Generation of an In Vitro Cartilage Aging Model Using Human Sera from Old Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Expansion of Human MSCs (hMSCs)
2.2. Preparation of Gelatin Methacryloyl (GelMA)
2.3. Differentiation of hMSCs
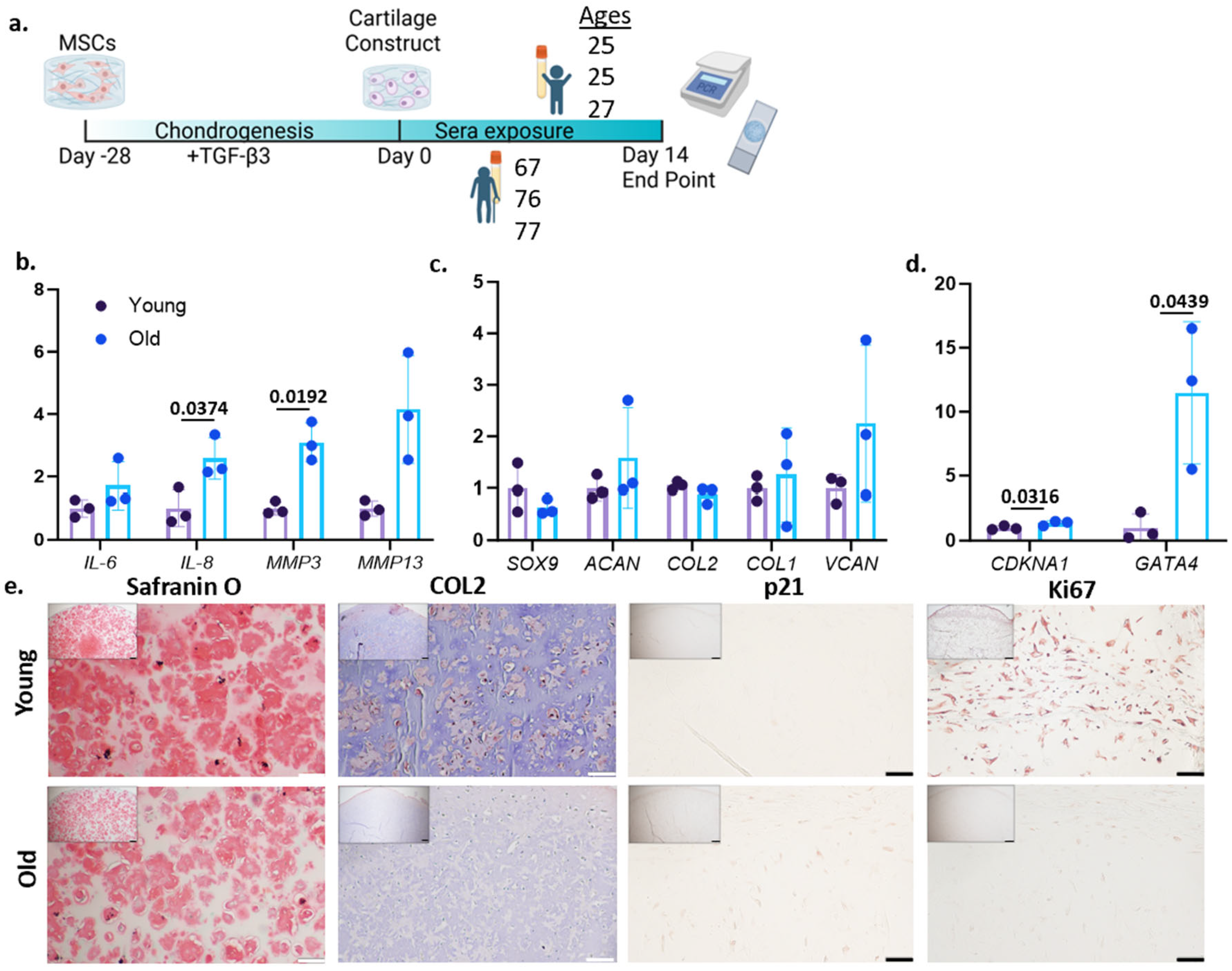
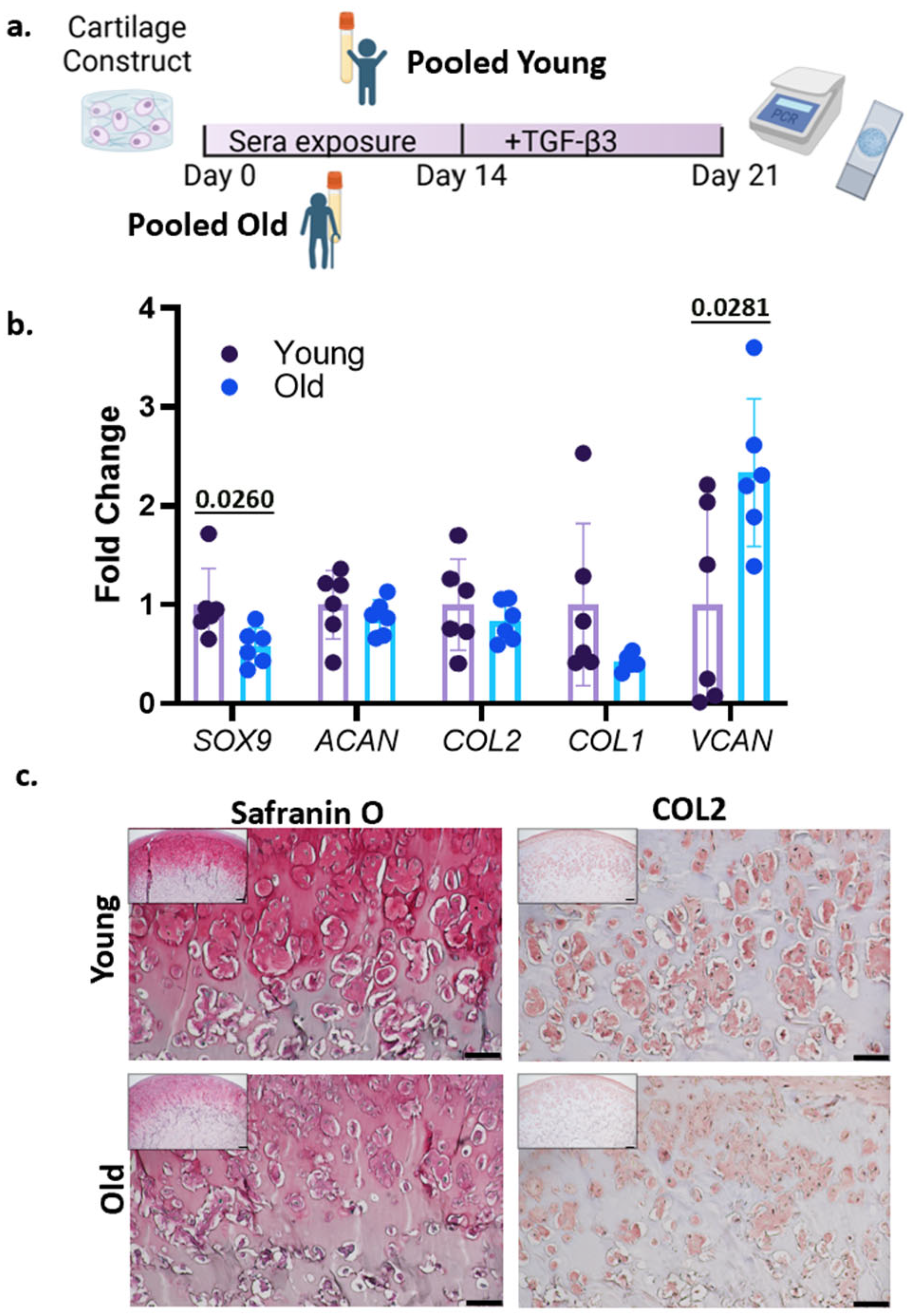
2.4. Human Serum Treatment and Chondrogenesis
2.5. Total RNA Isolation and Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
2.6. Histology
2.7. Immunohistochemistry (IHC)
2.8. Statistical Analysis
3. Results
3.1. Treatment of Older Sera Increases Aging-Associated Phenotypes in Engineered Cartilage
3.2. Treatment with Human Sera and TGF-β3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalhoub, M.; Anaya, M.; Deek, S.; Zaben, A.H.; Abdalla, M.A.; Jaber, M.M.; Koni, A.A.; Zyoud, S.H. The impact of pain on quality of life in patients with osteoarthritis: A cross-sectional study from Palestine. BMC Musculoskelet. Disord. 2022, 23, 248. [Google Scholar] [CrossRef]
- Losina, E.; Paltiel, A.D.; Weinstein, A.M.; Yelin, E.; Hunter, D.J.; Chen, S.P.; Klara, K.; Suter, L.G.; Solomon, D.H.; Burbine, S.A.; et al. Lifetime medical costs of knee osteoarthritis management in the United States: Impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015, 67, 203–215. [Google Scholar] [CrossRef]
- Loeser, R.F. The effects of aging on the development of osteoarthritis. HSS J. 2012, 8, 18–19. [Google Scholar] [CrossRef]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Rezus, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.D.; Dima, N.; Badescu, C.; Rezus, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Zhou, J.; Wei, L. The age-related changes in cartilage and osteoarthritis. Biomed. Res. Int. 2013, 2013, 916530. [Google Scholar] [CrossRef]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 492–496. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef]
- Copp, M.E.; Chubinskaya, S.; Bracey, D.N.; Shine, J.; Sessions, G.; Loeser, R.F.; Diekman, B.O. Comet assay for quantification of the increased DNA damage burden in primary human chondrocytes with aging and osteoarthritis. Aging Cell 2022, 21, e13698. [Google Scholar] [CrossRef]
- Ramasamy, T.S.; Yee, Y.M.; Khan, I.M. Chondrocyte Aging: The Molecular Determinants and Therapeutic Opportunities. Front. Cell Dev. Biol. 2021, 9, 625497. [Google Scholar] [CrossRef]
- Rios, J.L.; Sapede, D.; Djouad, F.; Rapp, A.E.; Lang, A.; Larkin, J.; Ladel, C.; Mobasheri, A. Animal Models of Osteoarthritis Part 1-Preclinical Small Animal Models: Challenges and Opportunities for Drug Development. Curr. Protoc. 2022, 2, e596. [Google Scholar] [CrossRef]
- Rinwa, P.; Eriksson, M.; Cotgreave, I.; Backberg, M. 3R-Refinement principles: Elevating rodent well-being and research quality. Lab. Anim. Res. 2024, 40, 11. [Google Scholar] [CrossRef]
- Shen, H.; He, Y.; Wang, N.; Fritch, M.R.; Li, X.; Lin, H.; Tuan, R.S. Enhancing the potential of aged human articular chondrocytes for high-quality cartilage regeneration. FASEB J. 2021, 35, e21410. [Google Scholar] [CrossRef]
- Lee, J.; Smeriglio, P.; Chu, C.R.; Bhutani, N. Human iPSC-derived chondrocytes mimic juvenile chondrocyte function for the dual advantage of increased proliferation and resistance to IL-1beta. Stem Cell Res. Ther. 2017, 8, 244. [Google Scholar] [CrossRef]
- Georget, M.; Defois, A.; Guiho, R.; Bon, N.; Allain, S.; Boyer, C.; Halgand, B.; Waast, D.; Grimandi, G.; Fouasson-Chailloux, A.; et al. Development of a DNA damage-induced senescence model in osteoarthritic chondrocytes. Aging 2023, 15, 8576–8593. [Google Scholar] [CrossRef]
- Yagi, M.; Endo, K.; Komori, K.; Sekiya, I. Comparison of the effects of oxidative and inflammatory stresses on rat chondrocyte senescence. Sci. Rep. 2023, 13, 7697. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.; Geng, X.; Duan, Z.; Wang, X.; Li, P.; Wang, C.; Wei, L. Impairment of chondrocyte proliferation after exposure of young murine cartilage to an aged systemic environment in a heterochronic parabiosis model. Swiss Med. Wkly. 2018, 148, w14607. [Google Scholar]
- Gonzalez-Armenta, J.L.; Li, N.; Lee, R.L.; Lu, B.; Molina, A.J.A. Heterochronic Parabiosis: Old Blood Induces Changes in Mitochondrial Structure and Function of Young Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 434–439. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Aging insights from heterochronic parabiosis models. npj Aging 2024, 10, 38. [Google Scholar] [CrossRef]
- Carson, B.P.; Patel, B.; Amigo-Benavent, M.; Pauk, M.; Kumar Gujulla, S.; Murphy, S.M.; Kiely, P.A.; Jakeman, P.M. Regulation of muscle protein synthesis in an in vitro cell model using ex vivo human serum. Exp. Physiol. 2018, 103, 783–789. [Google Scholar] [CrossRef]
- Catteau, M.; Gouzi, F.; Blervaque, L.; Passerieux, E.; Blaquiere, M.; Ayoub, B.; Bughin, F.; Mercier, J.; Hayot, M.; Pomies, P. Effects of a human microenvironment on the differentiation of human myoblasts. Biochem. Biophys. Res. Commun. 2020, 525, 968–973. [Google Scholar] [CrossRef]
- George, T.; Velloso, C.P.; Alsharidah, M.; Lazarus, N.R.; Harridge, S.D. Sera from young and older humans equally sustain proliferation and differentiation of human myoblasts. Exp. Gerontol. 2010, 45, 875–881. [Google Scholar] [CrossRef]
- Carlson, M.E.; Conboy, I.M. Loss of stem cell regenerative capacity within aged niches. Aging Cell 2007, 6, 371–382. [Google Scholar] [CrossRef]
- Lin, H.; Yang, G.; Tan, J.; Tuan, R.S. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials 2012, 33, 4480–4489. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Liu, S.; Yagi, H.; Zhang, X.; Yocum, L.; Romero-Lopez, M.; Rhee, C.; Makarcyzk, M.J.; Yu, I.; et al. Human Mesenchymal Stem Cell-Derived Miniature Joint System for Disease Modeling and Drug Testing. Adv. Sci. 2022, 9, e2105909. [Google Scholar] [CrossRef]
- Wight, T.N.; Kang, I.; Merrilees, M.J. Versican and the control of inflammation. Matrix Biol. 2014, 35, 152–161. [Google Scholar] [CrossRef]
- Xiong, H.; Hua, F.; Dong, Y.; Lin, Y.; Ying, J.; Liu, J.; Wang, X.; Zhang, L.; Zhang, J. DNA damage response and GATA4 signaling in cellular senescence and aging-related pathology. Front. Aging Neurosci. 2022, 14, 933015. [Google Scholar] [CrossRef]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; Demaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Vanyushin, B.F. The Effects of Parabiosis on Aging and Age-Related Diseases. Adv. Exp. Med. Biol. 2020, 1260, 107–122. [Google Scholar]
- Lei, C.; Colangelo, D.; Patil, P.; Li, V.; Ngo, K.; Wang, D.; Dong, Q.; Yousefzadeh, M.J.; Lin, H.; Lee, J.; et al. Influences of circulatory factors on intervertebral disc aging phenotype. Aging 2020, 12, 12285–12304. [Google Scholar] [CrossRef]
- Allen, S.L.; Marshall, R.N.; Edwards, S.J.; Lord, J.M.; Lavery, G.G.; Breen, L. The effect of young and old ex vivo human serum on cellular protein synthesis and growth in an in vitro model of aging. Am. J. Physiol. Cell Physiol. 2021, 321, C26–C37. [Google Scholar] [CrossRef]
- Voirin, A.C.; Celle, S.; Perek, N.; Roche, F. Sera of elderly obstructive sleep apnea patients alter blood-brain barrier integrity in vitro: A pilot study. Sci. Rep. 2020, 10, 11309. [Google Scholar] [CrossRef]
- Early prediction of healthy ageing and age-related diseases using blood protein biomarkers. Nat. Metab. 2025, 7, 14–15. [CrossRef]
- Skowronska-Krawczyk, D. Hallmarks of Aging: Causes and Consequences. Aging Biol. 2023, 1, 20230011. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Hagg, S.; Jylhava, J. Sex differences in biological aging with a focus on human studies. elife 2021, 10, e63425. [Google Scholar] [CrossRef]
- Kirsch, V.; Ramge, J.M.; Schoppa, A.; Ignatius, A.; Riegger, J. In Vitro Characterization of Doxorubicin-Mediated Stress-Induced Premature Senescence in Human Chondrocytes. Cells 2022, 11, 1106. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef]
| Age | 26 | 28 | 25 | 32 | 34 | 69 | 76 | 70 | 73 | 77 | 84 | 48 |
| Sex | F | F | M | M | M | F | F | F | M | M | M | F |
| Age | Sex |
|---|---|
| 24 | Male |
| 25 | Female |
| 25 | Female |
| 26 | Male |
| 27 | Female |
| 30 | Male |
| 67 | Female |
| 67 | Male |
| 73 | Male |
| 76 | Female |
| 76 | Male |
| 77 | Female |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hines, S.; Makarczyk, M.J.; Garzia, J.; Lin, H. Generation of an In Vitro Cartilage Aging Model Using Human Sera from Old Donors. Bioengineering 2025, 12, 823. https://doi.org/10.3390/bioengineering12080823
Hines S, Makarczyk MJ, Garzia J, Lin H. Generation of an In Vitro Cartilage Aging Model Using Human Sera from Old Donors. Bioengineering. 2025; 12(8):823. https://doi.org/10.3390/bioengineering12080823
Chicago/Turabian StyleHines, Sophie, Meagan J. Makarczyk, Joseph Garzia, and Hang Lin. 2025. "Generation of an In Vitro Cartilage Aging Model Using Human Sera from Old Donors" Bioengineering 12, no. 8: 823. https://doi.org/10.3390/bioengineering12080823
APA StyleHines, S., Makarczyk, M. J., Garzia, J., & Lin, H. (2025). Generation of an In Vitro Cartilage Aging Model Using Human Sera from Old Donors. Bioengineering, 12(8), 823. https://doi.org/10.3390/bioengineering12080823








