Three-Dimensional Assessment of the Biological Periacetabular Defect Reconstruction in an Ovine Animal Model: A µ-CT Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Surgical Technique
2.1.1. Group Setup
2.1.2. Sample Retrieval and Preparation
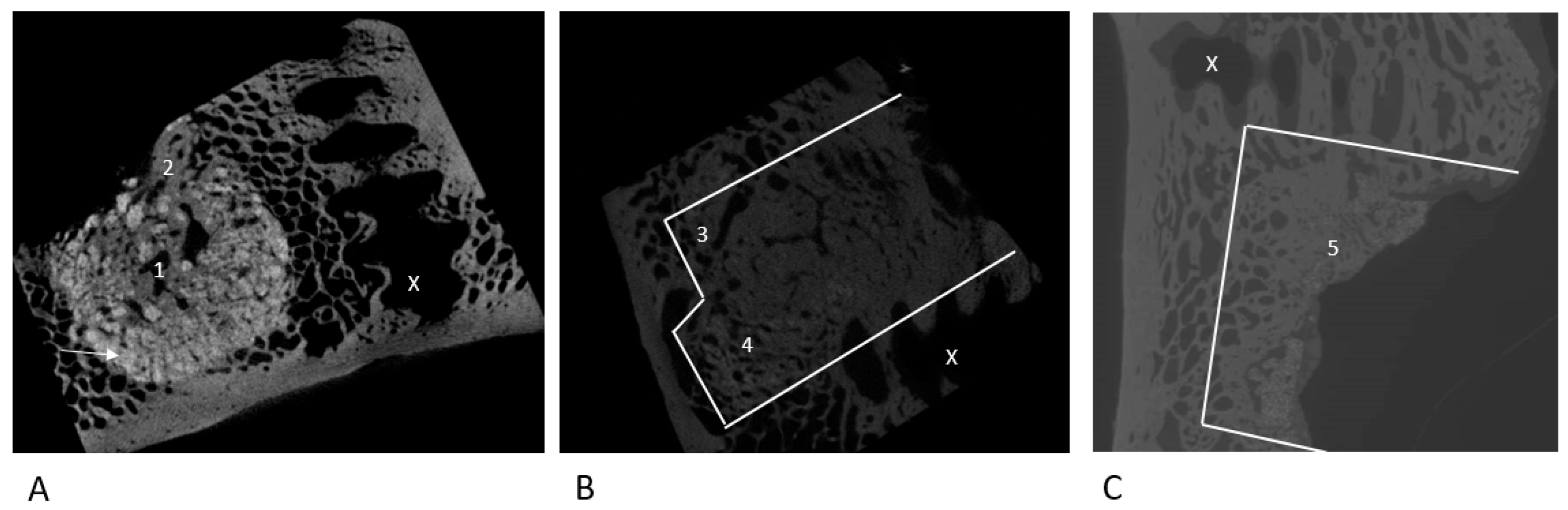
2.2. µ-CT Evaluation
2.3. Statistical Analysis
3. Results
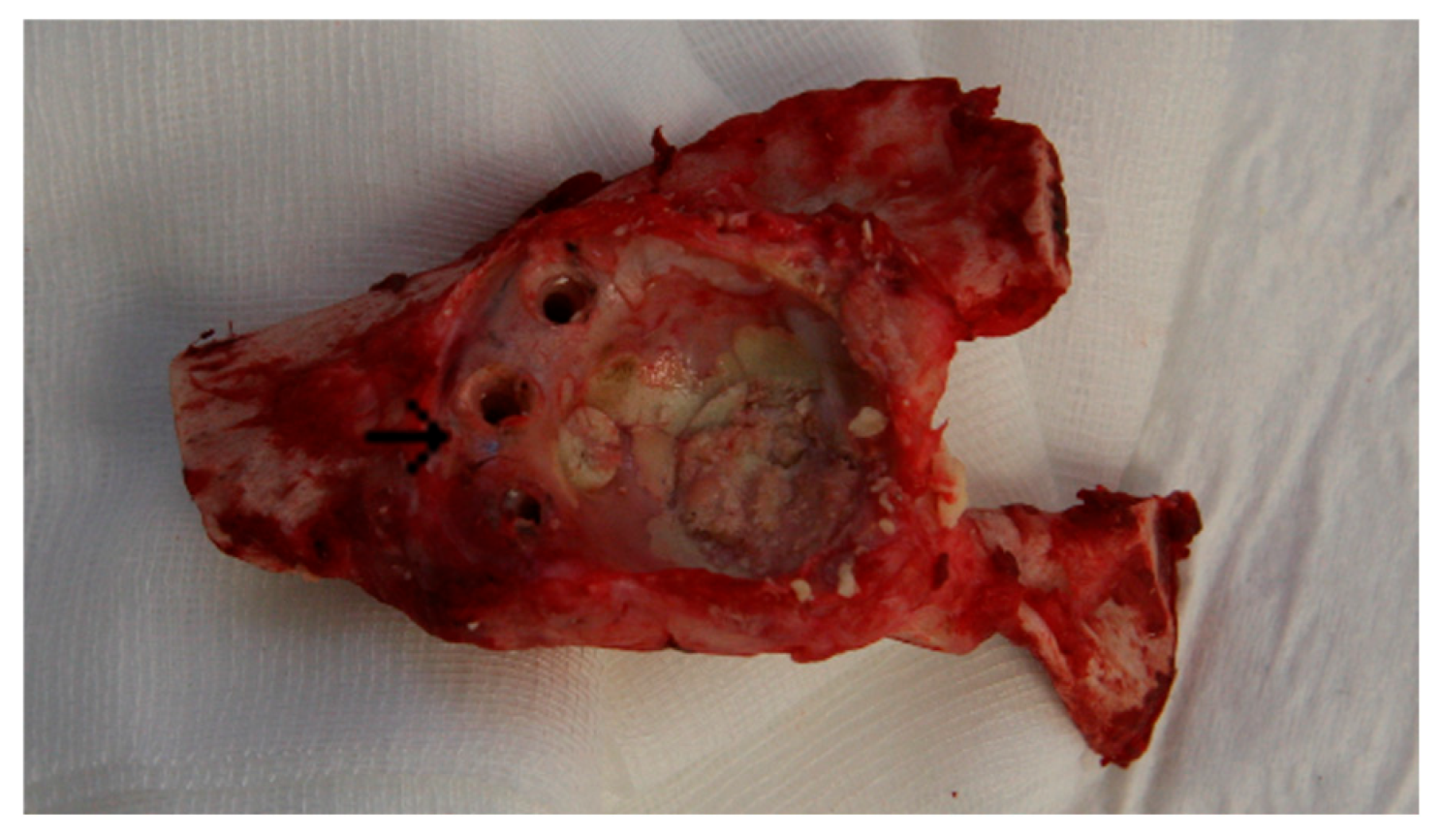
3.1. Animal Experiment: Macroscopic Assessment
3.2. Qualitative Marker Assessment
3.2.1. NanoBone®
3.2.2. Autologous Sheep Cancellous Bone
3.2.3. Tutoplast® Cancellous Bone
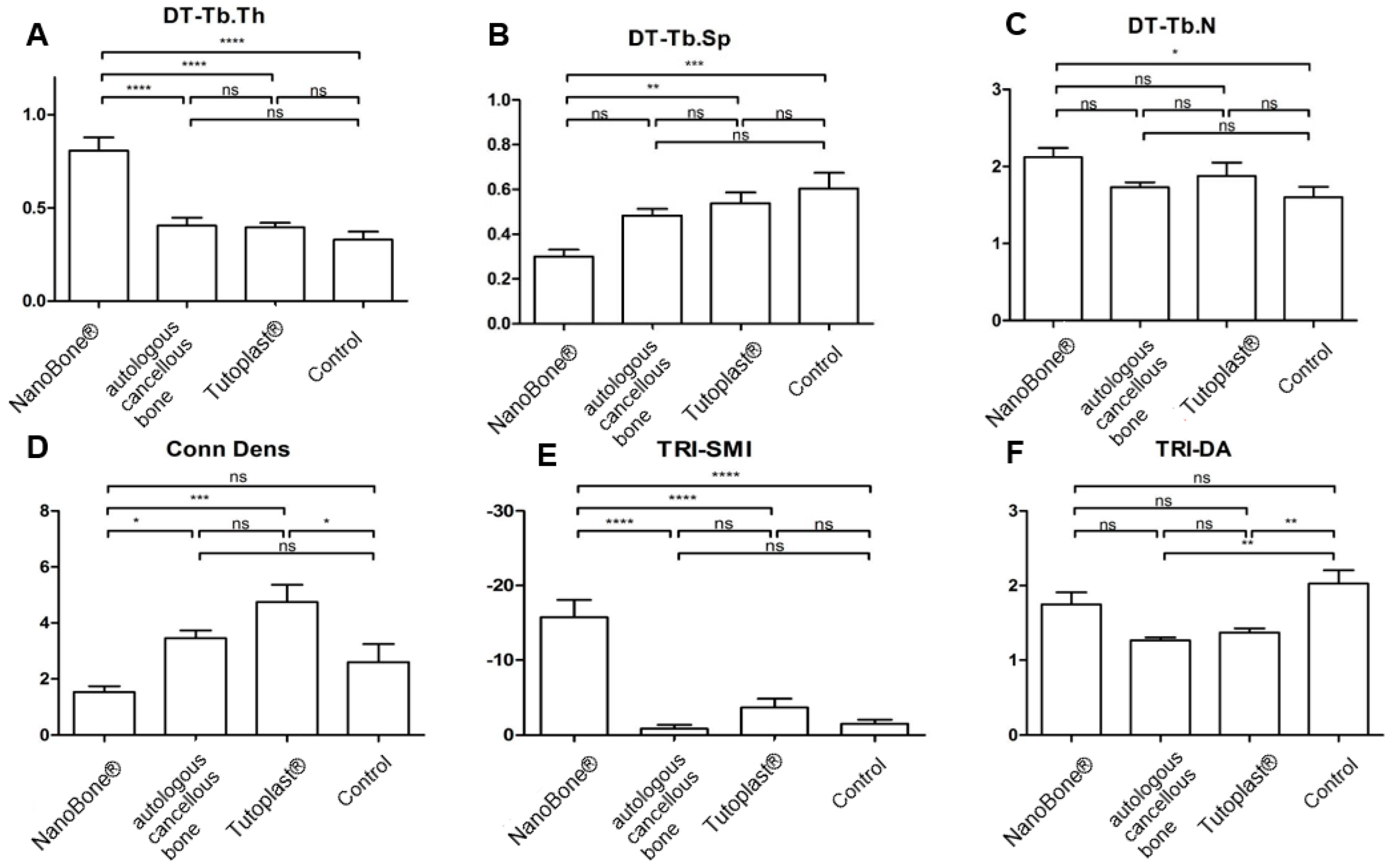
3.3. MV/TV; BV/TV; BSV/TV; BS/TV
3.4. Tb.Th, Tb.Sp, and Tb.N
3.5. Conn.D; SMI; DA
3.6. BMD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fröschen, F.S.; Randau, T.M.; Hischebeth, G.T.R.; Gravius, N.; Gravius, S.; Walter, S.G. Mid-Term Results after Revision Total Hip Arthroplasty with Custom-Made Acetabular Implants in Patients with Paprosky III Acetabular Bone Loss. Arch. Orthop. Trauma Surg. 2019, 140, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.G.; Randau, T.M.; Gravius, N.; Gravius, S.; Fröschen, F.S. Monoflanged Custom-Made Acetabular Components Promote Biomechanical Restoration of Severe Acetabular Bone Defects by Metallic Defect Reconstruction. J. Arthroplast. 2019, 35, 831–835. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Rueger, J.; Hägele, J.; Lehmann, W.; Rücker, A.; Schlickewei, C. Knochenaufbau—Knochenersatzmaterialien. Orthop. Unfallchir. Up2date 2010, 5, 295–314. [Google Scholar] [CrossRef]
- Niedhart, C.; Pingsmann, A.; Jürgens, C.; Marr, A.; Blatt, R.; Niethard, F. Donor Site Morbidity after Bone Graft Harvesting from the Ventral and DorsalIliac Crest—A Prospective, Controlled Study. Z. Orthop. Ihre Grenzgeb. 2003, 141, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Thomas, D.; Liang, C.T. Bone Progenitor Cell Deficits and the Age-Associated Decline in Bone Repair Capacity. Calcif. Tissue Int. 1995, 56, 123–129. [Google Scholar] [CrossRef]
- Gravius, S.; Pagenstert, G.; Weber, O.; Kraska, N.; Röhrig, H.; Wirtz, D.C. Acetabular defect reconstruction in revision surgery of the hip. Autologous, homologous or metal? Orthopäde 2009, 38, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wähnert, D.; Koettnitz, J.; Merten, M.; Kronenberg, D.; Stange, R.; Greiner, J.F.W.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. SpongostanTM Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse. Materials 2021, 14, 1961. [Google Scholar] [CrossRef]
- Götz, W.; Gerber, T.; Michel, B.; Lossdörfer, S.; Henkel, K.; Heinemann, F. Immunohistochemical Characterization of Nanocrystalline Hydroxyapatite Silica Gel (NanoBone®) Osteogenesis: A Study on Biopsies from Human Jaws. Clin. Oral Implants Res. 2008, 19, 1016–1026. [Google Scholar] [CrossRef]
- Heinemann, S.; Gelinsky, M.; Worch, H.; Hanke, T. Resorbable bone substitution materials. An overview of commercially available materials and new approaches in the field of composites. Orthopäde 2011, 40, 761–773. [Google Scholar] [CrossRef]
- Oonishi, H.; Iwaki, Y.; Kin, N.; Kushitani, S.; Murata, N.; Wakitani, S.; Imoto, K. Hydroxyapatite in Revision of Total Hip Replacements with Massive Acetabular Defects: 4- to 10-Year Clinical Results. J. Bone Jt. Surg. Br. Vol. 1997, 79-B, 87–92. [Google Scholar] [CrossRef]
- Tanaka, C.; Shikata, J.; Ikenaga, M.; Takahashi, M. Acetabular Reconstruction Using a Kerboull-Type Acetabular Reinforcement Device and Hydroxyapatite Granules. J. Arthroplast. 2003, 18, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip Joint Forces in Sheep. J. Biomech. 1999, 32, 769–777. [Google Scholar] [CrossRef]
- Phillips, T.W.; Johnston, G.; Wood, P. Selection of an Animal Model for Resurfacing Hip Arthroplasty. J. Arthroplast. 1987, 2, 111–117. [Google Scholar] [CrossRef]
- Phillips, T.W.; Gurr, K.R.; Rao, D.R. Hip Implant Evaluation in an Arthritic Animal Model. Arch. Orthop. Trauma Surg. 1990, 109, 194–196. [Google Scholar] [CrossRef]
- Phillips, T.W.; Gurr, K. A Preconditioned Arthritic Hip Model. J. Arthroplast. 1989, 4, 193–200. [Google Scholar] [CrossRef]
- Pearce, A.; Richards, R.; Milz, S.; Schneider, E.; Pearce, S. Animal Models for Implant Biomaterial Research in Bone: A Review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, J.; Liao, E.-Y.; Dai, R.-C.; Wu, X.-P.; Genant, H.K. Application of Micro-Ct Assessment of 3-d Bone Microstructure in Preclinical and Clinical Studies. J. Bone Miner. Metab. 2005, 23, 122–131. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Laib, A.; Koller, B.; Prohaska, S.; Mosekilde, L.; Gowin, W. Stereological Measures of Trabecular Bone Structure: Comparison of 3D Micro Computed Tomography with 2D Histological Sections in Human Proximal Tibial Bone Biopsies. J. Microsc. 2005, 218, 171–179. [Google Scholar] [CrossRef]
- Aaron, J.E.; Skerry, T.M. Intramembranous Trabecular Generation in Normal Bone. Bone Miner. 1994, 25, 211–230. [Google Scholar] [CrossRef]
- Fröschen, F.S.; Randau, T.M.; Haddouti, E.-M.; Schildberg, F.A.; Müller-Broich, J.D.; Götz, W.; Reimann, S.; Wirtz, D.C.; Gravius, S. Establishment of a Periprosthetic Acetabular Bone Defect in an In Vivo Model. Appl. Sci. 2024, 14, 3375. [Google Scholar] [CrossRef]
- Gerber, T.; Traykova, T.; Henkel, K.O.; Bienengraeber, V. Development and In Vivo Test of Sol-Gel Derived Bone Grafting Materials. J. Sol-Gel Sci. Technol. 2003, 26, 1173–1178. [Google Scholar] [CrossRef]
- Traykova, T.; Bötcher, R.; Neumann, H.G.; Henkel, K.-O.; Bienengräber, V.; Gerber, T. Silica/Calcium Phosphate Sol-Gel Derived Bone Grafting Material—From Animal Tests to First Clinical Experience. Key Eng. Mater. 2004, 254–256, 679–682. [Google Scholar] [CrossRef]
- Henkel, K.-O.; Lenz, J.-H.; Gerber, T.; Bienengräber, V. Ein qualitativ neuartiges Knochenaufbaumaterial auf Hydroxylapatit-Xerogel-Basis. ZWR 2005, 114, 416–418. [Google Scholar] [CrossRef]
- Gerber, T.; Ter, G.H.; Knoblich, B.; Dörfling, P.; Bienengräber, V.; Henkel, K.-O. Development of Bioactive Sol-Gel Material Template for In Vitro and In Vivo Synthesis of Bone Material. J. Sol-Gel Sci. Technol. 2000, 19, 441–445. [Google Scholar] [CrossRef]
- Henkel, K.-O.; Gerber, T.; Dietrich, W.; Bienengräber, V. Novel calcium phosphate formula for filling bone defects. Initial in vivo long-term result. Mund Kiefer Gesichtschirurgie 2004, 8, 277–281. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone Histomorphometry: Standardization of Nomenclature, Symbols, and Units: Report of the Asbmr Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chen, M.Y.; Hsu, J.-T.; Li, Y.-F.; Chang, C.-H.; Chen, K.-T. Three-Dimensional Bone Structure and Bone Mineral Density Evaluations of Autogenous Bone Graft after Sinus Augmentation: A Microcomputed Tomography Analysis. Clin. Oral Implants Res. 2012, 23, 1098–1103. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, J.; Liu, T.; Gao, L.; Gu, Z.; Wu, G. Low-Dose rhBMP2/7 Heterodimer to Reconstruct Peri-Implant Bone Defects: A Micro-CT Evaluation. J. Clin. Periodontol. 2012, 39, 98–105. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hauser, J.; Ring, A. Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research. Bioengineering 2022, 9, 298. [Google Scholar] [CrossRef]
- Lu, S.; Lam, J.; Trachtenberg, J.E.; Lee, E.J.; Seyednejad, H.; van den Beucken, J.J.J.P.; Tabata, Y.; Kasper, F.K.; Scott, D.W.; Wong, M.E.; et al. Technical Report: Correlation Between the Repair of Cartilage and Subchondral Bone in an Osteochondral Defect Using Bilayered, Biodegradable Hydrogel Composites. Tissue Eng. Part C Methods 2015, 21, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Lucke, M.; Schwabe, P.; Raschke, M.; Haas, N.P.; Wildemann, B. Collective Review: Bioactive Implants Coated with Poly (D,L-Lactide) and Growth Factors IGF-I, TGF-β1, or BMP-2 for Stimulation of Fracture Healing. J. Long-Term Eff. Med. Implant. 2006, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.; Gross, G.J.; Dufresne, T.E.; Smith, T.S.; Cockman, M.D.; Chmielewski, P.A.; Lundy, M.W.; Hartke, J.R.; Sod, E.W. Three-Dimensional Microimaging (MRµI and µCT), Finite Element Modeling, and Rapid Prototyping Provide Unique Insights into Bone Architecture in Osteoporosis. Anat. Rec. 2001, 265, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Efeoglu, C.; Burke, J.L.; Parsons, A.J.; Aitchison, G.A.; Scotchford, C.; Rudd, C.; Vikram, A.; Fisher, S.E. Analysis of Calvarial Bone Defects in Rats Using Microcomputed Tomography: Potential for a Novel Composite Material and a New Quantitative Measurement. Br. J. Oral Maxillofac. Surg. 2009, 47, 616–621. [Google Scholar] [CrossRef]
- Engelke, K.; Karolczak, M.; Lutz, A.; Seibert, U.; Schaller, S.; Kalender, W. Micro-CT. Technology and Application for Assessing Bone Structure. Radiologe 1999, 39, 203–212. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Goldstein, S.A.; Parfitt, M.A.; Jesion, G.; Kleerekoper, M. The Direct Examination of Three-Dimensional Bone Architecture in Vitro by Computed Tomography. J. Bone Miner. Res. 1989, 4, 3–11. [Google Scholar] [CrossRef]
- Goulet, R.W.; Goldstein, S.A.; Ciarelli, M.J.; Kuhn, J.L.; Brown, M.B.; Feldkamp, L.A. The Relationship between the Structural and Orthogonal Compressive Properties of Trabecular Bone. J. Biomech. 1994, 27, 375–389. [Google Scholar] [CrossRef]
- Kinney, J.H.; Ladd, A.J.C. The Relationship Between Three-Dimensional Connectivity and the Elastic Properties of Trabecular Bone. J. Bone Miner. Res. 1998, 13, 839–845. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput. Methods Biomech. Biomed. Eng. 1997, 1, 15–23. [Google Scholar] [CrossRef]
- Yamada, M.; Egusa, H. Current Bone Substitutes for Implant Dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef]
- Harms, C.; Helms, K.; Taschner, T.; Stratos, I.; Ignatius, A.; Gerber, T.; Lenz, S.; Rammelt, S.; Vollmar, B.; Mittlmeier, T. Osteogenic Capacity of Nanocrystalline Bone Cement in a Weight-Bearing Defect at the Ovine Tibial Metaphysis. Int. J. Nanomed. 2012, 7, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.; Fernández, A.B.; Feito, M.J.; Matesanz, M.C.; Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regí, M.; Rojo, J.M.; Portolés, M.T. Effects of Nanocrystalline Hydroxyapatites on Macrophage Polarization. J. Mater. Chem. B 2016, 4, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, C.; Zhang, B.; Yao, C.; Zhang, Y. Advances in 3D-Printed Scaffold Technologies for Bone Defect Repair: Materials, Biomechanics, and Clinical Prospects. BioMed. Eng. OnLine 2025, 24, 51. [Google Scholar] [CrossRef] [PubMed]


| NanoBone® | Autologous Cancellous Bone | Tutoplast® | Control Group | |
|---|---|---|---|---|
| MV/TV [%] | 85.74 ± 2.69 | 49.97 ± 4.54 | 64.49 ± 5.63 | 42.94 ± 4.6 |
| BV/TV [%] | 68.85 ± 3.76 | 45.4 ± 4.3 | 55.42 ± 5.33 | 42.94 ± 4.6 |
| BSV/TV [%] | 16.9 ± 1.75 | 4.57 ± 0.55 | 9.07 ± 0.53 | |
| BS/BV [mm−1] | 2.17 ± 0.22 | 6.47 ± 0.78 | 5.95 ± 0.53 | 7.62 ± 0.96 |
| Tb.Th [µm] | 0.81 ± 0.07 | 0.39 ± 0.05 | 0.39 ± 0.02 | 0.33 ± 0.04 |
| Th.SP [µm] | 0.3 ± 0.03 | 0.48 ± 0.03 | 0.54 ± 0.05 | 0.6 ± 0.07 |
| Tb.N [1/cm] | 2.12 ± 0.12 | 1.73 ± 0.06 | 1.88 ± 0.17 | 1.6 ± 0.13 |
| Conn. D [1/mm3] | 1.53 ± 0.21 | 3.46 ± 0.27 | 4.75 ± 0.62 | 2.6 ± 0.66 |
| SMI | −15.77 ± 2.29 | −0.86 ± 0.49 | −3.65 ± 1.19 | −1.51 ± 0.54 |
| DA | 1.75 ± 0.45 | 1.27 ± 0.11 | 1.37 ± 0.16 | 2.03 ± 0.5 |
| BMD [mg HA/cm3) | 133.78 ± 6.55 | 73.32 ± 15.81 | 92.32 ± 32.92 | 69.68 ± 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröschen, F.S.; Randau, T.M.; Haddouti, E.-M.; Müller-Broich, J.D.; Schildberg, F.A.; Götz, W.; John, D.; Reimann, S.; Wirtz, D.C.; Gravius, S. Three-Dimensional Assessment of the Biological Periacetabular Defect Reconstruction in an Ovine Animal Model: A µ-CT Analysis. Bioengineering 2025, 12, 729. https://doi.org/10.3390/bioengineering12070729
Fröschen FS, Randau TM, Haddouti E-M, Müller-Broich JD, Schildberg FA, Götz W, John D, Reimann S, Wirtz DC, Gravius S. Three-Dimensional Assessment of the Biological Periacetabular Defect Reconstruction in an Ovine Animal Model: A µ-CT Analysis. Bioengineering. 2025; 12(7):729. https://doi.org/10.3390/bioengineering12070729
Chicago/Turabian StyleFröschen, Frank Sebastian, Thomas Martin Randau, El-Mustapha Haddouti, Jacques Dominik Müller-Broich, Frank Alexander Schildberg, Werner Götz, Dominik John, Susanne Reimann, Dieter Christian Wirtz, and Sascha Gravius. 2025. "Three-Dimensional Assessment of the Biological Periacetabular Defect Reconstruction in an Ovine Animal Model: A µ-CT Analysis" Bioengineering 12, no. 7: 729. https://doi.org/10.3390/bioengineering12070729
APA StyleFröschen, F. S., Randau, T. M., Haddouti, E.-M., Müller-Broich, J. D., Schildberg, F. A., Götz, W., John, D., Reimann, S., Wirtz, D. C., & Gravius, S. (2025). Three-Dimensional Assessment of the Biological Periacetabular Defect Reconstruction in an Ovine Animal Model: A µ-CT Analysis. Bioengineering, 12(7), 729. https://doi.org/10.3390/bioengineering12070729







