Complete Protocol and Guidelines for the Implementation and Manufacturing of the Tübingen Palatal Plate—An Interdisciplinary Technical Note on the Tübingen Approach for Infants with Robin Sequence
Abstract
1. Introduction
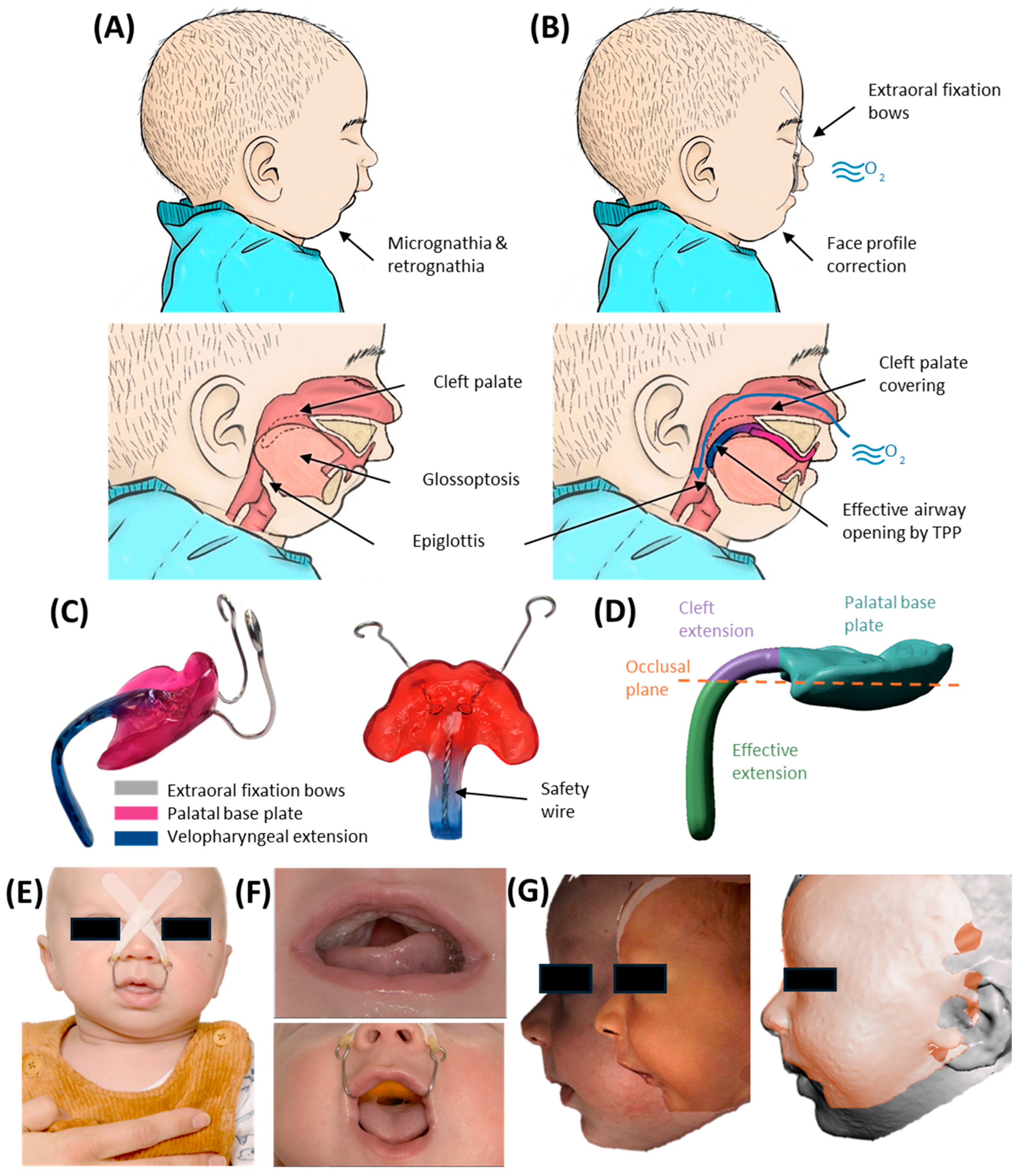
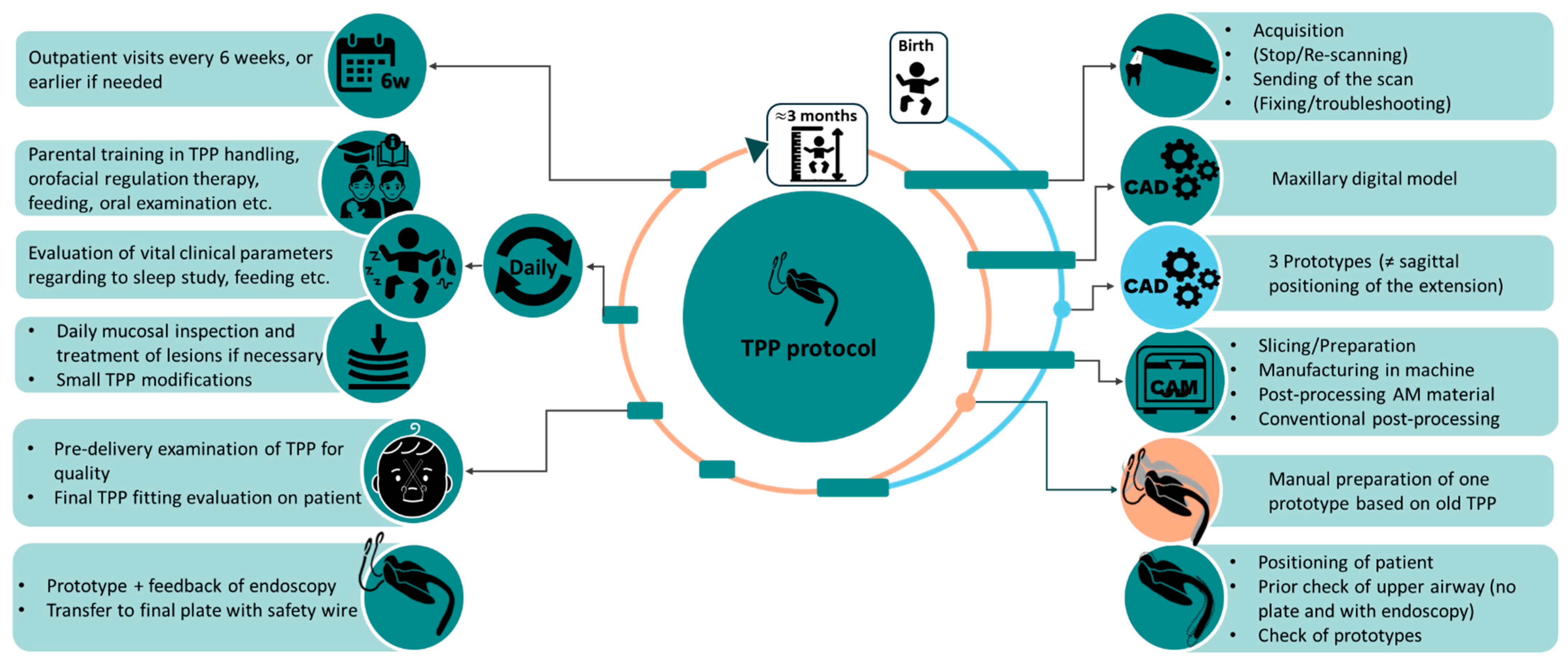
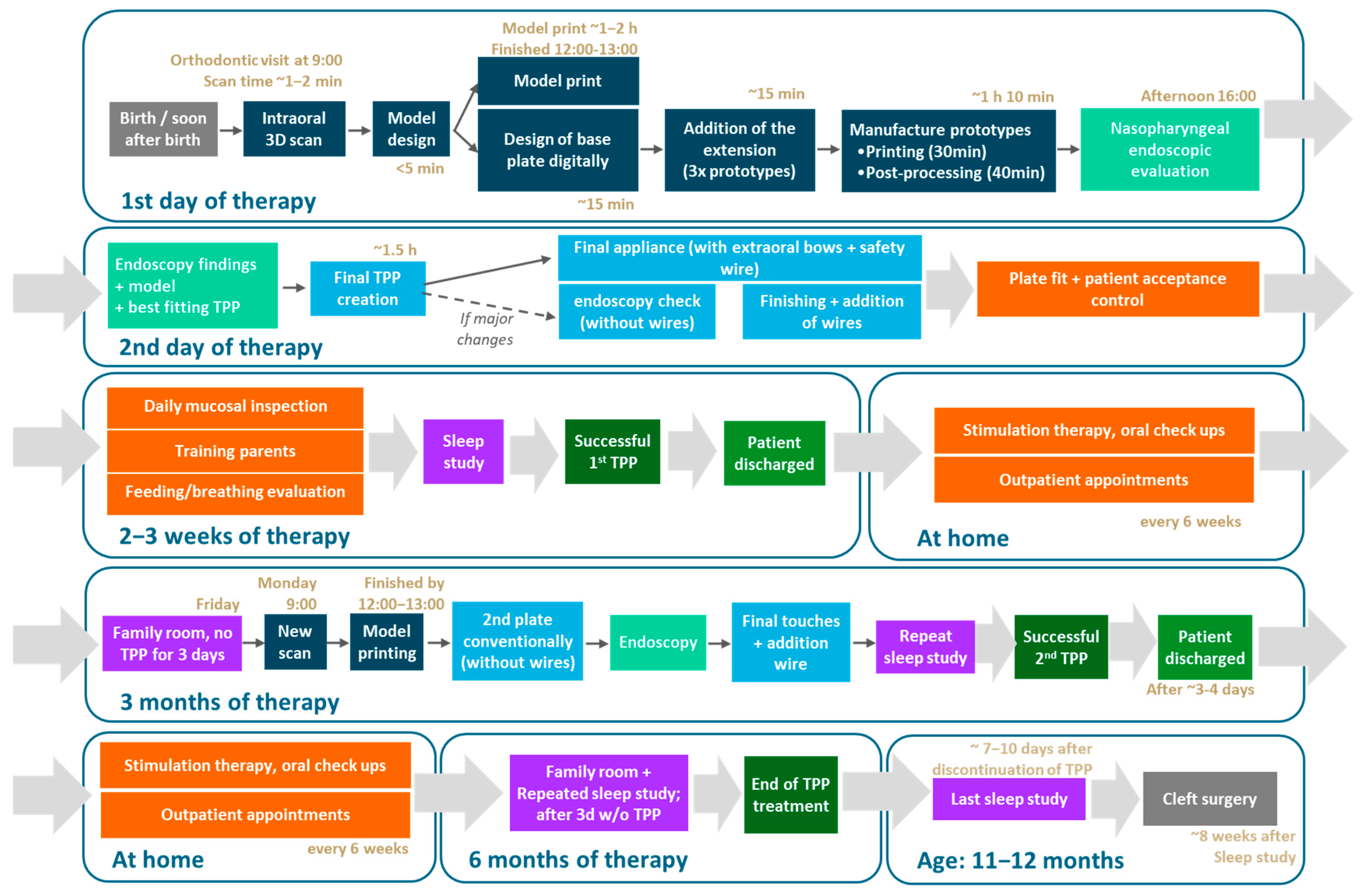
2. Resume of TPP Treatment Workflow
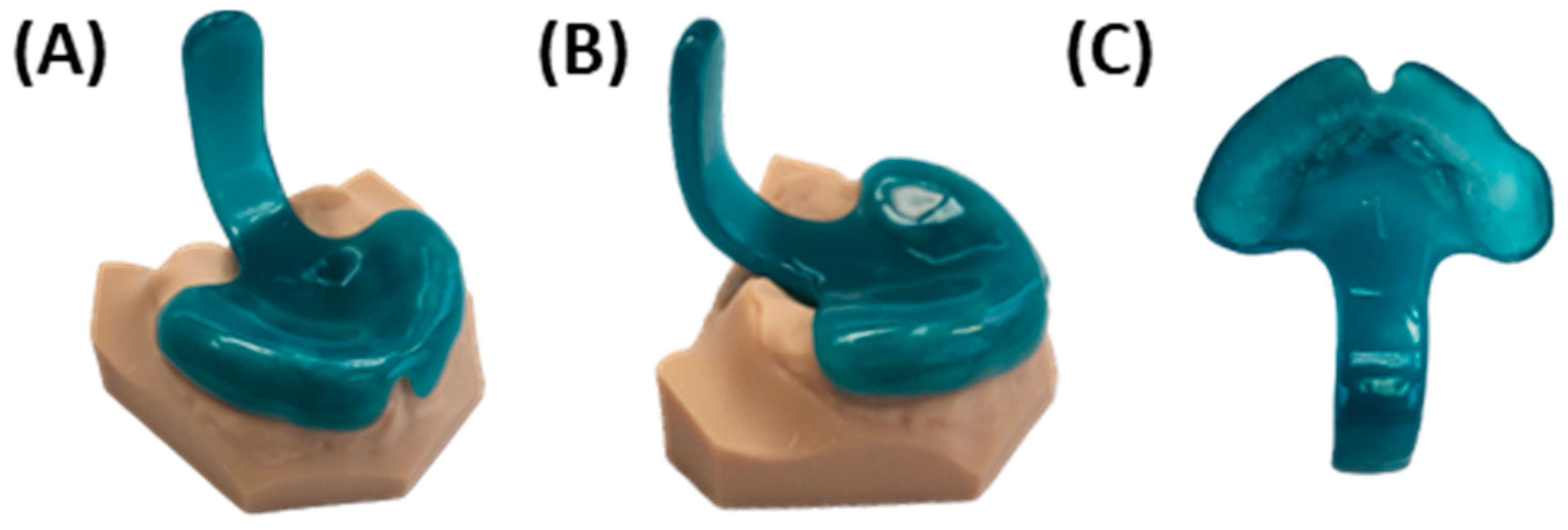
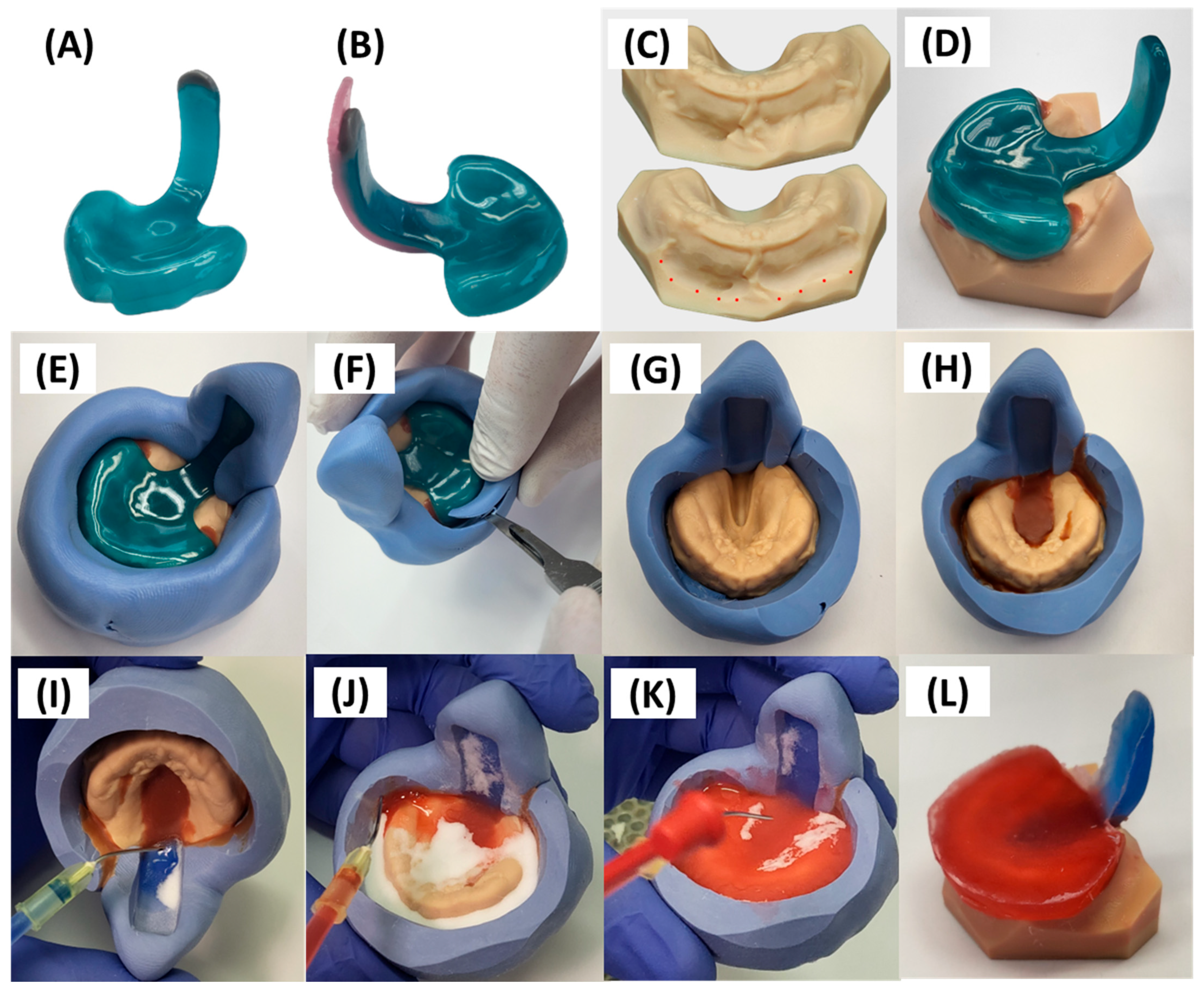
3. Prototyping Stage for the First Patient-Specific TPP
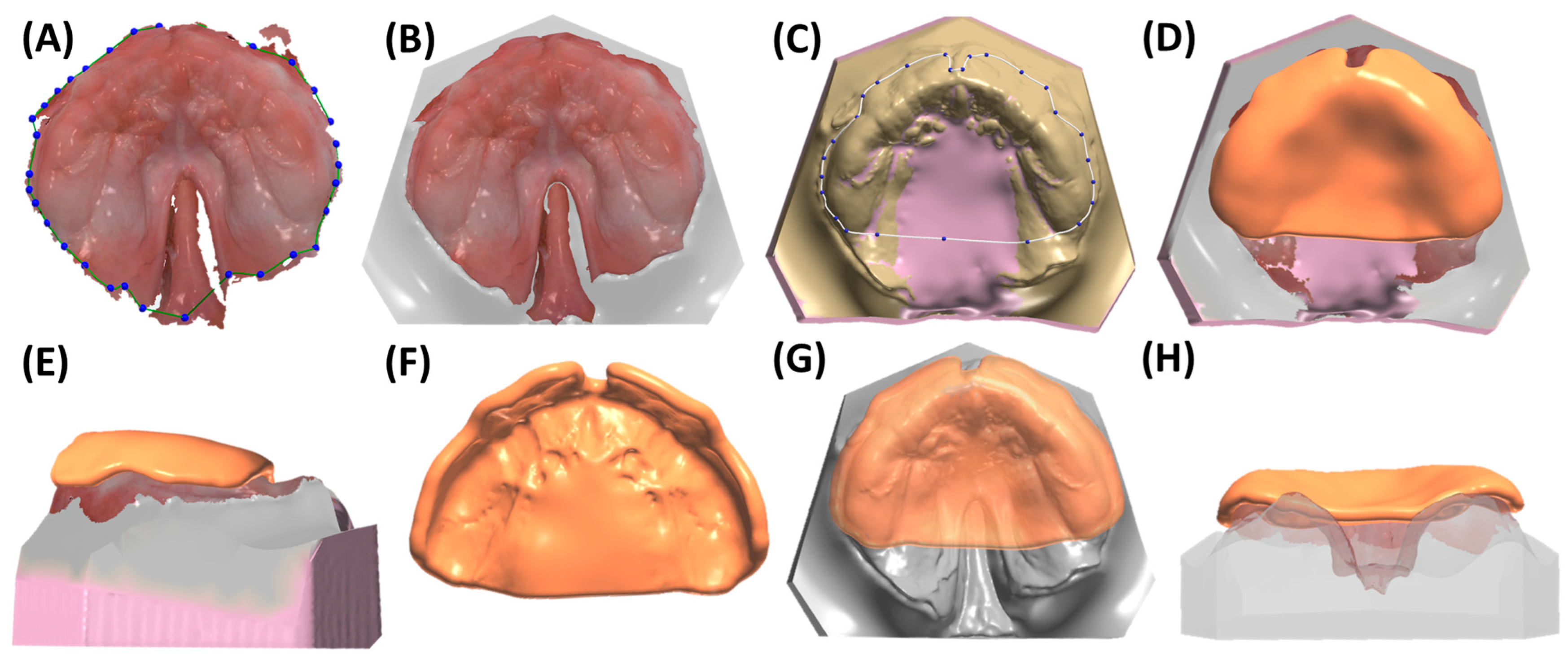
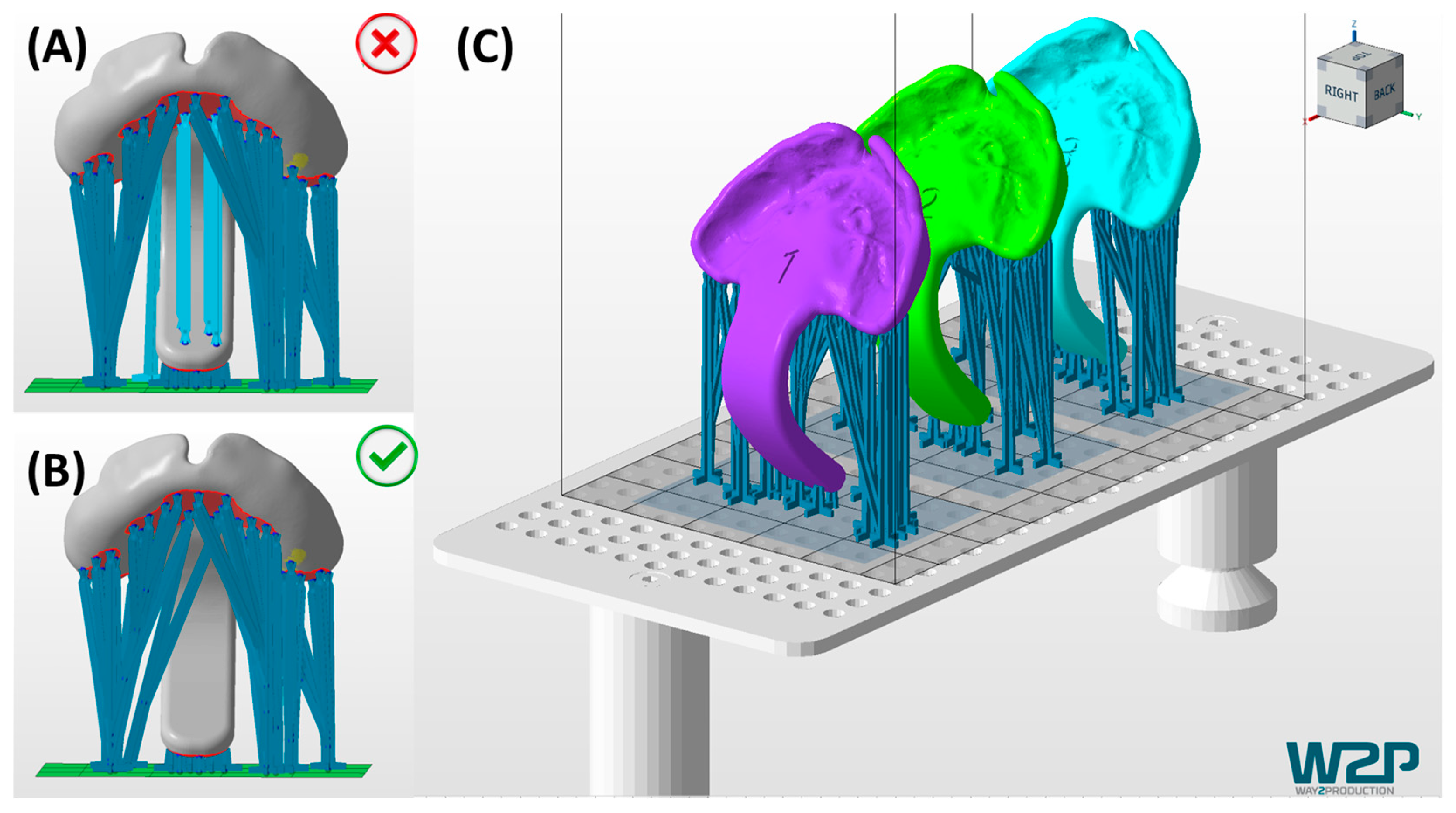
3.1. Digital Manufacturing Through CAD/CAM
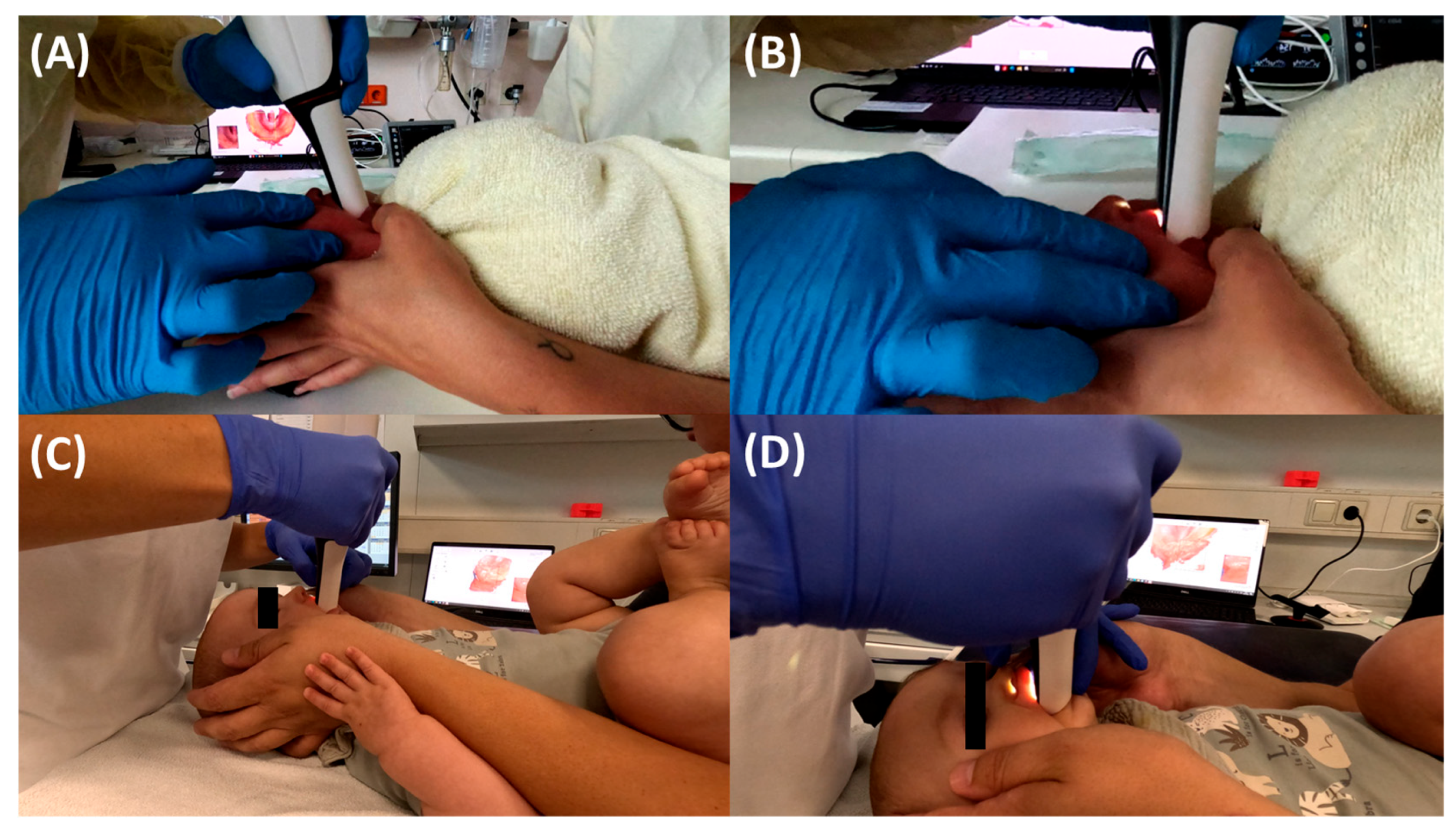
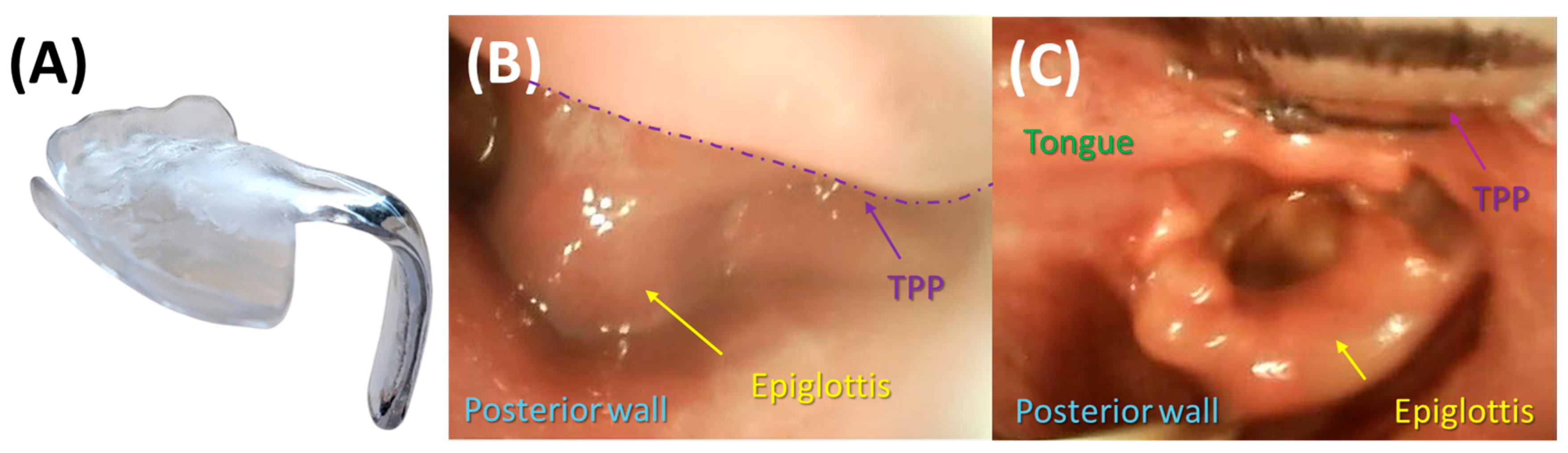
3.2. Endoscopic Exploration of the Prototypes
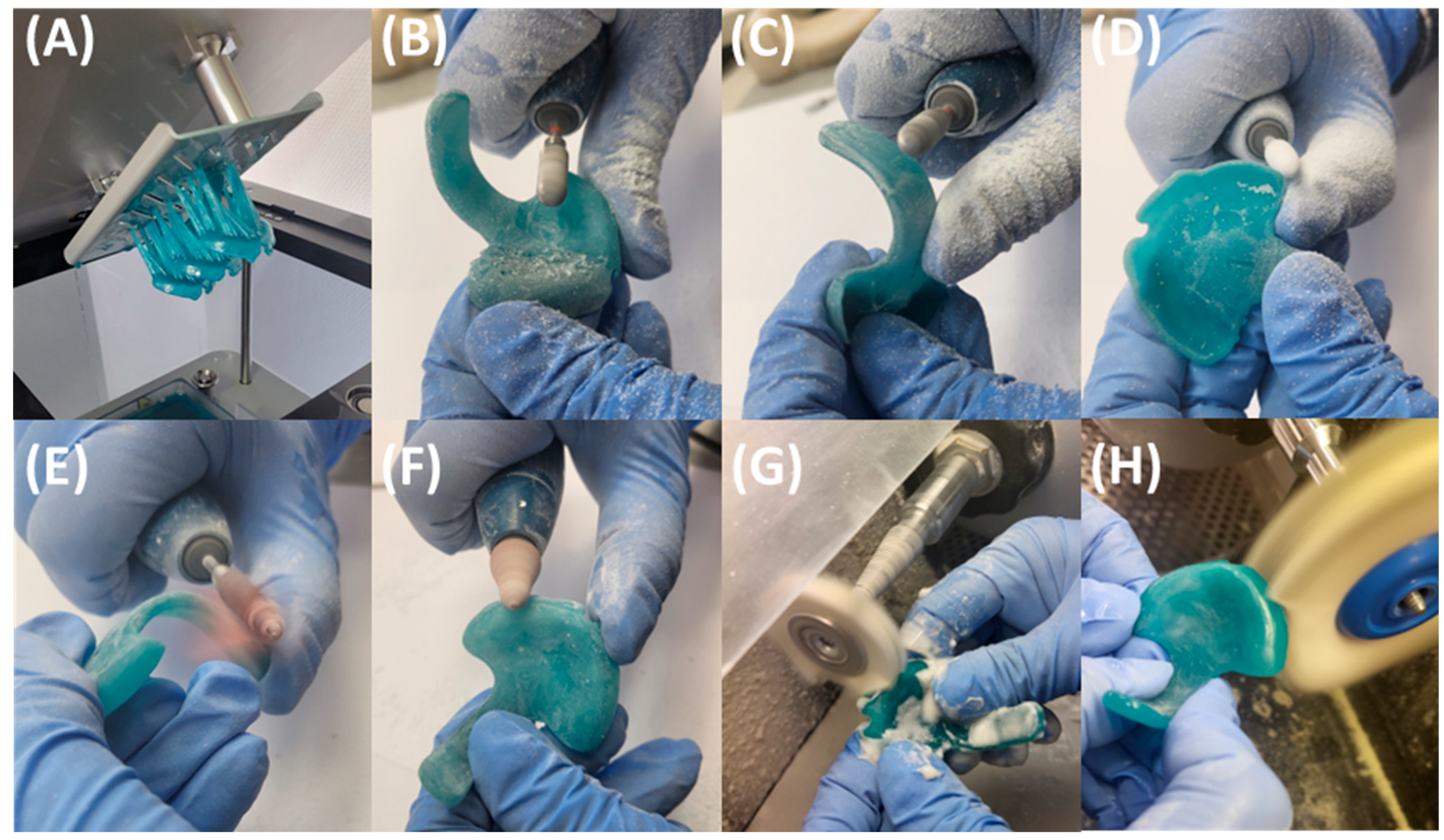
4. Transfer of Prototype to Final Appliance
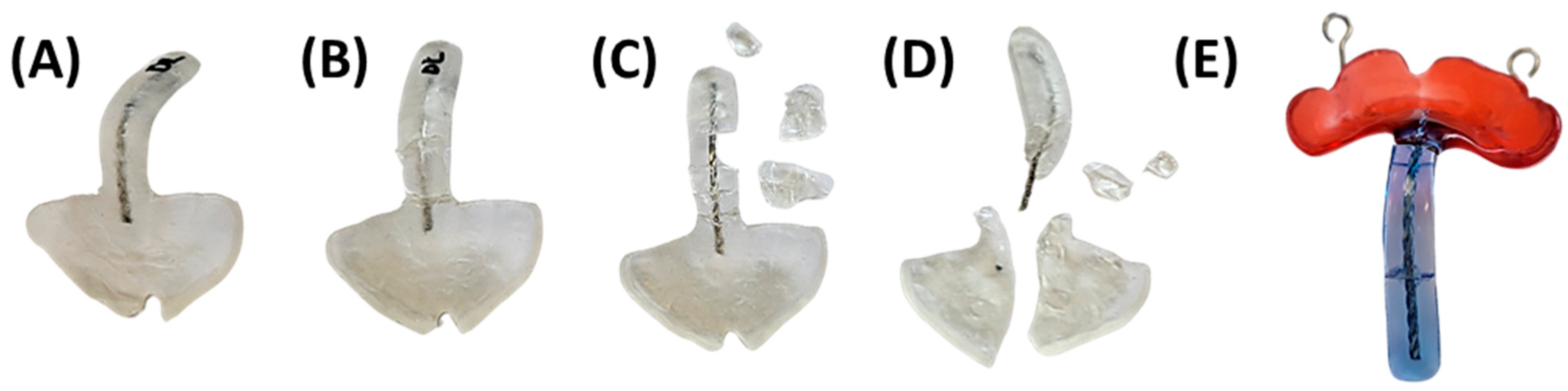
5. Quality Control, Potential Dangers and Side Effects
5.1. Appliance Quality Control Pre-Delivery
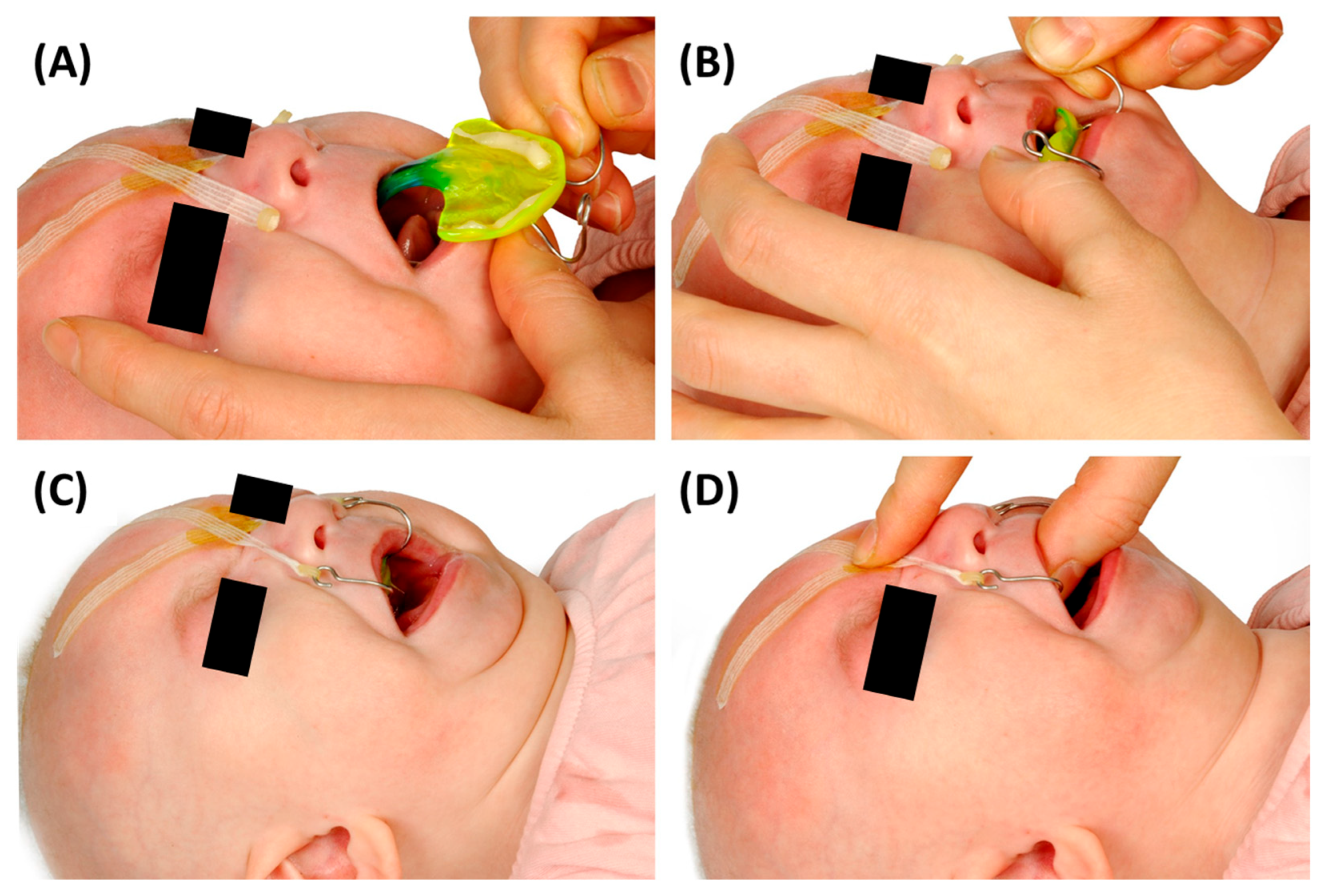
5.2. Final Fitting Appliance Evaluation, TPP Insertion, Adjustment and Initial Handling Protocol
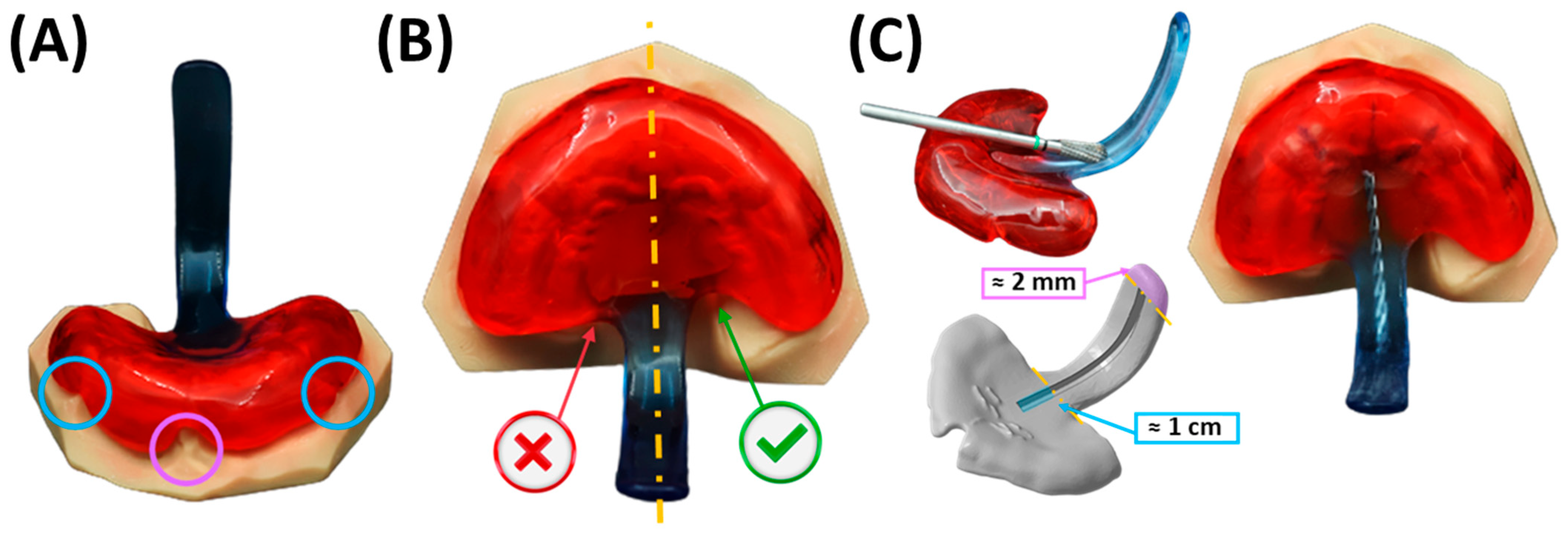
5.2.1. Adjustment of the Extraoral Fixation Bows and Frenula Sparing of the Base Palatal Plate
5.2.2. Final Insertion
5.2.3. Fixation Technique
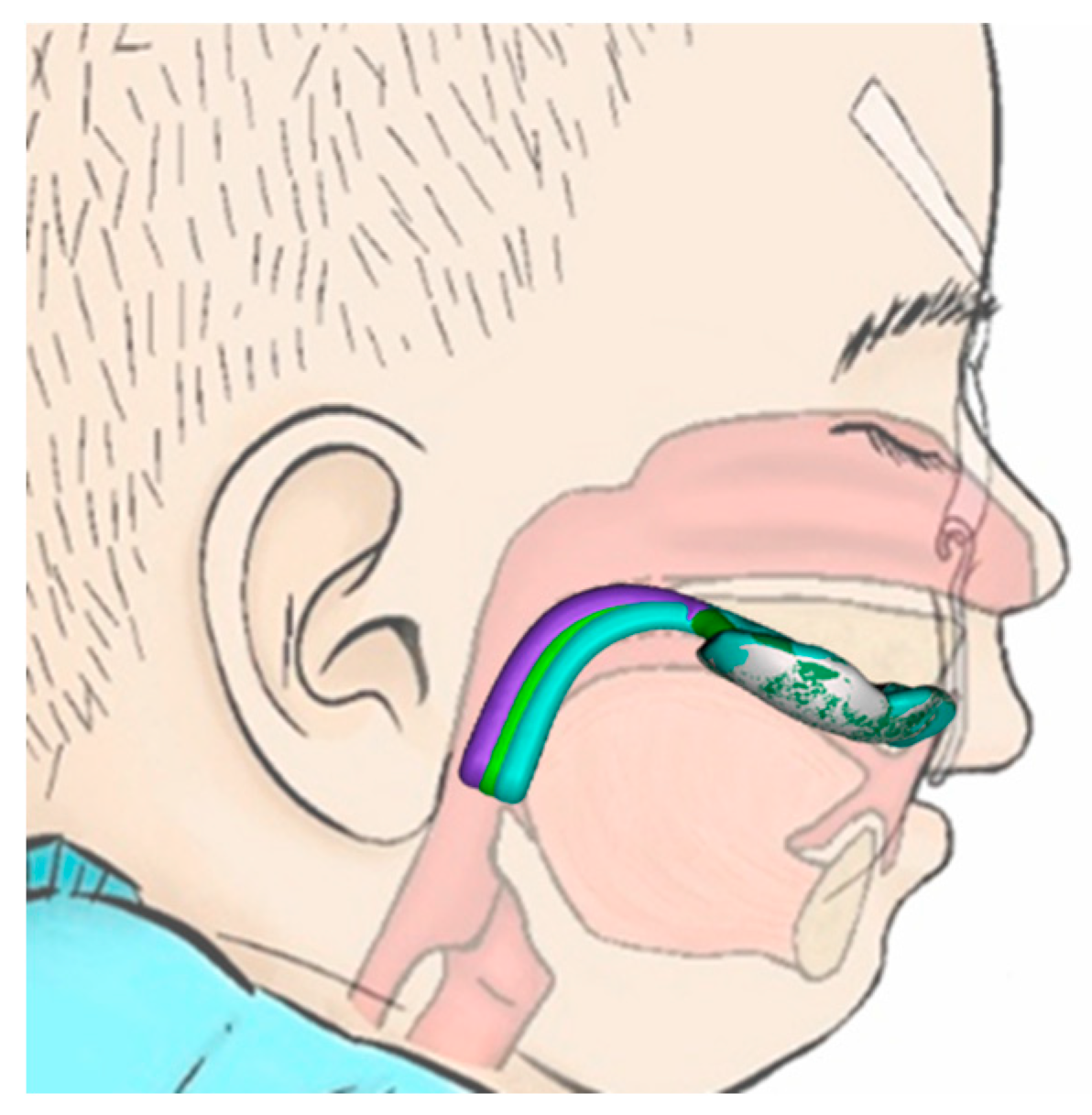
5.2.4. Position of the Velopharyngeal Extension
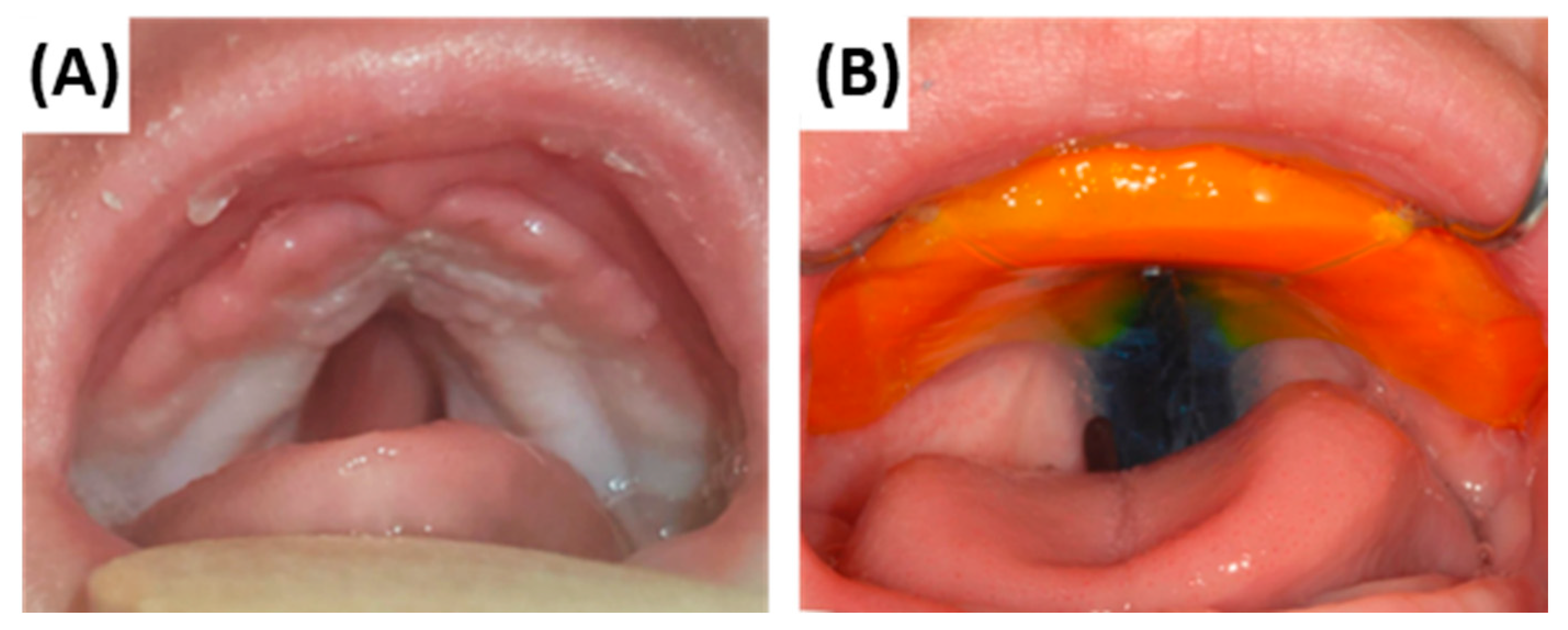
5.2.5. Daily Maintenance and Reapplication
5.3. Follow-Up Controls and Addressing Potential Side Effects
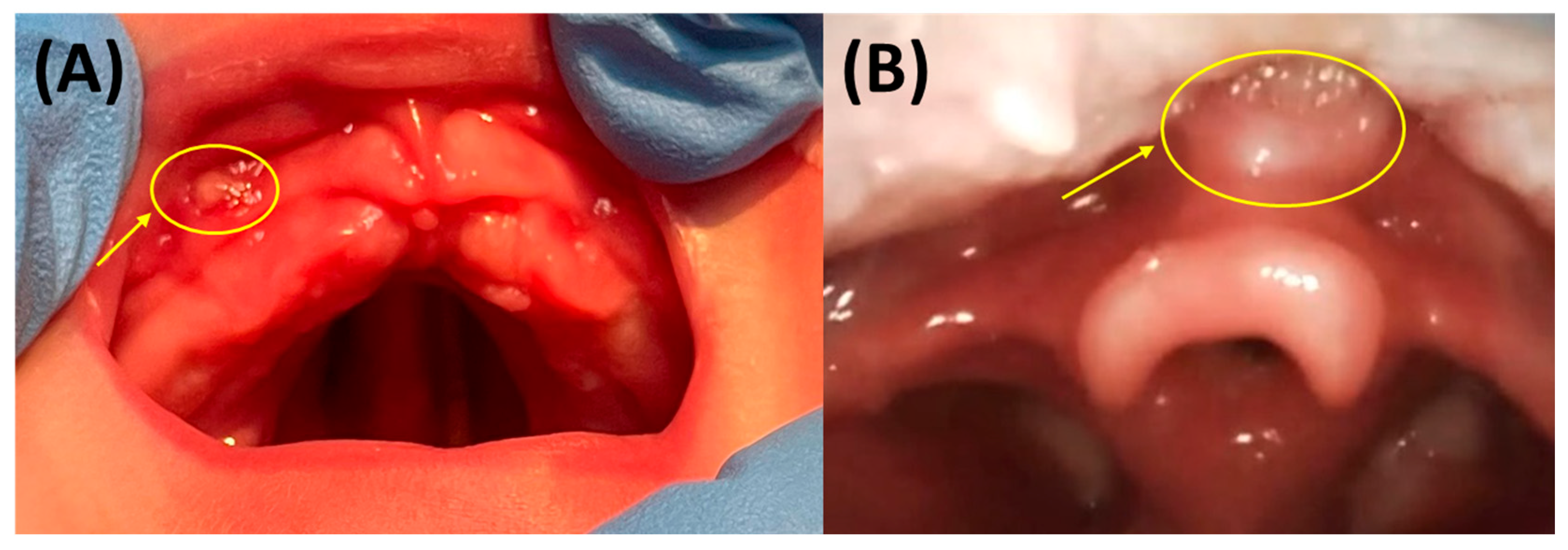
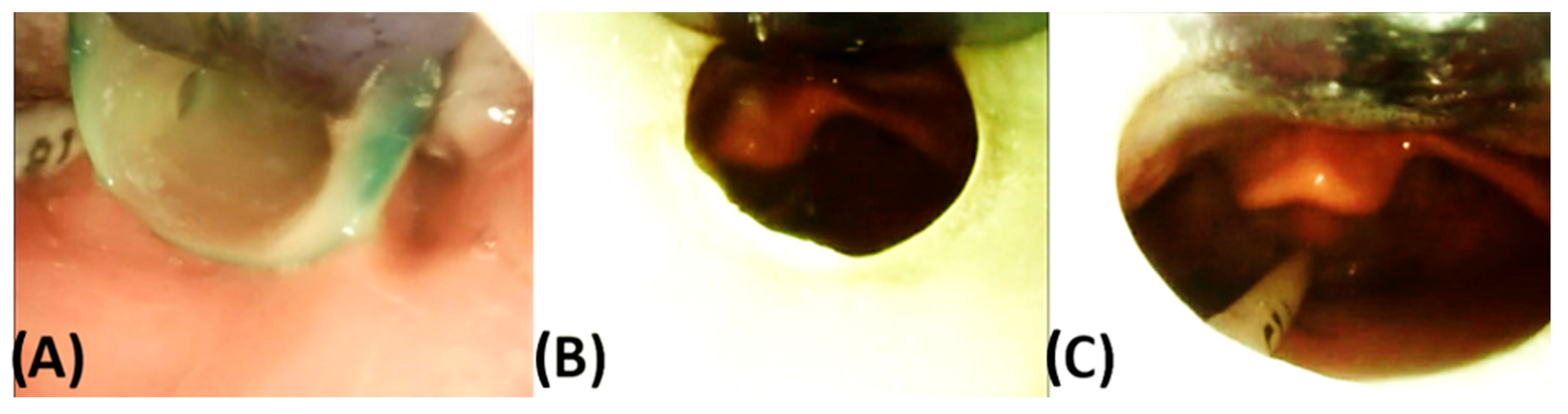
5.3.1. Oral Mucosal Lesions (OMLs) or Mucosal Ulcerations (MUs)
5.3.2. Mucosal Indentations or So-Called “Dents”
5.4. Checklist for the TPP Protocol
6. Reasoning for the Current TPP Approach and Workflow in Our Centre
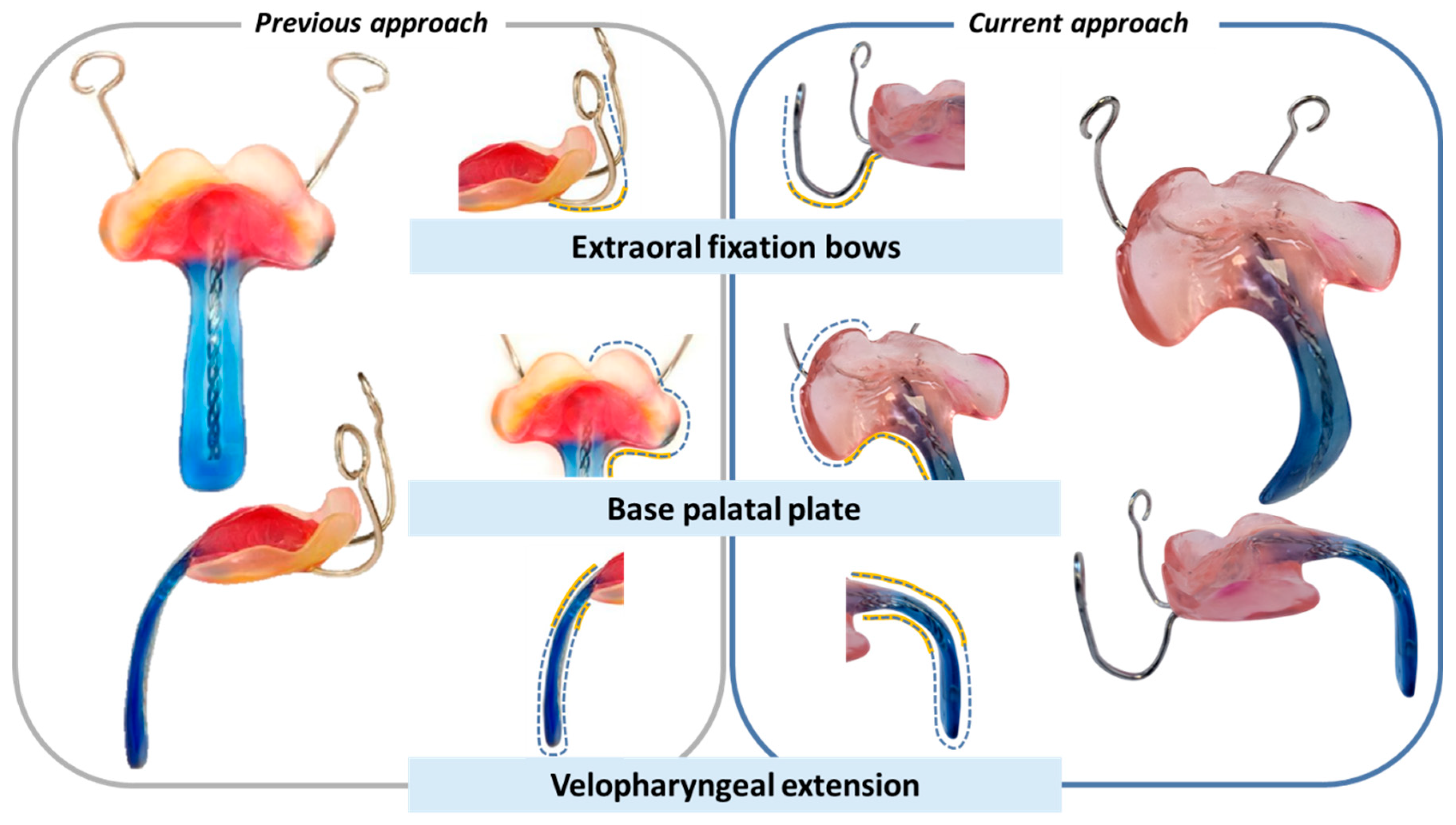
6.1. Reasoning for the Presented Prototyping Approach
6.2. Reasons for a Conventional Manufacturing Approach for the Final TPP Appliance
7. Other Workflow Approaches
8. Special Velopharyngeal Extension Configurations for Non-Standard RS Cases
8.1. No Hard and Soft Cleft Palate
8.2. Hypotonic Oropharyngeal Musculature and/or Challenging Anatomical Structures
9. Subsequent TPPs
10. Other TPP Treatment Success Factors
11. End of Treatment and Next Stages
12. Orthodontic Treatment Considerations Following TPP Therapy
13. Research Supporting the TPP Approach from Our Centre
| Authors | Shortened Title | Key Findings | Main Topics/Disciplines |
|---|---|---|---|
| Weismann et al. (Under review) [40] | Orthodontic Appliance-Related MUs in Infants with Craniofacial Disorder (CD) | In patients with palatal plates and CD were evaluated, where 88% of RS patients developed MUs. Plate type significantly influenced rates; however, sex, cleft location, syndrome, and morphology did not. | Orthodontics, Craniofacial Anomalies, Mucosal Pathology |
| Wiechers et al. (2025) [83] | Positioning and OSA in RS | Prone position improves but does not eliminate OSA and may worsen symptoms in some cases. | Sleep Medicine, Pediatric OSA, Positioning Therapy |
| Wiechers et al. (2024) [69] | Facial Profile Changes in RS with TPP and Controls | TPP supports mandibular catch-up growth; ANB’ angle and Jaw Index improved over time. | Orthodontics, Craniofacial Growth and Development |
| Wiechers (2023) [62] | Sleep and neurocognitive outcomes in primary school children with RS | Children with RS had higher risk of OSA and behavioural problems, while neurocognitive outcomes were normal. Regular OSA screening throughout childhood is recommended. | Sleep Medicine, Pediatric OSA |
| Effert et al. (2023) [9] | Prospective Orthodontic Evaluation Post-TPP | School-aged RS children post-TPP showed normal skeletal/dentoalveolar parameters, PAS, with stimulated mandibular/TMJ (temporomandibular joint) growth but a more convex soft tissue profile. | Orthodontics, Craniofacial Growth, Airway Assessment |
| Knechtel et al. (2023) [14] | Caring for Infants with TPP | Practical tips from 20 years’ experience on TPP feeding, cleaning, and placement. | Nursing Practice, Feeding Management, Medical Device Care |
| Effert et al. (2023) [15] | Orthodontic Needs after TPP | RS patients show higher orthodontic needs regardless of cleft status. | Orthodontics, Craniofacial Anomalies |
| Oechsle et al. (2022) [74] | Multicentre Registry for RS Treatment | Prospective multinational registry collects standardized data on treatments, complications, and outcomes to improve personalized care and long-term understanding. | Clinical Research, Multicentre Registry, Evidence-Based Medicine |
| Aretxabaleta et al. (2022) [8] | CAD Measurement semi-automation for TPPs | CAD-based semi-automatic method enables fast, accurate, reproducible TPP measurements. | Digital Dentistry, CAD/CAM Technology, Orthodontics |
| Naros et al. (2022) [70] | Neurocognitive Development in Isolated RS Treated with TPP | Children with isolated RS treated early with TPP showed normal cognitive development by age 5–6, comparable to cleft-only peers. | Pediatric Neurodevelopment, Craniofacial Anomalies, Early Intervention |
| Naros et al. (2022) [71] | Speech Development in Cleft Palate with/without RS | Good speech outcomes in RS and cleft-palate-only children; isolated RS, surgery timing, and cleft severity did not worsen results after TPP treatment. | Speech–Language Pathology, Craniofacial Speech Outcomes |
| Resnick et al. (2025) [73] | Comparison of TPP and MDO | Both MDO and TPP improve airway, feeding, and growth; MDO superior for severe UAO, whereas TPP shows better early feeding and weight gain. | Comparative Treatment Outcomes, Airway Management, Feeding, Craniofacial Surgery |
| Wiechers et al. (2021) [7] | Evidence and Practice with TPP | Review highlighting clinical effectiveness and practical aspects of TPP treatment. | Clinical Review, Orthodontics, Craniofacial Treatment |
| Wiechers et al. (2021) [10] | Growth Outcomes after TPP in RS | Catch-up growth after initial weight loss; tube feeding decreased post-TPP. | Feeding Management, Growth Monitoring, Craniofacial Treatment |
| Naros et al. (2021) [72] | Perioperative Complications in RS Cleft Palate Repair Post-TPP | Early TPP treatment corrects UAO and reduces perioperative complications in RS cleft palate repair. | Surgery, Perioperative Care, Airway Management |
| Aretxabaleta et al. (2021) [25] | Accuracy of Additive vs. Subtractive Palatal Plates | Subtractive methods showed higher accuracy than additive ones; DLP at 100 µm balances accuracy and efficiency for TPP production. | Manufacturing Technology, Digital Dentistry, Material Science |
| Authors | Shortened Title | Key Findings | Main Topics/Disciplines |
|---|---|---|---|
| Aretxabaleta et al. (2021) [24] | Fracture Load of Digital TPP Appliances | CAD/CAM improves TPP production; Freeprint tray recommended for prototyping, Smile PEEK for the final TPP for maximum safety. | Materials Science, Device Safety, Digital Manufacturing |
| Aretxabaleta et al. (2021) [23] | CAD/CAM Materials for TPP: Flexural Strength Study | SLA and SM methods had the highest flexural strength under standardized testing. | Material Testing, Digital Dentistry, Biomechanics |
| Wiechers et al. (2021) [84] | Treatment of Infants with Craniofacial Malformations | Infants with CD/RS at high risk for respiratory, feeding, and developmental issues requiring early multidisciplinary care and prenatal planning. | Multidisciplinary Care, Neonatology, Craniofacial Medicine |
| Weise et al. (2021) [18] | IOS of Neonates and Infants with Craniofacial Disorders | IOS is a fast, safe, feasible procedure for neonates and infants with craniofacial malformations like RS. | Digital Dentistry, Clinical Workflow, Pediatric Care |
| Xepapadeas et al. (2020) [22] | Digital Workflow for RS: Technical Note Part II | Digital workflow enabled successful TPP prototype production from intraoral scans. | Clinical Workflow, Digital Dentistry, Orthodontics |
| Müller-Hagedorn et al. (2020) [85] | TPP Prototype Method | Thermoplastic adjustable spur improves airway and stimulates mandibular growth. | Orthodontics, Device Design |
| Xepapadeas et al. (2020) [21] | Digital Workflow for Trisomy 21 Plates: Technical Note Part I | Workflow for base palatal plate production. | Clinical Workflow, Digital Dentistry |
| Wiechers et al. (2019) [11] | Mandibular Growth in Infants Treated with TPP | Jaw Index and apnoea scores improved; tube feeding dropped from 84% to 8%; no craniofacial surgery needed; mandibular growth promoted. | Orthodontics, Feeding Management, Craniofacial Growth |
| Poets et al. (2019) [12] | Summary of Current Evidence on TPP | TPP effective in relieving UAO, stimulating growth, and improving cleft outcomes; alternative to surgery. | Review, Airway Management, Craniofacial Treatment |
| Müller-Hagedorn et al. (2017) [59] | TPP for Syndromic RS | Modified TPP designs including ring and tube solutions proposed. | Orthodontics, Syndromic Craniofacial Disorders, Device Innovation |
| Poets et al. (2017) [5] | Multicentre Study on PEBP/TPP | PEBP treatment significantly reduced apnoea index and oxygen desaturation; tube feeding dropped from 74% to 14% with stable weight. | Multicentre Clinical Study, Airway Management, Feeding |
| Buchenau et al. (2017) [4] | Functional Treatment Using PEBP/TPP | PEBP reduced apnoea index, improved weight gain, and lowered tube feeding in isolated RS without surgery or tracheostomy. | Airway Management, Feeding, Non-invasive Treatment |
| Maas et al. (2014) [86] | Prospective Study on Initial Treatment and Early Weight Gain in Germany | Prone positioning (61%) and functional therapy (57%) common; PEBP used in 23%; surgery rare (6%), favouring non-invasive treatments. | Epidemiology, Treatment Patterns, Feeding Management |
| Vatlach et al. (2014) [1] | Birth Prevalence and Treatments in Germany | RS prevalence 12.4/100,000; majority treated conservatively; few required surgery. | Epidemiology, Treatment Trends |
| Poets et al. (2011) [87] | Treatment of UAO and Feeding in RS-Like Phenotype | Overview of treatment strategies for RS-like conditions. | Treatment Strategies, Airway and Feeding Management |
| Bacher et al. (2010) [6] | Treatment of Infants with RS | Overview of conservative vs. surgical options; PEBP introduced as an effective non-invasive alternative. | Airway Management, Feeding, Treatment Options |
| Buchenau et al. (2007) [57] | Randomized Trial of New Orthodontic Appliance for RS | Appliance with velar extension reduced apnoea index by 71% without adverse effects. | Sleep Medicine, Orthodontics, Clinical Trial |
| Bodman et al. (2003) [16] | The Tübingen palatal plate, an innovative therapy concept for RS (in German) | Appliance with extension effectively treats UAO, reducing hypoxemia and supporting mandibular growth | Sleep Medicine, Neonatology, Orthodontics, Clinical Trial |
14. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RS | Robin Sequence |
| UAO | Upper Airway Obstruction |
| TPP | Tübingen Palatal Plate |
| PEBP | Pre-epiglottic Baton Plate |
| IOS | Intraoral Scanning |
| CAD/CAM | Computer-Aided Design and Computer-Aided Manufacturing |
| AM | Additive Manufacturing |
| OAI | Mixed-Obstructive Apnoea Index |
| STL | Standard Tessellation Language |
| PLY | Polygon File Format |
| DCM | Digital Imaging and Communications in Medicine |
| SLA | Stereolithography |
| IPA | Isopropyl Alcohol |
| DLP | Direct Light Processing |
| MDR | Medical Device Regulation |
| PMMA | Polymethyl methacrylate |
| MU | Mucosal Ulceration |
| OML | Oral Mucosal Lesion |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| SM | Subtractive Manufacturing |
| PEEK | Polyetheretherketone |
| MPP | Modified palatal Plate |
| OAP | Orthodontic Airway Plate |
| MDO | Mandibular Distraction Osteogenesis |
| PAS | Posterior Airway Space |
| TMJ | Temporomandibular joint |
| CD | Craniofacial Disorder |
| ERN | European Reference Network |
References
- Vatlach, S.; Maas, C.; Poets, C.F. Birth prevalence and initial treatment of Robin sequence in Germany: A prospective epidemiologic study. Orphanet J. Rare Dis. 2014, 9, 9. [Google Scholar] [CrossRef]
- Côte, A.; Fanous, A.; Almajed, A.; Lacroix, Y. Pierre Robin sequence: Review of diagnostic and treatment challenges. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 451–464. [Google Scholar] [CrossRef]
- Holder-Espinasse, M.; Abadie, V.; Cormier-Daire, V.; Beyler, C.; Manach, Y.; Munnich, A.; Lyonnet, S.; Couly, G.; Amiel, J. Pierre Robin sequence: A series of 117 consecutive cases. J. Pediatr. 2001, 139, 588–590. [Google Scholar] [CrossRef]
- Buchenau, W.; Wenzel, S.; Bacher, M.; Müller-Hagedorn, S.; Arand, J.; Poets, C.F. Functional treatment of airway obstruction and feeding problems in infants with Robin sequence. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 102, F142–F146. [Google Scholar] [PubMed]
- Poets, C.F.; Maas, C.; Buchenau, W.; Arand, J.; Vierzig, A.; Braumann, B.; Müller-Hagedorn, S. Multicenter study on the effectiveness of the pre-epiglottic baton plate for airway obstruction and feeding problems in Robin sequence. Orphanet J. Rare Dis. 2017, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Bacher, M.; Linz, A.; Buchenau, W.; Arand, J.; Krimmel, M.; Poets, C. Treatment of infants with Pierre Robin sequence. Laryngo-Rhino-Otol. 2010, 89, 621–629. [Google Scholar]
- Wiechers, C.; Arand, J.; Koos, B.; Poets, C.F. Evidence and practical aspects of treatment with the Tübingen palatal plate. Semin. Fetal Neonatal Med. 2021, 26, 101281. [Google Scholar] [CrossRef] [PubMed]
- Aretxabaleta, M.; Roehler, A.; Poets, C.F.; Xepapadeas, A.B.; Koos, B.; Weise, C. Automation of Measurements for Personalized Medical Appliances by Means of CAD Software—Application in Robin Sequence Orthodontic Appliances. Bioengineering 2022, 9, 773. [Google Scholar] [CrossRef]
- Effert, J.; Uhlig, S.; Wiechers, C.; Quante, M.; Poets, C.F.; Schulz, M.C.; Reinert, S.; Krimmel, M.; Koos, B.; Weise, C. Prospective Evaluation of Children with Robin Sequence following Tübingen Palatal Plate Therapy. J. Clin. Med. 2023, 12, 448. [Google Scholar] [CrossRef]
- Wiechers, C.; Iffländer, R.; Gerdes, R.; Ciuffolotti, M.; Arand, J.; Weise, C.; Peters, K.; Grandke, B.; Reinert, S.; Koos, B. Retrospective study on growth in infants with isolated Robin sequence treated with the Tuebingen Palate Plate. Orphanet J. Rare Dis. 2021, 16, 338. [Google Scholar] [CrossRef]
- Wiechers, C.; Buchenau, W.; Arand, J.; Oertel, A.-F.; Peters, K.; Müller-Hagedorn, S.; Koos, B.; Poets, C.F. Mandibular growth in infants with Robin sequence treated with the Tübingen palatal plate. Head Face Med. 2019, 15, 17. [Google Scholar] [CrossRef]
- Poets, C.F.; Koos, B.; Reinert, S.; Wiechers, C. The Tübingen palatal plate approach to Robin sequence: Summary of current evidence. J. Cranio-Maxillofac. Surg. 2019, 47, 1699–1705. [Google Scholar] [CrossRef]
- Roux, W. Über die Selbstregulation der Lebewesen. Arch. Entwicklungsmechanik Org. 1901, 13, 610–650. [Google Scholar] [CrossRef]
- Knechtel, P.; Weismann, C.; Poets, C.F. Caring for Infants with Robin Sequence Treated with the Tübingen Palatal Plate: A Review of Personal Practice. Children 2023, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Effert, J.; Wiechers, C.; Kreutzer, K.; Poets, C.; Schulz, M.; Krimmel, M.; Aretxabaleta, M.; Finke, H.; Koos, B.; Weise, C. Retrospective evaluation of the orthodontic treatment needs in primary school children with Robin sequence following Tübingen palatal plate therapy in infancy. J. Cranio-Maxillofac. Surg. 2023, 51, 528–535. [Google Scholar]
- Von Bodman, A.; Buchenau, W.; Bacher, M.; Arand, J.; Urschitz, M.S.; Poets, C.F. Die Tübinger Gaumenplatte—Ein innovatives Therapiekonzept bei Pierre-Robin-Sequenz. Wien. Klin. Wochenschr. 2003, 115, 871–873. [Google Scholar] [CrossRef]
- Bacher, M.; Linz, A.; Buchenau, W.; Arand, J.; Krimmel, M.; Poets, C. Therapeutisches Vorgehen bei Pierre-Robin-Sequenz. Laryngo-Rhino-Otol. 2010, 89, 621–629. [Google Scholar]
- Weise, C.; Frank, K.; Wiechers, C.; Weise, H.; Reinert, S.; Koos, B.; Xepapadeas, A.B. Intraoral scanning of neonates and infants with craniofacial disorders: Feasibility, scanning duration, and clinical experience. Eur. J. Orthod. 2022, 44, 279–286. [Google Scholar] [CrossRef]
- Reichert, F.; Amrhein, P.; Uhlemann, F. Unnoticed aspiration of palate plate impression material in a neonate: Diagnosis, therapy, outcome. Pediatr. Pulmonol. 2017, 52, E58–E60. [Google Scholar] [CrossRef]
- Chate, R. A report on the hazards encountered when taking neonatal cleft palate impressions (1983–1992). Br. J. Orthod. 1995, 22, 299–307. [Google Scholar] [CrossRef]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.; Wiechers, C.; Arand, J.; Koos, B. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans-part I: 3D printed and milled palatal stimulation plate for trisomy 21. BMC Oral Health 2020, 20, 20. [Google Scholar]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.; Wiechers, C.; Arand, J.; Koos, B. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans-part II: 3D printed Tübingen palatal plate prototype for newborns with Robin sequence. BMC Oral Health 2020, 20, 171. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Comparison of additive and subtractive CAD/CAM materials for their potential use as Tübingen Palatal Plate: An in-vitro study on flexural strength. Addit. Manuf. 2021, 37, 101693. [Google Scholar]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Fracture load of an Orthodontic appliance for Robin Sequence Treatment in a digital workflow. Materials 2021, 14, 344. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Unkovskiy, A.; Koos, B.; Spintzyk, S.; Xepapadeas, A.B. Accuracy Evaluation of Additively and Subtractively Fabricated Palatal Plate Orthodontic Appliances for Newborns and Infants–An In Vitro Study. Materials 2021, 14, 4103. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Šufliarsky, B.; Urbanová, W.; Čverha, M.; Strunga, M.; Varga, I. Pierre Robin Sequence and 3D Printed Personalized Composite Appliances in Interdisciplinary Approach. Polymers 2022, 14, 3858. [Google Scholar] [CrossRef]
- Morales-Konzept, C. Das Castillo Morales-Konzept. Sprache·Stimme·Gehör 2021, 45, 108–109. [Google Scholar]
- Castillo-Morales, R.; Brondo, J.; Hoyer, H.; Limbrock, G. Die Behandlung von Kau-, Schluck-und Sprechstörungen bei behinderten Kindern mit der orofazialen Regulationstherapie nach Castillo-Morales: Aufgabe für Pädiater und Zahnarzt. Zahnärztl Mitt 1985, 75, 935–942. [Google Scholar]
- Morales, R.C. Die Orofaziale Regulationstherapie; Pflaum: Waterford, CT, USA, 1998. [Google Scholar]
- Schiewack, M. Ganzheitliche Behandlung–Castillo Morales. Ergopraxis 2021, 14, 51. [Google Scholar]
- Limbrock, G.; Hesse, A.; Hoyer, H. Orofaziale Regulationstherapie nach Castillo-Morales bei Kindern mit zerebralen Läsionen. Fortschritte Kieferorthopädie 1987, 48, 335–339. [Google Scholar]
- Weismann, C.; Xepapadeas, A.B.; Bockstedte, M.; Koos, B.; Krimmel, M.; Poets, C.F.; Aretxabaleta, M. Complete Digital Workflow for Manufacturing Presurgical Orthodontic Palatal Plates in Newborns and Infants with Cleft Lip and/or Palate. J. Funct. Biomater. 2024, 15, 301. [Google Scholar] [CrossRef]
- Sundman, E.; Witt, H.; Sandin, R.; Kuylenstierna, R.; Bodén, K.; Ekberg, O.; Eriksson, L.I. Pharyngeal function and airway protection during subhypnotic concentrations of propofol, isoflurane, and sevoflurane: Volunteers examined by pharyngeal videoradiography and simultaneous manometry. Anesthesiology 2001, 95, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, M.; Russo, F.; Iemma, M. Awake versus drug-induced sleep endoscopy: Evaluation of airway obstruction in obstructive sleep apnea/hypopnoea syndrome. Laryngoscope 2013, 123, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Shorten, G.; Opie, N.; Graziotti, P.; Morris, I.; Khangure, M. Assessment of upper airway anatomy in awake, sedated and anaesthetised patients using magnetic resonance imaging. Anaesth. Intensive Care 1994, 22, 165–169. [Google Scholar] [CrossRef]
- Sher, A.E.; Shprintzen, R.J.; Thorpy, M.J. Endoscopic observations of obstructive sleep apnea in children with anomalous upper airways: Predictive and therapeutic value. Int. J. Pediatr. Otorhinolaryngol. 1986, 11, 135–146. [Google Scholar] [CrossRef] [PubMed]
- KG, D.G.C. Instructions of Use-Orthocryl Dentaurum: 2021. Available online: https://www.dentaurum.de/files/989-554-00.pdf (accessed on 26 August 2025).
- Charavet, C.; Graveline, L.; Gourdain, Z.; Lupi, L. What are the cleaning and disinfection methods for acrylic orthodontic removable appliance? A systematic review. Children 2021, 8, 967. [Google Scholar] [CrossRef]
- Tsolakis, A.Ι.; Kakali, L.; Prevezanos, P.; Bitsanis, I.; Polyzois, G. Use of different cleaning methods for removable orthodontic appliances: A questionnaire study. Oral Health Prev. Dent. 2019, 17, 299–302. [Google Scholar]
- Weismann, C.; Heise, K.; Peters, K.; Bockstedte, M.; Schulz, M.C.; Wiechers, C.; Quante, M.; Poets, C.F.; Koos, B.; Aretxabaleta, M. Orthodontic appliance-related mucosal ulcerations in newborns and infants with craniofacial disorders. Clin. Exp. Dent. Res. 2025; under review. [Google Scholar]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Yuan, M.-K.; Tsai, D.-C.; Chang, S.-C.; Yuan, M.-C.; Chang, S.-J.; Chen, H.-W.; Leu, H.-B. The risk of cataract associated with repeated head and neck CT studies: A nationwide population-based study. Am. J. Roentgenol. 2013, 201, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.P. The lens is more sensitive to radiation than we had believed. Br. J. Ophthalmol. 1997, 81, 257. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Glasl, B.; Sader, R.; Schopf, P. Conservative orthodontic primary care of four newborns with the Pierre-Robin sequence triad. J. Orofac. Orthop./Fortschritte Kieferorthopädie 2007, 68, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kochel, J.; Meyer-Marcotty, P.; Wirbelauer, J.; Böhm, H.; Kochel, M.; Thomas, W.; Bareis, U.; Hebestreit, H.; Speer, C.; Stellzig-Eisenhauer, A. Treatment modalities of infants with upper airway obstruction—Review of the literature and presentation of novel orthopedic appliances. Cleft Palate-Craniofacial J. 2011, 48, 44–55. [Google Scholar] [CrossRef]
- Gerzanic, L.; Feichtinger, M.; Kärcher, H. The influence of the Tübingen soft palate plate and early cleft closure on the nasopharyngeal airway for the management of airway obstruction in an infant with Pierre Robin sequence: A case report. Int. J. Surg. Case Rep. 2012, 3, 608–610. [Google Scholar] [CrossRef]
- Ho, A.C.; Wong, R.W.; Cheung, T.; Ng, D.K.; Siu, K.; Fung, S. Orthodontic plate for management of obstructive sleep apnoea in infants with Pierre Robin sequence: Experience and protocol in Hong Kong. J. Orthod. 2019, 46, 367–373. [Google Scholar] [CrossRef]
- Tomic, J.; Metzler, P.; Alcon, A.; Jakse, N.; Zemann, W.; Schanbacher, M.; Zrnc, T.A. Weight gain in infants with Pierre Robin sequence. J. Cranio-Maxillofac. Surg. 2020, 48, 555–559. [Google Scholar] [CrossRef]
- Schmidt, G.; Hirschfelder, A.; Heiland, M.; Matuschek, C. Customized pre-epiglottic baton plate—A practical guide for successful, patient-specific, noninvasive treatment of neonates with Robin Sequence. Cleft Palate-Craniofacial J. 2021, 58, 1063–1069. [Google Scholar] [CrossRef]
- Goryachkina, Y.A.; Goryachkin, A. Treatment of infants with Pierre Robin syndrome using the orthodontic device PEBP (pre-epiglottic baton plate). Stomatologiia 2020, 99, 29–32. [Google Scholar] [CrossRef]
- Choo, H.; Khosla, R.K.; Meister, K.D.; Wan, D.C.; Lin, H.-F.C.; Feczko, R.; Bruckman, K.; Hopkins, E.; Truong, M.T.; Lorenz, H.P. Nonsurgical orthodontic airway plate treatment for newborns with Robin sequence. Cleft Palate-Craniofacial J. 2022, 59, 403–410. [Google Scholar]
- Choo, H.; Kim, S.-H.; Ahn, H.-W.; Poets, C.F.; Chung, K.-R. Split orthodontic airway plate: An innovation to the utilization method of conventional orthodontic airway plate for neonates with Robin sequence. Korean J. Orthod. 2022, 52, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Benitez, B.K.; Rasadurai, A.; Boccalatte, L.A.; Mueller, A.A. Pre-Epiglottic Baton Plate in Newborns with Pierre Robin Sequence: Revisiting the Practical Workflow. Laryngoscope 2024, 134, 4766. [Google Scholar] [CrossRef]
- Poets, C.F.; Aretxabaleta, M.; Weismann, C.; Quante, M.; Wiechers, C.; Koos, B. Palatal plates for treating infants with Robin sequence. J. Clin. Sleep Med. 2025, 21, 735–736. [Google Scholar] [CrossRef]
- Buchenau, W.; Urschitz, M.S.; Sautermeister, J.; Bacher, M.; Herberts, T.; Arand, J.; Poets, C.F. A randomized clinical trial of a new orthodontic appliance to improve upper airway obstruction in infants with Pierre Robin sequence. J. Pediatr. 2007, 151, 145–149. [Google Scholar] [CrossRef]
- Paes, E.C.; van Nunen, D.P.; Basart, H.; Don Griot, J.P.W.; van Hagen, J.M.; van der Horst, C.M.; van den Boogaard, M.J.H.; Breugem, C.C. Birth prevalence of Robin sequence in the Netherlands from 2000-2010: A retrospective population-based study in a large Dutch cohort and review of the literature. Am. J. Med. Genet. Part A 2015, 167, 1972–1982. [Google Scholar] [CrossRef]
- Müller-Hagedorn, S.; Buchenau, W.; Arand, J.; Bacher, M.; Poets, C.F. Treatment of infants with Syndromic Robin sequence with modified palatal plates: A minimally invasive treatment option. Head Face Med. 2017, 13, 4. [Google Scholar] [CrossRef]
- Dhillon, R. The middle ear in cleft palate children pre and post palatal closure. J. R. Soc. Med. 1988, 81, 710–713. [Google Scholar] [CrossRef]
- Sharma, R.K.; Nanda, V. Problems of middle ear and hearing in cleft children. Indian J. Plast. Surg. 2009, 42, S144–S148. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Uhlig, S.; Poets, A.; Weise, C.; Naros, A.; Krimmel, M.; Koos, B.; Poets, C.F.; Quante, M. Sleep and neurocognitive outcome in primary school children with Robin Sequence. Sleep 2023, 46, zsac317. [Google Scholar] [CrossRef]
- Weise, C.; Lehmann, M.; Schulz, M.; Reinert, S.; Koos, B.; Weise, H. Tooth agenesis in German orthodontic patients with non-syndromic craniofacial disorder: A retrospective evaluation of panoramic radiographs. Clin. Oral Investig. 2022, 26, 5823–5832. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, S.H.; Ranta, R.E. Cephalometric measurements in patients with Pierre Robin syndrome and isolated cleft palate. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1992, 26, 177–183. [Google Scholar] [CrossRef]
- Laitinen, S.H.; Heliövaara, A.; Ranta, R.E. Craniofacial morphology in young adults with the Pierre Robin sequence and isolated cleft palate. Acta Odontol. Scand. 1997, 55, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Suda, N.; Motohashi, N.; Tsuji, M.; Ohyama, K. Skeletal characteristics and treatment outcome of five patients with Robin sequence. Angle Orthod. 2006, 76, 898–908. [Google Scholar] [CrossRef]
- Suri, S.; Ross, R.B.; Tompson, B.D. Craniofacial morphology and adolescent facial growth in Pierre Robin sequence. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 763–774. [Google Scholar] [CrossRef]
- Suri, S.; Ross, R.B.; Tompson, B.D. Mandibular morphology and growth with and without hypodontia in subjects with Pierre Robin sequence. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 37–46.e1. [Google Scholar] [CrossRef]
- Wiechers, C.; Sowula, J.; Kreutzer, K.; Schwarz, C.E.; Weismann, C.; Krimmel, M.; Poets, C.F.; Koos, B. Prospective cohort study on facial profile changes in infants with Robin sequence and healthy controls. World J. Pediatr. 2024, 20, 581–589. [Google Scholar] [CrossRef]
- Naros, A.; Steiner-Wilke, I.; Kaiser, N.; Bacher, M.; Koos, B.; Blumenstock, G.; Wiechers, C.; Poets, C.F.; Reinert, S.; Krimmel, M. Neurocognitive development in isolated Robin sequence treated with the Tuebingen palatal plate. Clin. Oral Investig. 2022, 26, 4817–4823. [Google Scholar] [CrossRef] [PubMed]
- Naros, A.; Bartel, S.; Bacher, M.; Koos, B.; Blumenstock, G.; Wiechers, C.; Poets, C.F.; Reinert, S.; Krimmel, M. Speech development in cleft palate with and without Robin sequence. Plast. Reconstr. Surg. 2022, 149, 443–452. [Google Scholar]
- Naros, A.; Krimmel, M.; Zengerle, F.; Bacher, M.; Koos, B.; Mack, U.; Wiechers, C.; Poets, C.F.; Reinert, S. Perioperative complications in cleft palate repair with Robin sequence following Tuebingen palatal plate treatment. J. Cranio-Maxillofac. Surg. 2021, 49, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Resnick, C.M.; de Gijsel, J.; Jindal, S.; Quante, M.; Poets, C.; Katz, E.; Wiechers, C. Comparative Effectiveness of Preepiglottic Baton Plates and Mandibular Distraction in Infants with Robin Sequence. Plast. Reconstr. Surg. 2025, 156, 267e–278e. [Google Scholar] [CrossRef]
- Oechsle, A.-L.; Wiechers, C.; Abadie, V.; Abel, F.; Breugem, C.; Poets, C.F. Study protocol for a multicenter, multinational, observational registry of epidemiology, treatment and outcome of patients with Robin sequence. Head Face Med. 2023, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Bulstrode, N.; Mathijssen, I.; van der Molen, A.M.; Wolvius, E.; Khan, A.; Koudstaal, M.; Bouter, A.; El Ghoul, K.; Logjes, B.; van der Plas, P. European Guideline Robin Sequence An Initiative from the European Reference Network for Rare Craniofacial Anomalies and Ear, Nose and Throat Disorders (ERN-CRANIO). J. Craniofacial Surg. 2024, 35, 279–361. [Google Scholar]
- Castillo-Morales, R. Orofaziale Regulation beim Down Syndrome durch Gaumenplatte. Sazialpadiatrie 1982, 4, 10–17. [Google Scholar]
- Krimmel, M.; Kluba, S.; Breidt, M.; Bacher, M.; Dietz, K.; Buelthoff, H.; Reinert, S. Three-dimensional assessment of facial development in children with Pierre Robin sequence. J. Craniofacial Surg. 2009, 20, 2055–2060. [Google Scholar] [CrossRef]
- Hermann, N.; Kreiborg, S.; Darvann, T.A.; Jensen, B.; Dahl, E.; Bolund, S. Craniofacial morphology and growth comparisons in children with Robin Sequence, isolated cleft palate, and unilateral complete cleft lip and palate. Cleft Palate-Craniofacial J. 2003, 40, 373–396. [Google Scholar]
- Hermann, N.; Kreiborg, S.; Darvann, T.A.; Jensen, B.; Dahl, E.; Bolund, S. Early craniofacial morphology and growth in children with nonsyndromic Robin Sequence. Cleft Palate-Craniofacial J. 2003, 40, 131–143. [Google Scholar]
- Hotz, M. Clefts of the secondary palate associated with the “Pierre-Robin syndrome”. Swed. Dent. J. Suppl. 1982, 15, 89–98. [Google Scholar]
- Figueroa, A.A.; Glupker, T.J.; Fitz, M.G.; Begole, E.A. Mandible, tongue, and airway in Pierre Robin sequence: A longitudinal cephalometric study. Cleft Palate-Craniofacial J. 1991, 28, 425–434. [Google Scholar]
- Daskalogiannakis, J.; Ross, R.B.; Tompson, B.D. The mandibular catch-up growth controversy in Pierre Robin sequence. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Goetz, S.; Kreutzer, K.; Weismann, C.; LeClair, J.; McGee, G.; Poets, C.F.; Quante, M. A Randomized Crossover Trial to Evaluate the Effect of Positioning on Obstructive Sleep Apnea in Infants with Robin Sequence. Children 2025, 12, 389. [Google Scholar] [CrossRef]
- Wiechers, C.; Thjen, T.; Koos, B.; Reinert, S.; Poets, C.F. Treatment of infants with craniofacial malformations. Arch. Dis. Child.-Fetal Neonatal Ed. 2021, 106, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Müller-Hagedorn, S.; Arand, J.; Scholz, T.; Poets, C.F.; Wiechers, C. An innovative method for manufacturing the Tuebingen palatal plate for infants with Robin sequence. BMC Pediatr. 2020, 20, 103. [Google Scholar] [CrossRef]
- Maas, C.; Poets, C.F. Initial treatment and early weight gain of children with Robin Sequence in Germany: A prospective epidemiological study. Arch. Dis. Child.-Fetal Neonatal Ed. 2014, 99, F491–F494. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F.; Bacher, M. Treatment of upper airway obstruction and feeding problems in Robin-like phenotype. J. Pediatr. 2011, 159, 887–892. [Google Scholar] [CrossRef] [PubMed]











| Main Step | Substeps | Checklist Item |
|---|---|---|
| Scanning |
| |
| TPP prototyping | Design and manufacturing |
|
| Endoscopic fit evaluation |
| |
| Final TPP | General |
|
| Safety Wire |
| |
| Base Plate |
| |
| Extension |
| |
| Extraoral Fixation Bows |
| |
| Final fitting on patient |
| |
| Follow-up | Evaluation |
|
| Caregiver Training |
| |
| Regular Monitoring |
|
| Author | Country | Used Name | Article Type | Type of TPP |
|---|---|---|---|---|
| Ludwig et al. (2006) [46] | Germany | TPP | Case report | Dynamic extension with two rounded single rods for both prototype and final |
| Kochel et al. (2011) [47] | Germany | TPP | Review and methodology | Two options, dynamic and static extension. Dynamic by a so-called “omega” shaped twisting of a single rod orthodontic wire for dynamic changes. Alternatively, a static version uses a polymer extension but without safety wire. |
| Gerzanic et al. (2012) [48] | Austria | TPP | Case report | Dynamic extension with two rounded single rods for both prototype and final. Improperly designed bows—too short and poorly positioned—leave insufficient space hindering feeding. |
| Ho et al. (2019) [49] | China | PEBP | Technical note with case report | Dynamic polymer extension setup. Combination of two rounded single rods to angle the extension and an orthodontic expansion screw in the middle half of the extension to allow length adjustments |
| Tomic et al. (2020) [50] | Austria | MPP | Original article | Dynamic extension with two rounded single rods for both prototype and final. Substantially shorter length of extension compared to other approaches, possibly insufficient to relieve the UAO for most cases. |
| Schmidt et al. (2020) [51] | Germany | PEBP | Report | Dynamic extension with two rounded single rods for both prototype and final. Double wire for prototype and final. Extraoral retention bows extend from inside the plate and surround the vestibular rim, making bedside adjustment of the edge difficult or impossible (potential increasing risk of OMLs). Rods only in top portion of extension, do not run down the length of the extension. |
| Goryachkina et al. (2020) [52] | Russia | PEBP | Original article (in Russian) | Dynamic extension with two rounded single rods for both prototype and final |
| Thurzo et al. (2022) [26] | Slovakia | TPP | Original article | Proof-of-concept using CT for a completely 3D-printed prototype with wires. |
| Cho et al. (2022) [53] | USA | OAP | Case report | Rigid extension with no safety wire; only polymer transparent extension |
| Cho et al. (2022) [54] | USA/Korea | OAP | Brief report | Split plate with expansion screw in palatal plate + thermoplastic extension |
| Benitez et al. (2024) [55] | Switzerland | PEBP | Technical report | Dynamic polymer extension setup + double wire for prototype. Rigid final appliance (different safety wire) |
| Dentition Stage | Age (Years) | Orthodontic Measures |
|---|---|---|
| Primary dentition | 4–6 | Early management of transverse maxillary deficiency and mandibular retrognathia using Class II functional appliances with expansion to guide mandibular growth.
|
| Early mixed dentition | 8–9 |
|
| Late mixed dentition | 10–12 |
|
| Pubertal growth | ♀: 10–14 ♂︎: 12–16 | Functional appliances designed to utilize the pubertal growth spurt for guiding craniofacial growth in a physiological direction |
| After full permanent dentition eruption | Ca. 12 |
|
| Skeletal dysgnathia | End of skeletal growth | Severe skeletal dysgnathia may require corrective osteotomy in collaboration with maxillofacial surgery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aretxabaleta, M.; Bockstedte, M.; Heise, K.; Theis, L.; Raible, C.; Peters, K.; Wiechers, C.; Koos, B.; Poets, C.F.; Weismann, C. Complete Protocol and Guidelines for the Implementation and Manufacturing of the Tübingen Palatal Plate—An Interdisciplinary Technical Note on the Tübingen Approach for Infants with Robin Sequence. Bioengineering 2025, 12, 1063. https://doi.org/10.3390/bioengineering12101063
Aretxabaleta M, Bockstedte M, Heise K, Theis L, Raible C, Peters K, Wiechers C, Koos B, Poets CF, Weismann C. Complete Protocol and Guidelines for the Implementation and Manufacturing of the Tübingen Palatal Plate—An Interdisciplinary Technical Note on the Tübingen Approach for Infants with Robin Sequence. Bioengineering. 2025; 12(10):1063. https://doi.org/10.3390/bioengineering12101063
Chicago/Turabian StyleAretxabaleta, Maite, Marit Bockstedte, Kathrin Heise, Lisa Theis, Christoph Raible, Katharina Peters, Cornelia Wiechers, Bernd Koos, Christian F. Poets, and Christina Weismann. 2025. "Complete Protocol and Guidelines for the Implementation and Manufacturing of the Tübingen Palatal Plate—An Interdisciplinary Technical Note on the Tübingen Approach for Infants with Robin Sequence" Bioengineering 12, no. 10: 1063. https://doi.org/10.3390/bioengineering12101063
APA StyleAretxabaleta, M., Bockstedte, M., Heise, K., Theis, L., Raible, C., Peters, K., Wiechers, C., Koos, B., Poets, C. F., & Weismann, C. (2025). Complete Protocol and Guidelines for the Implementation and Manufacturing of the Tübingen Palatal Plate—An Interdisciplinary Technical Note on the Tübingen Approach for Infants with Robin Sequence. Bioengineering, 12(10), 1063. https://doi.org/10.3390/bioengineering12101063








