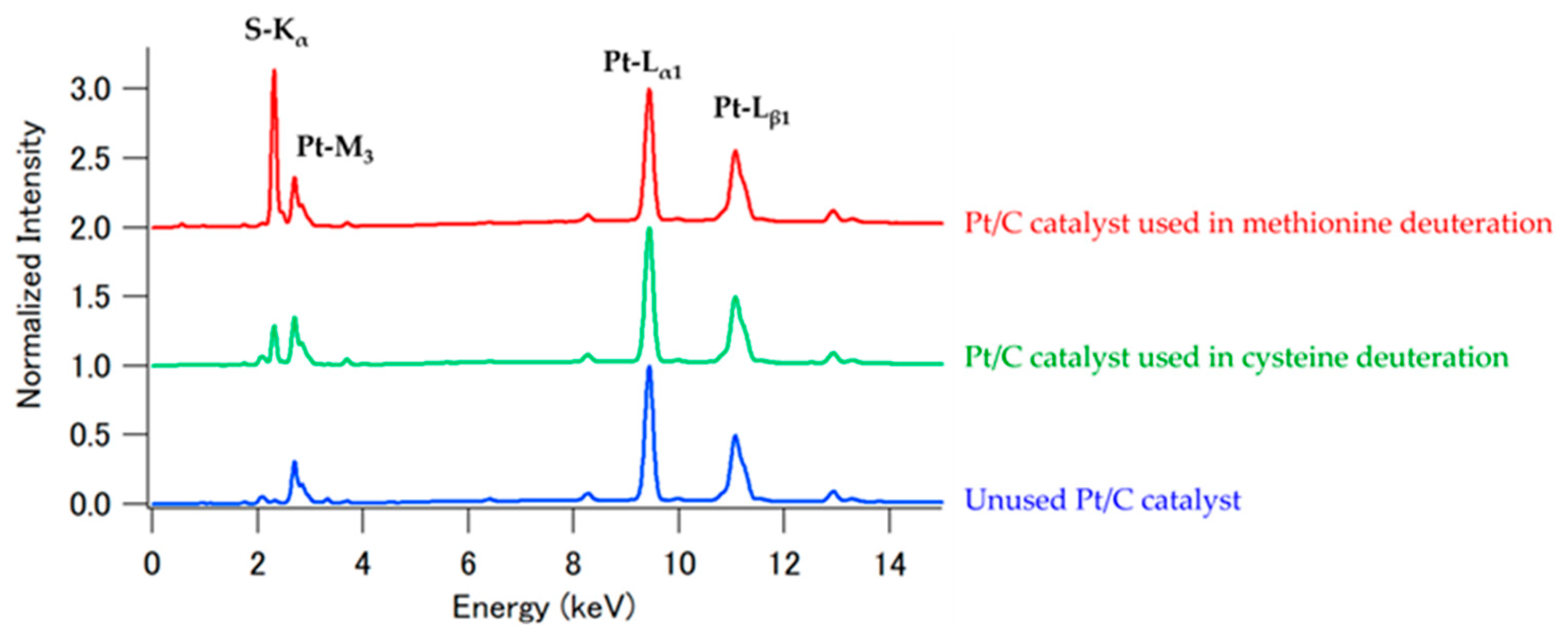

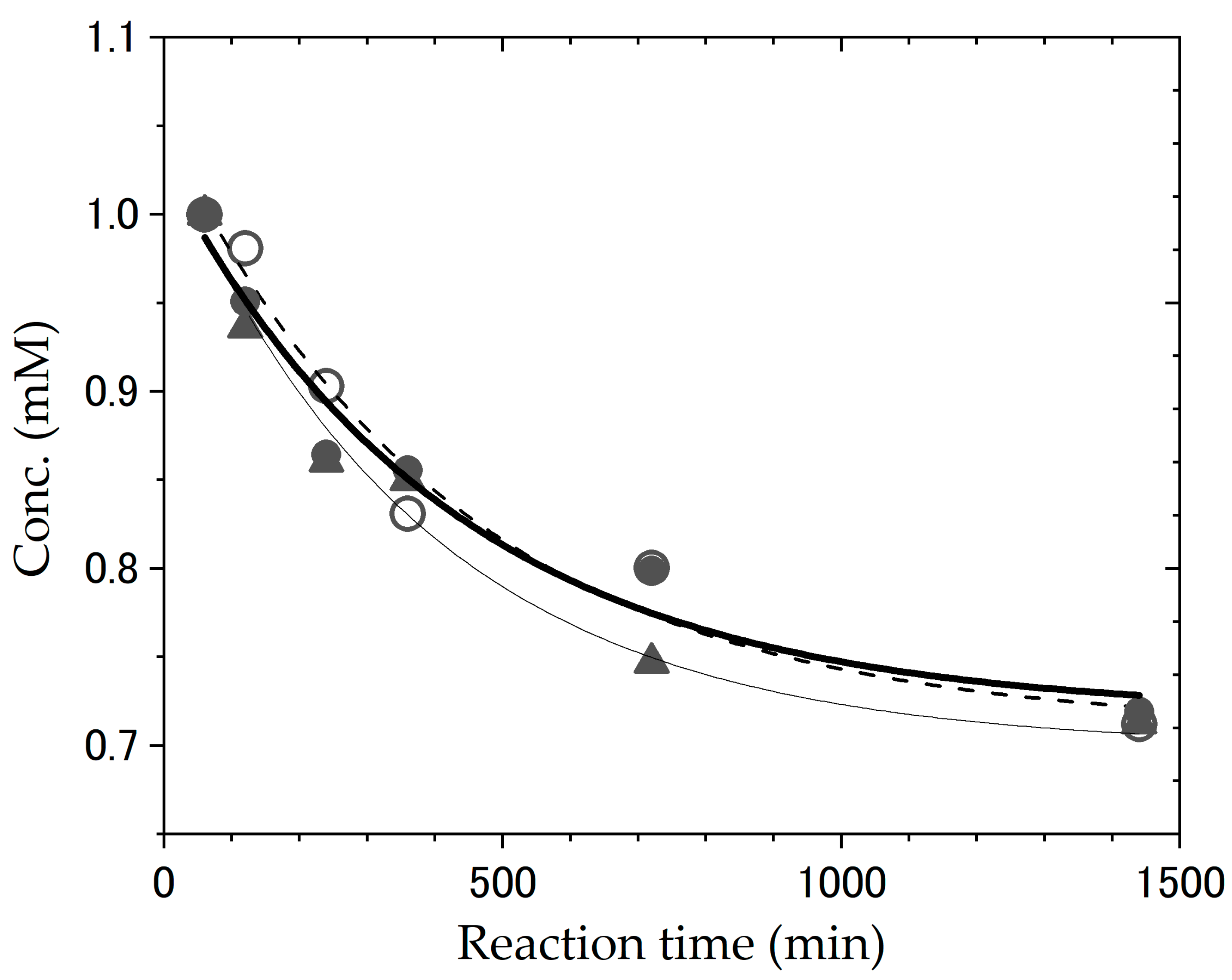
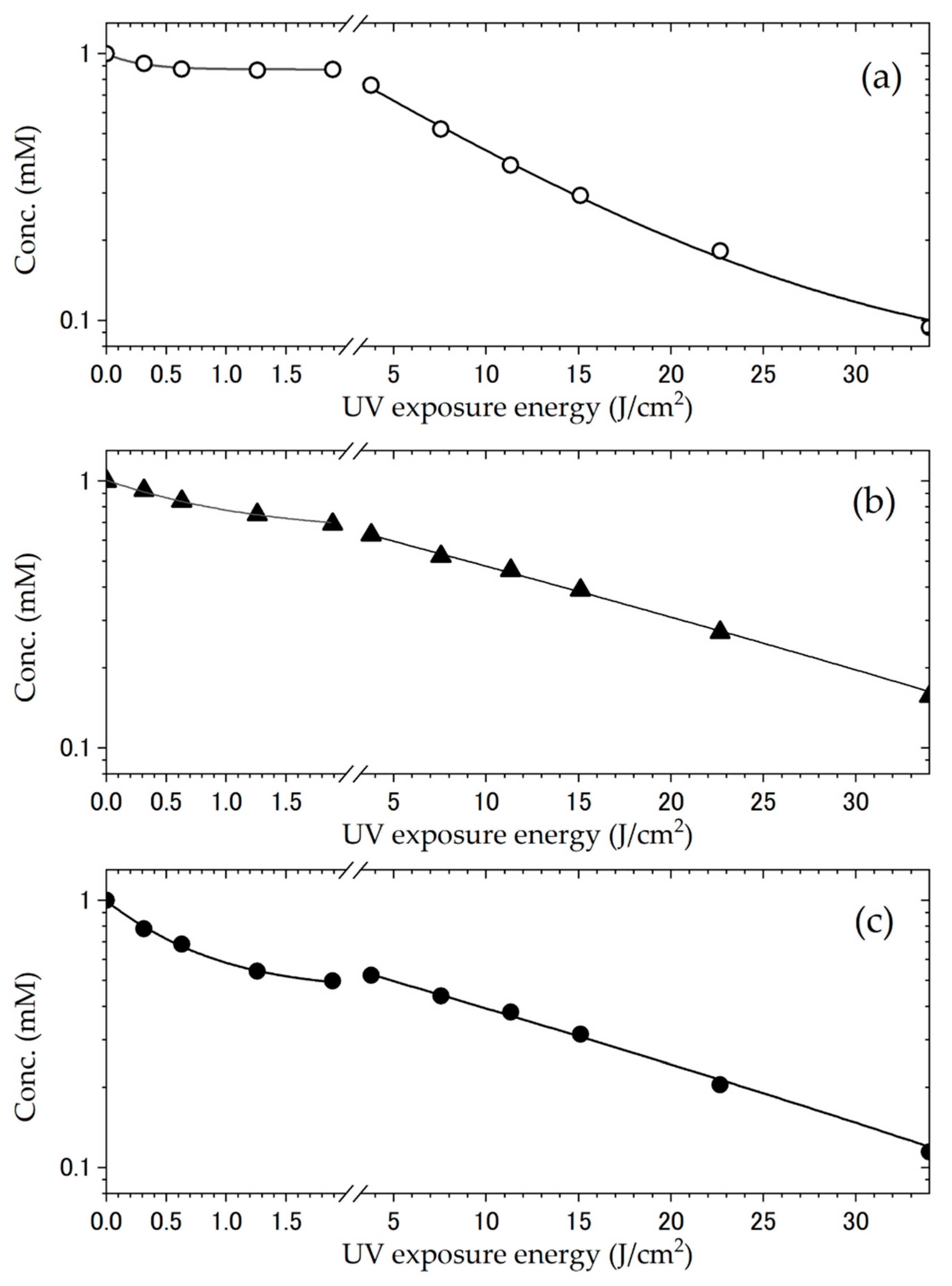
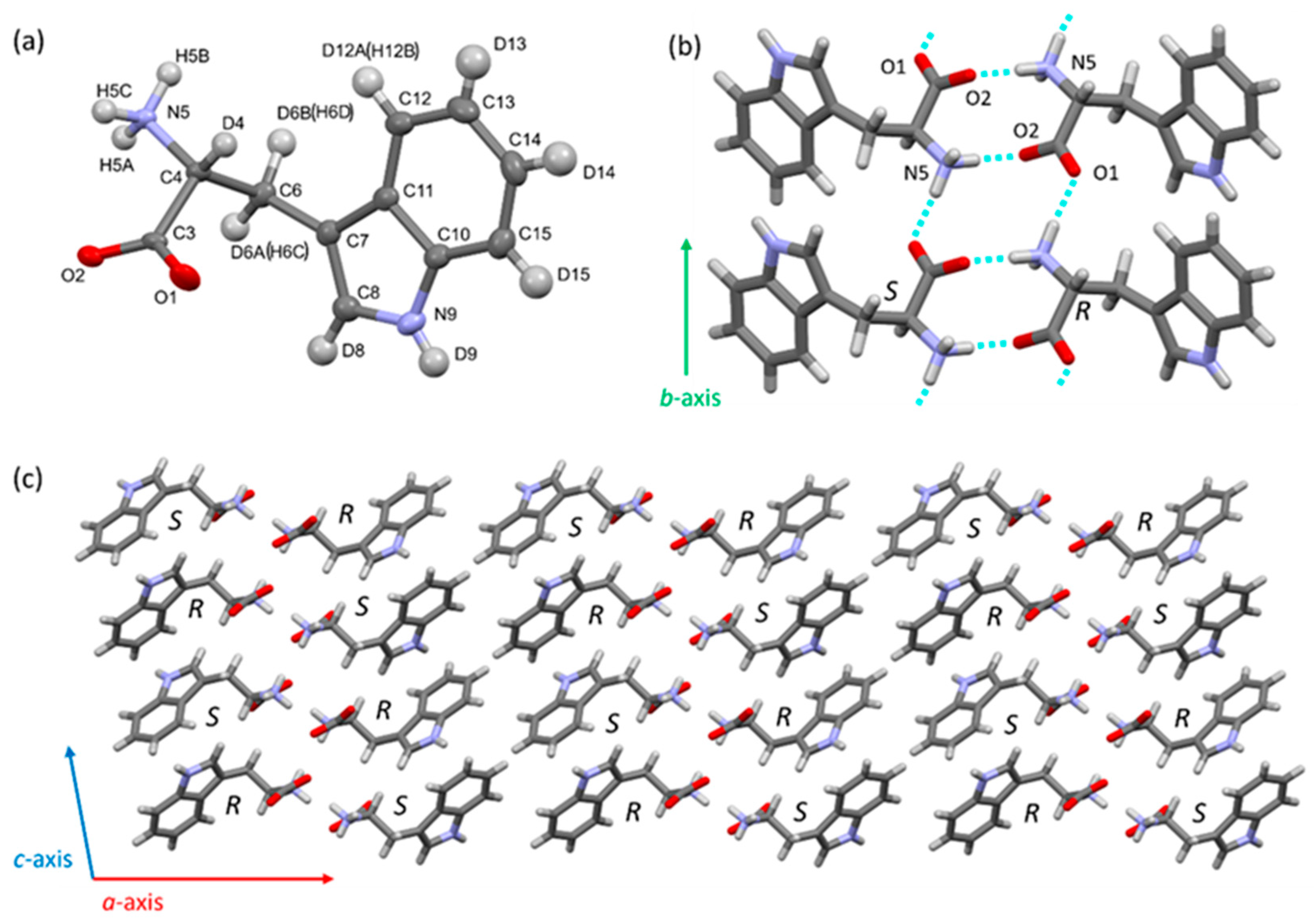
| Reaction Temperature | Racemization | Deuterated Site | Catalyst |
|---|---|---|---|
| 37 °C | Non-racemizing | Partially deuterated (α- and/or β-position) | Enzyme [18,19,20] |
| 70–135 °C | Non-racemizing | Partially deuterated (α-position) | Ru catalysts [14,15] |
| Room temperature | Racemizing | Partially deuterated (α-position) | Organocatalyst [23] |
| 100–230 °C | Racemizing | Fully deuterated | Pt/C (This work) |
| Sample | [α]D20 (deg·mL·g−1·dm−1) ± SD (20 °C, 598 nm) |
|---|---|
| L-Tryptophan (h-L-Trp) | −32.5925 ± 0.0199 |
| Deuterated tryptophan (d-Trp) | −1.6525 ± 0.2656 |
| Sample | kobs (First Reaction) and ΔEa | kobs (Second Reaction) and ΔEa |
|---|---|---|
| h-L-Trp | 0.230 min−1 | 0.0061 min−1 |
| d40-Trp (40% deuterated) | 0.065 min−1, 0.74 kcal/mol | 0.0024 min−1, 0.61 kcal/mol |
| d70-Trp (70% deuterated) | 0.080 min−1, 0.55 kcal/mol | 0.0027 min−1, 0.48 kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibazaki, C.; Sugiyama, H.; Ueda, M.; Oku, T.; Adachi, M.; Fisher, Z.; Akutsu-Suyama, K. Development of the Direct Deuteration Method for Amino Acids and Characterization of Deuterated Tryptophan. Bioengineering 2025, 12, 981. https://doi.org/10.3390/bioengineering12090981
Shibazaki C, Sugiyama H, Ueda M, Oku T, Adachi M, Fisher Z, Akutsu-Suyama K. Development of the Direct Deuteration Method for Amino Acids and Characterization of Deuterated Tryptophan. Bioengineering. 2025; 12(9):981. https://doi.org/10.3390/bioengineering12090981
Chicago/Turabian StyleShibazaki, Chie, Haruki Sugiyama, Misaki Ueda, Takayuki Oku, Motoyasu Adachi, Zoë Fisher, and Kazuhiro Akutsu-Suyama. 2025. "Development of the Direct Deuteration Method for Amino Acids and Characterization of Deuterated Tryptophan" Bioengineering 12, no. 9: 981. https://doi.org/10.3390/bioengineering12090981
APA StyleShibazaki, C., Sugiyama, H., Ueda, M., Oku, T., Adachi, M., Fisher, Z., & Akutsu-Suyama, K. (2025). Development of the Direct Deuteration Method for Amino Acids and Characterization of Deuterated Tryptophan. Bioengineering, 12(9), 981. https://doi.org/10.3390/bioengineering12090981







