Comprehensive Analysis of Chronic Low Back Pain: Morphological and Functional Impairments, Physical Activity Patterns, and Epidemiology in a German Population-Based Cross-Sectional Study
Abstract
1. Introduction
- To characterize structural and functional impairments of the spine/back and levels of functional activity in individuals with and without LBP.
- To analyze demographic factors (age, sex, Body Mass Index (BMI)) and clinical characteristics (pain intensity, pain duration).
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Quantitative Variables and Data Collection
2.4. Questionnaires
2.5. Physical Examination
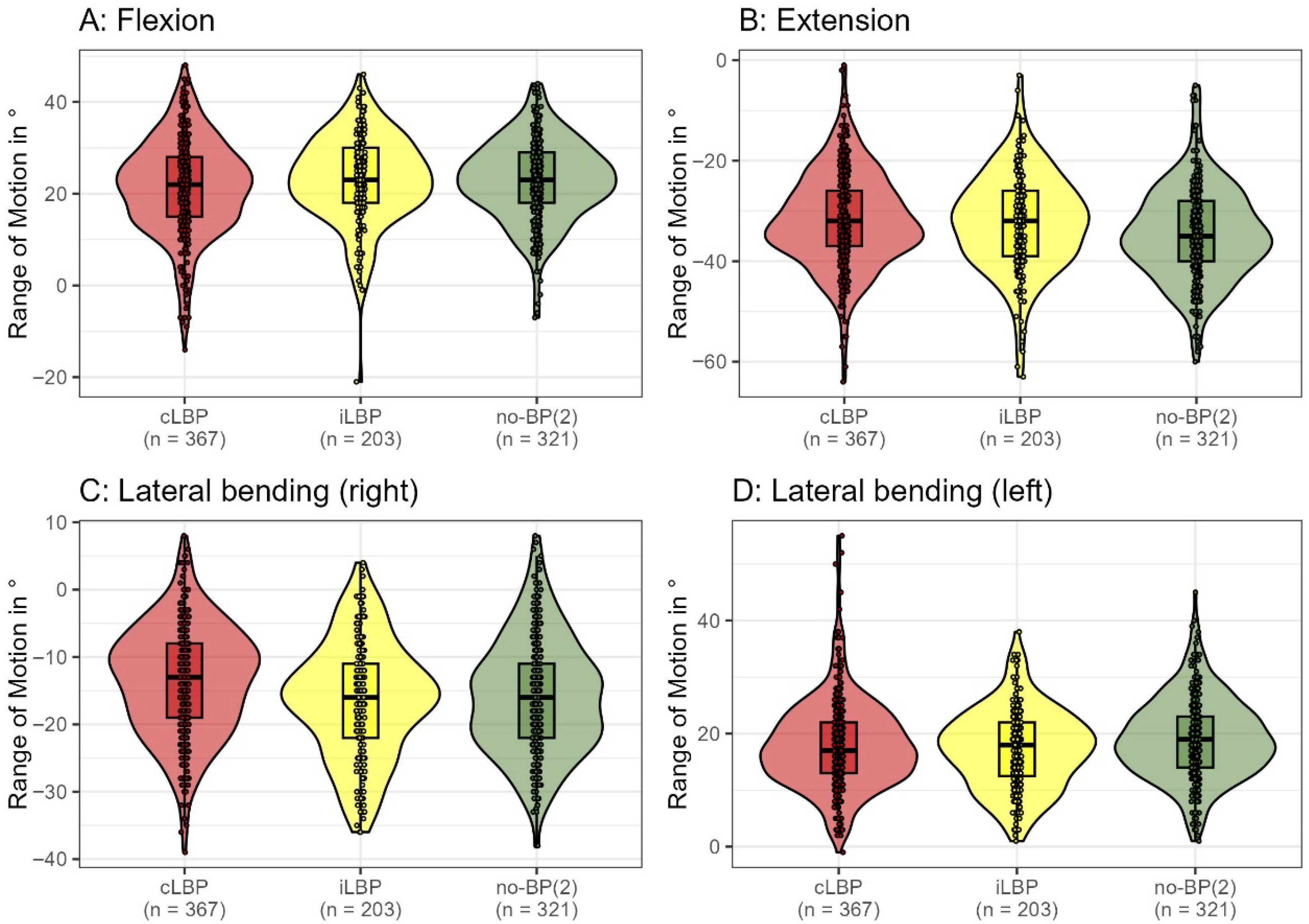
2.6. Back Morphology and Motion Analysis
2.7. Spino-Pelvic MRI
2.8. Definition of Pain Status
2.9. Characterization of Pain Status by Subgroups
2.9.1. Morphological Impairments
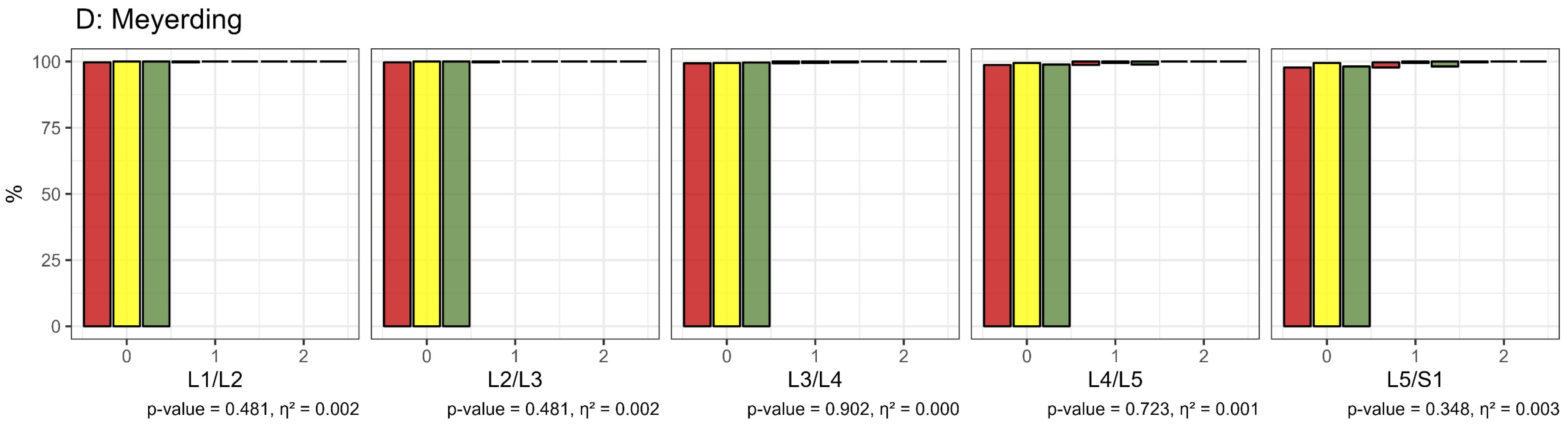
- MRI Analysis: Intervertebral discs and facet joints were graded using established degeneration scores. According to Pfirrmann et al., discs were categorized into five grades: grades 1 and 2 indicated no to minor changes and were considered non-impaired, while grades 3 to 5, reflecting moderate to severe degeneration, were deemed impaired [34,35]. Facet joints were evaluated based on Krämer et al.’s classification, with grades 0 to 2 indicating no to minor changes (non-impaired) and grades 3 to 5 indicating significant degeneration (impaired) [35,41]. Facet joint degeneration was also classified into four grades by Fujiwara et al., where grades 1 and 2 were non-impaired and grades 3 and 4 indicated impairment [37]. Spondylolisthesis was assessed using Meyerding’s classification, with grade 1 (spondylolisthesis ≤25%) considered non-impaired and grades 2 to 5 (spondylolisthesis >25%) considered impaired [38].
- Back Morphology Measurements: Segmental angles of the lumbar spine in an upright standing position were compared to a reference database of healthy individuals. Participants exhibiting deviations beyond two standard deviations from the norm were classified as impaired.
2.9.2. Functional Impairments
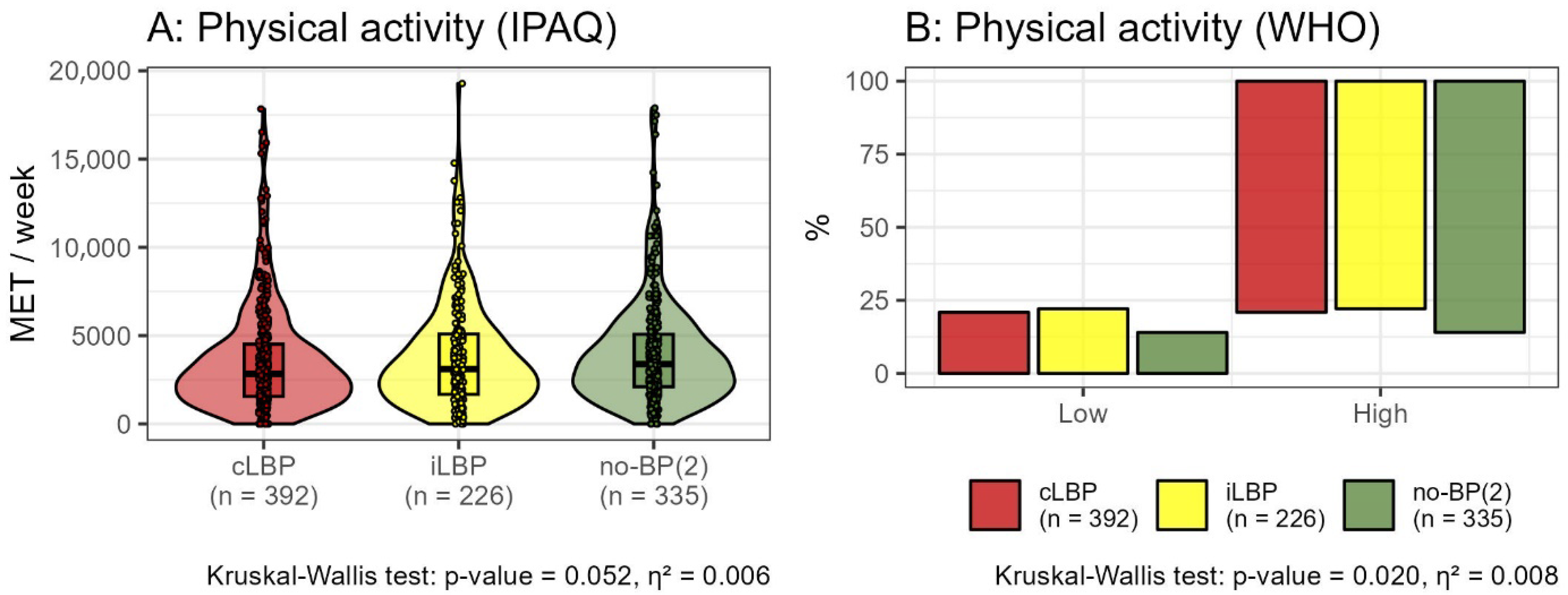
2.9.3. Level of Physical Activity
2.10. Analysis of Demographic and Clinical Characteristics
2.11. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characterization of Pain Status to Subgroups
3.2.1. Morphological Impairment
3.2.2. Functional Impairment
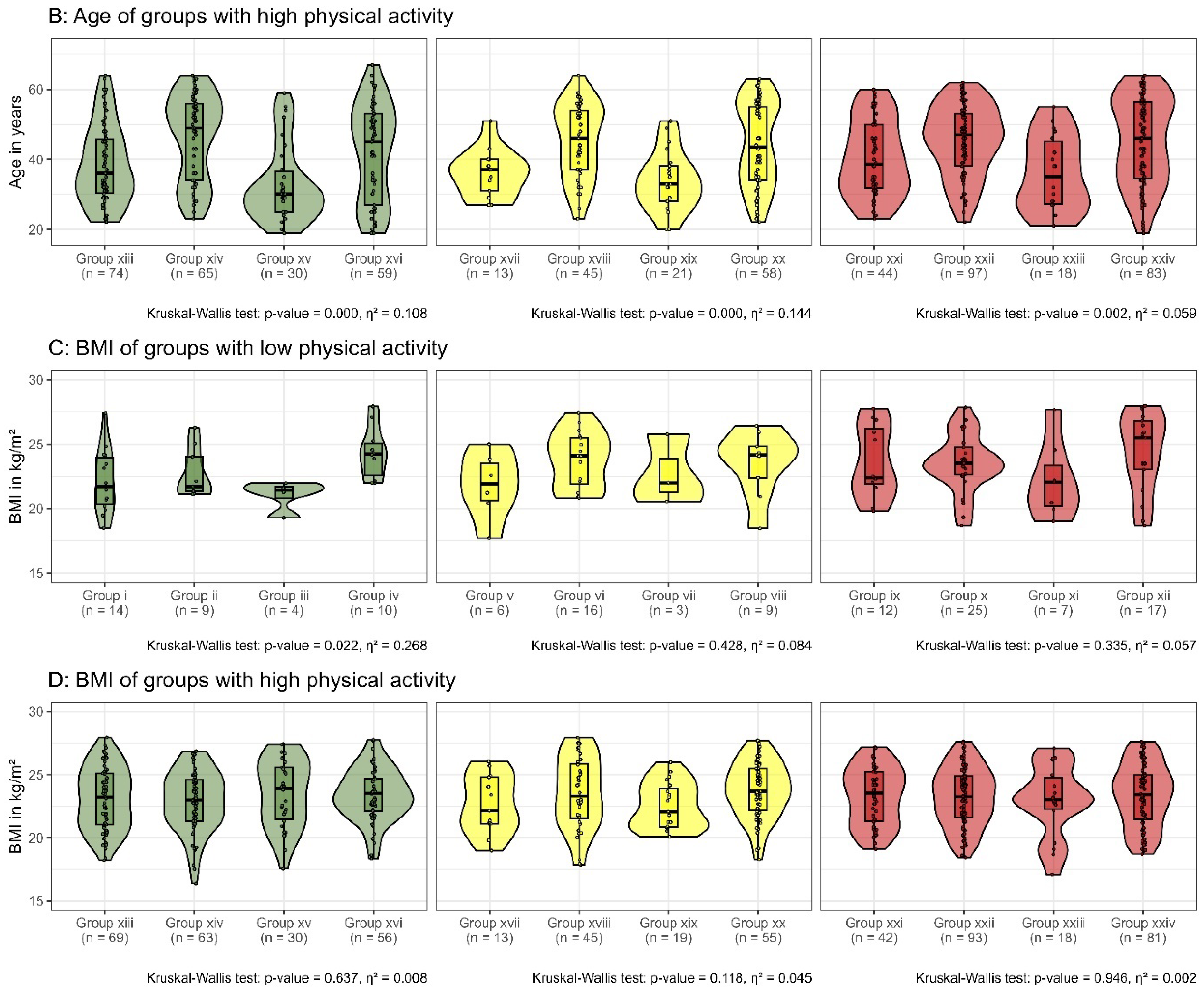
3.2.3. Physical Activity
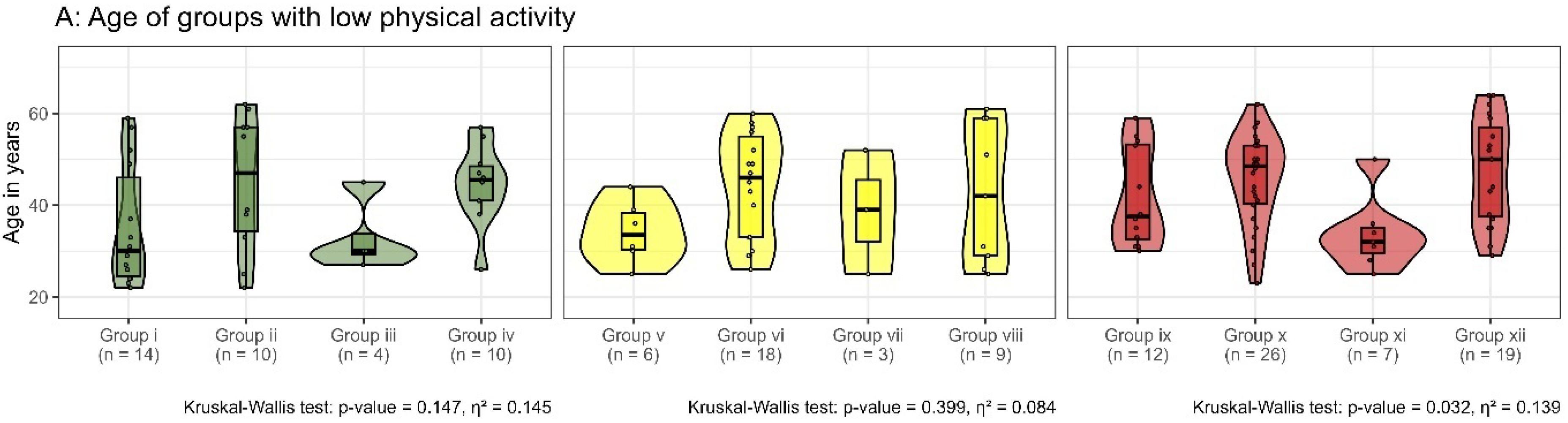
3.2.4. Assigning Participants to Subgroups
3.3. Impact of Demographic and Clinical Characteristics on Subgroup Differentiation
3.4. Impact of Physical Activity on Subgroup Differentiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| cLBP | chronic Low Back Pain |
| DRKS | German Clinical Trial Register |
| iLBP | intermittent Low Back Pain |
| IPAQ | International Physical Activity Questionnaire |
| IQR | Interquartile Range |
| LBP | Low Back Pain |
| MET | Metabolic Equivalent of Task |
| MRI | Magnetic Resonance Imaging |
| no-BP(2) | pain-free |
| ROM | Range of Motion |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| WHO | World Health Organization |
| YLD | Years Lived with Disability |
References
- Vos, T.; Abajobir, A.; Abbafati, C.; Abbas, K.; Abate, K.; Abd-Allah, F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Koes, B.W.; van Tulder, M.W.; Thomas, S. Diagnosis and treatment of low back pain. BMJ 2006, 332, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Meucci, R.D.; Fassa, A.G.; Faria, N.M. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Di, J.; Wang, W.; Feng, H. Global burden of low back pain from 1990 to 2021: A comprehensive analysis of risk factors and trends using the Global Burden of Disease Study 2021. BMC Public Health 2025, 25, 1886. [Google Scholar] [CrossRef]
- Jenkins, H.J.; Ferreira, G.; Downie, A.; Maher, C.; Buchbinder, R.; Hancock, M.J. The available evidence on the effectiveness of 10 common approaches to the management of non-specific low back pain: An evidence map. Eur. J. Pain 2022, 26, 1399–1411. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P. Low back pain: A call for action. Lancet 2018, 391, 2384–2388. [Google Scholar] [CrossRef]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Bishop, F.L.; Dima, A.L.; Ngui, J.; Little, P.; Moss-Morris, R.; Foster, N.E.; Lewith, G.T. “Lovely Pie in the Sky Plans”: A Qualitative Study of Clinicians’ Perspectives on Guidelines for Managing Low Back Pain in Primary Care in England. Spine 2015, 40, 1842–1850. [Google Scholar] [CrossRef]
- Wirth, B.; Schweinhardt, P. Personalized assessment and management of non-specific low back pain. Eur. J. Pain 2024, 28, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.; Pagano, M.; Cappadona, I.; Cardile, D.; Calabrò, R.S.; Corallo, F. Psychological Support for Chronic Low Back Pain: A Systematic Review on the Validity of a Growing Remote Approach. Curr. Pain Headache Rep. 2025, 29, 51. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Hancock, M.J.; Maher, C.G.; Latimer, J.; Spindler, M.F.; McAuley, J.H.; Laslett, M.; Bogduk, N. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur. Spine J. 2007, 16, 1539–1550. [Google Scholar] [CrossRef]
- Deyo, R.A.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef]
- Errabity, A.; Calmels, P.; Han, W.S.; Bonnaire, R.; Pannetier, R.; Convert, R.; Molimard, J. The effect of low back pain on spine kinematics: A systematic review and meta-analysis. Clin. Biomech. 2023, 108, 106070. [Google Scholar] [CrossRef]
- Steiger, F.; Wirth, B.; de Bruin, E.D.; Mannion, A.F. Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. Eur. Spine J. 2012, 21, 575–598. [Google Scholar] [CrossRef]
- Wernli, K.; Tan, J.S.; O’Sullivan, P.; Smith, A.; Campbell, A.; Kent, P. Does Movement Change When Low Back Pain Changes? A Systematic Review. J. Orthop. Sports Phys. Ther. 2020, 50, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.; Mackey, M.; Stamatakis, E.; Zadro, J.R.; Shirley, D. The association between physical activity and low back pain: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 8244. [Google Scholar] [CrossRef]
- Shiri, R.; Falah-Hassani, K. Does leisure time physical activity protect against low back pain? Systematic review and meta-analysis of 36 prospective cohort studies. Br. J. Sports Med. 2017, 51, 1410–1418. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain. 1992, 50, 133–149. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Guermazi, M.; Ghroubi, S.; Kassis, M.; Jaziri, O.; Keskes, H.; Kessomtini, W.; Ben Hammouda, I.; Elleuch, M.H. Validity and reliability of Spinal Mouse to assess lumbar flexion. Ann. Readapt. Med. Phys. 2006, 49, 172–177. [Google Scholar] [CrossRef]
- Post, R.B.; Leferink, V.J. Spinal mobility: Sagittal range of motion measured with the SpinalMouse, a new non-invasive device. Arch. Orthop. Trauma. Surg. 2004, 124, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Knecht, K.; Balaban, G.; Dvorak, J.; Grob, D. A new skin-surface device for measuring the curvature and global and segmental ranges of motion of the spine: Reliability of measurements and comparison with data reviewed from the literature. Eur. Spine J. 2004, 13, 122–136. [Google Scholar] [CrossRef]
- Topalidou, A.; Tzagarakis, G.; Souvatzis, X.; Kontakis, G.; Katonis, P. Evaluation of the reliability of a new non-invasive method for assessing the functionality and mobility of the spine. Acta Bioeng. Biomech. 2014, 16, 117–124. [Google Scholar]
- Barrett, E.; McCreesh, K.; Lewis, J. Reliability and validity of non-radiographic methods of thoracic kyphosis measurement: A systematic review. Man. Ther. 2014, 19, 10–17. [Google Scholar] [CrossRef]
- Dreischarf, B.; Koch, E.; Dreischarf, M.; Schmidt, H.; Pumberger, M.; Becker, L. Comparison of three validated systems to analyse spinal shape and motion. Sci. Rep. 2022, 12, 10222. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Hancock, M.J.; Sharma, S.; Sharma, S.; Harris, I.A.; Cohen, S.P.; Magnussen, J.; Maher, C.G.; Traeger, A.C. Low back pain of disc, sacroiliac joint, or facet joint origin: A diagnostic accuracy systematic review. eClinicalMedicine 2023, 59, 101960. [Google Scholar] [CrossRef]
- Kraemer, R.; Wild, A.; Haak, H.; Herdmann, J.; Krauspe, R.; Kraemer, J. Classification and management of early complications in open lumbar microdiscectomy. Eur. Spine J. 2003, 12, 239–246. [Google Scholar] [CrossRef]
- Fujiwara, A.; Tamai, K.; Yamato, M.; An, H.S.; Yoshida, H.; Saotome, K.; Kurihashi, A. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: An MRI study. Eur. Spine J. 1999, 8, 396–401. [Google Scholar] [CrossRef]
- Meyerding, H. Spondylolisthesis. Surg. Gynecol. Obs. 1932, 54, 371–377. [Google Scholar]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef]
- Schizas, C.; Theumann, N.; Burn, A.; Tansey, R.; Wardlaw, D.; Smith, F.W.; Kulik, G. Qualitative Grading of Severity of Lumbar Spinal Stenosis Based on the Morphology of the Dural Sac on Magnetic Resonance Images. Spine 2010, 35, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Ludwig, J. Surgical treatment of lumbar disc herniation. Indication and methods. Orthopade 1999, 28, 579–584. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak-Łukaszewska, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Sport Sci. 2014, 21, 19–25. [Google Scholar]
- Cohen, J.; Cohen, J.W. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.R-project.org/ (accessed on 3 June 2024).
- Posit Team. RStudio: Integrated Development Environment for R. 2024. Available online: http://www.posit.co/ (accessed on 3 June 2024).
- Liuke, M.; Solovieva, S.; Lamminen, A.; Luoma, K.; Leino-Arjas, P.; Luukkonen, R.; Riihimäki, H. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes. 2005, 29, 903–908. [Google Scholar] [CrossRef]
- Chen, X.-l.; Li, X.-y.; Wang, Y.; Lu, S.-b. Relation of lumbar intervertebral disc height and severity of disc degeneration based on Pfirrmann scores. Heliyon 2023, 9, e20764. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2015, 36, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Otani, K.; Sekiguchi, M.; Konno, S.I. Relationship between lumbar disc degeneration on MRI and low back pain: A cross-sectional community study. Fukushima J. Med. Sci. 2022, 68, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rahyussalim, A.J.; Zufar, M.L.L.; Kurniawati, T. Significance of the Association between Disc Degeneration Changes on Imaging and Low Back Pain: A Review Article. Asian Spine J. 2020, 14, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Suri, P.; Naeini, M.K.; Heagerty, P.J.; Freidin, M.B.; Smith, I.G.; Elgaeva, E.E.; Compte, R.; Tsepilov, Y.A.; Williams, F.M.K. The association of lumbar intervertebral disc degeneration with low back pain is modified by underlying genetic propensity to pain. Spine J. 2025, 25, 8–17. [Google Scholar] [CrossRef]


| Low Physical Activity | no-BP(2) | Group i | Group ii | Group iii | Group iv | |
| Morphology | ✓ | ✗ | ✓ | ✗ | ||
| Function | ✓ | ✓ | ✗ | ✗ | ||
| iLBP | Group v | Group vi | Group vii | Group viii | ||
| Morphology | ✓ | ✗ | ✓ | ✗ | ||
| Function | ✓ | ✓ | ✗ | ✗ | ||
| cLBP | Group ix | Group x | Group xi | Group xii | ||
| Morphology | ✓ | ✗ | ✓ | ✗ | ||
| Function | ✓ | ✓ | ✗ | ✗ | ||
| High Physical Activity | no-BP(2) | Group xiii | Group xiv | Group xv | Group xvi | |
| Morphology | ✓ | ✗ | ✓ | ✗ | ||
| Function | ✓ | ✓ | ✗ | ✗ | ||
| iLBP | Group xvii | Group xviii | Group xix | Group xx | ||
| Morphology | ✓ | ✗ | ✓ | ✗ | ||
| Function | ✓ | ✓ | ✗ | ✗ | ||
| cLBP | Group xxi | Group xxii | Group xxiii | Group xxiv | ||
| Morphology | ✓ | ✗ | ✓ | ✗ | ||
| Function | ✓ | ✓ | ✗ | ✗ |
| Low Physical Activity | no-BP(2) n = 47 (17, 30) (4.9%) | Group i | Group ii | Group iii | Group iv | Missing Data |
| n = 14 (6, 8) 10%/1.9% | n = 10 (4, 6) 7.2%/1.3% | n = 4 (1, 3) 2.9%/0.5% | n = 10 (3, 7) 7.2%/1.3% | n = 9 (3, 6) | ||
| iLBP n = 50 (18, 32) (5.2%) | Group v | Group vi | Group vii | Group viii | ||
| n = 6 (2, 4) 4.3% / 0.8% | n = 18 (4, 14) 13% / 2.4% | n = 3 (0, 3) 2.1% / 0.4% | n = 9 (6, 3) 6.5%/1.2% | n = 14 (6, 8) | ||
| cLBP n = 82 (38, 44) (8.6%) | Group ix | Group x | Group xi | Group xii | ||
| n = 12 (7, 5) 8.7%/1.6% | n = 26 (12, 14) 19%/13.5% | n = 7 (1, 6) 5.1%/0.9% | n = 19 (12, 7) 13.8%/2.6% | n = 18 (6, 12) | ||
| n = 138 (19% of the entire group) + 41 with missing data (MRI or SpinalMouse) | ||||||
| High Physical Activity | no-BP(2) n = 288 (123, 165) (30%) | Group xiii | Group xiv | Group xv | Group xvi | Missing Data |
| n = 74 (40, 34) 12%/10% | n = 65 (22, 43) 11%/8.7% | n = 30 (15, 15) 4.9%/4% | n = 59 (22, 37) 9.7%/7.9% | n = 60 (24, 36) | ||
| iLBP n = 176 (86, 90) (18%) | Group xvii | Group xviii | Group xix | Group xx | ||
| n = 13 (8, 5) 2.1%/1.7% | n = 45 (18, 27) 7.4%/6% | n = 21 (10, 11) 3.5% / 2.8% | n = 58 (34, 24) 9.6%/7.8% | n = 39 (16, 23) | ||
| cLBP n = 310 (134, 176) (33%) | Group xxi | Group xxii | Group xxiii | Group xxiv | ||
| n = 44 (20, 24) 7.2%/5.9% | n = 97 (39, 58) 16%/13% | n = 18 (7, 11) 3%/2.4% | n = 83 (36, 47) 14%/11% | n = 68 (32, 36) | ||
| n = 607 (81% of the entire group) + 167 with missing data (MRI or SpinalMouse) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoehl, B.U.; Taheri, N.; Schönnagel, L.; Becker, L.A.; Mödl, L.; Reitmaier, S.; Pumberger, M.; Schmidt, H. Comprehensive Analysis of Chronic Low Back Pain: Morphological and Functional Impairments, Physical Activity Patterns, and Epidemiology in a German Population-Based Cross-Sectional Study. Bioengineering 2025, 12, 878. https://doi.org/10.3390/bioengineering12080878
Hoehl BU, Taheri N, Schönnagel L, Becker LA, Mödl L, Reitmaier S, Pumberger M, Schmidt H. Comprehensive Analysis of Chronic Low Back Pain: Morphological and Functional Impairments, Physical Activity Patterns, and Epidemiology in a German Population-Based Cross-Sectional Study. Bioengineering. 2025; 12(8):878. https://doi.org/10.3390/bioengineering12080878
Chicago/Turabian StyleHoehl, Bernhard Ulrich, Nima Taheri, Lukas Schönnagel, Luis Alexander Becker, Lukas Mödl, Sandra Reitmaier, Matthias Pumberger, and Hendrik Schmidt. 2025. "Comprehensive Analysis of Chronic Low Back Pain: Morphological and Functional Impairments, Physical Activity Patterns, and Epidemiology in a German Population-Based Cross-Sectional Study" Bioengineering 12, no. 8: 878. https://doi.org/10.3390/bioengineering12080878
APA StyleHoehl, B. U., Taheri, N., Schönnagel, L., Becker, L. A., Mödl, L., Reitmaier, S., Pumberger, M., & Schmidt, H. (2025). Comprehensive Analysis of Chronic Low Back Pain: Morphological and Functional Impairments, Physical Activity Patterns, and Epidemiology in a German Population-Based Cross-Sectional Study. Bioengineering, 12(8), 878. https://doi.org/10.3390/bioengineering12080878







