OCT in Oncology and Precision Medicine: From Nanoparticles to Advanced Technologies and AI
Abstract
1. Introduction and Background
2. Basics of OCT Imaging in Pathology
2.1. OCT’s Role in Pathological Diagnosis
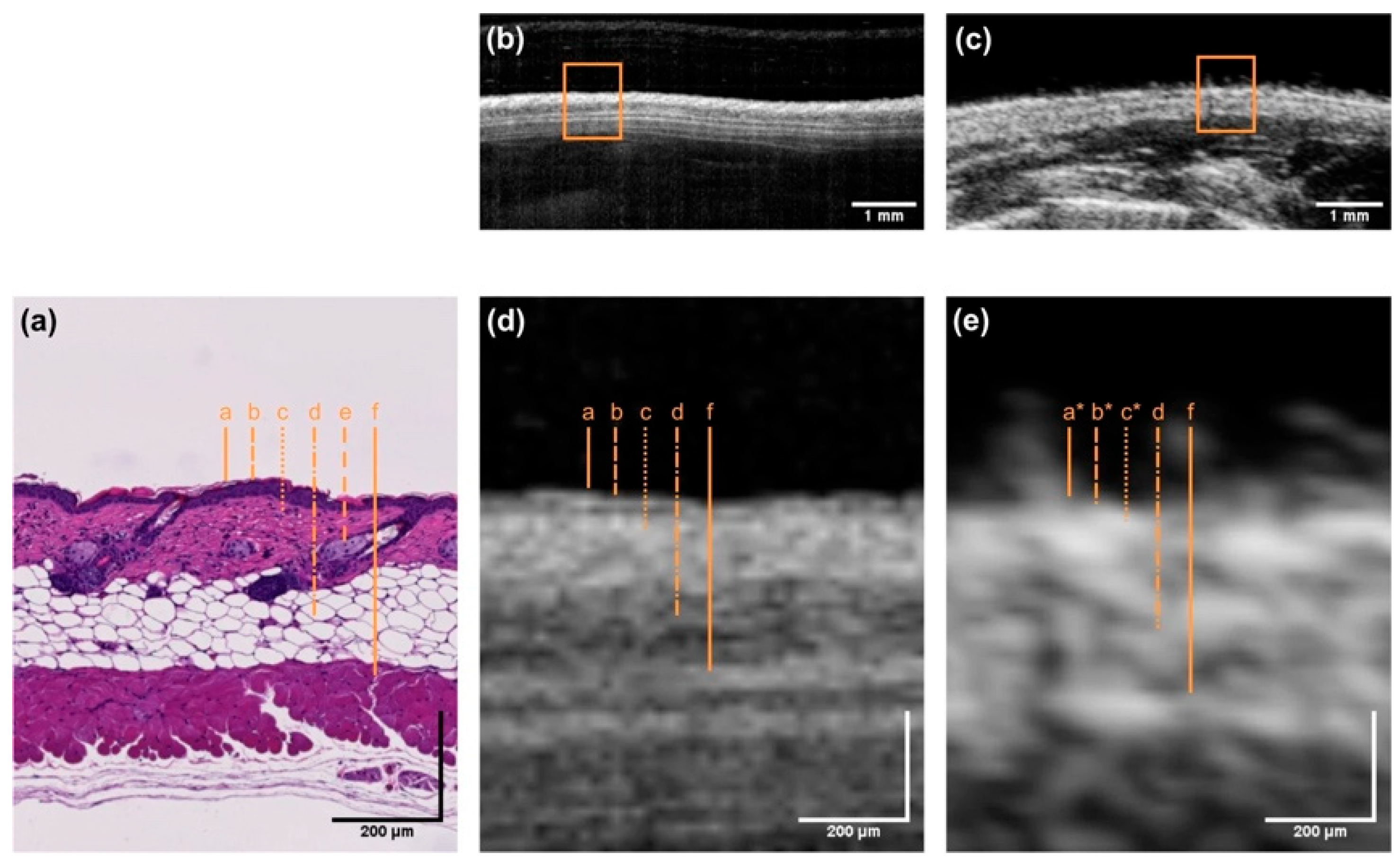
2.2. OCT for Tissue Characterization and Guiding Biopsies
2.3. Clinical Applications of OCT
3. OCT in Oncology: Tumor Markers, Personalized Medicine, and Real-Time Treatment
3.1. The Role of OCT in Tumor Detection and Tumor Microenvironment Analysis
3.2. OCT-Guided Personalized Cancer Treatment
3.3. Key Limitations of OCT in Oncology
4. Nanoparticles in OCT Imaging: Enhancing Diagnostic and Therapeutic Capabilities
4.1. The Role of Nanoparticles in OCT Contrast Enhancement
4.2. Types of Nanoparticles for OCT Applications
4.3. Tumor-Targeting Nanoparticles in OCT Imaging for Personalized Medicine
5. Future Directions in OCT Imaging for Pathology and Oncology
5.1. Overcoming OCT Limitations with Nanotechnology
5.2. Multimodal Imaging: Integrating OCT with MRI, PET, and Ultrasound
5.3. Personalized Medicine: Combination of AI and OCT-Guided Precision Oncology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1979, 254, 1178–1181. [Google Scholar] [CrossRef]
- Swanson, E.A.; Izatt, J.A.; Lin, C.P.; Fujimoto, J.G.; Schuman, J.S.; Hee, M.R.; Huang, D.; Puliafito, C.A. In vivo retinal imaging by optical coherence tomography. Opt. Lett. 1993, 18, 1864. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F.; Hitzenberger, C.K.; Drexler, W.; Kamp, G.; Sattmann, H. In Vivo Optical Coherence Tomography. Am. J. Ophthalmol. 1993, 116, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Sakata, L.M.; DeLeon-Ortega, J.; Sakata, V.; Girkin, C.A. Optical coherence tomography of the retina and optic nerve—A review. Clin. Exp. Ophthalmol. 2009, 37, 90–99. [Google Scholar] [CrossRef]
- Mokhtari, A.; Maris, B.M.; Fiorini, P. A Survey on Optical Coherence Tomography—Technology and Application. Bioengineering 2025, 12, 65. [Google Scholar] [CrossRef]
- Pizurica, A.; Jovanov, L.; Huysmans, B.; Zlokolica, V.; De Keyser, P.; Dhaenens, F.; Philips, W. Multiresolution Denoising for Optical Coherence Tomography: A Review and Evaluation. Curr. Med. Imaging Rev. 2008, 4, 270–284. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Swanson, E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2016, 57, OCT1–OCT13. [Google Scholar] [CrossRef]
- Fercher, A.F.; Hitzenberger, C.K.; Kamp, G.; El-Zaiat, S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995, 117, 43–48. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Leitgeb, R.; Kowalczyk, A.; Bajraszewski, T.; Fercher, A.F. In vivo human retinal imaging by Fourier domain optical coherence tomography. J. Biomed. Opt. 2002, 7, 457. [Google Scholar] [CrossRef]
- Choma, M.; Sarunic, M.; Yang, C.; Izatt, J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007. [Google Scholar] [CrossRef] [PubMed]
- Manapuram, R.K.; Manne, V.G.R.; Larin, K.V. Development of phase-stabilized swept-source OCT for the ultrasensitive quantification of microbubbles. Laser Phys. 2008, 18, 1080–1086. [Google Scholar] [CrossRef]
- Caujolle, S.; Cernat, R.; Silvestri, G.; Marques, M.J.; Bradu, A.; Feuchter, T.; Robinson, G.; Griffin, D.K.; Podoleanu, A. Speckle variance OCT for depth resolved assessment of the viability of bovine embryos. Biomed. Opt. Express 2017, 8, 5139. [Google Scholar] [CrossRef]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.; Patalay, R.; Lynch, M.D. Applications and future directions for optical coherence tomography in dermatology*. Br. J. Dermatol. 2021, 184, 1014–1022. [Google Scholar] [CrossRef]
- Schuh, S.; Holmes, J.; Ulrich, M.; Themstrup, L.; Jemec, G.B.E.; De Carvalho, N.; Pellacani, G.; Welzel, J. Imaging Blood Vessel Morphology in Skin: Dynamic Optical Coherence Tomography as a Novel Potential Diagnostic Tool in Dermatology. Dermatol. Ther. 2017, 7, 187–202. [Google Scholar] [CrossRef]
- Maltais-Tariant, R.; Becerra-Deana, R.I.; Brais-Brunet, S.; Dehaes, M.; Boudoux, C. Speckle contrast reduction through the use of a modally-specific photonic lantern for optical coherence tomography. Biomed. Opt. Express 2023, 14, 6250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ling, Y.; Mao, J.; Su, Y. Robust and automated dispersion compensation for FD-OCT using fractional Fourier transform. In Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXVIII; Izatt, J.A., Fujimoto, J.G., Eds.; SPIE: Bellingham, WA, USA, 2024; p. 85. [Google Scholar] [CrossRef]
- Avanaki, M.R.N.; Marques, M.J.; Bradu, A.; Hojjatoleslami, A.; Podoleanu, A.G. A New Algorithm for Speckle Reduction of Optical Coherence Tomography Images; Izatt, J.A., Fujimoto, J.G., Tuchin, V.V., Eds.; SPIE BiOS: San Francisco, CA, USA, 2014; p. 893437. [Google Scholar] [CrossRef]
- Lotz, S.; Göb, M.; Böttger, S.; Ha-Wissel, L.; Hundt, J.; Ernst, F.; Huber, R. Large area robotically assisted optical coherence tomography (LARA-OCT). Biomed. Opt. Express 2024, 15, 3993. [Google Scholar] [CrossRef]
- Medina, T.P.; Kolb, J.P.; Hüttmann, G.; Huber, R.; Medina, O.P.; Ha, L.; Ulloa, P.; Larsen, N.; Ferrari, A.; Rafecas, M.; et al. Imaging Inflammation—From Whole Body Imaging to Cellular Resolution. Front. Immunol. 2021, 12, 692222. [Google Scholar] [CrossRef]
- McNamara, M.; Subhash, H.M.; Leahy, M.J. In vivo full-field en face correlation mapping optical coherence tomography. J. Biomed. Opt. 2013, 18, 126008. [Google Scholar] [CrossRef]
- Brown, W.J.; Kim, S.; Wax, A. Noise characterization of supercontinuum sources for low-coherence interferometry applications. J. Opt. Soc. Am. A 2014, 31, 2703. [Google Scholar] [CrossRef]
- Angmo, D.; Nongpiur, M.; Sharma, R.; Sidhu, T.; Sihota, R.; Dada, T. Clinical utility of anterior segment swept-source optical coherence tomography in glaucoma. Oman J. Ophthalmol. 2016, 9, 3–10. [Google Scholar] [CrossRef]
- Srinivasan, V.J.; Adler, D.C.; Chen, Y.; Gorczynska, I.; Huber, R.; Duker, J.S.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-Speed Optical Coherence Tomography for Three-Dimensional and En Face Imaging of the Retina and Optic Nerve Head. Investig. Opthalmology Vis. Sci. 2008, 49, 5103. [Google Scholar] [CrossRef]
- del Río-Sancho, S.; Gallay, C.; Ventéjou, S.; Christen-Zaech, S. In vivo evaluation of skin of children with LC-OCT: An objective assessment. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Rivero, F.; Torres, R.A.; Ramírez, H.L.; Rodríguez, E.M.; Alfonso, F.; Solé, J.G.; Jaque, D. Dynamic single gold nanoparticle visualization by clinical intracoronary optical coherence tomography. J. Biophotonics 2017, 10, 674–682. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Adie, S.G.; Chaney, E.J.; Marjanovic, M.; Tangella, K.V.; Boppart, S.A. Three-Dimensional Optical Coherence Tomography for Optical Biopsy of Lymph Nodes and Assessment of Metastatic Disease. Ann. Surg. Oncol. 2013, 20, 3685–3693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Xu, Y.; Boppart, S.A. Review of optical coherence tomography in oncology. J. Biomed. Opt. 2017, 22, 121711. [Google Scholar] [CrossRef]
- Jabbar, A.; Khalid, R.; Cabrera, H.; Mahmood, N.; Mehmood, M.Q. Revolutionizing endoscopy: Non-invasive high-resolution imaging with time-domain optical coherence tomography. In Unconventional Optical Imaging IV; Georges, M., Verrier, N., Georgakoudi, I., Eds.; SPIE: Bellingham, WA, USA, 2024; p. 77. [Google Scholar] [CrossRef]
- Holm, K.B.E.; Nielsen, L.J.; Lock-Andersen, J.; Behrendt, N.; Svensson, M.S.; Themstrup, L.; Jemec, G.B.E. Optical coherence tomography for presurgical delineation of basal cell carcinomas on the face—A comparison with histopathology. J. Cutan. Pathol. 2023, 50, 441–449. [Google Scholar] [CrossRef]
- Schuetzenberger, K.; Pfister, M.; Messner, A.; Froehlich, V.; Garhoefer, G.; Hohenadl, C.; Schmetterer, L.; Werkmeister, R.M. Comparison of optical coherence tomography and high frequency ultrasound imaging in mice for the assessment of skin morphology and intradermal volumes. Sci. Rep. 2019, 9, 13643. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Grover, S.; Dominguez, J.M., II; Balaiya, S.; Chalam, K.V. Retinal Thickness Measurement Obtained with Spectral Domain Optical Coherence Tomography Assisted Optical Biopsy Accurately Correlates with Ex Vivo Histology. PLoS ONE 2014, 9, e111203. [Google Scholar] [CrossRef]
- RMcLaughlin, A.; Scolaro, L.; Robbins; Hamza, S.; Saunders, C.; Sampson, D.D. Imaging of Human Lymph Nodes Using Optical Coherence Tomography: Potential for Staging Cancer. Cancer Res. 2010, 70, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.P.; Agarwal, S.; James, B.L.; Heidari, E.; Muralidharan, A.; Yadav, V.; Pillai, V.; Shetty, V.; Chen, Z.; Hedne, N.; et al. Intra-operative point-of-procedure delineation of oral cancer margins using optical coherence tomography. Oral. Oncol. 2019, 92, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Steen, D.W. Limitations in imaging common conjunctival and corneal pathologies with Fourier-domain optical coherence tomography. Middle East. Afr. J. Ophthalmol. 2014, 21, 220–224. [Google Scholar] [CrossRef]
- Linehan, J.A.; Bracamonte, E.R.; Hariri, L.P.; Sokoloff, M.H.; Rice, P.S.; Barton, J.K.; Nguyen, M.M. Feasibility of optical coherence tomography imaging to characterize renal neoplasms: Limitations in resolution and depth of penetration. BJU Int. 2011, 108, 1820–1824. [Google Scholar] [CrossRef]
- Wang, H.-W.; Chen, Y. Clinical applications of optical coherence tomography in urology. Intravital 2014, 3, e28770. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Leggett, C.L.; Trindade, A.J. Optical coherence tomography in gastroenterology: A review and future outlook. J. Biomed. Opt. 2017, 22, 121716. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, H.; Liang, Z.; Zhao, W.; Zhu, M.; Xu, Y.; Guo, M.; Jia, Y.; Zou, C.; Yang, Z.; et al. Novel image features of optical coherence tomography for pathological classification of lung cancer: Results from a prospective clinical trial. Front. Oncol. 2022, 12, 870556. [Google Scholar] [CrossRef]
- Sun, J.G.; Adie, S.G.; Chaney, E.J.; Boppart, S.A. Segmentation and correlation of optical coherence tomography and X-ray images for breast cancer diagnostics. J. Innov. Opt. Health Sci. 2013, 06, 1350015. [Google Scholar] [CrossRef]
- Gladkova, N.; Streltsova, O.; Zagaynova, E.; Kiseleva, E.; Gelikonov, V.; Gelikonov, G.; Karabut, M.; Yunusova, K.; Evdokimova, O. Cross-polarization optical coherence tomography for early bladder-cancer detection: Statistical study. J. Biophotonics 2011, 4, 519–532. [Google Scholar] [CrossRef]
- Schlegl, T.; Waldstein, S.M.; Bogunovic, H.; Endstraßer, F.; Sadeghipour, A.; Philip, A.-M.; Podkowinski, D.; Gerendas, B.S.; Langs, G.; Schmidt-Erfurth, U. Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology 2018, 125, 549–558. [Google Scholar] [CrossRef]
- Yeu, E.; Berdahl, J.; Gupta, K.; Patterson, M. Sensitivity and specificity of SS-OCT for detecting macular pathologies vs SD-OCT. J. Cataract. Refract. Surg. 2024, 50, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ye, S.; Liu, L.; Wu, M. Impact of lens opacity and axial length on concomitant screening of maculopathy by swept-source optical coherence tomography-based optical biometer. Ann. Transl. Med. 2022, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, F.; Jampol, L.M.; Linsenmeier, R.A.; Fawzi, A.A. Association of Diabetic Macular Nonperfusion with Outer Retinal Disruption on Optical Coherence Tomography. JAMA Ophthalmol. 2015, 133, 1036–1044. [Google Scholar] [CrossRef]
- Ulrich, M.; Themstrup, L.; de Carvalho, N.; Manfredi, M.; Grana, C.; Ciardo, S.; Kästle, R.; Holmes, J.; Whitehead, R.; Jemec, G.B.; et al. Dynamic Optical Coherence Tomography in Dermatology. Dermatology 2016, 232, 298–311. [Google Scholar] [CrossRef]
- González, J.J.V.; Berger, M.; Schiele, S.; Rubeck, A.; Müller, G.; Welzel, J.; Schuh, S. Dynamic optical coherence tomography of chronic venous ulcers. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Won, J.; Monroy, G.L.; Dsouza, R.I.; Spillman, D.R.; McJunkin, J.; Porter, R.G.; Shi, J.; Aksamitiene, E.; Sherwood, M.; Stiger, L.; et al. Handheld Briefcase Optical Coherence Tomography with Real-Time Machine Learning Classifier for Middle Ear Infections. Biosensors 2021, 11, 143. [Google Scholar] [CrossRef]
- Song, G.; Jelly, E.T.; Chu, K.K.; Kendall, W.Y.; Wax, A. A review of low-cost and portable optical coherence tomography. Prog. Biomed. Eng. 2021, 3, 032002. [Google Scholar] [CrossRef]
- Wijesinghe, R.E.; Lee, S.-Y.; Ravichandran, N.K.; Han, S.; Jeong, H.; Han, Y.; Jung, H.-Y.; Kim, P.; Jeon, M.; Kim, J. Optical coherence tomography-integrated, wearable (backpack-type), compact diagnostic imaging modality for in situ leaf quality assessment. Appl. Opt. 2017, 56, D108. [Google Scholar] [CrossRef]
- Xin, N.; Zhong, H.; Zhang, X.; Li, Q. Portable wide-filed OCT/OCTA for retinal imaging. In Optics in Health Care and Biomedical Optics XIII; Luo, Q., Li, X., Gu, Y., Zhu, D., Eds.; SPIE: Bellingham, WA, USA, 2023; p. 93. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Ling, S.; Wang, J.; Wang, G.; Zhang, B.; Zhao, H.; Zhao, Q.; Mao, J. Research progress on the application of optical coherence tomography in the field of oncology. Front. Oncol. 2022, 12, 953934. [Google Scholar] [CrossRef]
- Başkan, C.; Kılıcarslan, A. How Can We Diagnose Ocular Surface Squamous Neoplasia with Optical Coherence Tomography? Cureus 2023, 15, e36320. [Google Scholar] [CrossRef] [PubMed]
- Aboumourad, R.J.; Galor, A.; Karp, C.L. Case Series: High-Resolution Optical Coherence Tomography as an Optical Biopsy in Ocular Surface Squamous Neoplasia. Optom. Vision. Sci. 2021, 98, 450–455. [Google Scholar] [CrossRef]
- South, F.A.; Chaney, E.J.; Marjanovic, M.; Adie, S.G.; Boppart, S.A. Differentiation of ex vivo human breast tissue using polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2014, 5, 3417. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Mesa, K.J.; South, F.A.; Chaney, E.J.; Spillman, D.R.; Barkalifa, R.; Marjanovic, M.; Carney, P.S.; Higham, A.M.; et al. Complementary use of polarization-sensitive and standard OCT metrics for enhanced intraoperative differentiation of breast cancer. Biomed. Opt. Express 2018, 9, 6519. [Google Scholar] [CrossRef] [PubMed]
- Gubarkova, E.V.; Kiseleva, E.B.; Sirotkina, M.A.; Vorontsov, D.A.; Achkasova, K.A.; Kuznetsov, S.S.; Yashin, K.S.; Matveyev, A.L.; Sovetsky, A.A.; Matveev, L.A.; et al. Diagnostic Accuracy of Cross-Polarization OCT and OCT-Elastography for Differentiation of Breast Cancer Subtypes: Comparative Study. Diagnostics 2020, 10, 994. [Google Scholar] [CrossRef]
- Hariri, L.; Mino-Kenudson, M.; Lanuti, M.; Miller, A.J.; Mark, E.J.; Suter, M.J. Diagnosing Lung Carcinomas with Optical Coherence Tomography. Ann. Am. Thorac. Soc. 2015, 12, 193–201. [Google Scholar] [CrossRef]
- van Manen, L.; Dijkstra, J.; Boccara, C.; Benoit, E.; Vahrmeijer, A.L.; Gora, M.J.; Mieog, J.S.D. The clinical usefulness of optical coherence tomography during cancer interventions. J. Cancer Res. Clin. Oncol. 2018, 144, 1967–1990. [Google Scholar] [CrossRef]
- Gallwas, J.; Mortensen, U.; Gaschler, R.; Ochsenkuehn, R.; Stepp, H.; Friese, K.; Dannecker, C. Validation of an ex vivo human cervical tissue model for optical imaging studies. Lasers Surg. Med. 2012, 44, 245–248. [Google Scholar] [CrossRef]
- Ren, C.; Zeng, X.; Shi, Z.; Wang, C.; Wang, H.; Wang, X.; Zhang, B.; Jiang, Z.; Ma, H.; Hu, H.; et al. Multi-center clinical study using optical coherence tomography for evaluation of cervical lesions in-vivo. Sci. Rep. 2021, 11, 7507. [Google Scholar] [CrossRef]
- Gallwas, J.; Jalilova, A.; Ladurner, R.; Kolben, T.M.; Kolben, T.; Ditsch, N.; Homann, C.; Lankenau, E.; Dannecker, C. Detection of cervical intraepithelial neoplasia by using optical coherence tomography in combination with microscopy. J. Biomed. Opt. 2017, 22, 016013. [Google Scholar] [CrossRef]
- Law, T.S.M.; Cheung, W.C.; Wu, F.; Zhang, R.; Chung, J.P.W.; Wang, C.C.; Chen, X.; Li, T.C. Endometrial Vascularization Characterized by Optical Coherence Tomography and Immunohistochemistry in Women Undergoing In Vitro Fertilization-Embryo Transfer Treatment. Medicina 2019, 55, 81. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, A.A.; Grechkanev, G.O.; Avetisyan, E.A.; Loginova, M.M.; Kiseleva, E.B.; Shepeleva, A.A.; Moiseev, A.A.; Sovetsky, A.A.; Gubarkova, E.V.; Anina, A.A.; et al. Quantitative Assessment of Polarization and Elastic Properties of Endometrial Tissue for Precancer/Cancer Diagnostics Using Multimodal Optical Coherence Tomography. Diagnostics 2024, 14, 2131. [Google Scholar] [CrossRef] [PubMed]
- Assayag, O.; Grieve, K.; Devaux, B.; Harms, F.; Pallud, J.; Chretien, F.; Boccara, C.; Varlet, P. Imaging of non-tumorous and tumorous human brain tissues with full-field optical coherence tomography. Neuroimage Clin. 2013, 2, 549–557. [Google Scholar] [CrossRef]
- Muller, B.G.; Swaan, A.; de Bruin, D.M.; van den Bos, W.; Schreurs, A.W.; Faber, D.J.; Zwartkruis, E.C.H.; Rozendaal, L.; Vis, A.N.; Nieuwenhuijzen, J.A.; et al. Customized Tool for the Validation of Optical Coherence Tomography in Differentiation of Prostate Cancer. Technol. Cancer Res. Treat. 2017, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Freund, J.E.; Buijs, M.; Savci-Heijink, C.D.; de Bruin, D.M.; de la Rosette, J.J.M.C.H.; van Leeuwen, T.G.; Laguna, M.P. Optical Coherence Tomography in Urologic Oncology: A Comprehensive Review. SN Compr. Clin. Med. 2019, 1, 67–84. [Google Scholar] [CrossRef]
- Takae, S.; Tsukada, K.; Sato, Y.; Okamoto, N.; Kawahara, T.; Suzuki, N. Accuracy and safety verification of ovarian reserve assessment technique for ovarian tissue transplantation using optical coherence tomography in mice ovary. Sci. Rep. 2017, 7, 43550. [Google Scholar] [CrossRef]
- Peters, I.T.A.; Stegehuis, P.L.; Peek, R.; Boer, F.L.; van Zwet, E.W.; Eggermont, J.; Westphal, J.R.; Kuppen, P.J.; Trimbos, J.B.; Hilders, C.G.; et al. Noninvasive Detection of Metastases and Follicle Density in Ovarian Tissue Using Full-Field Optical Coherence Tomography. Clin. Cancer Res. 2016, 22, 5506–5513. [Google Scholar] [CrossRef]
- Schwartz, D.; Sawyer, T.W.; Thurston, N.; Barton, J.; Ditzler, G. Ovarian cancer detection using optical coherence tomography and convolutional neural networks. Neural Comput. Appl. 2022, 34, 8977–8987. [Google Scholar] [CrossRef]
- Roy, S.; Dukic, T.; Keepers, Z.; Bhandary, B.; Lamichhane, N.; Molitoris, J.; Ko, Y.H.; Banerjee, A.; Shukla, H.D. SOX2 and OCT4 mediate radiation and drug resistance in pancreatic tumor organoids. Cell Death Discov. 2024, 10, 106. [Google Scholar] [CrossRef]
- Perwein, M.K.E.; Welzel, J.; De Carvalho, N.; Pellacani, G.; Schuh, S. Dynamic Optical Coherence Tomography: A Non-Invasive Imaging Tool for the Distinction of Nevi and Melanomas. Cancers 2022, 15, 20. [Google Scholar] [CrossRef]
- Cappilli, S.; Paradisi, A.; Di Stefani, A.; Palmisano, G.; Pellegrino, L.; D’onghia, M.; Ricci, C.; Tognetti, L.; Verzì, A.E.; Rubegni, P.; et al. Line-Field Confocal Optical Coherence Tomography: A New Skin Imaging Technique Reproducing a ‘Virtual Biopsy’ with Evolving Clinical Applications in Dermatology. Diagnostics 2024, 14, 1821. [Google Scholar] [CrossRef] [PubMed]
- Yarovaya, V.; Sioufi, K.; Shields, C.L. Parafoveolar retinoblastoma regression with foveal preservation following intra-arterial chemotherapy documented on hand-held optical coherence tomography in a newborn. Int. J. Retin. Vitr. 2017, 3, 43. [Google Scholar] [CrossRef]
- Zysk, A.M.; Chen, K.; Gabrielson, E.; Tafra, L.; Gonzalez, E.A.M.; Canner, J.K.; Schneider, E.B.; Cittadine, A.J.; Carney, P.S.; Boppart, S.A.; et al. Intraoperative Assessment of Final Margins with a Handheld Optical Imaging Probe During Breast-Conserving Surgery Reduce the Reoperation Rate: Results of a Multicenter Study. Ann. Surg. Oncol. 2015, 22, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.P.; Ni, S.; Khan, S.; Wei, X.; Ostmo, S.; Chiang, M.F.; Jia, Y.; Huang, D.; Jian, Y.; Campbell, J.P. Advantages of Widefield Optical Coherence Tomography in the Diagnosis of Retinopathy of Prematurity. Front. Pediatr. 2022, 9, 797684. [Google Scholar] [CrossRef]
- Say, E.A.T.; Shah, S.U.; Ferenczy, S.; Shields, C.L. Optical Coherence Tomography of Retinal and Choroidal Tumors. J. Ophthalmol. 2011, 2011, 385058. [Google Scholar] [CrossRef][Green Version]
- Karl, A.; Stepp, H.; Willmann, E.; Buchner, A.; Hocaoglu, Y.; Stief, C.; Tritschler, S. Optical coherence tomography for bladder cancer—Ready as a surrogate for optical biopsy?—Results of a prospective mono-centre study. Eur. J. Med. Res. 2010, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tsai, T.-H.; Adler, D.C.; Lee, H.-C.; Cohen, D.W.; Mondelblatt, A.; Wang, Y.; Connolly, J.L.; Fujimoto, J.G. Photothermal optical coherence tomography in ex vivo human breast tissues using gold nanoshells. Opt. Lett. 2010, 35, 700. [Google Scholar] [CrossRef]
- Nguyen, V.; Qian, W.; Li, Y.; Liu, B.; Aaberg, M.; Henry, J.; Zhang, W.; Wang, X.; Paulus, Y.M. Chain-like gold nanoparticle clusters for multimodal photoacoustic microscopy and optical coherence tomography enhanced molecular imaging. Nat. Commun. 2021, 12, 34. [Google Scholar] [CrossRef]
- Kirillin, M.Y.; A Sergeeva, E.; Agrba, P.D.; Krainov, A.D.; A Ezhov, A.; Shuleiko, D.V.; Kashkarov, P.K.; Zabotnov, S.V. Laser-ablated silicon nanoparticles: Optical properties and perspectives in optical coherence tomography. Laser Phys. 2015, 25, 075604. [Google Scholar] [CrossRef]
- Si, P.; Yuan, E.; Liba, O.; Winetraub, Y.; Yousefi, S.; SoRelle, E.D.; Yecies, D.W.; Dutta, R.; de la Zerda, A. Gold Nanoprisms as Optical Coherence Tomography Contrast Agents in the Second Near-Infrared Window for Enhanced Angiography in Live Animals. ACS Nano 2018, 12, 11986–11994. [Google Scholar] [CrossRef]
- Tucker-Schwartz, J.M.; Beavers, K.R.; Sit, W.W.; Shah, A.T.; Duvall, C.L.; Skala, M.C. In vivo imaging of nanoparticle delivery and tumor microvasculature with multimodal optical coherence tomography. Biomed. Opt. Express 2014, 5, 1731. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Lifante, J.; Besteiro, L.V.; Wang, Z.; Govorov, A.O.; Rivero, F.; Alfonso, F.; Sanz-Rodríguez, F.; Jaque, D. Plasmonic Copper Sulfide Nanoparticles Enable Dark Contrast in Optical Coherence Tomography. Adv. Healthc. Mater. 2020, 9, e1901627. [Google Scholar] [CrossRef]
- Tang, P.; Jiang, X.; Wang, Y.; Chen, H.; Zhang, Y.S.; Gao, P.; Wang, H.; Li, X.; Zhou, J. Plasmonic Nanoprobe of (Gold Triangular Nanoprism Core)/(Polyaniline Shell) for Real-Time Three-Dimensional pH Imaging of Anterior Chamber. Anal. Chem. 2017, 89, 9758–9766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Wang, L.; Li, Z.; Yuan, Z. Retroreflective-type Janus microspheres as a novel contrast agent for enhanced optical coherence tomography. J. Biophotonics 2017, 10, 878–886. [Google Scholar] [CrossRef]
- Liba, O.; SoRelle, E.D.; Sen, D.; de la Zerda, A. Contrast-enhanced optical coherence tomography with picomolar sensitivity for functional in vivo imaging. Sci. Rep. 2016, 6, 23337. [Google Scholar] [CrossRef] [PubMed]
- Lapierre-Landry, M.; Gordon, A.Y.; Penn, J.S.; Skala, M.C. In vivo photothermal optical coherence tomography of endogenous and exogenous contrast agents in the eye. Sci. Rep. 2017, 7, 9228. [Google Scholar] [CrossRef]
- Hu, J.; Gorsak, T.; Rodríguez, E.M.; Calle, D.; Muñoz-Ortiz, T.; Jaque, D.; Fernández, N.; Cussó, L.; Rivero, F.; Torres, R.A.; et al. Magnetic Nanoplatelets for High Contrast Cardiovascular Imaging by Magnetically Modulated Optical Coherence Tomography. ChemPhotoChem 2019, 3, 529–539. [Google Scholar] [CrossRef]
- Xu, Q.; Jalilian, E.; Fakhoury, J.W.; Manwar, R.; Michniak-Kohn, B.; Elkin, K.B.; Avanaki, K. Monitoring the topical delivery of ultrasmall gold nanoparticles using optical coherence tomography. Skin. Res. Technol. 2020, 26, 263–268. [Google Scholar] [CrossRef]
- Mondal, I.; Raj, S.; Roy; Poddar, R. Silver nanoparticles (AgNPs) as a contrast agent for imaging of animal tissue using swept-source optical coherence tomography (SSOCT). Laser Phys. 2018, 28, 015601. [Google Scholar] [CrossRef]
- Zabotnov, S.V.; Kurakina, D.; Kashaev, F.; Skobelkina, A.; Kolchin, A.; Kaminskaya, T.; Agrba, P.; Sergeeva, E.; Kashkarov, P.; Kirillin, M.; et al. Structural and optical properties of nanoparticles formed by laser ablation of porous silicon in liquids: Perspectives in biophotonics. Quantum Electron. 2020, 50, 69–75. [Google Scholar] [CrossRef]
- de Schellenberger, A.A.; Poller, W.C.; Stangl, V.; Landmesser, U.; Schellenberger, E. Macrophage uptake switches on OCT contrast of superparamagnetic nanoparticles for imaging of atherosclerotic plaques. Int. J. Nanomed. 2018, 13, 7905–7913. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, A.L.; Blackmon, R.L.; Sierchio, J.M. Magnetic and Plasmonic Contrast Agents in Optical Coherence Tomography. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y.; Aguirre, A.D.; Tsai, T.-H.; Cohen, D.W.; Connolly, J.L.; Fujimoto, J.G. Ex vivo imaging of human thyroid pathology using integrated optical coherence tomography and optical coherence microscopy. J. Biomed. Opt. 2010, 15, 016001. [Google Scholar] [CrossRef]
- John, R.; Chaney, E.J.; Boppart, S.A. Dynamics of Magnetic Nanoparticle-Based Contrast Agents in Tissues Tracked Using Magnetomotive Optical Coherence Tomography. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 691–697. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Ortgies, D.H.; Torres, R.A.; Fernández, N.; Porto, L.; Rodríguez, E.M.; Solé, J.G.; Jaque, D.; Alfonso, F.; Rivero, F. Quantum Dots Emitting in the Third Biological Window as Bimodal Contrast Agents for Cardiovascular Imaging. Adv. Funct. Mater. 2017, 27, 1703276. [Google Scholar] [CrossRef]
- Papagiannaros, A.; Upponi, J.; Hartner, W.; Mongayt, D.; Levchenko, T.; Torchilin, V. Quantum dot loaded immunomicelles for tumor imaging. BMC Med. Imaging 2010, 10, 22. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef]
- Cordonnier, A.S.T. Protein Encapsulation into PLGA Nanoparticles by a Novel Phase Separation Method Using Non-Toxic Solvents. J. Nanomed. Nanotechnol. 2014, 5, 6. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Levin, T.; Sade, H.; Binyamini, R.B.-S.; Pour, M.; Nachman, I.; Lellouche, J.-P. Tungsten disulfide-based nanocomposites for photothermal therapy. Beilstein J. Nanotechnol. 2019, 10, 811–822. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, Z.; Niu, W.; Chen, M.; Wang, M.; Guo, Y.; Mao, C.; Lin, C.; Lei, B. Enhanced Physiological Stability and Long-Term Toxicity/Biodegradation In Vitro/In Vivo of Monodispersed Glycerolphosphate-Functionalized Bioactive Glass Nanoparticles. Part. Part. Syst. Charact. 2019, 36, 1800507. [Google Scholar] [CrossRef]
- Gul, A.; Ahmed, D.; Fazil, M.M.; Aslam, T.; Rashid, M.A.; Khan, H.; Ali, A.; Ali, S. Biofabrication of silver nanoparticles using Spirulina platensis: In vitro anti-coagulant, thrombolytic and catalytic dye degradation activity. Microsc. Res. Tech. 2023, 86, 823–833. [Google Scholar] [CrossRef]
- Sun, C.; Du, K.; Fang, C.; Bhattarai, N.; Veiseh, O.; Kievit, F.; Stephen, Z.; Lee, D.; Ellenbogen, R.G.; Ratner, B.; et al. PEG-Mediated Synthesis of Highly Dispersive Multifunctional Superparamagnetic Nanoparticles: Their Physicochemical Properties and Function In Vivo. ACS Nano 2010, 4, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Li, X.; Yang, M.; Liu, T.; Bao, J.; Jiang, M.; Hu, L.; Wang, Y.; Shao, P.; et al. Combined surface functionalization of MSC membrane and PDA inhibits neurotoxicity induced by Fe3O4 in mice based on apoptosis and autophagy through the ASK1/JNK signaling pathway. Aging 2023, 15, 6933–6949. [Google Scholar] [CrossRef]
- Reddy, N.; Shi, Z.; Xu, H.; Yang, Y. Development of wheat glutenin nanoparticles and their biodistribution in mice. J. Biomed. Mater. Res. A 2015, 103, 1653–1658. [Google Scholar] [CrossRef]
- Sastry, A.; Li, J.D.; Raynor, W.; Viehland, C.; Song, Z.; Xu, L.; Farsiu, S.; Izatt, J.A.; Toth, C.A.; Vajzovic, L. Microscope-Integrated OCT-Guided Volumetric Measurements of Subretinal Blebs Created by a Suprachoroidal Approach. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Chen, J.; Lu, B.; Xiong, H.; Tang, Z.; Ji, Y. In vivo monitoring of microneedle-based transdermal drug delivery of insulin. J. Innov. Opt. Health Sci. 2018, 11, 1850032. [Google Scholar] [CrossRef]
- Kavalaraki, A.; Spyratou, E.; Kouri, M.A.; Efstathopoulos, E. Gold Nanoparticles as Contrast Agents in Ophthalmic Imaging. Optics 2023, 4, 74–99. [Google Scholar] [CrossRef]
- Nguyen, V.; Hu, J.; Zhe, J.; Ramasamy, S.; Ahmed, U.; Paulus, Y.M. Advanced nanomaterials for imaging of eye diseases. ADMET DMPK 2024, 12, 269–298. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Narawane, A.; Trout, R.; Viehland, C.; Kuo, A.N.; Vajzovic, L.; Dhalla, A.-H.; Toth, C.A. Optical clearing with tartrazine enables deep transscleral imaging with optical coherence tomography. J. Biomed. Opt. 2024, 29, 120501. [Google Scholar] [CrossRef]
- Moothanchery, M.; Seeni, R.Z.; Xu, C.; Pramanik, M. Photoacoustic microscopy imaging for microneedle drug delivery. In Photons Plus Ultrasound: Imaging and Sensing 2018; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Bellingham, WA, USA, 2018; p. 199. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, J.; Liu, N.; Zhao, L.; Xu, B. Prognostic Implications of Molecular Subtypes in Primary Small Cell Lung Cancer and Their Correlation with Cancer Immunity. Front. Oncol. 2022, 12, 779276. [Google Scholar] [CrossRef] [PubMed]
- Harrington, B.S.; Kamdar, R.; Ning, F.; Korrapati, S.; Caminear, M.W.; Hernandez, L.F.; Butcher, D.; Edmondson, E.F.; Traficante, N.; Hendley, J.; et al. UGDH promotes tumor-initiating cells and a fibroinflammatory tumor microenvironment in ovarian cancer. J. Exp. Clin. Cancer Res. 2023, 42, 270. [Google Scholar] [CrossRef] [PubMed]
- Suman; Mishra, S.; Chander, H. High formin binding protein 17 (FBP17) expression indicates poor differentiation and invasiveness of ductal carcinomas. Sci. Rep. 2020, 10, 11543. [Google Scholar] [CrossRef]
- Bernardo, C.; Eriksson; Marzouka, N.; Liedberg, F.; Sjödahl, G.; Höglund, M. Molecular pathology of the luminal class of urothelial tumors. J. Pathol. 2019, 249, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Warrick, J.I.; Walter, V.; Yamashita, H.; Shuman, L.; Amponsa, V.O.; Zheng, Z.; Chan, W.; Whitcomb, T.L.; Yue, F.; Iyyanki, T.; et al. FOXA1, GATA3 and PPARɣ Cooperate to Drive Luminal Subtype in Bladder Cancer: A Molecular Analysis of Established Human Cell Lines. Sci. Rep. 2016, 6, 38531. [Google Scholar] [CrossRef]
- Geistlinger, L.; Oh, S.; Ramos, M.; Schiffer, L.; LaRue, R.S.; Henzler, C.M.; Munro, S.A.; Daughters, C.; Nelson, A.C.; Winterhoff, B.J.; et al. Multiomic analysis of subtype evolution and heterogeneity in high-grade serous ovarian carcinoma. Cancer Res. 2020, 80, 4335–4345. [Google Scholar] [CrossRef]
- Milani; Scotti, F.; Bergamini, F. Comment on: Diagnostic algorithm utilising multimodal imaging including optical coherence tomography angiography for the detection of myopic choroidal neovascularization. Eye 2021, 35, 349–350. [Google Scholar] [CrossRef]
- Usman, M.; Iqbal, K.; Ali, M.H.; Nafees, K. Features and Diagnostic Accuracy of Optical Coherence Tomography Angiography in Neovascular Age-related Macular Degeneration. Cureus 2019, 11, e6485. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, W.; Jiao, B.; Yang, Q.; Zhang, X.; Chen, R.; Wang, X.; Xiao, X.; Zhu, Y.; Liao, W.; et al. Correlation between retinal structure and brain multimodal magnetic resonance imaging in patients with Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1088829. [Google Scholar] [CrossRef]
- Aslam, N.; Khan, I.U.; Bashamakh, A.; Alghool, F.A.; Aboulnour, M.; Alsuwayan, N.M.; Alturaif, R.K.; Brahimi, S.; Aljameel, S.S.; Al Ghamdi, K. Multiple Sclerosis Diagnosis Using Machine Learning and Deep Learning: Challenges and Opportunities. Sensors 2022, 22, 7856. [Google Scholar] [CrossRef]
- Koulen; Schliesser, J.; Gallimore, G.; Kunjukunju, N.; Sabates, N.; Sabates, F. Clinical application of optical coherence tomography in combination with functional diagnostics: Advantages and limitations for diagnosis and assessment of therapy outcome in central serous chorioretinopathy. Clin. Ophthalmol. 2014, 8, 2337. [Google Scholar] [CrossRef][Green Version]
- Jerjes, W.; Stevenson, H.; Ramsay, D.; Hamdoon, Z. Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence. J. Clin. Med. 2024, 13, 5822. [Google Scholar] [CrossRef]
- Seth, N.; Seth, I.; Bulloch, G.; Siu, A.H.Y.; Guo, A.; Chatterjee, R.; MacManus, M.; Donnan, L. 18F-FDG PET and PET/CT as a diagnostic method for Ewing sarcoma: A systematic review and meta-analysis. Pediatr. Blood Cancer 2022, 69, e29415. [Google Scholar] [CrossRef]
- Leitgeb, R.A.; Baumann, B. Multimodal Optical Medical Imaging Concepts Based on Optical Coherence Tomography. Front. Phys. 2018, 6, 114. [Google Scholar] [CrossRef]
- Perini, G.; Rodriguez-Vieitez, E.; Kadir, A.; Sala, A.; Savitcheva, I.; Nordberg, A. Clinical impact of 18F-FDG-PET among memory clinic patients with uncertain diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 612–622. [Google Scholar] [CrossRef]
- Cerdá-Fuertes, N.; Stoessel, M.; Mickeliunas, G.; Pless, S.; Cagol, A.; Barakovic, M.; Maceski, A.M.; González, C.Á.; Souza, M.D.; Schaedlin, S.; et al. Optical coherence tomography versus other biomarkers: Associations with physical and cognitive disability in multiple sclerosis. Mult. Scler. J. 2023, 29, 1540–1550. [Google Scholar] [CrossRef]
- Caverzasi, E.; Cordano, C.; Zhu, A.H.; Zhao, C.; Bischof, A.; Kirkish, G.; Bennett, D.J.; Devereux, M.; Baker, N.; Inman, J.; et al. Imaging correlates of visual function in multiple sclerosis. PLoS ONE 2020, 15, e0235615. [Google Scholar] [CrossRef]
- Richter, G.M.; Zhang, X.; Tan, O.; Francis, B.A.; Chopra, V.; Greenfield, D.S.; Varma, R.; Schuman, J.S.; Huang, D. Regression Analysis of Optical Coherence Tomography Disc Variables for Glaucoma Diagnosis. J. Glaucoma 2016, 25, 634–642. [Google Scholar] [CrossRef]
- Mrejen, S.; Khan, S.; Gallego-Pinazo, R.; Jampol, L.M.; Yannuzzi, L.A. Acute Zonal Occult Outer Retinopathy. JAMA Ophthalmol. 2014, 132, 1089. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef]
- Yammouri, G.; Lahcen, A.A. AI-Reinforced Wearable Sensors and Intelligent Point-of-Care Tests. J. Pers. Med. 2024, 14, 1088. [Google Scholar] [CrossRef]
- Sahu, A.; Yélamos, O.; Iftimia, N.; Cordova, M.; Alessi-Fox, C.; Gill, M.; Maguluri, G.; Dusza, S.W.; Navarrete-Dechent, C.; González, S.; et al. Evaluation of a Combined Reflectance Confocal Microscopy–Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018, 154, 1175. [Google Scholar] [CrossRef]
- Niioka, H.; Kume, T.; Kubo, T.; Soeda, T.; Watanabe, M.; Yamada, R.; Sakata, Y.; Miyamoto, Y.; Wang, B.; Nagahara, H.; et al. Automated diagnosis of optical coherence tomography imaging on plaque vulnerability and its relation to clinical outcomes in coronary artery disease. Sci. Rep. 2022, 12, 14067. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Efficacy of optical coherence tomography in the diagnosing of oral cancerous lesion: Systematic review and meta-analysis. Head Neck 2023, 45, 473–481. [Google Scholar] [CrossRef]
- Sorkhabi, M.A.; Potapenko, I.O.; Ilginis, T.; Alberti, M.; Cabrerizo, J. Assessment of Anterior Uveitis Through Anterior-Segment Optical Coherence Tomography and Artificial Intelligence-Based Image Analyses. Transl. Vis. Sci. Technol. 2022, 11, 7. [Google Scholar] [CrossRef]
- Khalifa, A.K.M.; Kubo, T.; Shimamura, K.; Ino, Y.; Kishk, Y.T.; Hasan-Ali, H.; Abdel-Galeel, A.; Terada, K.; Emori, H.; Higashioka, D.; et al. Impact of Optical Coherence Tomography Imaging on Decision-Making During Percutaneous Coronary Intervention in Patients Presented with Acute Coronary Syndromes. Circ. J. 2021, 85, CJ-20-0942. [Google Scholar] [CrossRef]
- Ughi, G.J.; Marosfoi, M.G.; King, R.M.; Caroff, J.; Peterson, L.M.; Duncan, B.H.; Langan, E.T.; Collins, A.; Leporati, A.; Rousselle, S.; et al. A neurovascular high-frequency optical coherence tomography system enables in situ cerebrovascular volumetric microscopy. Nat. Commun. 2020, 11, 3851. [Google Scholar] [CrossRef]
- Panzarella, V.; Buttacavoli, F.; Rodolico, V.; Maniscalco, L.; Firenze, A.; De Caro, V.; Mauceri, R.; Rombo, S.E.; Campisi, G. Application of Targeted Optical Coherence Tomography in Oral Cancer: A Cross-Sectional Preliminary Study. Diagnostics 2024, 14, 2247. [Google Scholar] [CrossRef]
- Suciu, C.I.; Marginean, A.; Suciu, V.-I.; Muntean, G.A.; Nicoară, S.D. Diabetic Macular Edema Optical Coherence Tomography Biomarkers Detected with EfficientNetV2B1 and ConvNeXt. Diagnostics 2023, 14, 76. [Google Scholar] [CrossRef]
- Daxenberger, F.; Deußing, M.; Eijkenboom, Q.; Gust, C.; Thamm, J.; Hartmann, D.; French, L.E.; Welzel, J.; Schuh, S.; Sattler, E.C. Innovation in Actinic Keratosis Assessment: Artificial Intelligence-Based Approach to LC-OCT PRO Score Evaluation. Cancers 2023, 15, 4457. [Google Scholar] [CrossRef] [PubMed]
- van der Waerden, R.G.A.; A Volleberg, R.H.J.; Luttikholt, T.J.; Cancian, P.; van der Zande, J.L.; Stone, G.W.; Holm, N.R.; Kedhi, E.; Escaned, J.; Pellegrini, D.; et al. Artificial intelligence for the analysis of intracoronary optical coherence tomography images: A systematic review. Eur. Heart J.-Digit. Health 2025, 6, 270–284. [Google Scholar] [CrossRef]
- Arslan, J.; Samarasinghe, G.; Sowmya, A.; Benke, K.K.; Hodgson, L.A.; Guymer, R.H.; Baird, P.N. Deep Learning Applied to Automated Segmentation of Geographic Atrophy in Fundus Autofluorescence Images. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef]
- Carmichael, J.; Costanza, E.; Blandford, A.; Struyven, R.; Keane; Balaskas, K. Diagnostic decisions of specialist optometrists exposed to ambiguous deep-learning outputs. Sci. Rep. 2024, 14, 6775. [Google Scholar] [CrossRef]
- Labib, K.M.; Ghumman, H.; Jain, S.; Jarstad, J.S. A Review of the Utility and Limitations of Artificial Intelligence in Retinal Disorders and Pediatric Ophthalmology. Cureus 2024, 16, e71063. [Google Scholar] [CrossRef]
- Kodach, M.V.; Kalkman, J.; Faber, D.J.; van Leeuwen, T.G. Quantitative comparison of the OCT imaging depth at 1300 nm and 1600 nm. Biomed. Opt. Express 2010, 1, 176–185. [Google Scholar] [CrossRef]
- Subhash, H.M.; Wang, R.K. Overcoming the penetration depth limit in optical microscopy: Adaptive optics and wavefront shaping. J. Innov. Opt. Health Sci. 2019, 12, 1930002. [Google Scholar] [CrossRef]

| Nanoparticle Type | Optical Property | Application in OCT | Advantages | Challenges |
|---|---|---|---|---|
| AuNPs | SPR | Contrast Enhancement | High Biocompatibility | Cost, Aggregation Issues |
| AgNPs | Strong Scattering | Image Enhancement | High Stability | Toxicity Concerns |
| SiNPs | High Reflective Index | Deep Tissue Imaging | Biodegradable | Limited Studies |
| QDs | Fluorescence | Multiplex Imaging | Tunable Emission | Potential Cytotoxicity |
| Advantage | Description | Example Nanoparticles |
|---|---|---|
| High-Contrast Imaging | Improves visualization of microstructures | AuNPs, AgNPs and QDs |
| Theranostic Capabilities | Enables simultaneous diagnosis and treatment | Magnetic, Hybrid Nanoparticles |
| Real-Time Monitoring | Facilitates intraoperative tracking | Plasmonic Nanoparticles |
| Biodegradability | Minimizes long-term toxicity | PLGA, Chitosan |
| Clinical Application | AI Model(s) Used | Imaging Modality | Dataset Characteristics | Reported Outcome | Limitation |
|---|---|---|---|---|---|
| Diabetic Macular Edema Detection | EfficientNetV2, ConvNeXT | Retinal OCT | Custom-labled dataset, high-resolution OCT scans | High classification accuracy for edema biomarkers | Requires extensive training data; risk of overfitting on rare subtypes |
| Coronary Plaques Analysis | CNN-based model | Intravascular OCT | Small dataset (n < 300); manually labeled vulnerable plaques | 88.46% plaque detection accuracy | Limited generalizability; needs external validation |
| Lesions Differentiation | Deep CNN | Retinal OCT | Oral lesion dataset; biopsy-verified classes | >90% accuracy in lesion classification | Model interpretability and real-time clinical integration |
| Actinic Keratosis Evaluation | Automated ML pipeline | LC-OCT | Clinical image set with dermatological annotation | Increased diagnostic accuracy vs. visual assessment | Artifacts and variability in LC-OCT images |
| Coronary Disease Classification | Ensemble CNN with multimodal fusion | OCT + Angiography | OCT paired with angiographic images | Enhanced specificity in arterial disease detection | High computational cost for real-time screening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshpour Moghadam, S.; Maris, B.; Mokhtari, A.; Daffara, C.; Fiorini, P. OCT in Oncology and Precision Medicine: From Nanoparticles to Advanced Technologies and AI. Bioengineering 2025, 12, 650. https://doi.org/10.3390/bioengineering12060650
Daneshpour Moghadam S, Maris B, Mokhtari A, Daffara C, Fiorini P. OCT in Oncology and Precision Medicine: From Nanoparticles to Advanced Technologies and AI. Bioengineering. 2025; 12(6):650. https://doi.org/10.3390/bioengineering12060650
Chicago/Turabian StyleDaneshpour Moghadam, Sanam, Bogdan Maris, Ali Mokhtari, Claudia Daffara, and Paolo Fiorini. 2025. "OCT in Oncology and Precision Medicine: From Nanoparticles to Advanced Technologies and AI" Bioengineering 12, no. 6: 650. https://doi.org/10.3390/bioengineering12060650
APA StyleDaneshpour Moghadam, S., Maris, B., Mokhtari, A., Daffara, C., & Fiorini, P. (2025). OCT in Oncology and Precision Medicine: From Nanoparticles to Advanced Technologies and AI. Bioengineering, 12(6), 650. https://doi.org/10.3390/bioengineering12060650







