Unlocking Superior MFH Performance Below Hergt’s Biological Safety Limit: SPION-Based Magnetic Nanoplatforms Deliver High Heating Efficiency at Low AMF
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
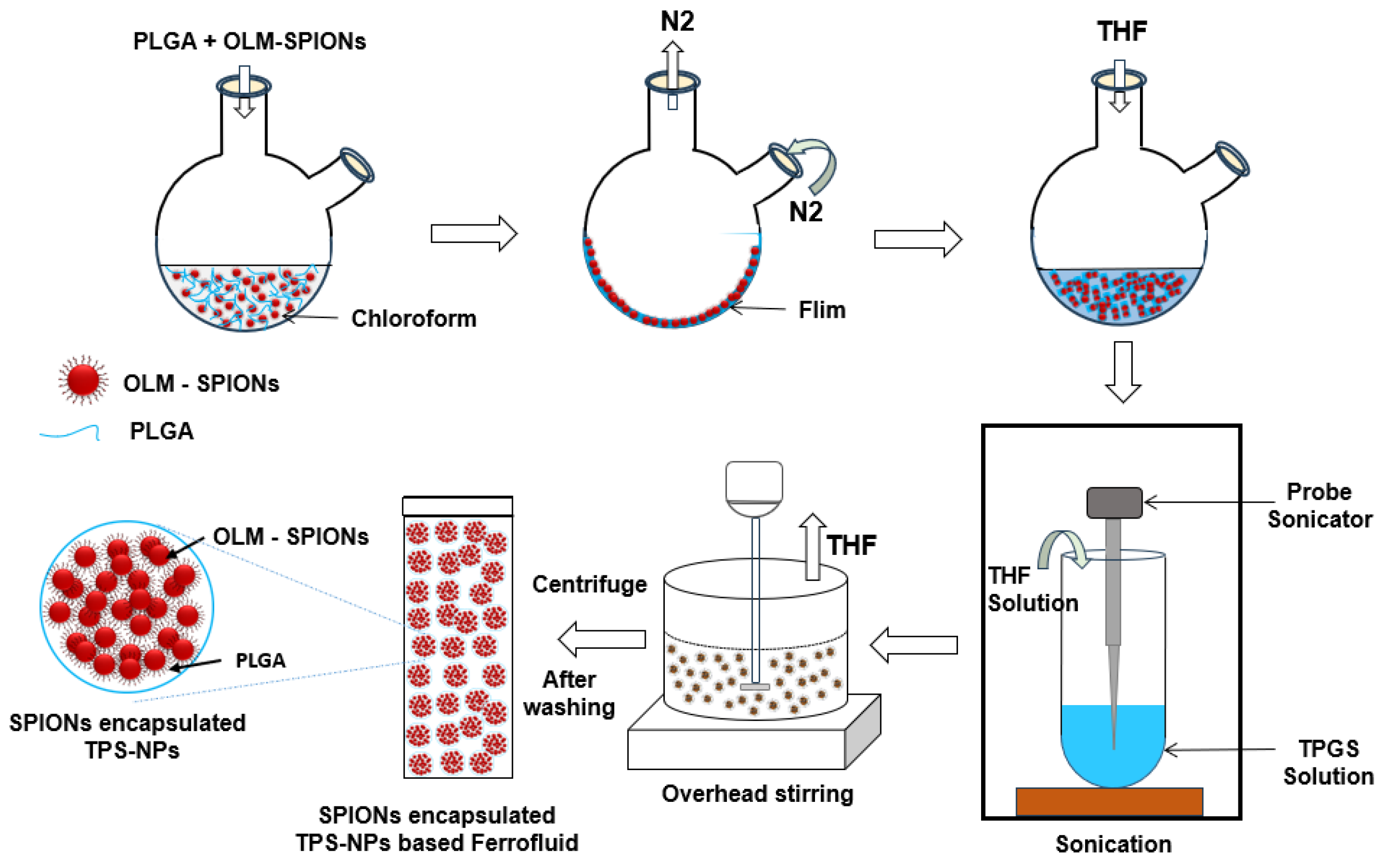
2.2. Synthesis of TPGS-Stabilized PLGA Nanoparticles (TPS-NPs)
2.3. Characterization
2.4. Calorimetric MFH
3. Results
3.1. OLM-SPIONs
3.2. TPGS-Stabilized PLGA Nanoparticles (TPS-NPs)
Optimization of Empty and SPION-Encapsulated TPS-NPs
- Effects of concentration of stabilizer
- Effects of OLM-SPION concentration
| Sr. No. | PLGA (mg) | TPGS % (w/v) | OLM-SPIONs (mgFe) | Mean Hydrodynamic Size (nm) | PDI | Fe (EE %) | Zeta Potential (ζ) (mv) |
|---|---|---|---|---|---|---|---|
| Empty TPS-NPs | |||||||
| S1 | 10 | 0 | NA | 176 ± 65 | 0.24 ± 0.12 | NA | - |
| S2 | 10 | 0.05 | NA | 168 ± 24 | 0.22 ± 0.15 | NA | - |
| S3 | 10 | 0.1 | NA | 156 ± 7 | 0.18 ± 0.02 | NA | − |
| S4 | 10 | 0.5 | NA | 151 ± 5 | 0.14 ± 0.05 | NA | −56.4 |
| S5 | 10 | 1 | NA | 166 ± 8 | 0.11± 0.04 | NA | −60.1 |
| S6 | 10 | 2 | NA | 175 ± 51 | 0.20 ± 0.11 | NA | − |
| SPION-encapsulated TPS-NPs (via 0.5 Wt % TPGS) | |||||||
| S7 | 10 | 0.5 | 2 | 106 ± 3 | 0.32 ± 0.02 | ~66.7 | −23.8 |
| S8 | 10 | 0.5 | 4 | 125 ± 2 | 0.20 ± 0.03 | ~68.9 | −28.0 |
| S9 | 10 | 0.5 | 6 | 144 ± 3 | 0.13 ± 0.02 | ~65.4 | −9.7 |
| S10 | 10 | 0.5 | 8 | 151 ± 5 | 0.32 ± 0.08 | ~62.5 | −2.2 |
| SPION-encapsulated TPS-NPs (via 1 Wt % TPGS) | |||||||
| S11 | 10 | 1 | 2 | 113 ± 4 | 0.21 ± 0.03 | ~70.8 | −34.4 |
| S12 | 10 | 1 | 4 | 127 ± 6 | 0.20 ± 0.04 | ~69.6 | −29.2 |
| S13 | 10 | 1 | 6 | 149 ± 2 | 0.15 ± 0.04 | ~67.1 | −10.6 |
| S14 | 10 | 1 | 8 | 171 ± 7 | 0.35 ± 0.11 | ~64.8 | −3.7 |
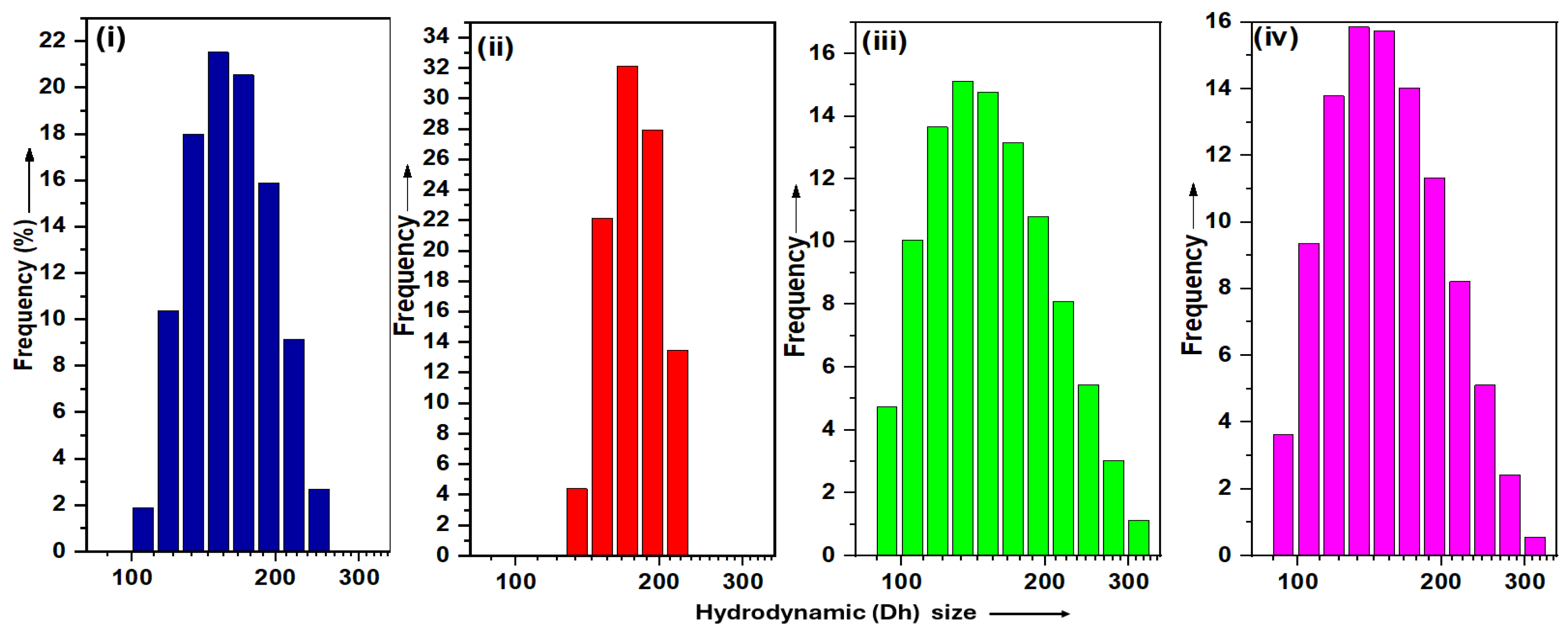
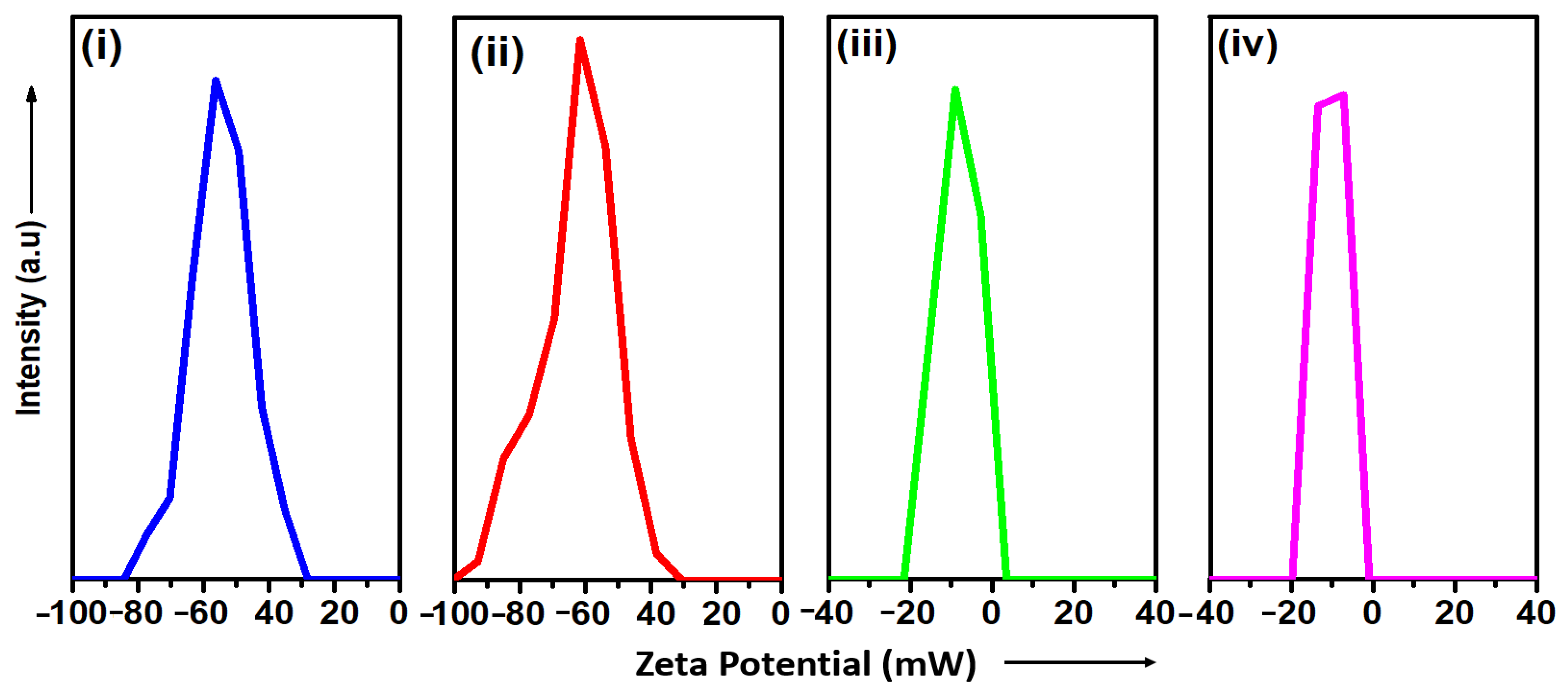
3.3. DLS
3.3.1. Hydrodynamic Size
3.3.2. Water Dispersibility
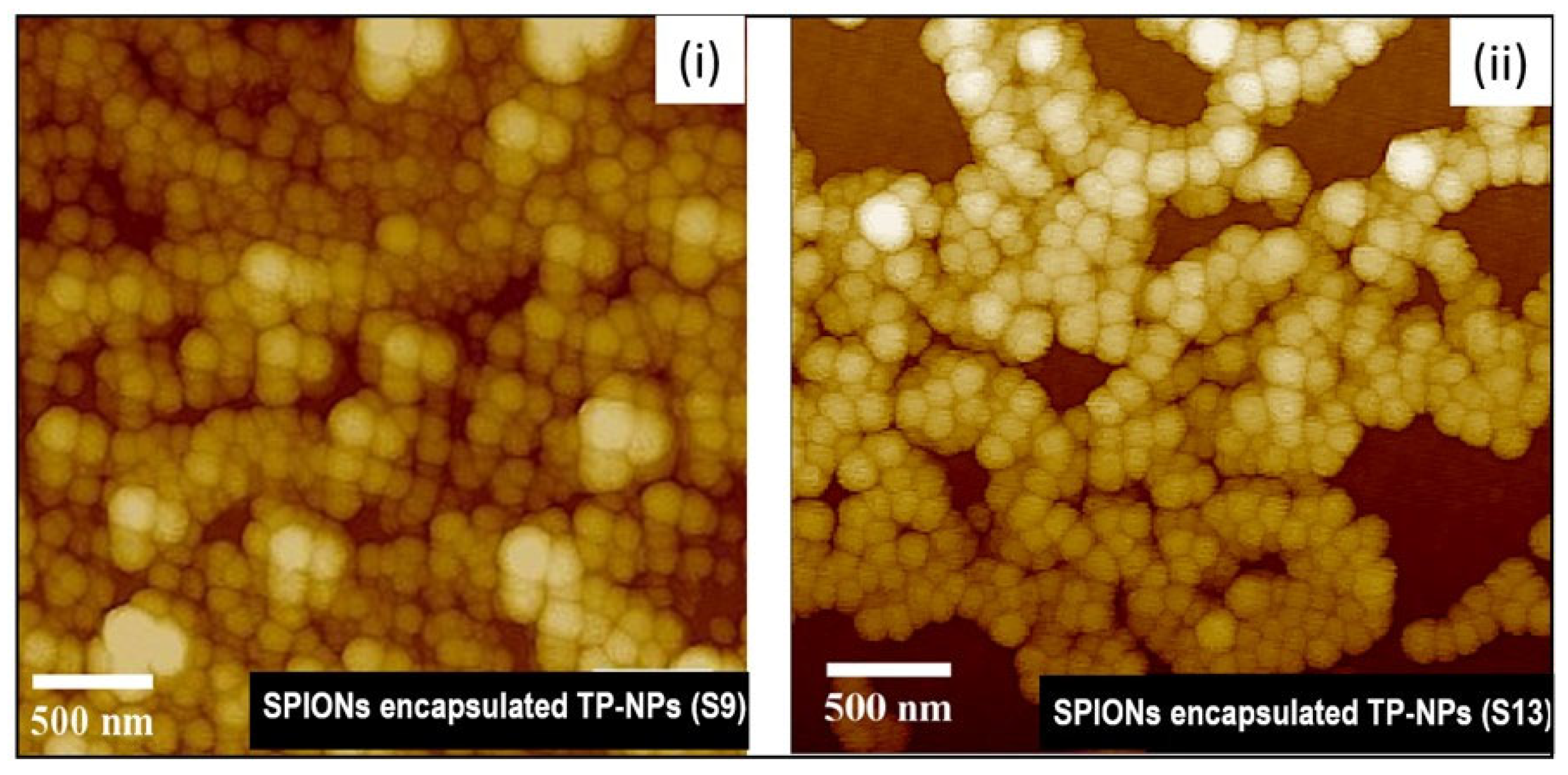
3.4. Morphology—AFM and TEM
3.5. Magnetic Properties—SQUID
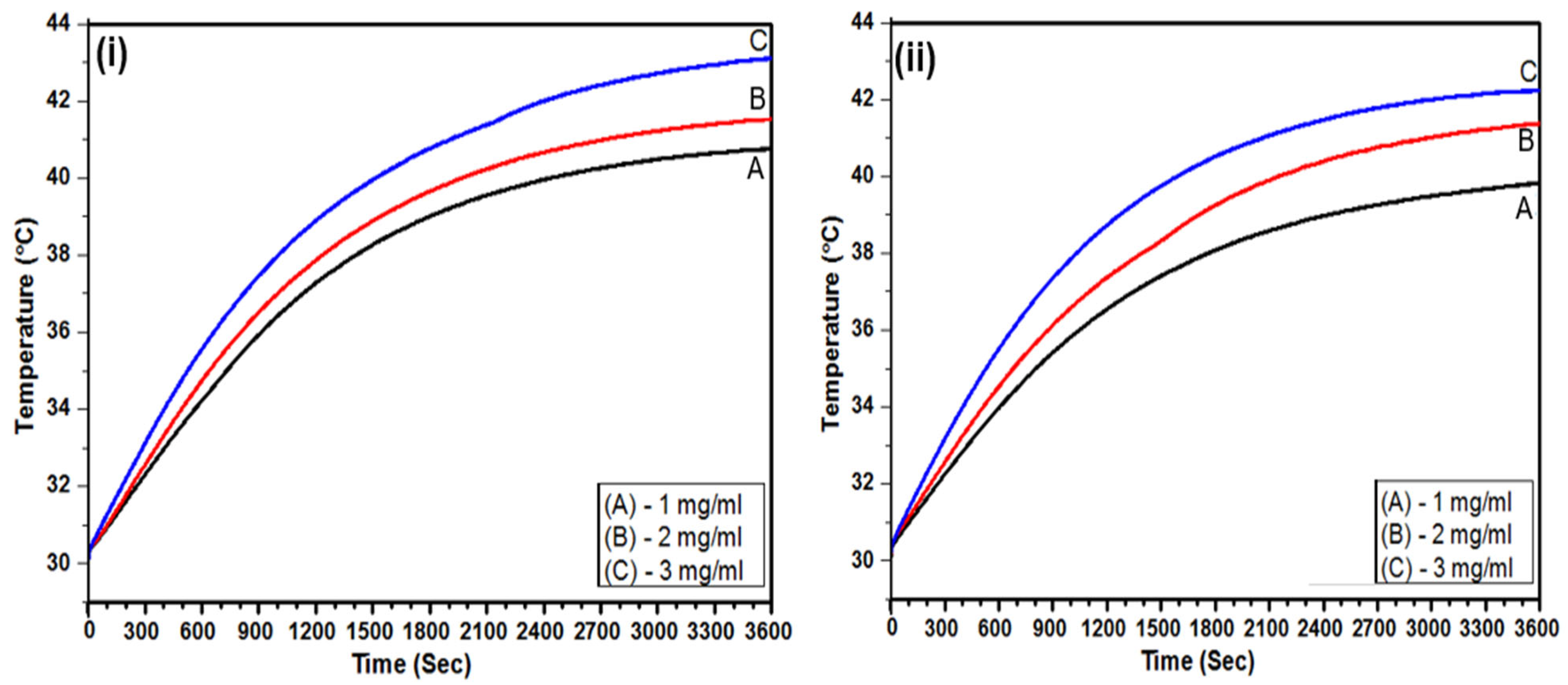
3.6. Calorimetric MFH
3.7. Thermal Analysis
3.7.1. Thermogravimetric Analysis (TGA)
3.7.2. Differential Scanning Calorimetry (DSC)
4. Discussion
| Sr. No | Samples | Dh (nm) | Particle Size (nm) | OLM-SPIONs /Fe (EE %) | AMF (H (kA/m) & f (KHz)) | H × f (GAm−1 s−1) | Ms (emu/g) | SAR (W/gFe) | ILP (nHm2kg−1) | Mini. Particles Concern. mgFe/mL) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SPION-encapsulated TPS-NPs | 144 ± 3 | 140 ± 27.71 | ~ | H = 8.6, f = 475.5 | 4.1 | 25.1 | 48.8 ± 2.3 | 1.39± 0.07 | 3 | This work |
| 2 ∞ | CCM-NIF-SPION-encapsulated TPS-NPs | 218 ± 3 | 194±14 | 73.2 | H = 11.5, f = 751.5 | 8.6 | 9.1 | 62.8 | 0.6 | 0.5 | [25] |
| 3 a | CCM-Ver-SPION-encapsulated PV-NPs | 282 ± 2.4 | 250-280 | 64.9 | H = 10.9, f = 751.5 | 8.2 | 8.2 | 8.5 | 0.1 | 6 | [27] |
| 4 | CCM-5Fu-SPION-encapsulated PV-NPs | - | 150 | - | H = 18.03, f = 305 | 5.5 | - | - | - | 0.5 | [51] |
| 5 | CCM-SPION-encapsulated. PV-NPs | 72 | 250 | 65 | H = 11.7, f = 427 | 5 | 6 | 59 | - | 5 | [64] |
| 6 | SPION-encapsulated. TPGS-NPs | - | 178.2 | - | H = 89, f = 240 | 21.3 | 73.9 | 51.4 | - | 1 | [65] |
| 7 | Aminosilane-coated SPIONs | 100 | - | H = 16, f = 874 | 14 | - | 194 | - | 0.6 | [66,67] | |
| 0.1 | |||||||||||
| 8 | Aminosilane-coated SPIONs | - | 12 | - | H = 2-15, f = 100 | 1.5 | - | - | - | 112 * | [68] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| DLS | Dynamic Light Scattering |
| DSC | Differential Scanning Calorimetry |
| Dh | Hydrodynamic Diameter |
| ILP | Intrinsic Loss Power |
| KTP | Potassium Thiocyanate-Based Protocol |
| M-SESE | Modified Single Emulsion Solvent Evaporation |
| MFH | Magnetic Fluid Hyperthermia |
| OLM | Oleic Acid and Oleylamine |
| PLGA | Poly (D, L-lactide-co-glycolide) |
| PV-NPs | PVA-Stabilized PLGA Nanoparticles |
| SAR | Specific Absorption Rate |
| SPIONs | Superparamagnetic Iron Oxide Nanoparticles |
| OLM-SPIONs | Oleic-Acid-and-Oleylamine-coated SPIONs |
| SQUID | Superconducting Quantum Interference Device |
| TB | Blocking Temperatures |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TPGS | D-α-Tocopheryl Polyethylene Glycol Succinate |
| TPS-NPs | TPGS-stabilized PLGA nanoparticles |
| UV–Vis | Ultraviolet–Visible |
| ζ | Zeta Potential |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Paresishvili, T.; Kakabadze, Z. Challenges and Opportunities Associated With Drug Delivery for the Treatment of Solid Tumors. Oncol. Rev. 2023, 17, 10577. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for Advancing Cancer Nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Motelica, L.; Ficai, D.; Semenescu, A.; Oprea, O.-C.; Ficai, A. Smart Magnetic Drug Delivery Systems for the Treatment of Cancer. Nanomaterials 2023, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Zhang, S. Recent Advances in PLGA Polymer Nanocarriers for Ovarian Cancer Therapy. Front. Oncol. 2025, 15, 1526718. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic Particle Hyperthermia: Nanoparticle Magnetism and Materials Development for Cancer Therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Lu, M. Advances in Magnetic Induction Hyperthermia. Front. Bioeng. Biotechnol. 2024, 12, 1432189. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H. Roland Felix Magnetic Fluid Hyperthermia (MFH): Cancer Treatment with AC Magnetic Field Induced Excitation of Biocompatible Superparamagnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sahraian, M.A.; Shokrgozar, M.A.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles: Promises for Diagnosis and Treatment of Multiple Sclerosis. ACS Chem. Neurosci. 2011, 2, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zaloga, J.; Ding, Y.; Liu, Y.; Janko, C.; Pischetsrieder, M.; Alexiou, C.; Boccaccini, A.R. Facile Preparation of Multifunctional Superparamagnetic PHBV Microspheres Containing SPIONs for Biomedical Applications. Sci. Rep. 2016, 6, 23140. [Google Scholar] [CrossRef]
- Rajan, A.; Laha, S.S.; Sahu, N.K.; Thorat, N.D.; Shankar, B. Recent Advancements and Clinical Aspects of Engineered Iron Oxide Nanoplatforms for Magnetic Hyperthermia-Induced Cancer Therapy. Mater. Today Bio 2024, 29, 101348. [Google Scholar] [CrossRef]
- Etemadi, H.; Buchanan, J.K.; Kandile, N.G.; Plieger, P.G. Iron Oxide Nanoparticles: Physicochemical Characteristics and Historical Developments to Commercialization for Potential Technological Applications. ACS Biomater. Sci. Eng. 2021, 7, 5432–5450. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic Particle Hyperthermia—Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Mehata, A.K.; Setia, A.; Vikas, V.; Malik, A.K.; Hassani, R.; Dailah, H.G.; Alhazmi, H.A.; Albarraq, A.A.; Mohan, S.; Muthu, M.S. Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future. Pharmaceutics 2023, 15, 722. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.; Yu, Z.; Gao-wei, L. Formulation, Characterization and Evaluation of Curcumin-Loaded PLGA-TPGS Nanoparticles For Liver Cancer Treatment. Drug Des. Devel. Ther. 2019, 13, 3569–3578. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Sebastian, V.; Larrea, A.; Marquina, C.; Blanco-Prieto, M.J. Co-Encapsulation of Superparamagnetic Nanoparticles and Doxorubicin in PLGA Nanocarriers: Development, Characterization and in Vitro Antitumor Efficacy in Glioma Cells. Eur. J. Pharm. Biopharm. 2019, 145, 65–75. [Google Scholar] [CrossRef]
- Sudame, A.; Kandasamy, G.; Singh, D.; Tomy, C.V.; Maity, D. Symbiotic Thermo-Chemotherapy for Enhanced HepG2 Cancer Treatment via Magneto-Drugs Encapsulated Polymeric Nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125355. [Google Scholar] [CrossRef]
- Das, M.; Mishra, D.; Dhak, P.; Gupta, S.; Maiti, T.K.; Basak, A.; Pramanik, P. Biofunctionalized, Phosphonate-Grafted, Ultrasmall Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Multimodal Imaging. Small 2009, 5, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Sudame, A.; Maity, D.; Soni, S.; Sushmita, K.; Veerapu, N.S.; Bose, S.; Tomy, C.V. Multifunctional Magnetic-Polymeric Nanoparticles Based Ferrofluids for Multi-Modal in Vitro Cancer Treatment Using Thermotherapy and Chemotherapy. J. Mol. Liq. 2019, 293, 111549. [Google Scholar] [CrossRef]
- Riedinger, A.; Guardia, P.; Curcio, A.; Garcia, M.A.; Cingolani, R.; Manna, L.; Pellegrino, T. Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Lett. 2013, 13, 2399–2406. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of Commercial Colloids for Magnetic Hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Bhati, P.; Chakrabarty, A.; Maity, D. Systematic Investigations on Heating Effects of Carboxyl-Amine Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Based Ferrofluids for in Vitro Cancer Hyperthermia Therapy. J. Mol. Liq. 2018, 256, 224–237. [Google Scholar] [CrossRef]
- Maity, D.; Kale, S.N.; Kaul-Ghanekar, R.; Xue, J.-M.; Ding, J. Studies of Magnetite Nanoparticles Synthesized by Thermal Decomposition of Iron (III) Acetylacetonate in Tri(Ethylene Glycol). J. Magn. Magn. Mater. 2009, 321, 3093–3098. [Google Scholar] [CrossRef]
- Maity, D.; Choo, S.G.; Yi, J.; Ding, J.; Xue, J.M. Synthesis of Magnetite Nanoparticles via a Solvent-Free Thermal Decomposition Route. J. Magn. Magn. Mater. 2009, 321, 1256–1259. [Google Scholar] [CrossRef]
- Shang, Q.; Zhai, J.; Tian, R.; Zheng, T.; Zhang, X.; Liang, X.; Zhang, J. Fabrication, Characterization, and Controlled Release of Eprinomectin from Injectable Mesoporous PLGA Microspheres. RSC Adv. 2015, 5, 75025–75032. [Google Scholar] [CrossRef]
- Yu, M.; Yao, Q.; Zhang, Y.; Chen, H.; He, H.; Zhang, Y.; Yin, T.; Tang, X.; Xu, H. Core/Shell PLGA Microspheres with Controllable in Vivo Release Profile via Rational Core Phase Design. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Tu, P. The Mechanism of Self-Assembled Mixed Micelles in Improving Curcumin Oral Absorption: In Vitro and in Vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Okassa, L.N.; Marchais, H.; Douziech-Eyrolles, L.; Hervé, K.; Cohen-Jonathan, S.; Munnier, E.; Soucé, M.; Linassier, C.; Dubois, P.; Chourpa, I. Optimization of Iron Oxide Nanoparticles Encapsulation within Poly(d,l-Lactide-Co-Glycolide) Sub-Micron Particles. Eur. J. Pharm. Biopharm. 2007, 67, 31–38. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. A Novel Controlled Release Formulation for the Anticancer Drug Paclitaxel (Taxol®): PLGA Nanoparticles Containing Vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.I.; Sambamoorthy, U.; Kumar, S.A. D-ɑ-Tocopheryl Polyethylene Glycol Succinate: A Review of Multifarious Applications in Nanomedicines. OpenNano 2022, 6, 100036. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Development, Surface Modification and Applications in Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, I.; Chakrabarti, M.; Mukherjee, A. Genotoxicity and Biocompatibility of Superparamagnetic Iron Oxide Nanoparticles: Influence of Surface Modification on Biodistribution, Retention, DNA Damage and Oxidative Stress. Food Chem. Toxicol. 2020, 136, 110989. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, R.H.; Ganguly, S.; Dewanjee, S.; Sinha, S.; Gupta, A.; Ganguly, S.; Chattopadhyay, D.; Chatterjee Debnath, M. Garcinol Loaded Vitamin E TPGS Emulsified PLGA Nanoparticles: Preparation, Physicochemical Characterization, in Vitro and in Vivo Studies. Sci. Rep. 2017, 7, 530. [Google Scholar] [CrossRef] [PubMed]
- Şengel, C.T.; Hasçiçek, C.; Gönül, N. Design of Vitamin E D-α-Tocopheryl Polyethylene Glycol 1000 Succinate-Emulsified Poly (D,L–Lactide–Co-Glycolide) Nanoparticles: Influence of Duration of Ultrasonication Energy. J. Young Pharm. 2011, 3, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, W.; Chen, Y.; Chang, D.; Wang, T.; Zhang, X.; Mei, L.; Zeng, X.; Huang, L. Doxorubicin-Loaded Star-Shaped Copolymer PLGA-Vitamin E TPGS Nanoparticles for Lung Cancer Therapy. J. Mater. Sci. Mater. Med. 2015, 26, 165. [Google Scholar] [CrossRef]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-Free Nanodrugs with Efficient Drug Delivery and Release for Cancer Therapy: From Intrinsic Physicochemical Properties to External Modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef]
- Haripriyaa, M.; Suthindhiran, K. Pharmacokinetics of Nanoparticles: Current Knowledge, Future Directions and Its Implications in Drug Delivery. Futur. J. Pharm. Sci. 2023, 9, 113. [Google Scholar] [CrossRef]
- Kim, D.-H.; Vitol, E.A.; Liu, J.; Balasubramanian, S.; Gosztola, D.J.; Cohen, E.E.; Novosad, V.; Rozhkova, E.A. Stimuli-Responsive Magnetic Nanomicelles as Multifunctional Heat and Cargo Delivery Vehicles. Langmuir 2013, 29, 7425–7432. [Google Scholar] [CrossRef]
- Ai, H.; Flask, C.; Weinberg, B.; Shuai, X.-T.; Pagel, M.D.; Farrell, D.; Duerk, J.; Gao, J. Magnetite-Loaded Polymeric Micelles as Ultrasensitive Magnetic-Resonance Probes. Adv. Mater. 2005, 17, 1949–1952. [Google Scholar] [CrossRef]
- Vidawati, S.; Barbosa, S.; Taboada, P.; Mosquera, V. Effects of Sonication Processing on the Behavior of the Synthesis Human Serum Albumin-SPIONs Loaded PLGA Nanoparticles. Adv. Biol. Chem. 2019, 9, 179–188. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Girija, A.R.; Nagaoka, Y.; Iwai, S.; Suzuki, M.; Kizhikkilot, V.; Yoshinda, Y.; Maekawa, T.; Nair, S.D. Curcumin and 5-Fluorouracil-Loaded, Folate- and Transferrin-Decorated Polymeric Magnetic Nanoformulation: A Synergistic Cancer Therapeutic Approach, Accelerated by Magnetic Hyperthermia. Int. J. Nanomed. 2014, 9, 437–459. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of Iron Oxide Nanoparticles under Oxidizing Environment and Their Stabilization in Aqueous and Non-Aqueous Media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Maity, D. Recent Advances in Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for in Vitro and in Vivo Cancer Nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Zhang, Y.; García-Gabilondo, M.; Rosell, A.; Roig, A. MRI/Photoluminescence Dual-Modal Imaging Magnetic PLGA Nanocapsules for Theranostics. Pharmaceutics 2019, 12, 16. [Google Scholar] [CrossRef]
- Ferreira-Filho, V.C.; Morais, B.; Vieira, B.J.C.; Waerenborgh, J.C.; Carmezim, M.J.; Tóth, C.N.; Même, S.; Lacerda, S.; Jaque, D.; Sousa, C.T.; et al. Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile. Molecules 2024, 29, 1824. [Google Scholar] [CrossRef]
- Davarnejad, R.; Barati, S.; Kooshki, M. CFD Simulation of the Effect of Particle Size on the Nanofluids Convective Heat Transfer in the Developed Region in a Circular Tube. Springerplus 2013, 2, 192. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Sas, W.; Jasiurkowska-Delaporte, M.; Czaja, P.; Zieliński, P.M.; Fitta, M. Magnetic Properties Study of Iron Oxide Nanoparticles-Loaded Poly(ε-Caprolactone) Nanofibres. Magnetochemistry 2021, 7, 61. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Jafarzadeh, H.; Ramezani, M. Preparation and Characterization of Uniform-Sized PLGA Nanospheres Encapsulated with Oleic Acid-Coated Magnetic-Fe3O4 Nanoparticles for Simultaneous Diagnostic and Therapeutic Applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 146–154. [Google Scholar] [CrossRef]
- Shim, H.; Sah, H. Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry. Pharmaceutics 2020, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Mokhtarzadeh, A.; Ramezani, M. In Vitro and in Vivo Evaluation of Anti-Nucleolin-Targeted Magnetic PLGA Nanoparticles Loaded with Doxorubicin as a Theranostic Agent for Enhanced Targeted Cancer Imaging and Therapy. Eur. J. Pharm. Biopharm. 2017, 113, 60–74. [Google Scholar] [CrossRef]
- Kini, S.; Bahadur, D.; Panda, D. Magnetic PLGA Nanospheres: A Dual Therapy for Cancer. IEEE Trans. Magn. 2011, 47, 2882–2886. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Maity, D.; Yong, C.X.; Chuang, K.-H.; Ding, J.; Feng, S.-S. Vitamin E (d-Alpha-Tocopheryl-Co-Poly(Ethylene Glycol) 1000 Succinate) Micelles-Superparamagnetic Iron Oxide Nanoparticles for Enhanced Thermotherapy and MRI. Biomaterials 2011, 32, 5663–5672. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The Effect of Thermotherapy Using Magnetic Nanoparticles on Rat Malignant Glioma. J. Neurooncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Rego, G.N.D.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Silva, H.R.D.; Gamarra, L.F. Therapeutic Evaluation of Magnetic Hyperthermia Using Fe3O4-Aminosilane-Coated Iron Oxide Nanoparticles in Glioblastoma Animal Model. Einstein (São Paulo) 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy Using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
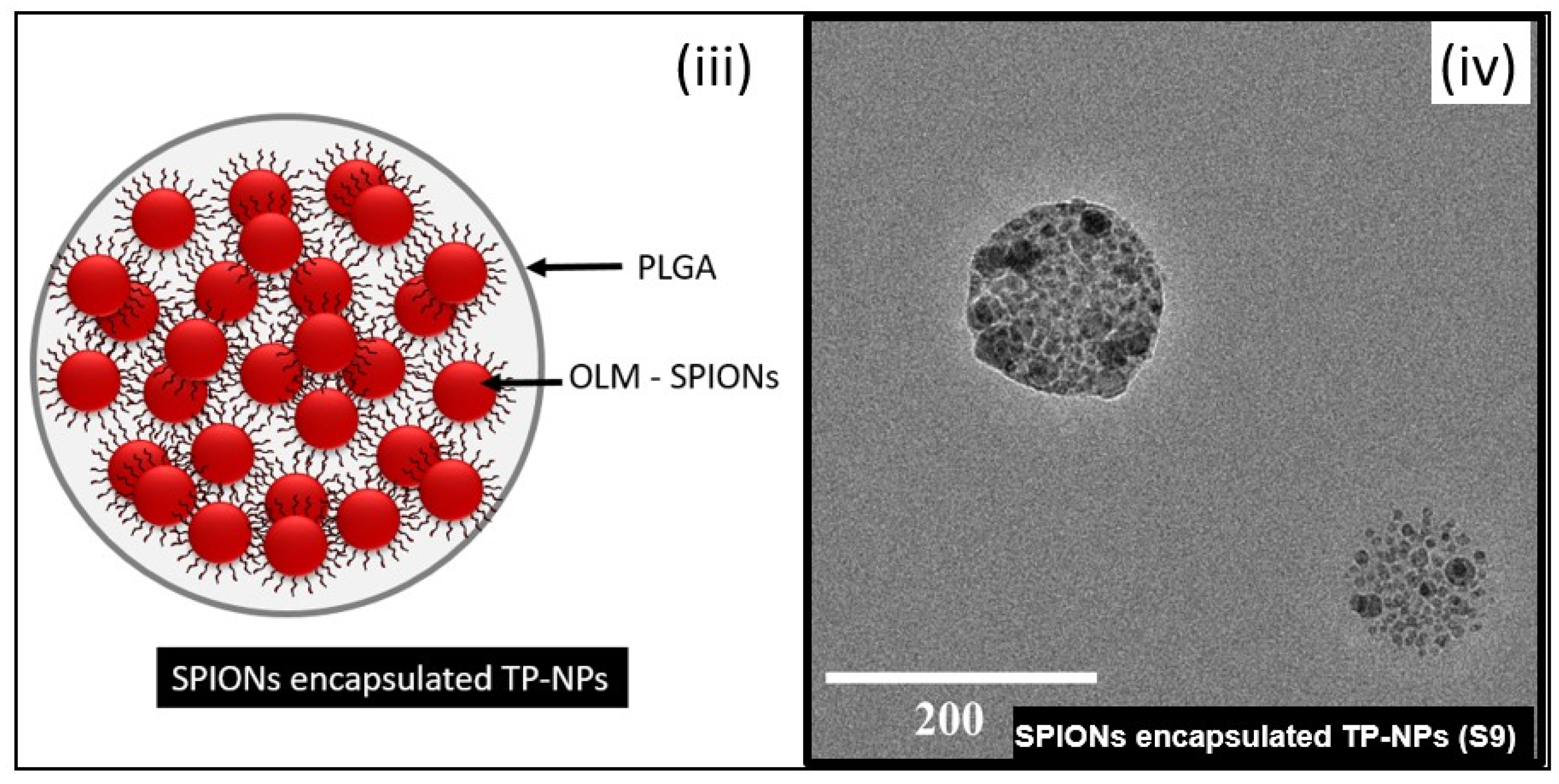
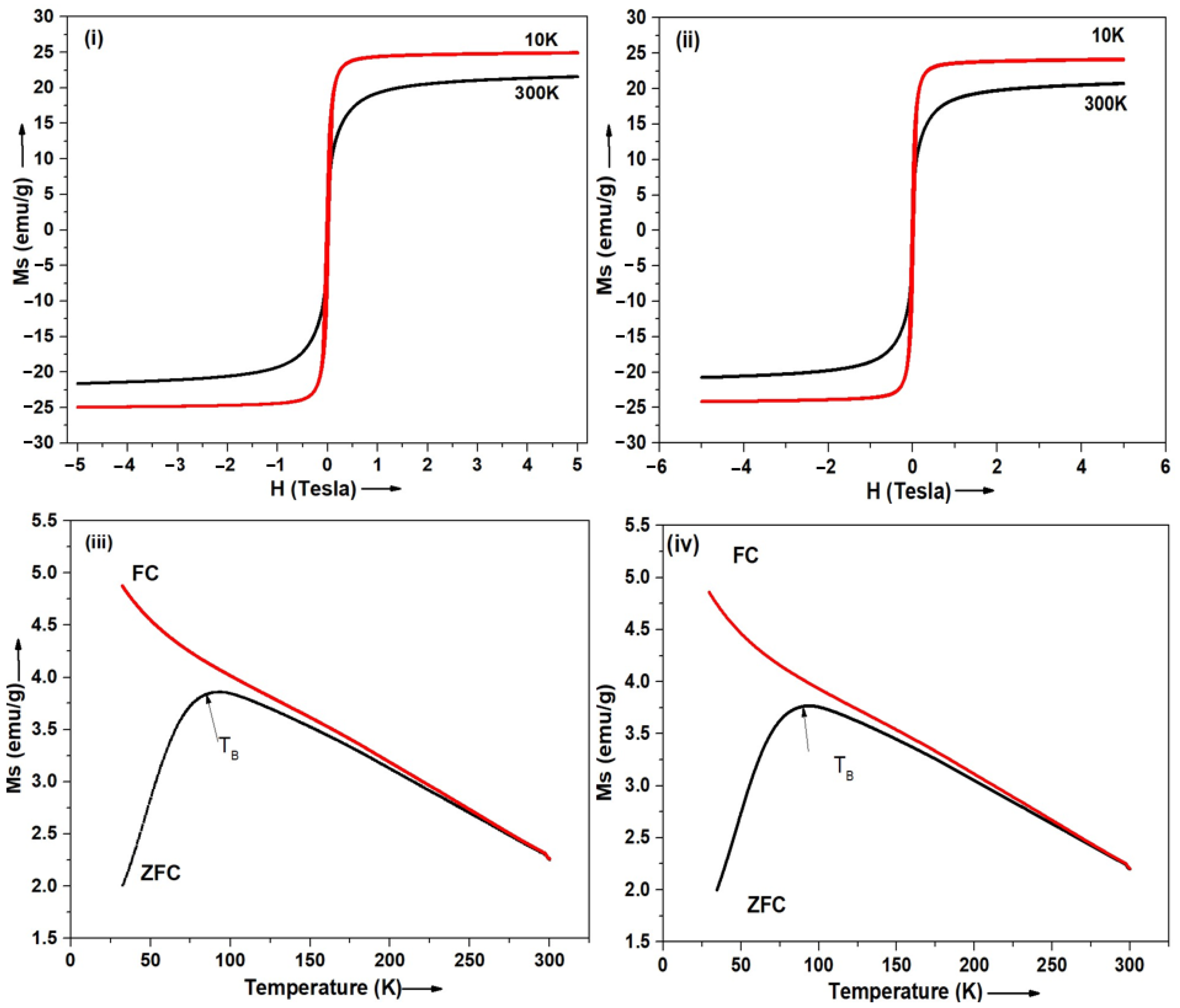
| Sr. No. | Samples | Sample Code | Ms (emu/g) | Blocking Temperature (TB) | |
|---|---|---|---|---|---|
| At 10 K | At 300 K | ||||
| 1 | OLM-SPIONs | - | 66.6 * | 59.1 * | 100 K |
| 2 | SPION-encapsulated TPS-NPs (0.5 Wt % TPGS) | S9 | 25.09 ± 0.07 | 21.66 ± 0.03 | 86.16 K |
| 3 | SPION-encapsulated TPS-NPs (1 Wt % TPGS) | S13 | 24.13 ± 0.06 | 20.64 ± 0.02 | 90.25 K |
| Sr. No. | Sample Code | OLM-SPIONs Concentration (mgFe) | PLGA (mg) | TPGS % (w/v) | Time (mins) | Temperature (°C) | ∆T (°C) | SAR (W/gFe) | ILP (nHm2kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | S9 | 3 mgFe | 10 | 0.5 | 39.1 | 42.0 | ~12 | 48.8 ± 2.3 ± 2.3 ± 2.3 ± 2.3 | 1.39 ± 0.07 |
| S13 | 10 | 1 | 49.8 | 42.0 | 46.1 ± 2.1 | 1.31 ± 0.06 | |||
| 2 | S9 | 2 mgFe | 10 | 0.5 | 60 | 41.5 | ~10 | - | - |
| S13 | 10 | 1 | 60 | 41.2 | - | - | |||
| 3 | S9 | 1 mgFe | 10 | 0.5 | 60 | 40.7 | ~10 | - | - |
| S13 | 10 | 1 | 60 | 39.8 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudame, A.; Maity, D. Unlocking Superior MFH Performance Below Hergt’s Biological Safety Limit: SPION-Based Magnetic Nanoplatforms Deliver High Heating Efficiency at Low AMF. Bioengineering 2025, 12, 715. https://doi.org/10.3390/bioengineering12070715
Sudame A, Maity D. Unlocking Superior MFH Performance Below Hergt’s Biological Safety Limit: SPION-Based Magnetic Nanoplatforms Deliver High Heating Efficiency at Low AMF. Bioengineering. 2025; 12(7):715. https://doi.org/10.3390/bioengineering12070715
Chicago/Turabian StyleSudame, Atul, and Dipak Maity. 2025. "Unlocking Superior MFH Performance Below Hergt’s Biological Safety Limit: SPION-Based Magnetic Nanoplatforms Deliver High Heating Efficiency at Low AMF" Bioengineering 12, no. 7: 715. https://doi.org/10.3390/bioengineering12070715
APA StyleSudame, A., & Maity, D. (2025). Unlocking Superior MFH Performance Below Hergt’s Biological Safety Limit: SPION-Based Magnetic Nanoplatforms Deliver High Heating Efficiency at Low AMF. Bioengineering, 12(7), 715. https://doi.org/10.3390/bioengineering12070715






