Evaluation of Surgical Protocols for Speech Improvement in Children with Cleft Palate: A Systematic Review and Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
- -
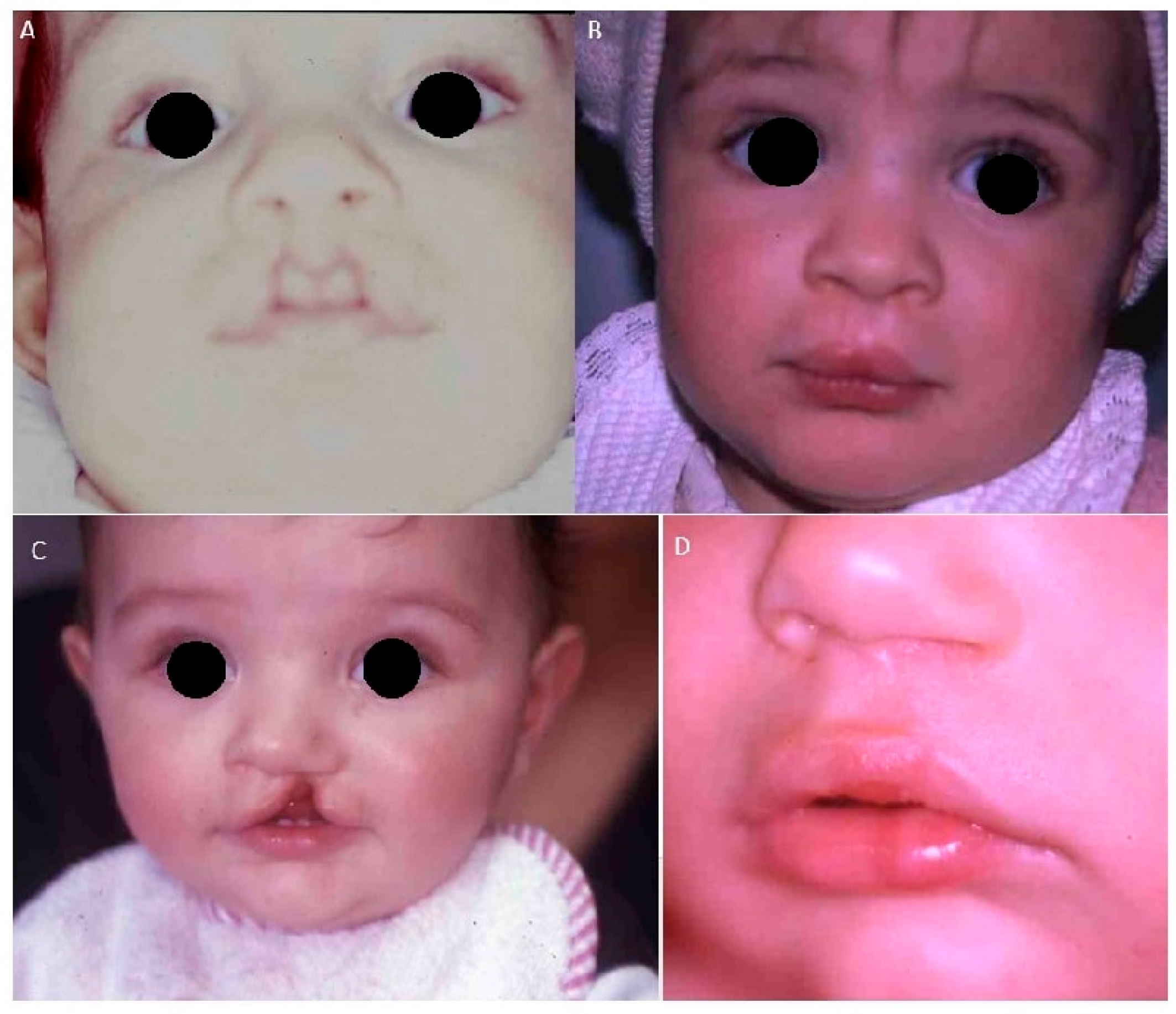
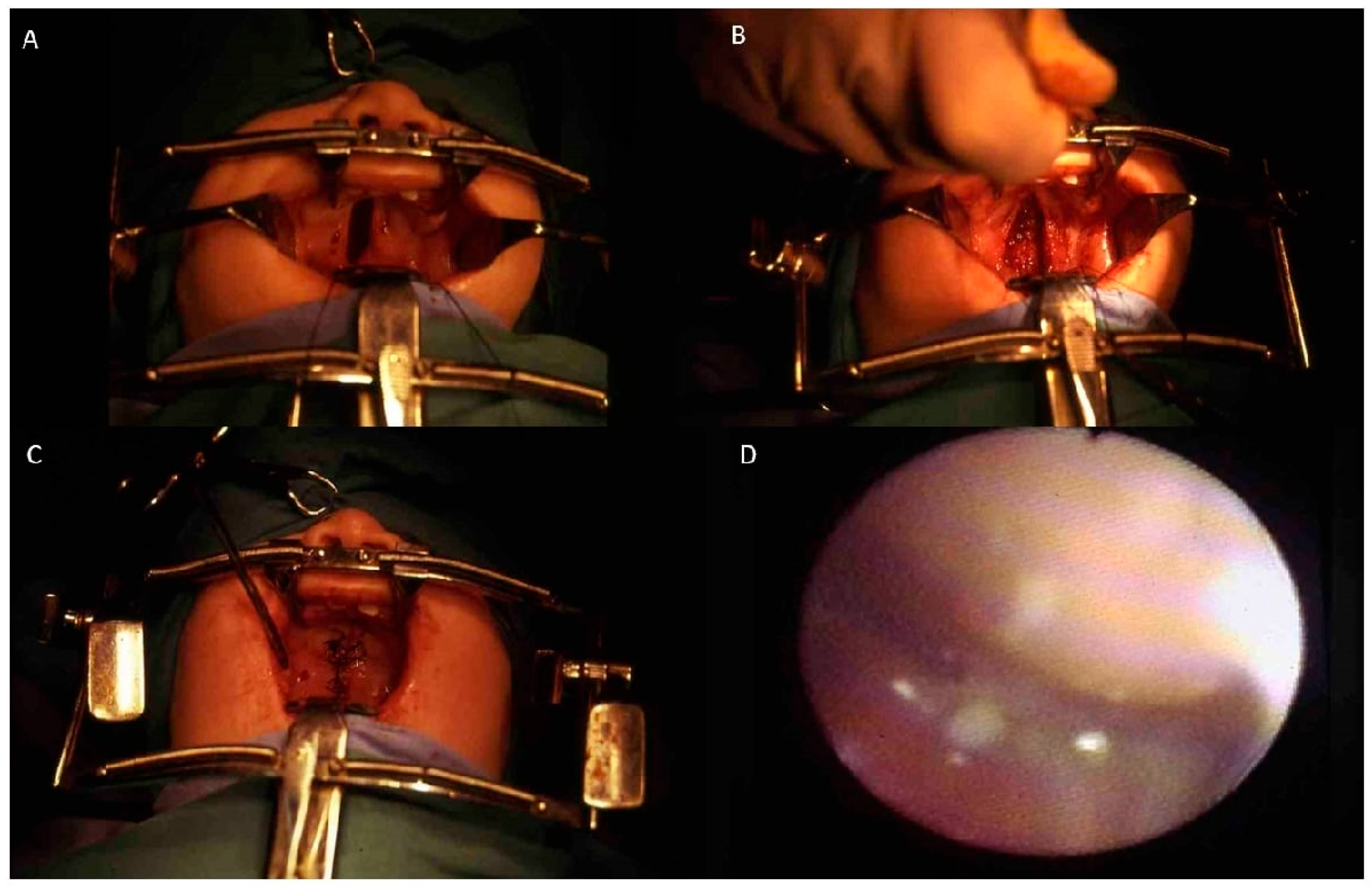
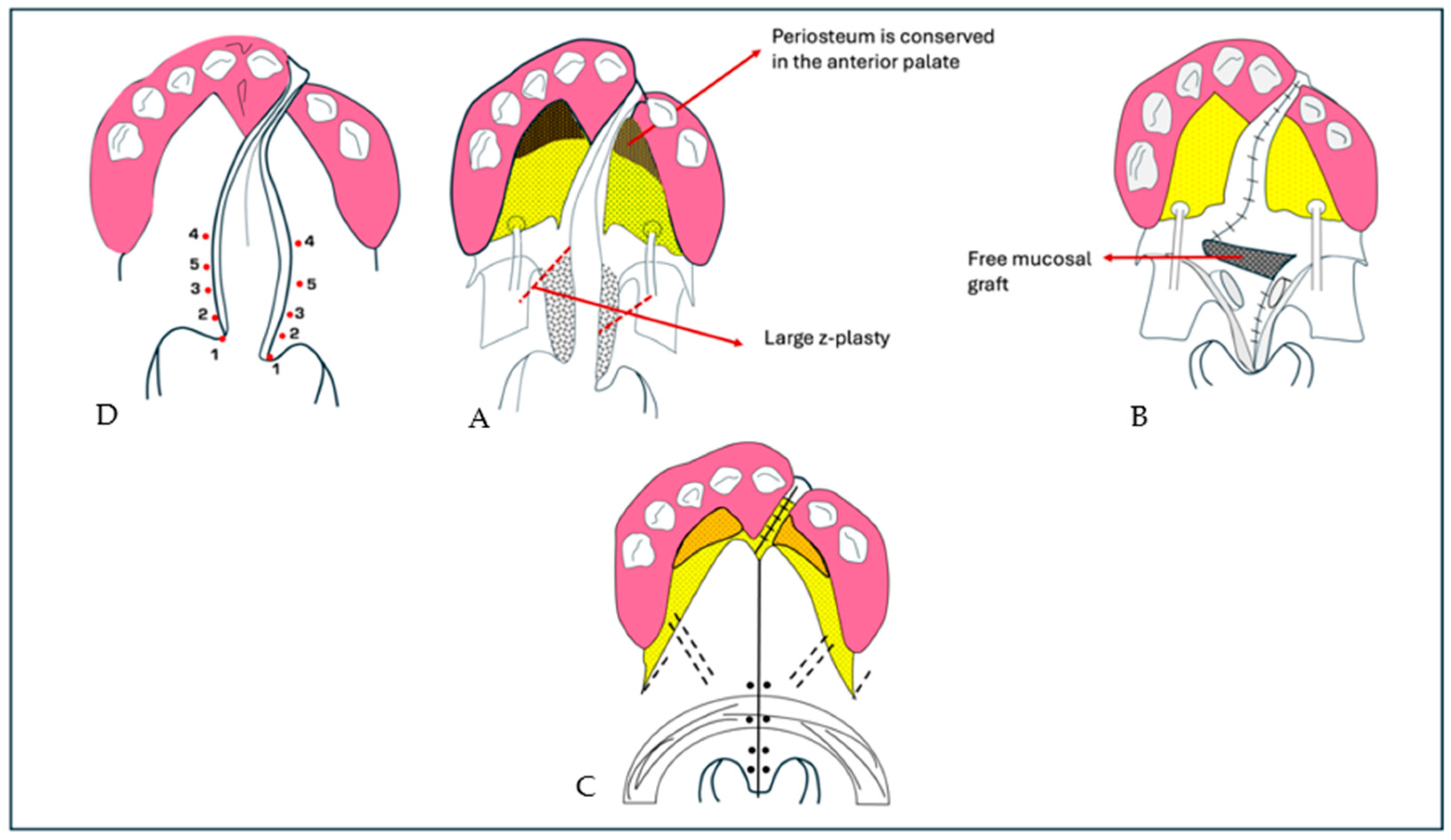
- Case 1: Two 6-month-old patients with cleft lip underwent cleft repair surgery using a modified Millard technique. At one-year follow-up, good-quality scars without retraction were observed, indicating a successful outcome.
- -
- Case 2: A 6-month-old patient with unilateral cleft of the soft palate underwent cleft repair surgery using intravelar veloplasty. Postoperative evaluation confirmed complete closure of the soft palate and adequate velopharyngeal competence.
2.2. Search Strategy

2.3. Inclusion and Exclusion Criteria
- Population (P): Children with cleft palate undergoing surgical intervention for speech improvement.
- Intervention (I): Different surgical protocols for cleft palate correction, including the following:
- -
- Sommerlad technique;
- -
- Von Langenbeck palatoplasty;
- -
- Furlow’s double-opposing Z-plasty;
- -
- Modified V-Y palatoplasty;
- -
- Two-stage palatoplasty (IVVP and Von Langenbeck);
- -
- Vomeroplasty.
- Comparison (C): Comparison between different surgical techniques or timing of interventions:
- -
- Single-stage vs. two-stage palatoplasty;
- -
- Early vs. delayed hard palate closure;
- -
- Techniques with mucosal graft vs. without mucosal graft.
- Outcome (O): Speech outcomes including the following:
- -
- Velopharyngeal insufficiency (VPI);
- -
- Nasal resonance;
- -
- Consonant competence (PCC);
- -
- Speech intelligibility;
- -
- Postoperative complications (oronasal fistulas, wound dehiscence);
- -
- Need for secondary surgical interventions.
2.4. Data Screening and Selection
3. Results
Quality Assessment
- Case Series
4. Discussion
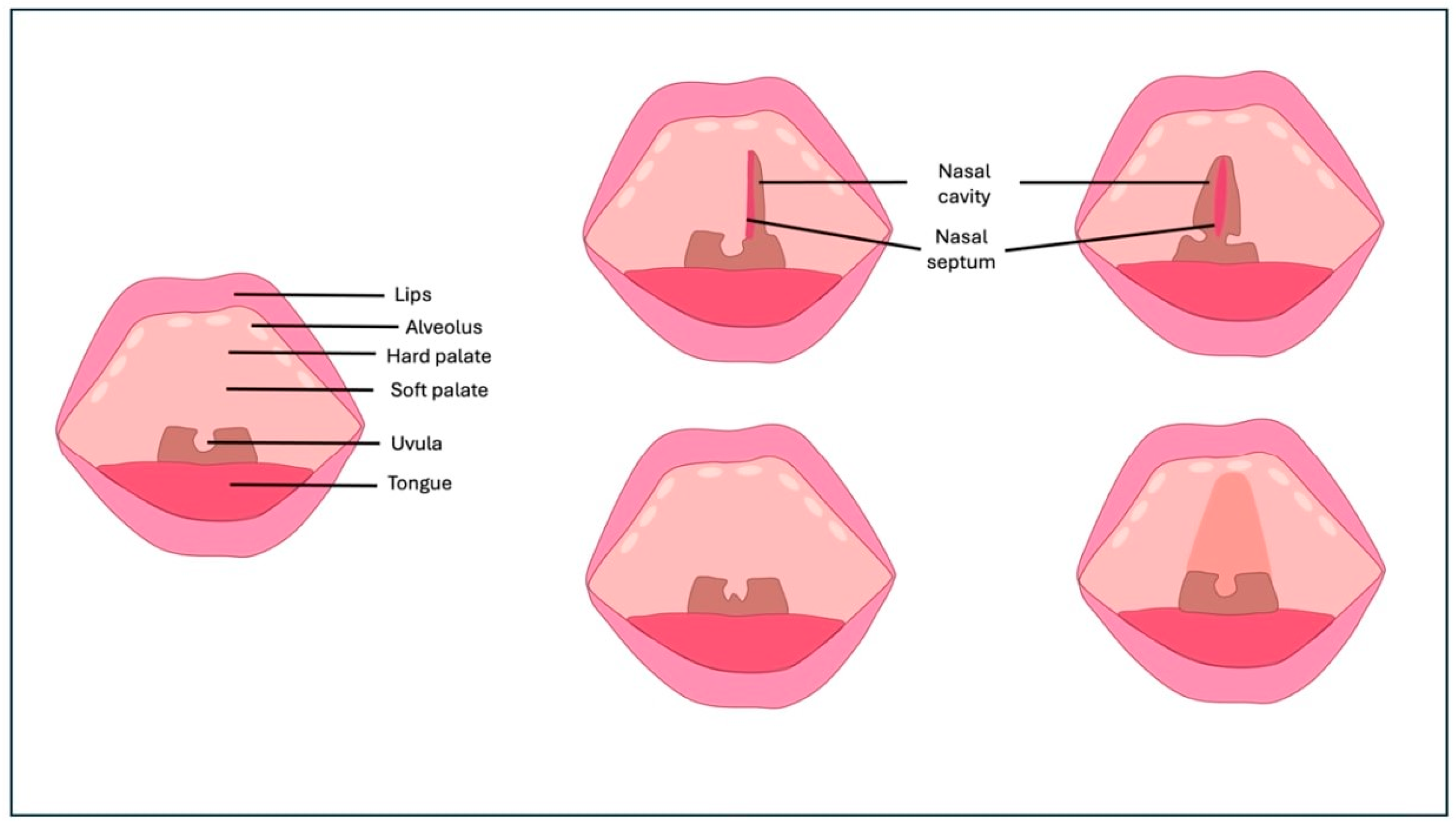
4.1. CP and Speech
4.2. Palatoplasty Techniques and Speech Outcomes
4.3. Timing of CP Treatment and Speech Outcomes
5. Conclusions
5.1. Limitations of the Study
5.2. Future Objectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OFC | Orofacial cleft |
| CL | Cleft lip |
| CP | Cleft palate |
| CL/P | Cleft lip and palate |
| VPI | Velopharyngeal insufficiency |
| IVVP | Intravelar veloplasty |
| PCC | Percent consonant correct |
| FON | Oronasal fistulas |
| NSI | Nasal Severity Index |
| UCLP | Unilateral cleft lip and palate |
| RCT | Randomized controlled trial |
References
- Abdel-Aziz, M.; El-Hoshy, H.; Ghandour, H. Treatment of Velopharyngeal Insufficiency after Cleft Palate Repair Depending on the Velopharyngeal Closure Pattern. J. Craniofac. Surg. 2011, 22, 813–817. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Nassar, A.; Rashed, M.; Naguib, N.; El-Tahan, A.-R. Furlow Palatoplasty for Previously Repaired Cleft Palate with Velopharyngeal Insufficiency. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1748–1751. [Google Scholar] [CrossRef]
- Agrawal, K. Cleft Palate Repair and Variations. Indian J. Plast. Surg. 2009, 42, S102–S109. [Google Scholar] [CrossRef]
- Alaluusua, S.; Hölttä, E.; Saarikko, A.; Geneid, A.; Leikola, J.; Heliövaara, A. Speech Symptoms of Velopharyngeal Insufficiency and the Incidence of Secondary Speech Surgery in 10-Year-Old Children with Unilateral Cleft Lip and Palate: Comparison of 2 Randomized Surgical Methods for Primary Palatal Surgery. J. Craniofac. Surg. 2023, 34, 461–466. [Google Scholar] [CrossRef]
- Allori, A.C.; Kelley, T.; Meara, J.G.; Albert, A.; Bonanthaya, K.; Chapman, K.; Cunningham, M.; Daskalogiannakis, J.; De Gier, H.; Heggie, A.A.; et al. A Standard Set of Outcome Measures for the Comprehensive Appraisal of Cleft Care. Cleft Palate Craniofac. J. 2017, 54, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, A.; Inchingolo, A.D.; Riccaldo, L.; Avantario, P.; Buongiorno, S.; Malcangi, G.; Bordea, I.R.; Palermo, A.; Inchingolo, F.; Inchingolo, A.M.; et al. The Use of Platelet-Rich Fibrin (PRF) in the Management of Dry Socket: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10069. [Google Scholar] [CrossRef]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of Probiotics on the Occurrence of Nutrition Absorption Capacities in Healthy Children: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.D.; Guglielmo, M.; Morolla, R.; Palumbo, I.; Riccaldo, L.; Mancini, A.; Palermo, A.; Malcangi, G.; Inchingolo, A.M.; et al. Nanotechnology and Its Application in Dentistry: A Systematic Review of Recent Advances and Innovations. J. Clin. Med. 2024, 13, 5268. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Carpentiere, V.; Garofoli, G.; Azzollini, D.; Campanelli, M.; Paduanelli, G.; Palermo, A.; et al. Artificial Intelligence and Its Clinical Applications in Orthodontics: A Systematic Review. Diagnostics 2023, 13, 3677. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.M.; Trilli, I.; Ferrante, L.; Noia, A.D.; de Ruvo, E.; Inchingolo, F.; Mancini, A.; Cocis, S.; Palermo, A.; et al. Management of Oro-Antral Communication: A Systemic Review of Diagnostic and Therapeutic Strategies. Diagnostics 2025, 15, 194. [Google Scholar] [CrossRef]
- Andrades, P.; Espinosa-de-Los-Monteros, A.; Shell, D.H.; Thurston, T.E.; Fowler, J.S.; Xavier, S.T.; Ray, P.D.; Grant, J.H. The Importance of Radical Intravelar Veloplasty during Two-Flap Palatoplasty. Plast. Reconstr. Surg. 2008, 122, 1121–1130. [Google Scholar] [CrossRef]
- Baillie, L.; Sell, D. Benchmarking Speech, Velopharyngeal Function Outcomes and Surgical Characteristics Following the Sommerlad Protocol and Palate Repair Technique. Cleft Palate Craniofac. J. 2020, 57, 1197–1215. [Google Scholar] [CrossRef]
- Bardach, J.; Morris, H.L.; Olin, W.H. Late Results of Primary Veloplasty: The Marburg Project. Plast. Reconstr. Surg. 1984, 73, 207–218. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bettens, K.; Alighieri, C.; Bruneel, L.; D’haeseleer, E.; Luyten, A.; Sseremba, D.; Musasizib, D.; Ojok, I.; Hodges, A.; Galiwango, G.; et al. Better Speech Outcomes after Very Early Palatal Repair?—A Longitudinal Case-Control Study in Ugandan Children with Cleft Palate. J. Commun. Disord. 2022, 96, 106198. [Google Scholar] [CrossRef]
- Bettens, K.; Bruneel, L.; Alighieri, C.; Sseremba, D.; Musasizib, D.; Ojok, I.; Hodges, A.; Galiwango, G.; Adriaansen, A.; D’haeseleer, E.; et al. Perceptual Speech Outcomes After Early Primary Palatal Repair in Ugandan Patients with Cleft Palate. Cleft Palate Craniofac. J. 2021, 58, 999–1011. [Google Scholar] [CrossRef]
- Bhuskute, A.; Skirko, J.R.; Roth, C.; Bayoumi, A.; Durbin-Johnson, B.; Tollefson, T.T. Association of Velopharyngeal Insufficiency with Quality of Life and Patient-Reported Outcomes After Speech Surgery. JAMA Facial Plast. Surg. 2017, 19, 406–412. [Google Scholar] [CrossRef]
- Broen, P.A.; Devers, M.C.; Doyle, S.S.; Prouty, J.M.; Moller, K.T. Acquisition of Linguistic and Cognitive Skills by Children with Cleft Palate. J. Speech Lang. Hear. Res. 1998, 41, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, A.; Inchingolo, A.D.; Piras, F.; Colonna, V.; Giorgio, R.V.; Carone, C.; Rapone, B.; Malcangi, G.; Inchingolo, A.M.; Inchingolo, F.; et al. Therapeutic Strategies and Genetic Implications for Periodontal Disease Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7217. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, A.; Inchingolo, A.M.; Inchingolo, F.; Sardano, R.; Trilli, I.; Di Noia, A.; Ferrante, L.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. Paediatric Dental Trauma: Insights from Epidemiological Studies and Management Recommendations. BMC Oral Health 2025, 25, 6. [Google Scholar] [CrossRef]
- Mancini, A.; Chirico, F.; Colella, G.; Piras, F.; Colonna, V.; Marotti, P.; Carone, C.; Inchingolo, A.D.; Inchingolo, A.M.; Inchingolo, F.; et al. Evaluating the Success Rates and Effectiveness of Surgical and Orthodontic Interventions for Impacted Canines: A Systematic Review of Surgical and Orthodontic Interventions and a Case Series. BMC Oral Health 2025, 25, 295. [Google Scholar] [CrossRef]
- Muntean, A.; Mzoughi, S.M.; Pacurar, M.; Candrea, S.; Inchingolo, A.D.; Inchingolo, A.M.; Ferrante, L.; Dipalma, G.; Inchingolo, F.; Palermo, A.; et al. Silver Diamine Fluoride in Pediatric Dentistry: Effectiveness in Preventing and Arresting Dental Caries—A Systematic Review. Children 2024, 11, 499. [Google Scholar] [CrossRef]
- Joos, U.; Wermker, K.; Kruse-Löesler, B.; Kleinheinz, J. Influence of Treatment Concept, Velopharyngoplasty, Gender and Age on Hypernasality in Patients with Cleft Lip, Alveolus and Palate. J. Craniomaxillofac. Surg. 2006, 34, 472–477. [Google Scholar] [CrossRef]
- Katzel, E.B.; Basile, P.; Koltz, P.F.; Marcus, J.R.; Girotto, J.A. Current Surgical Practices in Cleft Care: Cleft Palate Repair Techniques and Postoperative Care. Plast. Reconstr. Surg. 2009, 124, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Keuning, K.H.D.M.; Wieneke, G.H.; van Wijngaarden, H.A.; Dejonckere, P.H. The Correlation between Nasalance and a Differentiated Perceptual Rating of Speech in Dutch Patients with Velopharyngeal Insufficiency. Cleft Palate Craniofac. J. 2002, 39, 277–284. [Google Scholar] [CrossRef]
- Keuning, K.H.D.M.; Wieneke, G.H.; Dejonckere, P.H. Correlation between the Perceptual Rating of Speech in Dutch Patients with Velopharyngeal Insufficiency and Composite Measures Derived from Mean Nasalance Scores. Folia Phoniatr. Et Logop. 2004, 56, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Klintö, K.; Brunnegård, K.; Havstam, C.; Appelqvist, M.; Hagberg, E.; Taleman, A.-S.; Lohmander, A. Speech in 5-Year-Olds Born with Unilateral Cleft Lip and Palate: A Prospective Swedish Intercenter Study. J. Plast. Surg. Hand Surg. 2019, 53, 309–315. [Google Scholar] [CrossRef]
- Klintö, K.; Eva-Kristina-Salameh; Olsson, M.; Flynn, T.; Svensson, H.; Lohmander, A. Phonology in Swedish-Speaking 3-Year-Olds Born with Cleft Lip and Palate and the Relationship with Consonant Production at 18 Months. Int. J. Lang. Commun. Disord. 2014, 49, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Klintö, K.; Svensson, H.; Elander, A.; Lohmander, A. Speech and Phonology in Swedish-Speaking 3-Year-Olds with Unilateral Complete Cleft Lip and Palate Following Different Methods for Primary Palatal Surgery. Cleft Palate Craniofac. J. 2014, 51, 274–282. [Google Scholar] [CrossRef]
- Larsson, A.; Miniscalco, C.; Mark, H.; Schölin, J.S.; Jönsson, R.; Persson, C. Internationally Adopted Children with Unilateral Cleft Lip and Palate-Consonant Proficiency and Perceived Velopharyngeal Competence at the Age of 5. Cleft Palate Craniofac. J. 2020, 57, 849–859. [Google Scholar] [CrossRef]
- Larsson, A.; Miniscalco, C.; Mark, H.; Jönsson, R.; Persson, C. Persisting Speech Difficulties at 7–8 Years of Age–a Longitudinal Study of Speech Production in Internationally Adopted Children with Cleft Lip and Palate. Logop. Phoniatr. Vocol. 2024, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Schölin, J.; Mark, H.; Jönsson, R.; Persson, C. Speech Production in 3-Year-Old Internationally Adopted Children with Unilateral Cleft Lip and Palate. Int. J. Lang. Commun. Disord. 2017, 52, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, H.-T.; Chen, Y.-Y.; Wu, W.-L.; Liu, J.-Y.; Hao, J.-S.; Luo, D.-Y. Cleft Relapse and Oronasal Fistula after Furlow Palatoplasty in Infants with Cleft Palate: Incidence and Risk Factors. Int. J. Oral Maxillofac. Surg. 2017, 46, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-F.; Lee, Y.-H.; Wang, R.; Huang, C.-S.; Chen, P.K.-T.; Lo, L.-J.; Chen, Y.-R. Vomer Flap for Hard Palate Repair Is Related to Favorable Maxillary Growth in Unilateral Cleft Lip and Palate. Clin. Oral Investig. 2014, 18, 1269–1276. [Google Scholar] [CrossRef]
- Liu, B.Y.; Chen, X.X.; Cao, J.; Lu, Y. Analysis of Velopharyngeal Function and Speech Outcomes of Sommerlad Palatoplasty Combined with Sphincter Pharyngoplasty in Surgical Repair of Older Patients with Cleft Palate: Experience from a Major Craniofacial Surgery Centre in Eastern China. Br. J. Oral. Maxillofac. Surg. 2020, 58, 819–823. [Google Scholar] [CrossRef]
- Tache, A.; Maryn, Y.; Mommaerts, M.Y. Need for Velopharyngeal Surgery after Primary Palatoplasty in Cleft Patients. A Retrospective Cohort Study and Review of Literature. Ann. Med. Surg. 2021, 69, 102707. [Google Scholar] [CrossRef]
- Sweeney, T.; Hegarty, F.; Powell, K.; Deasy, L.; Regan, M.O.; Sell, D. Randomized Controlled Trial Comparing Parent Led Therapist Supervised Articulation Therapy (PLAT) with Routine Intervention for Children with Speech Disorders Associated with Cleft Palate. Int. J. Lang. Commun. Disord. 2020, 55, 639–660. [Google Scholar] [CrossRef]
- Sullivan, S.R.; Jung, Y.-S.; Mulliken, J.B. Outcomes of Cleft Palatal Repair for Internationally Adopted Children. Plast. Reconstr. Surg. 2014, 133, 1445–1452. [Google Scholar] [CrossRef]
- Stock, N.M.; Feragen, K.B. Psychological Adjustment to Cleft Lip and/or Palate: A Narrative Review of the Literature. Psychol. Health 2016, 31, 777–813. [Google Scholar] [CrossRef]
- Spruijt, N.E.; Beenakker, M.; Verbeek, M.; Heinze, Z.C.M.; Breugem, C.C.; van der Molen, A.B.M. Reliability of the Dutch Cleft Speech Evaluation Test and Conversion to the Proposed Universal Scale. J. Craniofac. Surg. 2018, 29, 390–395. [Google Scholar] [CrossRef]
- Southby, L.; Harding, S.; Phillips, V.; Wren, Y.; Joinson, C. Speech Input Processing in Children Born with Cleft Palate: A Systematic Literature Review with Narrative Synthesis. Int. J. Lang. Commun. Disord. 2021, 56, 668–693. [Google Scholar] [CrossRef] [PubMed]
- Sommerlad, B.C. A Technique for Cleft Palate Repair. Plast. Reconstr. Surg. 2003, 112, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.C.; Semb, G.; Nelson, P.; Brattström, V.; Mølsted, K.; Prahl-Andersen, B.; Gundlach, K.K. The Eurocleft Project 1996–2000: Overview. J. Craniomaxillofac. Surg. 2001, 29, 131–140; discussion 141–142. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.; Grunwell, P.; Mildinhall, S.; Murphy, T.; Cornish, T.A.; Bearn, D.; Shaw, W.C.; Murray, J.J.; Williams, A.C.; Sandy, J.R. Cleft Lip and Palate Care in the United Kingdom–the Clinical Standards Advisory Group (CSAG) Study. Part 3: Speech Outcomes. Cleft Palate Craniofac. J. 2001, 38, 30–37. [Google Scholar] [CrossRef]
- Semb, G.; Enemark, H.; Friede, H.; Paulin, G.; Lilja, J.; Rautio, J.; Andersen, M.; Åbyholm, F.; Lohmander, A.; Shaw, W.; et al. A Scandcleft Randomised Trials of Primary Surgery for Unilateral Cleft Lip and Palate: 1. Planning and Management. J. Plast. Surg. Hand Surg. 2017, 51, 2–13. [Google Scholar] [CrossRef]
- Scherer, N.J.; Yamashita, R.; de Oliveira, D.N.; DiLallo, J.; Trindade, I.; Fukushiro, A.P.; Richards, K. Early Speech and Language Intervention in Brazilian-Portuguese Toddlers with Cleft Lip and/or Palate. Clin. Linguist. Phon. 2022, 36, 34–53. [Google Scholar] [CrossRef]
- Savaci, N.; Hoşnuter, M.; Tosun, Z.; Demir, A. Maxillofacial Morphology in Children with Complete Unilateral Cleft Lip and Palate Treated by One-Stage Simultaneous Repair. Plast. Reconstr. Surg. 2005, 115, 1509–1517. [Google Scholar] [CrossRef]
- Saothonglang, K.; Punyavong, P.; Winaikosol, K.; Jenwitheesuk, K.; Surakunprapha, P. Risk Factors of Fistula Following Primary Palatoplasty. J. Craniofac. Surg. 2021, 32, 587–590. [Google Scholar] [CrossRef]
- Sand, A.; Hagberg, E.; Lohmander, A. On the Benefits of Speech-Language Therapy for Individuals Born with Cleft Palate: A Systematic Review and Meta-Analysis of Individual Participant Data. J. Speech Lang. Hear. Res. 2022, 65, 555–573. [Google Scholar] [CrossRef]
- Rullo, R.; Maggio, D.D.; Addabbo, F.; Rullo, F.; Festa, V.M.; Perillo, L. Speech Outcome in Unilateral Complete Cleft Lip and Palate Patients: A Descriptive Study. Eur. J. Paediatr. Dent. 2014, 15, 293–296. [Google Scholar]
- Rintala, A.E.; Haapanen, M.L. The Correlation between Training and Skill of the Surgeon and Reoperation Rate for Persistent Cleft Palate Speech. Br. J. Oral Maxillofac. Surg. 1995, 33, 271–295; discussion 297–298. [Google Scholar] [CrossRef]
- Reddy, R.R.; Reddy, S.G.; Vaidhyanathan, A.; Bergé, S.J.; Kuijpers-Jagtman, A.M. Maxillofacial Growth and Speech Outcome after One-Stage or Two-Stage Palatoplasty in Unilateral Cleft Lip and Palate. A Systematic Review. J. Craniomaxillofac. Surg. 2017, 45, 995–1003. [Google Scholar] [CrossRef]
- Lohmander, A.; Olsson, M.; Flynn, T. Early Consonant Production in Swedish Infants with and without Unilateral Cleft Lip and Palate and Two-Stage Palatal Repair. Cleft Palate Craniofac. J. 2011, 48, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Luyten, A.; Bettens, K.; D’haeseleer, E.; De Ley, S.; Hodges, A.; Galiwango, G.; Vermeersch, H.; Van Lierde, K. Impact of Early Synchronous Lip and Palatal Repair on Speech. Folia Phoniatr. Logop. 2013, 65, 303–311. [Google Scholar] [CrossRef]
- Luyten, A.; Bettens, K.; D’haeseleer, E.; De Ley, S.; Hodges, A.; Galiwango, G.; Bonte, K.; Vermeersch, H.; Van Lierde, K. The Impact of Palatal Repair before and after 6 Months of Age on Speech Characteristics. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 787–798. [Google Scholar] [CrossRef]
- Mahoney, M.-H.; Swan, M.C.; Fisher, D.M. Prospective Analysis of Presurgical Risk Factors for Outcomes in Primary Palatoplasty. Plast. Reconstr. Surg. 2013, 132, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mapar, D.; Khanlar, F.; Sadeghi, S.; Abdali, H.; Memarzadeh, M.; Davari, H.A.; Derakhshandeh, F. The Incidence of Velopharyngeal Insufficiency and Oronasal Fistula after Primary Palatal Surgery with Sommerlad Intravelar Veloplasty: A Retrospective Study in Isfahan Cleft Care Team. Int. J. Pediatr. Otorhinolaryngol. 2019, 120, 6–10. [Google Scholar] [CrossRef]
- Menegueti, K.I.; Mangilli, L.D.; Alonso, N.; Andrade, C.R.F.d. Speech profile of patients undergoing primary palatoplasty. Codas 2017, 29, e20160146. [Google Scholar] [CrossRef]
- Mink van der Molen, A.B.; Janssen, K.; Specken, T.F.J.M.C.; Stubenitsky, B.M. The Modified Honig Velopharyngoplasty–a New Technique to Treat Hypernasality by Palatal Lengthening. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft Lip and Palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Naidu, P.; Yao, C.A.; Chong, D.K.; Magee, W.P. Cleft Palate Repair: A History of Techniques and Variations. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4019. [Google Scholar] [CrossRef]
- Nakamura, N.; Ogata, Y.; Sasaguri, M.; Suzuki, A.; Kikuta, R.; Ohishi, M. Aerodynamic and Cephalometric Analyses of Velopharyngeal Structure and Function Following Re-Pushback Surgery for Secondary Correction in Cleft Palate. Cleft Palate Craniofac. J. 2003, 40, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Naran, S.; Ford, M.; Losee, J.E. What’s New in Cleft Palate and Velopharyngeal Dysfunction Management? Plast. Reconstr. Surg. 2017, 139, 1343e–1355e. [Google Scholar] [CrossRef]
- Nyberg, J.; Peterson, P.; Lohmander, A. Speech Outcomes at Age 5 and 10 Years in Unilateral Cleft Lip and Palate after One-Stage Palatal Repair with Minimal Incision Technique–a Longitudinal Perspective. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1662–1670. [Google Scholar] [CrossRef]
- Okhiria, Å.; Persson, C.; Johansson, M.B.; Hakelius, M.; Nowinski, D. Longitudinal Data on Speech Outcomes in Internationally Adopted Children Compared with Non-Adopted Children with Cleft Lip and Palate. Int. J. Lang. Commun. Disord. 2023, 58, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Paliobei, V.; Psifidis, A.; Anagnostopoulos, D. Hearing and Speech Assessment of Cleft Palate Patients after Palatal Closure. Long-Term Results. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1373–1381. [Google Scholar] [CrossRef]
- Pereira, V.J.; Sell, D.; Tuomainen, J. Effect of Maxillary Osteotomy on Speech in Cleft Lip and Palate: Perceptual Outcomes of Velopharyngeal Function. Int. J. Lang. Commun. Disord. 2013, 48, 640–650. [Google Scholar] [CrossRef]
- Perko, M.A. Two-Stage Closure of Cleft Palate (Progress Report). J. Maxillofac. Surg. 1979, 7, 46–80. [Google Scholar] [CrossRef]
- Peterson, P.; Nyberg, J.; Persson, C.; Mark, H.; Lohmander, A. Speech Outcome and Self-Reported Communicative Ability in Young Adults Born with Unilateral Cleft Lip and Palate: Comparing Long-Term Results After 2 Different Surgical Methods for Palatal Repair. Cleft Palate Craniofac. J. 2022, 59, 751–764. [Google Scholar] [CrossRef]
- Raud Westberg, L.; Höglund Santamarta, L.; Karlsson, J.; Nyberg, J.; Neovius, E.; Lohmander, A. Speech Outcome in Young Children Born with Unilateral Cleft Lip and Palate Treated with One- or Two-Stage Palatal Repair and the Impact of Early Intervention. Logoped. Phoniatr. Vocol. 2019, 44, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, P.; Poorjavad, M.; Abdali, H. Speech Outcomes after Palatal Closure in 3–7-Year-Old Children. Braz. J. Otorhinolaryngol. 2022, 88, 594–601. [Google Scholar] [CrossRef]
- Ross, R.B. Treatment Variables Affecting Facial Growth in Complete Unilateral Cleft Lip and Palate. Cleft Palate J. 1987, 24, 5–77. [Google Scholar]
- Schaar Johansson, M.; Becker, M.; Eriksson, M.; Stiernman, M.; Klintö, K. Surgical Treatment of Velopharyngeal Dysfunction: Incidence and Associated Factors in the Swedish Cleft Palate Population. J. Plast. Reconstr. Aesthet. Surg. 2024, 90, 240–248. [Google Scholar] [CrossRef]
- Schweckendiek, H. The problem of early and late surgery in congenital fissure of the of the lips and palate. Z. Laryngol. Rhinol. Otol. 1951, 30, 51–56. [Google Scholar]
- Schweckendiek, W.; Doz, P. Primary Veloplasty: Long-Term Results without Maxillary Deformity. a Twenty-Five Year Report. Cleft Palate J. 1978, 15, 268–274. [Google Scholar] [PubMed]
- Willadsen, E.; Lohmander, A.; Persson, C.; Boers, M.; Kisling-Møller, M.; Havstam, C.; Elander, A.; Andersen, M. Scandcleft Project, Trial 1: Comparison of Speech Outcome in Relation to Timing of Hard Palate Closure in 5-Year-Olds With UCLP. Cleft Palate Craniofac. J. 2019, 56, 1276–1286. [Google Scholar] [CrossRef]
- Willadsen, E.; Lohmander, A.; Persson, C.; Lundeborg, I.; Alaluusua, S.; Aukner, R.; Bau, A.; Boers, M.; Bowden, M.; Davies, J.; et al. Scandcleft Randomised Trials of Primary Surgery for Unilateral Cleft Lip and Palate: 5. Speech Outcomes in 5-Year-Olds–Consonant Proficiency and Errors. J. Plast. Surg. Hand Surg. 2017, 51, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, E.; Jørgensen, L.D.; Alaluusua, S.; Pedersen, N.H.; Nielsen, J.B.; Hölttä, E.; Hide, Ø.; Hayden, C.; Havstam, C.; Hammarström, I.L.; et al. Scandcleft Randomized Trials of Primary Surgery for Unilateral Cleft Lip and Palate: Speech Proficiency at 10 Years of Age. Int. J. Lang. Commun. Disord. 2023, 58, 892–909. [Google Scholar] [CrossRef]
- Timbang, M.R.; Gharb, B.B.; Rampazzo, A.; Papay, F.; Zins, J.; Doumit, G. A Systematic Review Comparing Furlow Double-Opposing Z-Plasty and Straight-Line Intravelar Veloplasty Methods of Cleft Palate Repair. Plast. Reconstr. Surg. 2014, 134, 1014–1022. [Google Scholar] [CrossRef]
- Trotter, C.; Choi, D.G.; Roohani, I.; Alfeerawi, S.; Naidu, P.; Shakoori, P.; Fahradyan, A.; Lee, J.A.; Magee, W.P.; Urata, M.M.; et al. A Single Institution 19 Year Comparison of Furlow and Straight Line Palatoplasty Techniques in Bilateral Cleft Lip and Palate. Cleft Palate Craniofac. J. 2024, 62, 1134–1143. [Google Scholar] [CrossRef]
- Britton, L.; Albery, L.; Bowden, M.; Harding-Bell, A.; Phippen, G.; Sell, D. A Cross-Sectional Cohort Study of Speech in Five-Year-Olds with Cleft Palate ± Lip to Support Development of National Audit Standards: Benchmarking Speech Standards in the United Kingdom. Cleft Palate Craniofac. J. 2014, 51, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Brunnegård, K.; Lohmander, A. A Cross-Sectional Study of Speech in 10-Year-Old Children with Cleft Palate: Results and Issues of Rater Reliability. Cleft Palate Craniofac. J. 2007, 44, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.; Lohmander, A.; Moller, C.; Magnusson, L. A Longitudinal Study of Hearing and Middle Ear Status in Adolescents with Cleft Lip and Palate. Laryngoscope 2013, 123, 1374–1380. [Google Scholar] [CrossRef]
- Broen, P.A.; Moller, K.T.; Carlstrom, J.; Doyle, S.S.; Devers, M.; Keenan, K.M. Comparison of the Hearing Histories of Children with and without Cleft Palate. Cleft Palate Craniofac. J. 1996, 33, 127–133. [Google Scholar] [CrossRef]
- Lohmander, A.; Willadsen, E.; Persson, C.; Henningsson, G.; Bowden, M.; Hutters, B. Methodology for Speech Assessment in the Scandcleft Project—An International Randomized Clinical Trial on Palatal Surgery: Experiences from a Pilot Study. Cleft Palate Craniofac. J. 2009, 46, 347–362. [Google Scholar] [CrossRef]
- Liedman-Boshko, J.; Lohmander, A.; Persson, C.; Lith, A.; Elander, A. Perceptual Analysis of Speech and the Activity in the Lateral Pharyngeal Walls before and after Velopharyngeal Flap Surgery. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 22–32. [Google Scholar] [CrossRef]
- Bessell, A.; Sell, D.; Whiting, P.; Roulstone, S.; Albery, L.; Persson, M.; Verhoeven, A.; Burke, M.; Ness, A.R. Speech and Language Therapy Interventions for Children with Cleft Palate: A Systematic Review. Cleft Palate Craniofac. J. 2013, 50, e1–e17. [Google Scholar] [CrossRef]
- Lohmander, A.; Friede, H.; Elander, A.; Persson, C.; Lilja, J. Speech Development in Patients with Unilateral Cleft Lip and Palate Treated with Different Delays in Closure of the Hard Palate after Early Velar Repair: A Longitudinal Perspective. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2006, 40, 267–274. [Google Scholar] [CrossRef]
- Hutters, B.; Henningsson, G. Speech Outcome Following Treatment in Cross-Linguistic Cleft Palate Studies: Methodological Implications. Cleft Palate Craniofac. J. 2004, 41, 544–549. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Sell, D.; Sweeney, T.; Harding-Bell, A.; Williams, A. The Cleft Audit Protocol for Speech—Augmented: A Validated and Reliable Measure for Auditing Cleft Speech. Cleft Palate Craniofac. J. 2006, 43, 272–288. [Google Scholar] [CrossRef]
- Ha, S.; Koh, K.S.; Moon, H.; Jung, S.; Oh, T.S. Clinical Outcomes of Primary Palatal Surgery in Children with Nonsyndromic Cleft Palate with and without Lip. Biomed. Res. Int. 2015, 2015, 185459. [Google Scholar] [CrossRef]
- Kappen, I.F.P.M.; Bittermann, D.; Janssen, L.; Bittermann, G.K.P.; Boonacker, C.; Haverkamp, S.; de Wilde, H.; Van Der Heul, M.; Specken, T.F.; Koole, R.; et al. Long-Term Follow-Up Study of Young Adults Treated for Unilateral Complete Cleft Lip, Alveolus, and Palate by a Treatment Protocol Including Two-Stage Palatoplasty: Speech Outcomes. Arch. Plast. Surg. 2017, 44, 202–209. [Google Scholar] [CrossRef]
- Persson, C.; Davies, J.; Havstam, C.; Søgaard, H.; Bowden, M.; Boers, M.; Nielsen, J.B.; Alaluusua, S.; Lundeborg Hammarström, I.; Emborg, B.K.; et al. Longitudinal Speech Outcome at 5 and 10 Years in UCLP: Influence of Speech Therapy and Secondary Velopharyngeal Surgery. Cleft Palate Craniofac. J. 2024, 62, 772–785. [Google Scholar] [CrossRef]
- Hammarström, I.L.; Nyberg, J.; Alaluusua, S.; Rautio, J.; Neovius, E.; Berggren, A.; Persson, C.; Willadsen, E.; Lohmander, A. Scandcleft Project Trial 2-Comparison of Speech Outcome in 1- and 2-Stage Palatal Closure in 5-Year-Olds With UCLP. Cleft Palate Craniofac. J. 2020, 57, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.; Pedersen, N.-H.; Hayden, C.; Bowden, M.; Aukner, R.; Vindenes, H.A.; Åbyholm, F.; Withby, D.; Willadsen, E.; Lohmander, A. Scandcleft Project Trial 3: Comparison of Speech Outcomes in Relation to Sequence in 2-Stage Palatal Repair Procedures in 5-Year-Olds with Unilateral Cleft Lip and Palate. Cleft Palate Craniofac. J. 2020, 57, 352–363. [Google Scholar] [CrossRef]
- Bruneel, L.; Bettens, K.; De Bodt, M.; Roche, N.; Bonte, K.; Van Lierde, K. Speech Outcomes Following Sommerlad Primary Palatoplasty: Results of the Ghent University Hospital. J. Commun. Disord. 2018, 72, 111–121. [Google Scholar] [CrossRef]
- Jørgensen, L.D.; Willadsen, E. Longitudinal Study of the Development of Obstruent Correctness from Ages 3 to 5 Years in 108 Danish Children with Unilateral Cleft Lip and Palate: A Sub-Study within a Multicentre Randomized Controlled Trial. Int. J. Lang. Commun. Disord. 2020, 55, 121–135. Available online: https://pubmed.ncbi.nlm.nih.gov/31710176/ (accessed on 5 March 2025). [CrossRef]
- Mark, H.; Lilja, J.; Havstam, C. Long-Term Longitudinal Follow-up of Individuals with UCLP after Gothenburg Twostage Palate Closure: Surgical and Speech Outcomes. J. Plast. Surg. Hand Surg. 2023, 58, 19–25. [Google Scholar] [CrossRef]
- Chapman, K.L.; Sitzman, T.; Baylis, A.; Hardin-Jones, M.; Kirschner, R.; Temkit, M.H. A Comparative Effectiveness Study of Speech and Surgical Outcomes: Study Overview. Cleft Palate Craniofac. J. 2024, 4, 10556656241274242. Available online: https://www.unboundmedicine.com/medline/citation/39363863/A_Comparative_Effectiveness_Study_of_Speech_and_Surgical_Outcomes:_Study_Overview (accessed on 5 March 2025). [CrossRef] [PubMed]
- Hofman, L.; Paes, E.C.; Haverkamp, S.J.; Jenniskens, K.; Mink van der Molen, A.B. Long Term Speech Outcomes after Using the Sommerlad Technique for Primary Palatoplasty: A Retrospective Study in the Wilhelmina Children’s Hospital, Utrecht. Clin. Oral. Investig. 2024, 28, 441. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Nishihara, K.; Matsunaga, K.; Miura, N.; Kibe, T.; Nakamura, N. Perceptual-Speech, Nasometric, and Cephalometric Results After Modified V-Y Palatoplasties with or Without Mucosal Graft. Cleft Palate Craniofac. J. 2016, 53, 469–480. [Google Scholar] [CrossRef]
- Brudnicki, A.; Radkowska, E.; Sawicka, E.; Fudalej, P.S. Speech and Burden of Secondary Surgical Interventions Following One-Stage Repair of Unilateral Cleft Lip and Palate and Alveolar Bone Grafting Performed at Different Timings. J. Clin. Med. 2023, 12, 5545. [Google Scholar] [CrossRef]
- D’Esposito, V.; Lecce, M.; Marenzi, G.; Cabaro, S.; Ambrosio, M.R.; Sammartino, G.; Misso, S.; Migliaccio, T.; Liguoro, P.; Oriente, F.; et al. Platelet-Rich Plasma Counteracts Detrimental Effect of High-Glucose Concentrations on Mesenchymal Stem Cells from Bichat Fat Pad. J. Tissue Eng. Regen. Med. 2020, 14, 701–713. [Google Scholar] [CrossRef]
- Gasparro, R.; Qorri, E.; Valletta, A.; Masucci, M.; Sammartino, P.; Amato, A.; Marenzi, G. Non-Transfusional Hemocomponents: From Biology to the Clinic—A Literature Review. Bioengineering 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, K.; Yadalam, P.K.; Ramani, P.; Krishna, M.; Hafedh, S.; Badnjević, A.; Cervino, G.; Minervini, G. Light Gradient Boosting-Based Prediction of Quality of Life among Oral Cancer-Treated Patients. BMC Oral Health 2024, 24, 349. [Google Scholar] [CrossRef] [PubMed]
- Kosowski, T.R.; Weathers, W.M.; Wolfswinkel, E.M.; Ridgway, E.B. Cleft Palate. Semin. Plast. Surg. 2012, 26, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Madabhavi, I.; Niranjan, N.; Dogra, M. Auscultation of the Respiratory System. Ann. Thorac. Med. 2015, 10, 158–168. [Google Scholar] [CrossRef]

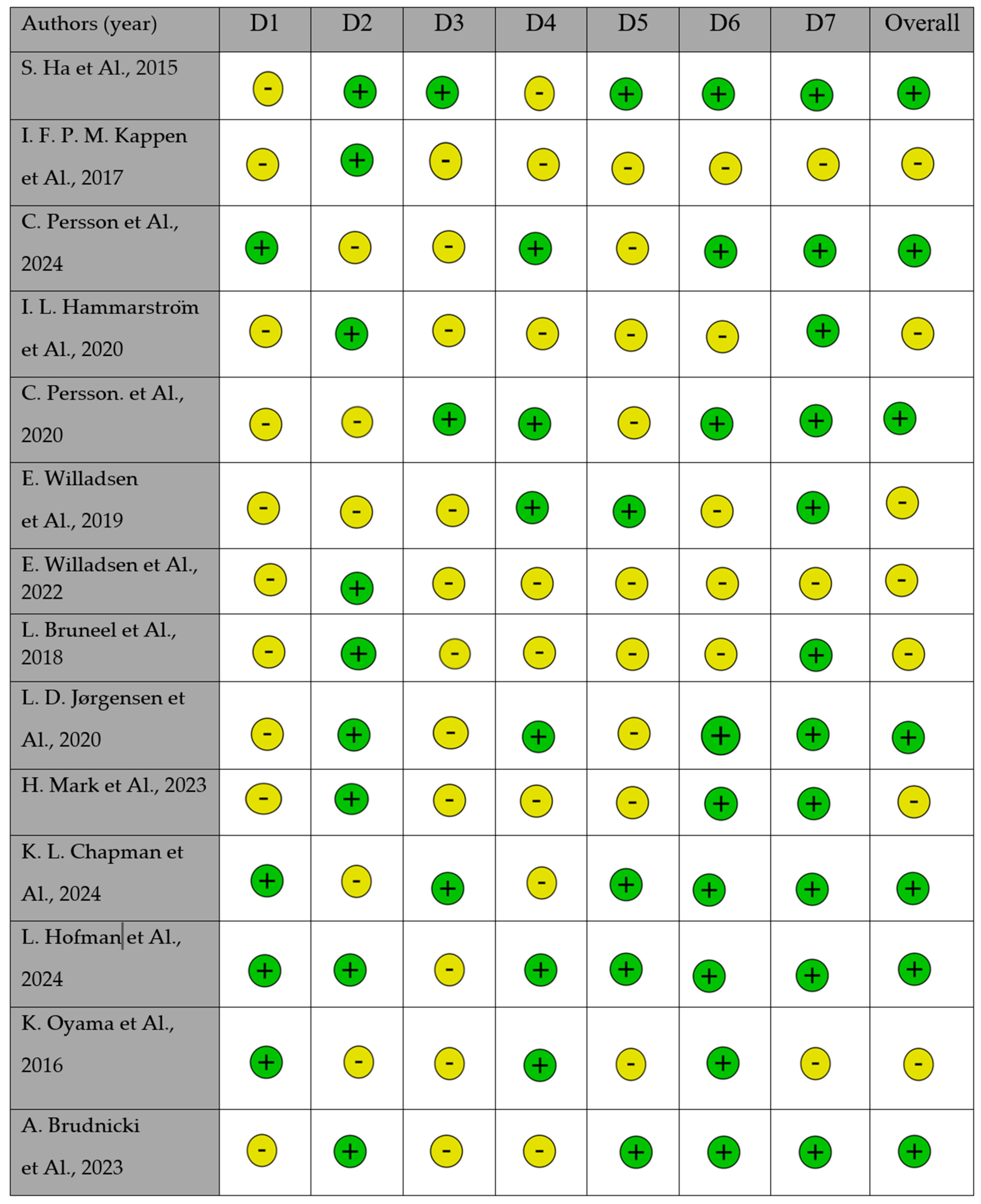
| Author | Type of Study | Outcome | Surgical Technique | Material and Methods | Results |
|---|---|---|---|---|---|
| Ha et al. (2015) [91] | Retrospective clinical study | To evaluate the clinical outcomes of primary cleft palate surgery, focusing on oronasal fistula rate, velopharyngeal insufficiency (VPI), and speech outcomes. | Furlow Z-plasty, two-flap palatoplasty, intravelar veloplasty (IVVP) | 292 patients with nonsyndromic cleft palate (±cleft lip) underwent primary palatoplasty at Seoul Asan Medical Center (2007–2012). Techniques included Furlow Z-plasty, two-flap palatoplasty, and intravelar veloplasty. Follow-up speech assessments were conducted at 12, 15, 24, and 36 months. | Oronasal fistula rate: 7.9%. VPI requiring secondary surgery: 19.2%. Speech therapy required in 50.3% of cases. Hypernasality in 28.8%, and articulatory deficits in 51.4%. Cleft type influenced speech outcomes and VPI rates. |
| Kappen et al. (2017) [92] | Retrospective follow-up study | To evaluate long-term speech outcomes in adults with unilateral complete cleft lip and palate (UCLP) treated with two-stage palatoplasty. | Two-stage palatoplasty (soft: IVVP, hard: Von Langenbeck) + pharyngoplasty if needed | 48 adults with UCLP who underwent two-stage palatal closure were assessed for speech outcomes. Medical history was reviewed, and speech evaluations were performed by a speech therapist. Nasometry and intelligibility scales were used. | 84.4% had intelligible speech (scores 1–2 on a 5-point scale). A total of 36% showed mild to moderate hypernasality. A total of 40% underwent pharyngoplasty. Higher incidence of articulation errors correlated with lower intelligibility scores. |
| Persson et al. (2024) [93] | Longitudinal study | Analyze speech development in children with unilateral cleft lip and palate (UCLP) at ages 5 and 10, evaluating the role of speech therapy and secondary velopharyngeal surgery. | Based on multicenter protocols | Data analysis from the Scandcleft project, including 320 children from five countries. Speech proficiency was assessed using velopharyngeal competence (VPC-Sum) and the percentage of consonants correct (PCC). The number of speech therapy sessions and secondary surgeries were also considered. | At age 5, only 23% of children had speech proficiency at “peer level,” increasing to 56% by age 10. The best predictor of speech competence at age 10 was proficiency at age 5. A high number of speech therapy sessions did not significantly improve outcomes. |
| Hammarström et al. (2020) [94] | Prospective RCT | Compare speech outcomes in 1-stage vs. 2-stage palatal closure in 5-year-olds with UCLP. | 1-stage vs. 2-stage palatal closure | 112 children (5 years old) from Sweden and Finland. Arm A: soft palate closure at 4 months, hard palate at 12 months. Arm C: both closures at 12 months. Evaluations: VPC, PCC, consonant errors, speech therapy visits. | No significant differences in VPC/VPI between groups. PCC scores were generally low, with Finnish center showing better results. Swedish centers had more consonant errors and speech therapy visits. |
| Persson et al. (2020) [95] | Prospective RCT | Compare speech outcomes in 2-stage palatal closure sequencing in 5-year-olds with UCLP. | Based on multicenter protocols | 136 children (5 years old) from Norway and UK. Arm A: lip and soft palate closure at 3–4 months, hard palate at 12 months. Arm D: lip and hard palate closure at 3–4 months, soft palate at 12 months. Evaluations: VPC, PCC, CSCs, and speech therapy visits. | No significant differences in VPC or PCC between groups. Some centers showed higher CSCs in Arm A. Wide variability in speech outcomes across centers. |
| Willadsen et al. (2017) [77] | Randomized controlled trial (RCT) | Evaluate speech outcomes in children with UCLP based on timing of hard palate closure. | Hard palate closure at 12 vs. 36 months | 143 children (5 years old), hard palate closure at 12 months (arm A) or 36 months (arm B), assessed using VPC, PCC, and speech therapy visits. | PCC scores ranged from 86 to 92%. VPC was achieved in 58–83% of participants. No single protocol proved superior. Girls had better speech outcomes. Factors like hearing level, speech therapy, and secondary surgeries influenced results. |
| Willadsen et al. (2019) [76] | Longitudinal randomized controlled trial (RCT) | To evaluate the development of obstruent correctness and speech error types in Danish children with unilateral cleft lip and palate (UCLP) from ages 3 to 5. | Hard palate closure at 12 vs. 36 months | 108 children with UCLP from the Scandcleft Project received early (12 months) or late (36 months) hard palate closure. Speech recordings at ages 3 and 5 were transcribed phonetically by blinded raters. Analyzed error types included cleft speech characteristics (CSCs) and developmental speech characteristics (DSCs). | PCC-obs scores improved from ages 3 to 5, but children with UCLP did not reach typical Danish children’s speech levels. Late closure group had significantly lower scores at age 5 than early closure group at age 3. CSCs at age 3 strongly predicted PCC-obs at age 5. |
| Bruneel et al. (2018) [96] | Retrospective cohort study | To evaluate speech outcomes following Sommerlad primary palatoplasty and compare them with an age- and gender-matched control group. | Sommerlad | 16 patients with cleft palate (mean age: 5.4 years) treated at Ghent University Hospital. Speech intelligibility, resonance, nasal airflow, and articulation were assessed perceptually. Nasalance values and NSI 2.0 were measured instrumentally. | The CPLE toothpaste/mouthwash provides an effective and natural alternative to SLS-free toothpaste +/− mouthwash containing EO when used as a complement to mechanical oral hygiene to reduce interdental gingival inflammation. |
| Jørgensen et al. (2020) [97] | Longitudinal study (RCT sub-study) | Evaluate obstruent correctness (PCC-obs) development and error patterns in Danish children with unilateral cleft lip and palate (UCLP) from ages 3 to 5, identifying predictors of PCC-obs at age 5. | Early vs. late hard palate closure (12 vs. 36 mo) | Analyzed data from the Scandcleft Project, including 108 Danish children with UCLP who underwent either early hard palate closure (EHPC at 12 months) or late hard palate closure (LHPC at 36 months). Phonetic transcription of speech samples from naming tests at ages 3 and 5 was performed by blinded raters. | PCC-obs significantly improved from ages 3 to 5, with greater gains in the LHPC group. However, at age 5, the LHPC group still did not reach the PCC-obs level of the EHPC group at age 3. Higher CSC and DSC frequencies at age 3 predicted lower PCC-obs at age 5. VPD and gender had minimal predictive impact. |
| Mark et al. (2023) [98] | Longitudinal follow-up study | To analyze speech outcomes in individuals with UCLP after Gothenburg two-stage palate closure (soft palate at 6 months, hard palate at 3 years). | Two-stage (soft at 6 months, hard at 3 years) | 28 patients underwent a two-stage closure. Speech samples recorded at 5, 10, 16, and 19 years were evaluated by three independent speech–language pathologists. Variables included hypernasality, articulation errors, and velopharyngeal function. | 25–30% of participants showed articulation disorders at 5 years, but most resolved later. Velopharyngeal incompetence in 20% at 5 years but none at 19 years. Fewer articulation errors compared to patients with later hard palate closure at 8 years. |
| Chapman et al. (2024) [99] | Prospective, longitudinal, observational, comparative effectiveness study | To compare speech outcomes and fistula rates between two palate repair techniques (IVVP vs. Furlow Z-plasty), evaluate early intervention speech–language (EI-SL) services, and analyze their impact on speech outcomes. | IVVP vs. Furlow Z-plasty | 1247 children with cleft palate (CP ± L) were enrolled from 20 sites in the US. Exclusion criteria included submucous cleft palate and bilateral sensorineural hearing loss. Primary outcome: perceptual ratings of hypernasality at age 3. Secondary outcomes: fistula rate, speech production, and quality of life. Statistical analysis used generalized estimating equations with propensity score weighting. | Recruitment completed in 2023 with 80% retention. In total, 562 children completed the final 3-year speech assessment. Final study activities to conclude in 2025. The study aims to resolve uncertainties about the effectiveness of IVVP vs. Furlow Z-plasty and improve cleft care research. |
| Hofman et al. (2024) [100] | Retrospective cohort study | To assess long-term speech outcomes and incidence of velopharyngeal insufficiency (VPI) after Sommerlad palatoplasty. | Sommerlad palatoplasty | 239 patients with cleft lip and/or palate (CL/P) treated at Wilhelmina Children’s Hospital (2008–2017) were reviewed. Inclusion criteria: Sommerlad palatoplasty, speech assessment at age 5 or older. Outcomes analyzed using chi-squared tests and odds ratios. | VPI rate: 52.7%. Speech correction surgery required in 49.8%. Higher Veau classification and cleft width >10 mm were significantly associated with worse speech outcomes. Fistula presence increased the likelihood of additional surgery. |
| Oyama et al. (2016) [101] | Retrospective cohort study | To compare speech, nasometric, and cephalometric outcomes after modified V-Y palatoplasty with or without a mucosal graft. | Modified V-Y palatoplasty with/without mucosal graft | 191 patients underwent primary palatoplasty (82 with mucosal graft, 109 without). Speech assessments included hypernasality rating, nasal emission, and nasometry. Cephalometric analysis evaluated velopharyngeal morphology and velar movement. | Normal resonance was significantly higher in the mucosal graft group (90.3%) vs. non-graft group (68.8%). Mean nasalance scores were lower in the graft group, approaching control levels. Cephalometry showed greater velar length and elevation in the graft group. |
| Brudnicki et al. (2023) [102] | Retrospective case–control study | To assess speech outcomes and the burden of secondary surgical interventions in UCLP patients undergoing one-stage repair and alveolar bone grafting at different ages. | Double-layer vomeroplasty; early vs. late ABG | 56 patients with unilateral cleft lip and palate (UCLP) were divided into early (<6 years) and late (>6 years) alveolar bone grafting (ABG) groups. Speech assessments were performed at age 10 using 27 standardized sentences. Secondary surgical interventions, such as pharyngoplasty and fistula repair, were recorded. | 7 patients had disordered speech intelligibility. A total of 12 had hypernasality, 13 had nasal emission, and 5 had nasal turbulence. Speech outcomes were significantly worse than the control group. A Dutch speech assessment protocol will be developed to standardize evaluations. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.M.; Dipalma, G.; Bassi, P.; Lagioia, R.; Cavino, M.; Colonna, V.; de Ruvo, E.; Inchingolo, F.; Giudice, G.; Palermo, A.; et al. Evaluation of Surgical Protocols for Speech Improvement in Children with Cleft Palate: A Systematic Review and Case Series. Bioengineering 2025, 12, 877. https://doi.org/10.3390/bioengineering12080877
Inchingolo AM, Dipalma G, Bassi P, Lagioia R, Cavino M, Colonna V, de Ruvo E, Inchingolo F, Giudice G, Palermo A, et al. Evaluation of Surgical Protocols for Speech Improvement in Children with Cleft Palate: A Systematic Review and Case Series. Bioengineering. 2025; 12(8):877. https://doi.org/10.3390/bioengineering12080877
Chicago/Turabian StyleInchingolo, Angelo Michele, Gianna Dipalma, Paola Bassi, Rosalba Lagioia, Mirka Cavino, Valeria Colonna, Elisabetta de Ruvo, Francesco Inchingolo, Giuseppe Giudice, Andrea Palermo, and et al. 2025. "Evaluation of Surgical Protocols for Speech Improvement in Children with Cleft Palate: A Systematic Review and Case Series" Bioengineering 12, no. 8: 877. https://doi.org/10.3390/bioengineering12080877
APA StyleInchingolo, A. M., Dipalma, G., Bassi, P., Lagioia, R., Cavino, M., Colonna, V., de Ruvo, E., Inchingolo, F., Giudice, G., Palermo, A., & Inchingolo, A. D. (2025). Evaluation of Surgical Protocols for Speech Improvement in Children with Cleft Palate: A Systematic Review and Case Series. Bioengineering, 12(8), 877. https://doi.org/10.3390/bioengineering12080877