Technology-Enabled Cognitive Strategy Intervention for Secondary Stroke Prevention: A Feasibility Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Intervention
2.3. Assessment
3. Data Analysis
4. Results
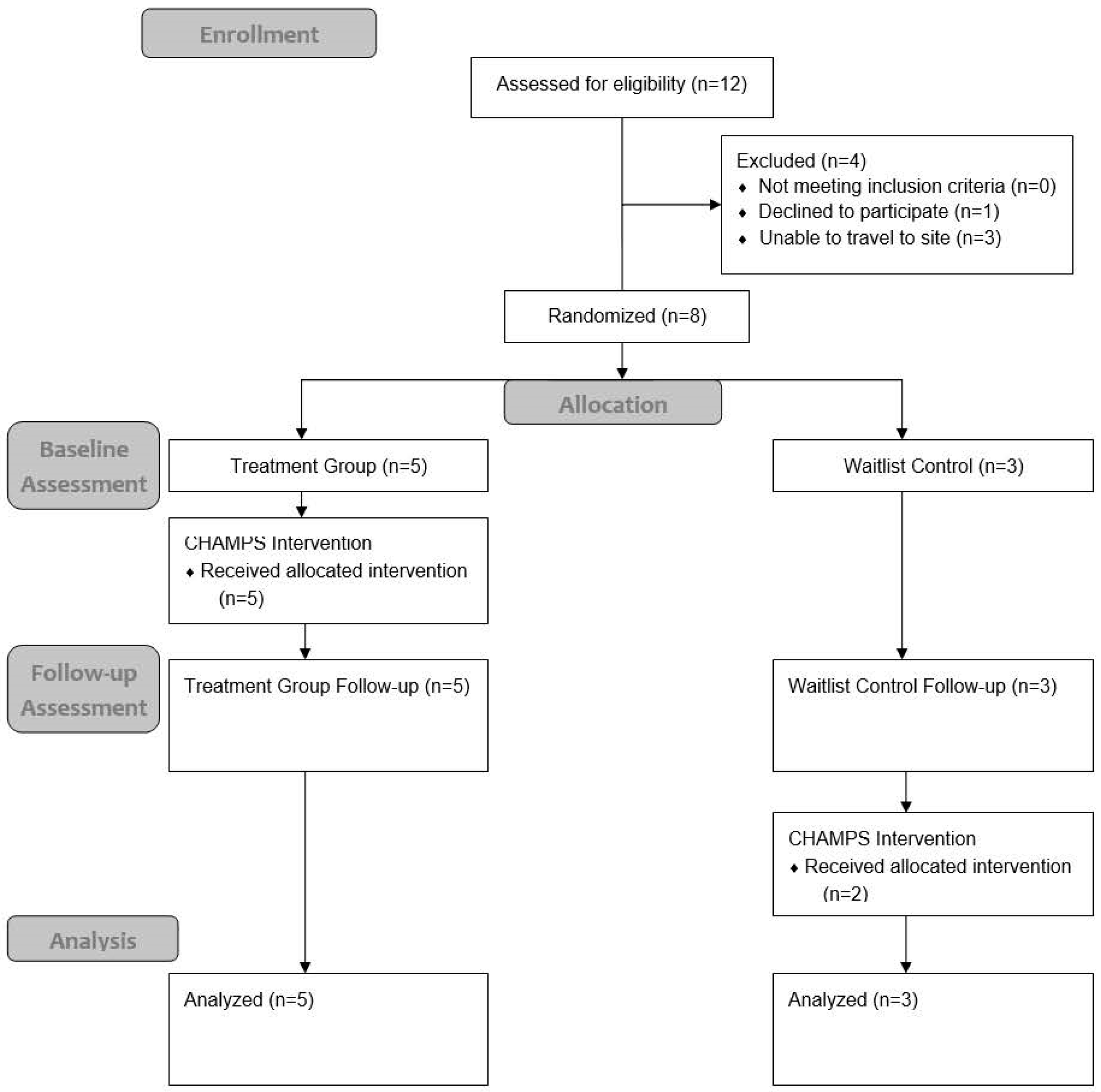
4.1. Participants
4.2. Feasibility
4.3. Safety
4.4. Delivery Characteristics
4.5. Potential Effects
5. Discussion
Broad Impacts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the Future of Stroke in the United States. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.P.; Schwartz, J.K.; Scott, S.L.; Devos, H.; Kovic, M.; Hong, I.; Akinwuntan, A. Interdisciplinary Approaches to Facilitate Return to Driving and Return to Work in Mild Stroke: A Position Paper. Arch. Phys. Med. Rehabil. 2018, 99, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.P.; Lutz, B.J.; Magwood, G.S. “Timing it Right”: Needs of African American adults with stroke and their caregivers across the care continuum. Ethn. Health 2019, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hotter, B.; Padberg, I.; Liebenau, A.; Knispel, P.; Heel, S.; Steube, D.; Wissel, J.; Wellwood, I.; Meisel, A. Identifying unmet needs in long-term stroke care using in-depth assessment and the Post-Stroke Checklist—The Managing Aftercare for Stroke (MAS-I) study. Eur. Stroke J. 2018, 3, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.C.; Neville, M. Cognitive Assessment Trends in Home Health Care for Adults with Mild Stroke. Am. J. Occup. Ther. 2016, 70, 7002290020p1–7002290020p8. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.P.; Mueller, M.; Magwood, G.; White, B.M.; Lackland, D.; Ellis, C. Racial and ethnic differences in post-stroke subjective cognitive decline exist. Disabil. Health J. 2019, 12, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Hartman-Maeir, A.; Soroker, N.; Ring, H.; Avni, N.; Katz, N. Activities, participation and satisfaction one-year post stroke. Disabil. Rehabil. 2007, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.S.; Krishnan, S.; Burns, S.P.; Ouellette, D.; Pappadis, M.R. Inconsistent Classification of Mild Stroke and Implications on Health Services Delivery. Arch. Phys. Med. Rehabil. 2020, 101, 1243–1259. [Google Scholar] [CrossRef] [PubMed]
- Magwood, G.S.; Ellis, C.; Nichols, M.; Burns, S.P.; Jenkins, C.; Woodbury, M.; Adams, R. Barriers and Facilitators of Stroke Recovery: Perspectives From African Americans With Stroke, Caregivers and Healthcare Professionals. J. Stroke Cerebrovasc. Dis. 2019, 28, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Saa, J.P.; Tse, T.; Baum, C.; Cumming, T.; Josman, N.; Rose, M.; Carey, L. Longitudinal evaluation of cognition after stroke—A systematic scoping review. PLoS ONE 2019, 14, e0221735. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, M.; Bak, T.; Czepiel, W.; Seniów, J.; Członkowska, A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement. Geriatr. Cogn. Disord. 2008, 26, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-H.; Tan, L.; Yu, J.-T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [CrossRef]
- Nys, G.M.S.; Van Zandvoort, M.J.E.; De Kort, P.L.M.; Jansen, B.P.W.; De Haan, E.H.F.; Kappelle, L.J. Cognitive disorders in acute stroke: Prevalence and clinical determinants. Cerebrovasc. Dis. 2007, 23, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.P.; Pickens, N.D.; Dawson, D.R.; Perea, J.D.; Vas, A.K.; Marquez de la Plata, C.; Neville, M. In-home contextual reality: A qualitative analysis using the Multiple Errands Test Home Version (MET-Home). Neuropsychol. Rehabil. 2020, 30, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Marteau, T.M. Executive function in the context of chronic disease prevention: Theory, research and practice. Prev. Med. 2014, 68, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Reimann, Z.; Miller, J.R.; Dahle, K.M.; Hooper, A.P.; Young, A.M.; Goates, M.C.; Magnusson, B.M.; Crandall, A. Executive functions and health behaviors associated with the leading causes of death in the United States: A systematic review. J. Health Psychol. 2020, 25, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Lipnicki, D.M.; Crawford, J.D.; Wen, W.; Brodaty, H. Progression of cognitive impairment in stroke/TIA patients over 3 years. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Haskins, E.C.; Cicerone, K.; Dams-O’Connor, K.; Eberle, R.; Langenbahn, D.; Shapiro-Rosenbaum, A. Translating Evidence-Based Recommendations into Practice; American Congress of Rehabilitation: Reston, VA, USA, 2012. [Google Scholar]
- Cicerone, K. The ACRM Cognitive Rehabilitation Manual and Textbook, 2nd ed.; American Congress of Rehabilitation: Reston, VA, USA, 2022. [Google Scholar]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Kingsley, K.; Nagele, D.; et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature From 2009 Through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.R.; McEwen, S.E.; Polatajko, H.J. Cognitive Orientation to Daily Occupational Performance in Occupational Therapy: Using the CO-OP Approach to Enable Participation Across the Lifespan; American Occupational Therapy Association: Bethesda, MD, USA, 2017. [Google Scholar]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. Available online: https://pubmed.ncbi.nlm.nih.gov/18230848/ (accessed on 15 October 2024). [CrossRef] [PubMed]
- Gorelick, P.B.; Furie, K.L.; Iadecola, C.; Smith, E.E.; Waddy, S.P.; Lloyd-Jones, D.M.; Bae, H.-J.; Bauman, M.A.; Dichgans, M.; Duncan, P.W.; et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke 2017, 48, e284–e303. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.L.; Gillett, S.R.; Wadley, V.G.; Unverzagt, F.W.; Judd, S.E.; McClure, L.A.; Howard, V.J.; Cushman, M. The American Heart Association Life’s Simple 7 and incident cognitive impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J. Am. Heart Assoc. 2014, 3, e000635. [Google Scholar] [CrossRef] [PubMed]
- Flach, C.; Muruet, W.; Wolfe, C.D.A.; Bhalla, A.; Douiri, A. Risk and Secondary Prevention of Stroke Recurrence. Stroke 2020, 51, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Arsava, E.M.; Kim, G.-M.; Oliveira-Filho, J.; Gungor, L.; Noh, H.J.; de Jesus Lordelo, M.; Avery, R.; Maier, I.L.; Ay, H. Prediction of Early Recurrence After Acute Ischemic Stroke. JAMA Neurol. 2016, 73, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Galecki, A.T.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Wadley, V.G. Trajectory of Cognitive Decline After Incident Stroke. JAMA 2015, 314, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hotz, I.; Deschwanden, P.F.; Liem, F.; Mérillat, S.; Malagurski, B.; Kollias, S.; Jäncke, L. Performance of three freely available methods for extracting white matter hyperintensities: FreeSurfer, UBO Detector, and BIANCA. Hum. Brain Mapp. 2022, 43, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sharby, N.; Martire, K.; Iversen, M.D. Decreasing health disparities for people with disabilities through improved communication strategies and awareness. Int. J. Environ. Res. Public. Health 2015, 12, 3301–3316. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.R.; Mahurin, R.K.; Gray, K.F. Bedside Assessment of Executive Cognitive Impairment: The Executive Interview. J. Am. Geriatr. Soc. 1992, 40, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.R.; Anderson, N.D.; Binns, M.; Bar, Y.; Chui, A.; Gill, N.; Linkewich, E.; McEwen, S.; Nalder, E.; Skidmore, E. Strategy-training post-stroke via tele-rehabilitation: A pilot randomized controlled trial. Disabil. Rehabil. 2024, 46, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Reinert, D.F.; Allen, J.P. The Alcohol Use Disorders Identification Test: An Update of Research Findings. Alcohol. Clin. Exp. Res. 2007, 31, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Shirinbayan, P.; Salavati, M.; Soleimani, F.; Saeedi, A.; Asghari-Jafarabadi, M.; Hemmati-Garakani, S.; Vameghi, R. The Psychometric Properties of the Drug Abuse Screening Test. Addict. Health 2020, 12, 25–33. [Google Scholar] [CrossRef] [PubMed]
- The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A Systematic Review—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0163834310000563?via%3Dihub (accessed on 10 December 2024).
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Okubo, P.C.M.I.; Fábio, S.R.C.; Domenis, D.R.; Takayanagui, O.M. Using the National Institute of Health Stroke Scale to predict dysphagia in acute ischemic stroke. Cerebrovasc. Dis. 2012, 33, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P.; Davis, P.H.; Leira, E.C.; Chang, K.-C.; Bendixen, B.H.; Clarke, W.R.; Woolson, R.F.; Hansen, M.D. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999, 53, 126. [Google Scholar] [CrossRef] [PubMed]
- Tickle-Degnen, L. Nuts and Bolts of Conducting Feasibility Studies. Am. J. Occup. Ther. 2013, 67, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Z.; Yu, D.; Chen, S.; Wang, A.; Wang, A.; Gao, X. Life’s Essential 8 and Risk of Stroke: A Prospective Community-Based Study. Stroke 2023, 54, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, X.; Jiang, J.; Zhao, M.; Peng, X.; Cui, F.; Wang, X.; Feng, J.; Chen, S.; Wu, S. Life’s Essential 8 Trajectories and Risk of Stroke: A Prospective Cohort Study. Stroke 2024, 55, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Carswell, A.; McColl, M.A.; Baptiste, S.; Law, M.; Polatajko, H.; Pollock, N. The Canadian Occupational Performance Measure: A Research and Clinical Literature Review. Can. J. Occup. Ther. 2004, 71, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.S.; Rogers, E.S.; Turner, L.W.; Keenum, A.J.; Weiss, B.D. Suitability of written supplemental materials available on the Internet for nonprescription medications. Am. J. Health. Syst. Pharm. 2006, 63, 71–78. [Google Scholar] [CrossRef] [PubMed]
- CDC-BRFSS-Questionnaires. 4 September 2024. Available online: https://www.cdc.gov/brfss/questionnaires/index.htm (accessed on 15 October 2024).
- Denboer, J.W.; Nicholls, C.; Corte, C.; Chestnut, K. National Institutes of Health Toolbox Cognition Battery. Arch. Clin. Neuropsychol. 2014, 29, 692–694. [Google Scholar] [CrossRef]
- Nitsch, K.P.; Casaletto, K.B.; Carlozzi, N.E.; Tulsky, D.S.; Heinemann, A.W.; Heaton, R.K. Uncorrected Versus Demographically-Corrected Scores on the NIH Toolbox Cognition Battery in Persons With Traumatic Brain Injury and Stroke. Rehabil. Psychol. 2017, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tulsky, D.S.; Holdnack, J.A.; Cohen, M.L.; Heaton, R.K.; Carlozzi, N.E.; Wong, A.W.; Boulton, A.J.; Heinemann, A.W. Factor structure of the NIH Toolbox Cognition Battery in individuals with acquired brain injury. Rehabil. Psychol. 2017, 62, 435. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, N.E.; Tulsky, D.S.; Wolf, T.J.; Goodnight, S.; Heaton, R.K.; Casaletto, K.B.; Wong, A.W.; Baum, C.M.; Gershon, R.C.; Heinemann, A.W. Construct validity of the NIH Toolbox Cognition Battery in individuals with stroke. Rehabil. Psychol. 2017, 62, 443. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.S.; Demeyere, N. Predictive validity of the Oxford digital multiple errands test (OxMET) for functional outcomes after stroke. Neuropsychol. Rehabil. 2024, 34, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.S.; Demeyere, N. Comparing the Oxford Digital Multiple Errands Test (OxMET) to a real-life version: Convergence, feasibility, and acceptability. Neuropsychol. Rehabil. 2024, 35, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.S.; Jespersen, A.; Chiu, E.G.; Payne, F.; Basting, R.; Duta, M.D.; Demeyere, N. The Oxford digital multiple errands test (OxMET): Validation of a simplified computer tablet based multiple errands test. Neuropsychol. Rehabil. 2022, 32, 1007–1032. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.S.; Burns, S.P.; Jespersen, A.; Demeyere, N. Cultural Adaptation of the Oxford Digital Multiple Errands Test (OxMET) for an English Speaking North American Population. Available online: https://www.resna.org/sites/default/files/conference/2022/AgingCognitiveSensory/77_Webb/77_Webb.pdf (accessed on 15 October 2024).
- Al-Heizan, M.O.; Marks, T.S.; Giles, G.M.; Edwards, D.F. Further Validation of the Menu Task: Functional Cognition Screening for Older Adults. OTJR Occup. Ther. J. Res. 2022, 42, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Marks, T.S.; Giles, G.M.; Kehl-Floberg, K.E.; Edwards, D.F. Screening older adults for potential deficits in IADL using the Menu Task and Medi-Cog-R. Alzheimers Dement. 2023, 19, e079778. [Google Scholar] [CrossRef]
- Edwards, D.F.; Wolf, T.J.; Marks, T.; Alter, S.; Larkin, V.; Padesky, B.L.; Spiers, M.; Al-Heizan, M.O.; Giles, G.M. Reliability and Validity of a Functional Cognition Screening Tool to Identify the Need for Occupational Therapy. Am. J. Occup. Ther. 2019, 73, 7302205050p1–7302205050p10. [Google Scholar] [CrossRef] [PubMed]
- Kurl, S.; Laukkanen, J.A. Life’s Essential 8 and ideal cardiovascular health. Int. J. Cardiol. 2024, 409, 132143. Available online: https://www.internationaljournalofcardiology.com/article/S0167-5273(24)00765-4/abstract (accessed on 15 October 2024). [CrossRef] [PubMed]
- Hill, E.B.; Cubellis, L.T.; Wexler, R.K.; Taylor, C.A.; Spees, C.K. Differences in Adherence to American Heart Association’s Life’s Essential 8, Diet Quality, and Weight Loss Strategies Between Those With and Without Recent Clinically Significant Weight Loss in a Nationally Representative Sample of US Adults. J. Am. Heart Assoc. 2023, 12, e026777. [Google Scholar] [CrossRef] [PubMed]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Marma Perak, A.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Tolea, M.I.; Heo, J.; Chrisphonte, S.; Galvin, J.E. A modified CAIDE (mCAIDE) risk score as a screening tool for cognitive impairment in older adults. J. Alzheimers Dis. JAD 2021, 82, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Baldini, A.L.; Velozo, C.; Romero, S.; Shulman, L.M. Validation of the PROMIS® measures of self-efficacy for managing chronic conditions. Qual. Life Res. 2017, 26, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Post, M.W.M.; Boosman, H.; van Zandvoort, M.M.; Passier, P.E.C.A.; Rinkel, G.J.E.; Visser-Meily, J.M.A. Development and validation of a short version of the Stroke Specific Quality of Life Scale. J. Neurol. Neurosurg. Psychiatry 2011, 82, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Kerber, K.A.; Brown, D.L.; Skolarus, L.E.; Morgenstern, L.B.; Smith, M.A.; Garcia, N.M.; Lisabeth, L.D. Validation of the 12-Item Stroke-Specific Quality of Life Scale in a Bi-ethnic Stroke Population. J. Stroke Cerebrovasc. Dis. 2013, 22, 1270–1272. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Baptiste, S.; McColl, M.; Opzoomer, A.; Polatajko, H.; Pollock, N. The Canadian Occupational Performance Measure: An Outcome Measure for Occupational Therapy. Can. J. Occup. Ther. 1990, 57, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.R.; Bar, Y.; Ajwani, F.; Rotenberg, S.; Atlas, B.; Ricupero, M.; Greewood, C.; Parrott, M.D. Combining elements of the CO-OP ApproachTM with education to promote healthy eating among older adults: A pilot study. Front. Rehabil. Sci. 2022, 3, 971300. [Google Scholar] [CrossRef] [PubMed]
- Koblinsky, N.; Anderson, N.; Ajwani, F.; Parrott, M.; Dawson, D.; Marzolini, S.; Oh, P.; MacIntosh, B.; Middleton, L.; Ferland, G. Feasibility and Preliminary Efficacy of the Lifestyle, Exercise and Diet (LEAD) Study: A Cluster Randomized Controlled Trial of a Combined Exercise and Diet Intervention in Older Adults with Vascular Risk Factors and Early Dementia Risk. Published Online 2021. Available online: https://www.researchsquare.com/article/rs-635502/latest (accessed on 27 November 2024).
- Wesselhoff, S.; Hanke, T.A.; Evans, C.C. Community mobility after stroke: A systematic review. Top. Stroke Rehabil. 2018, 25, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Shiggins, C.; Ryan, B.; Dewan, F.; Bernhardt, J.; O’Halloran, R.; Power, E.; Lindley, R.I.; McGurk, G.; Rose, M.L. Inclusion of People With Aphasia in Stroke Trials: A Systematic Search and Review. Arch. Phys. Med. Rehabil. 2024, 105, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Flowers, H.L.; Skoretz, S.A.; Silver, F.L.; Rochon, E.; Fang, J.; Flamand-Roze, C.; Martino, R. Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2016, 97, 2188–2201.e8. [Google Scholar] [CrossRef] [PubMed]
- Lecordier, S.; Manrique-Castano, D.; El Moghrabi, Y.; ElAli, A. Neurovascular alterations in vascular dementia: Emphasis on risk factors. Front. Aging Neurosci. 2021, 13, 727590. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Snyder, H.M.; Allegri, R.; Andrieu, S.; Arai, H.; Baker, L.; Belleville, S.; Brodaty, H.; Brucki, S.M.; et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020, 16, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Barber, P.A. Dementia risk and prevention by targeting modifiable vascular risk factors. J. Neurochem. 2018, 144, 565–581. [Google Scholar] [CrossRef] [PubMed]
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| Age | 48 | 53 | 61 | 50 | 55 | 75 | 66 | 86 |
| Sex | Female | Male | Male | Male | Male | Male | Female | Female |
| Race/Ethnicity | Hispanic/ White | Non-Hispanic White | Hispanic/ White | Non-Hispanic White | Hispanic/ White | Non-Hispanic White | Hispanic/White | Non-Hispanic White |
| Education Level | High School | High School | Some College | College Graduate | College Graduate | Some College | College Graduate | College Graduate |
| Marital Status | Never Married | Married | Couple | Married | Married | Married | Divorced | Widowed |
| Work Status | Unable to work | Retired | Retired | Employed | Retired | Unable to Work | Employed | Employed |
| Income Level | Unsure | 35 K to 50 K | 15 K to 35 K | 75 K or more | 75 K or more | 35 K to 50 K | 75 K or more | 75 K or more |
| Time since Onset | 7 years | 8 years | 8.5 years | 4 years | 8 years | 1.5 years | 1 year | <1 year |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| NIHSS Total Score | 7 | 1 | 5 | 2 | 2 | 1 | 0 | 0 |
| OxMET Accuracy Rule Breaks | −6 15 | 3 6 | −3 12 | 4 5 | 2 8 | 8 2 | 8 2 | 6 4 |
| Menu Task Assessment Total | 9 | 10 | 4 | 8 | 11 | 8 | 11 | 7 |
| NIH Toolbox Fully Corrected T-Score * | ||||||||
| Flanker Inhibitory Control Percentile | 20 5 | 32 4 | 31 5 | 28 3 | 38 16 | 50 38 | 55 69 | 20 |
| Dimensional Card Sort Percentile | 28 10 | 40 15 | 39 2 | 42 35 | 42 15 | 43 26 | 51 54 | 28 |
| List Sorting Working Memory Percentile | 48 39 | 36 5 | 47 48 | 32 4 | 17 80 | 42 23 | ||
| Pattern Comparison Processing Speed Percentile | 36 11 | 33 3 | 39 22 | 41 12 | 47 44 | 38 13 | 47 44 | 43 54 |
| Promis SE Managing Chronic Conditions T-score (SE) | 50.26 (2.23) | 48.18 (2.17) | 58.35 (3.41) | 50.26 (2.23) | 58.35 (3.41) | 46.27 (2.15) | 50.26 (2.23) | 63.85 (5.39) |
| Promis SE Managing Medications and Treatment T-score (SE) | 45.20 (2.80) | 38.30 (2.32) | 60.74 (6.31) | 54.95 (4.66) | 60.74 (6.31) | 30.44 (2.17) | 49.91 (3.73) | 60.74 (6.31) |
| SSQoL | 47 | 39 | 46 | 31 | 43 | 37 | 46 | 57 |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| LE8 Risk Score | 7 | 6 | 8 | 7 | 11 | 8 | 11 | 14 |
| mCAIDE Risk Score | 5 | 8 | 6 | 6 | 2 | 4 | 2 | 5 |
| Pre | Post | |
|---|---|---|
| Individual Scores | ||
| Case 1 | ||
| Performance | 3.67 ± 2.52 | 5.00 ± 2.64 |
| Satisfaction | 3.67 ± 2.31 | 5.33 ± 3.06 |
| Case 2 (lost to follow up) | ||
| Performance | - | - |
| Satisfaction | - | - |
| Case 3 | ||
| Performance | 5.00 ± 2.16 | 8.33 ± 1.53 |
| Satisfaction | 5.75 ± 1.26 | 9.33 ± 1.15 |
| Case 4 | ||
| Performance | 4.00 ± 0.00 | 3.67 ± 2.51 |
| Satisfaction | 7.00 ± 0.00 | 4.00 ± 1.00 |
| Case 5 | ||
| Performance | 6.00 ± 1.00 | 7.00 ± 1.00 |
| Satisfaction | 3.67 ± 2.31 | 4.67 ± 2.31 |
| Case 6 | ||
| Performance | 8.00 ± 0.00 | 8.00 ± 0.00 |
| Satisfaction | 8.00 ± 1.00 | 9.00 ± 0.00 |
| Case 7 | ||
| Performance | 6.00 ± 0.00 | 8.00 ± 0.00 |
| Satisfaction | 6.00 ± 0.00 | 9.00 ± 0.00 |
| Case 8 | ||
| Performance | 4.25 ± 2.52 | 5.50 ± 1.91 |
| Satisfaction | 3.50 ± 1.00 | 4.50 ± 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionne, T.; Richardson, J.D.; Quinn, D.; Luo, K.; Burns, S.P. Technology-Enabled Cognitive Strategy Intervention for Secondary Stroke Prevention: A Feasibility Study. Bioengineering 2025, 12, 778. https://doi.org/10.3390/bioengineering12070778
Dionne T, Richardson JD, Quinn D, Luo K, Burns SP. Technology-Enabled Cognitive Strategy Intervention for Secondary Stroke Prevention: A Feasibility Study. Bioengineering. 2025; 12(7):778. https://doi.org/10.3390/bioengineering12070778
Chicago/Turabian StyleDionne, Timothy, Jessica D. Richardson, Davin Quinn, Karen Luo, and Suzanne Perea Burns. 2025. "Technology-Enabled Cognitive Strategy Intervention for Secondary Stroke Prevention: A Feasibility Study" Bioengineering 12, no. 7: 778. https://doi.org/10.3390/bioengineering12070778
APA StyleDionne, T., Richardson, J. D., Quinn, D., Luo, K., & Burns, S. P. (2025). Technology-Enabled Cognitive Strategy Intervention for Secondary Stroke Prevention: A Feasibility Study. Bioengineering, 12(7), 778. https://doi.org/10.3390/bioengineering12070778






