Visible Light Optical Coherence Tomography: Technology and Biomedical Applications
Abstract
1. Introduction
- Superior resolution: Axial resolution is inversely proportional to the square of the center wavelength, while lateral resolution scales linearly with wavelength, indicating that vis-OCT can achieve significantly higher axial and lateral resolution than NIR-OCT;
- Hemoglobin absorption properties: The strong absorption of hemoglobin in the visible light range (especially 500–600 nm) enables quantitative measurement of microvascular oxygen saturation, making it a hallmark application of vis-OCT;
- Enhanced contrast in superficial tissues: Increased scattering at shorter wavelengths improves imaging contrast in shallow tissue structures such as retinal layers.
2. Theory and System Implementation of Vis-OCT
2.1. Basic Theory and Key Components of Vis-OCT
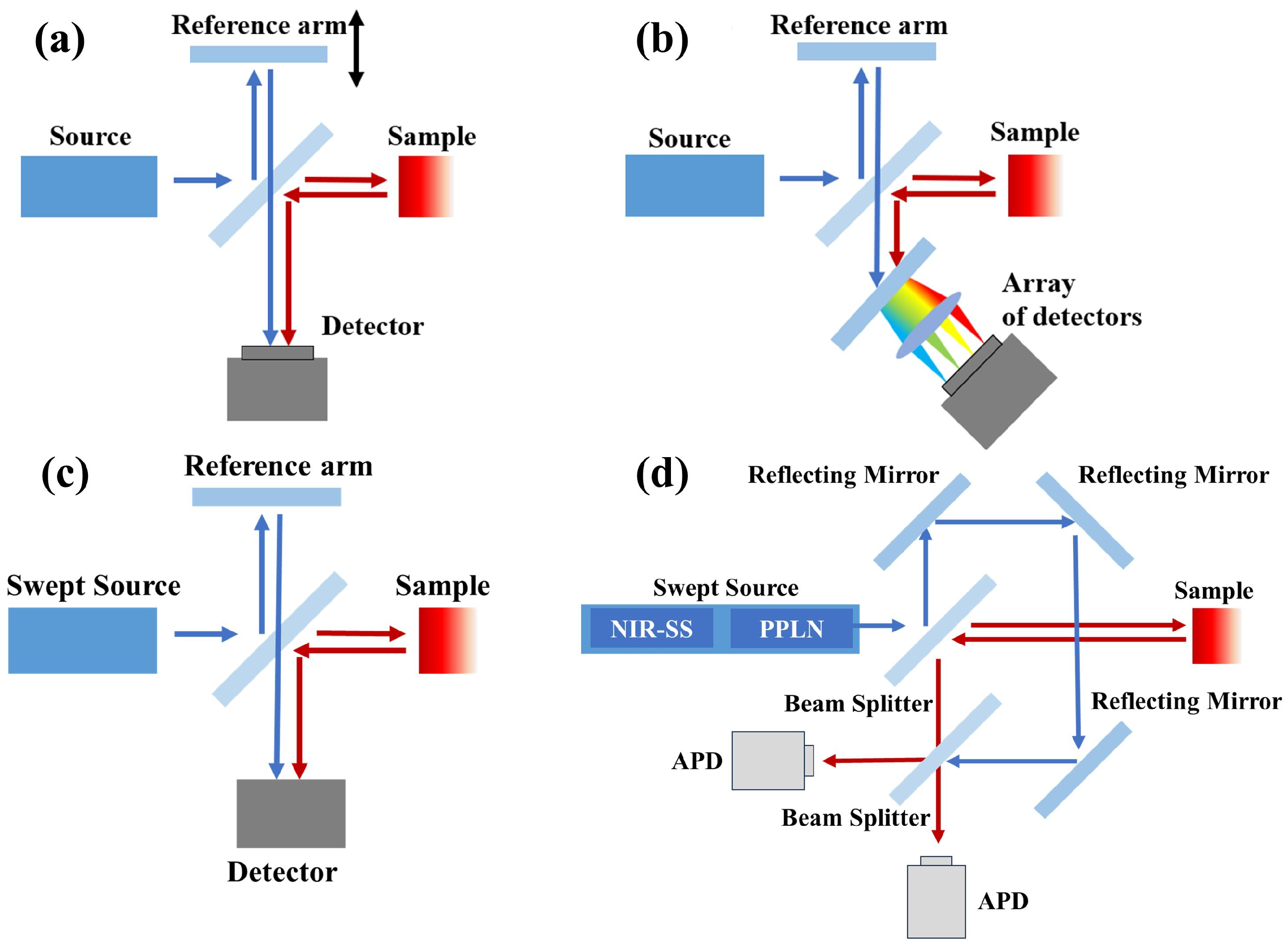
2.2. Evolution of Vis-OCT Technology
| Year | Authors | Contributions |
|---|---|---|
| 2002 | Povazay et al. [12] | Proposed vis-OCT concept; achieved 0.6 m resolution using a shaped visible supercontinuum light source. |
| 2004 | Dubois et al. [18] | Demonstrated white-light FF-OCT system with submicron axial resolution. |
| 2011 | Zhang et al. [21] | Developed the first dual-band OCT for in vivo rat retina imaging at visible and NIR wavelength range. |
| 2013 | Yi et al. [22] | Demonstrated the first in vivo retinal oximetry using vis-OCT and spectral modeling. |
| 2015 | Yi et al. [23] | Achieved the first human vis-OCT imaging with enhanced outer retina contrast. |
| 2017 | Chen et al. [24] | Enabled accurate human retinal mapping via statistical noise modeling and spectral fitting. |
| 2020 | Pi et al. [25] | Achieved sub-cellular of retinal microstructures in vivo. |
| 2020 | Miller et al. [26] | Introduced vis-OCT fibergraphy for RGC axon imaging. |
| 2024 | Wang et al. [27] | Built a dual-channel vis-OCT with full-range, wide-field, shot-noise-limited imaging capability. |
| 2025 | Fan et al. [16] | Developed swept source-based vis-OCT with deeper imaging depth and reduced sensitivity roll-off using a SHG-based swept laser. |
2.3. Vis-OCT Related Functional Imaging Methods
2.3.1. Doppler Flow Measurement
2.3.2. Vis-OCT Angiography (Vis-OCTA)
2.3.3. Vascular Oximetry
2.3.4. Vis-OCT for Nanoscale Sensing
2.4. Vis-OCT Technical Challenges
2.4.1. Broadband Visible Light Source
2.4.2. Dispersion Management
2.4.3. Limited Depth Penetration
2.4.4. System Noise Reduction
3. Biomedical Applications of Vis-OCT
3.1. Applications in Ophthalmologic Imaging
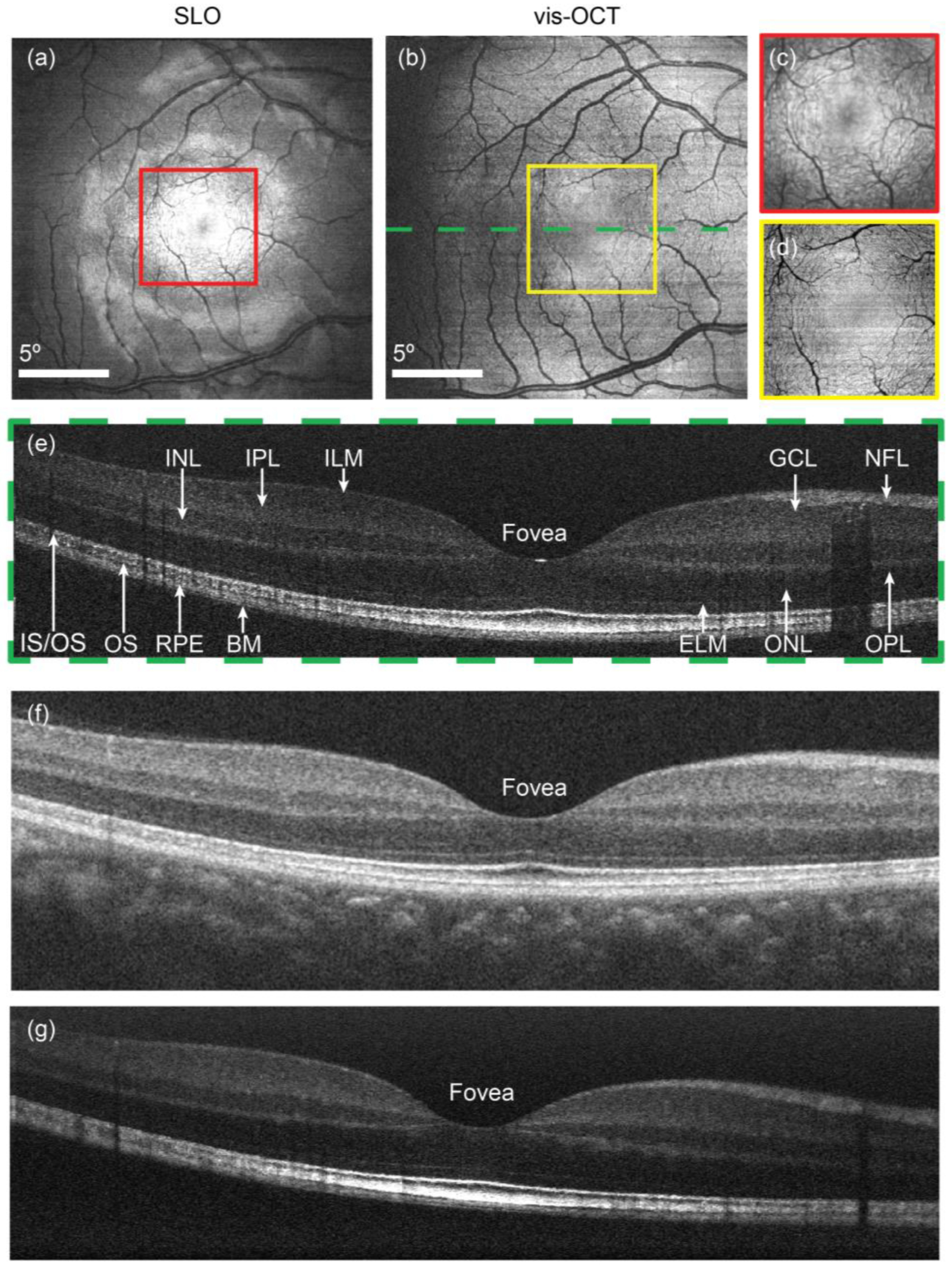
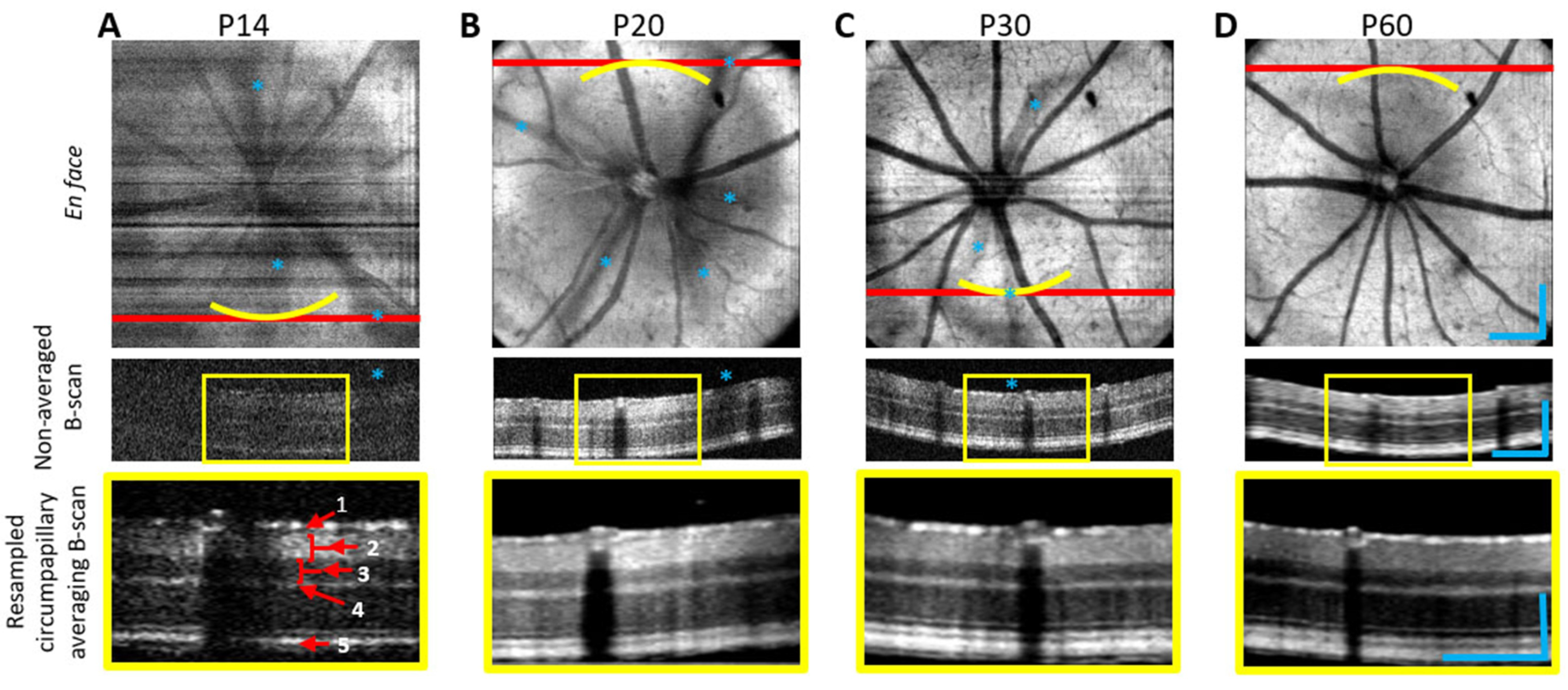
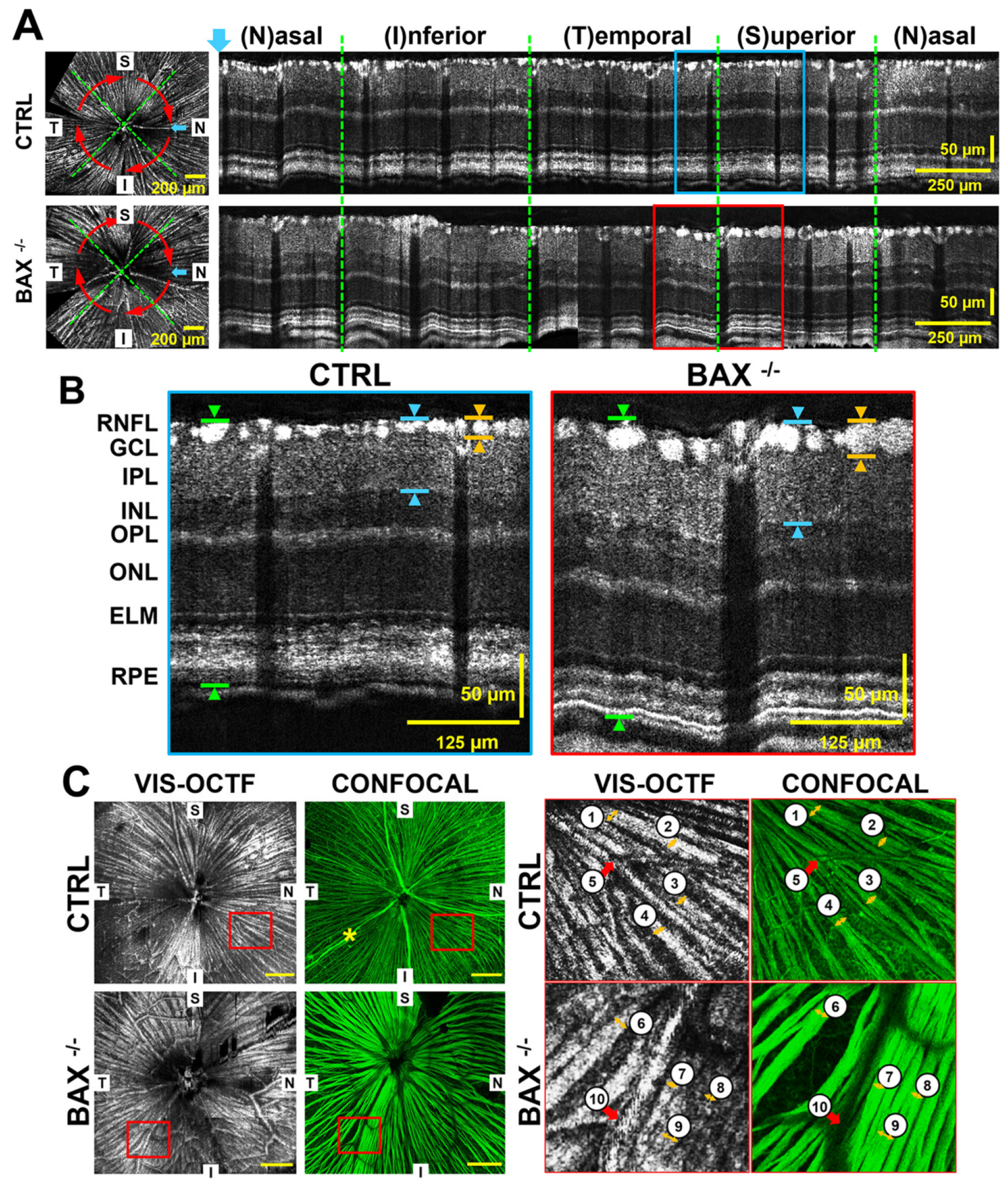
3.1.1. High-Resolution Structural Imaging
3.1.2. Retinal Oximetry
3.1.3. Clinical Application in Ophthalmology
3.2. Emerging Imaging Domains of vis-OCT
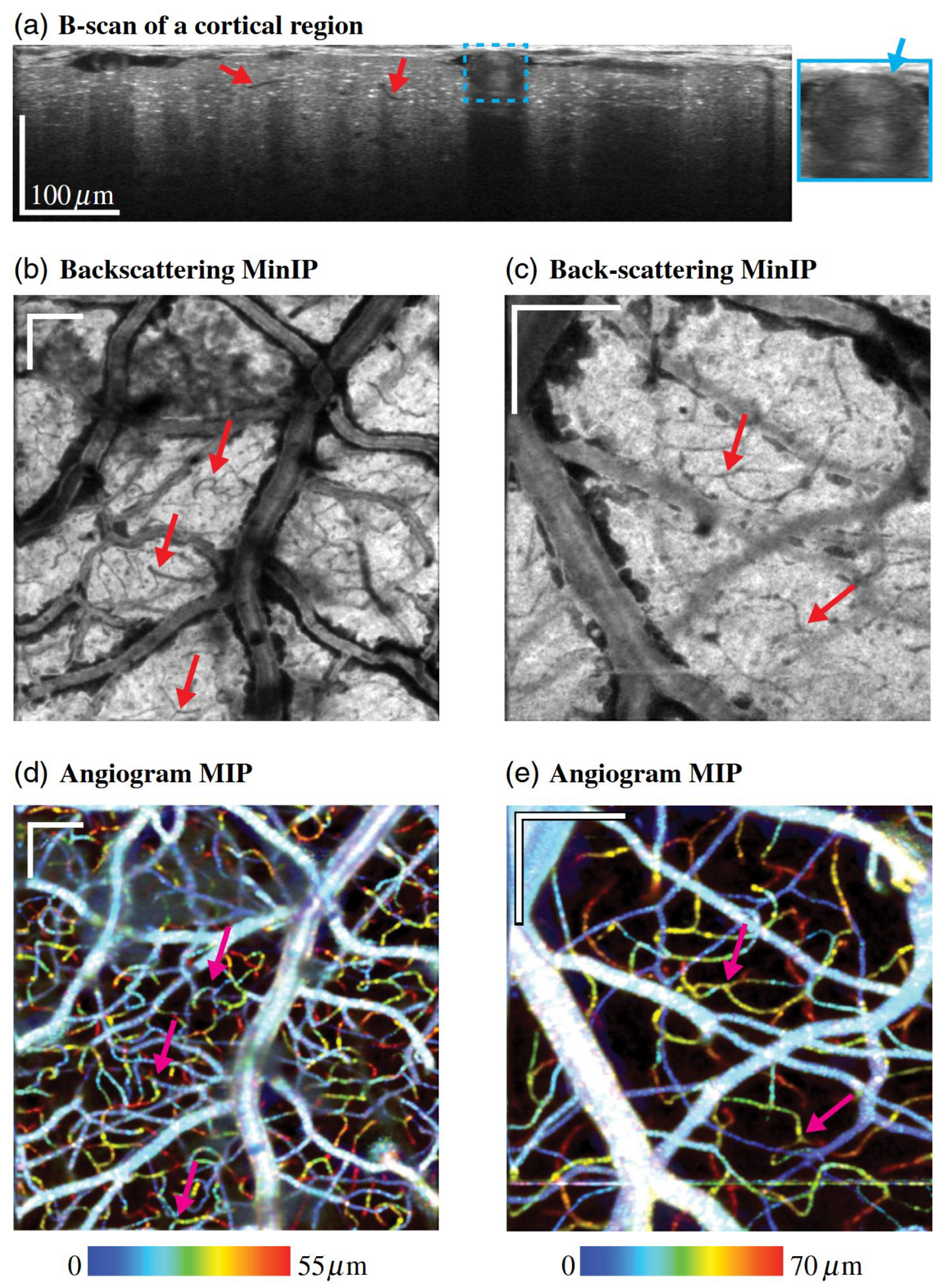
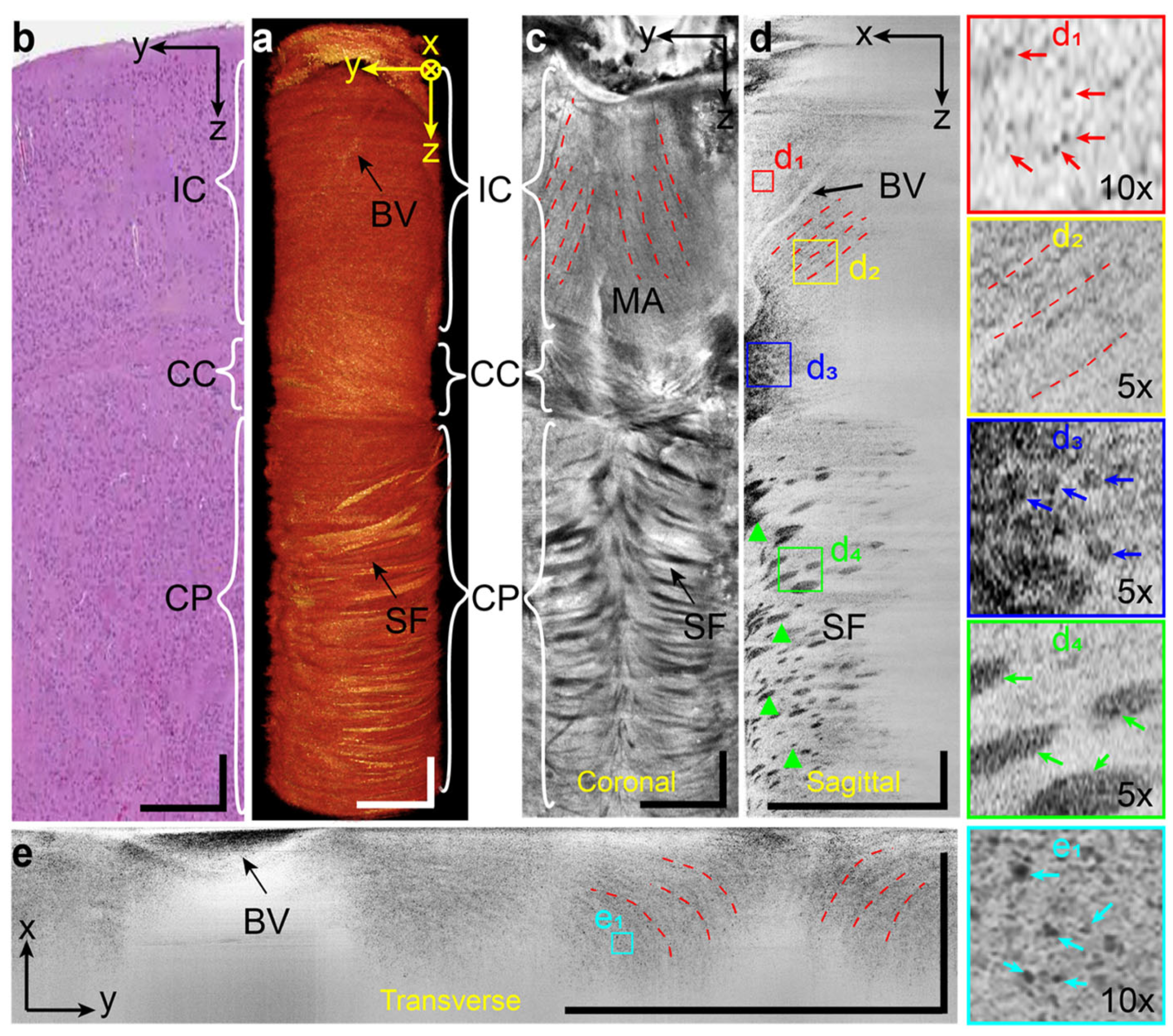
3.3. Multi-Modality Imaging with Vis-OCT
3.4. Other Functional Extensions of vis-OCT
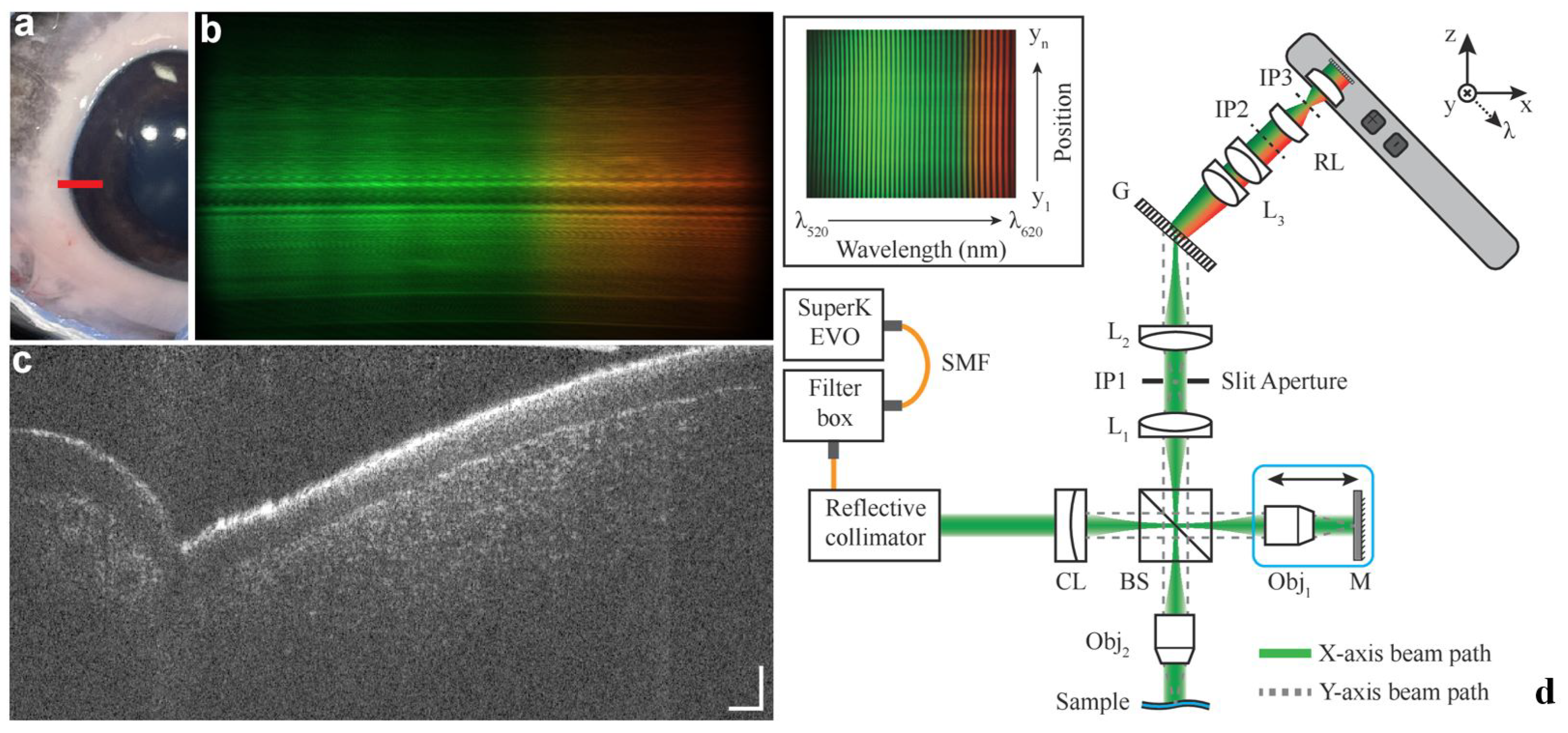
3.5. Image Processing and Artificial Intelligence Methods for Vis-OCT
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients With Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Jang, I.K.; Bouma, B.E.; Kang, D.H.; Park, S.J.; Park, S.W.; Seung, K.B.; Choi, K.B.; Shishkov, M.; Schlendorf, K.; Pomerantsev, E.; et al. Visualization of Coronary Atherosclerotic Plaques in Patients Using Optical Coherence Tomography: Comparison with Intravascular Ultrasound. J. Am. Coll. Cardiol. 2002, 39, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Welzel, J.; Lankenau, E.; Birngruber, R.; Engelhardt, R. Optical coherence tomography of the human skin. J. Am. Acad. Dermatol. 1997, 37, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.; Chopra, V.; Lu, A.T.H.; Schuman, J.S.; Ishikawa, H.; Wollstein, G.; Varma, R.; Huang, D. Detection of Macular Ganglion Cell Loss in Glaucoma by Fourier-Domain Optical Coherence Tomography. Ophthalmology 2009, 116, 2305–2314.e2. [Google Scholar] [CrossRef]
- Colston, B.W.; Everett, M.J.; Da Silva, L.B.; Otis, L.L.; Stroeve, P.; Nathel, H. Imaging of Hard- and Soft-Tissue Structure in the Oral Cavity by Optical Coherence Tomography. Appl. Opt. 1998, 37, 3582. [Google Scholar] [CrossRef]
- Tsai, T.H.; Lee, H.C.; Ahsen, O.O.; Liang, K.; Giacomelli, M.G.; Potsaid, B.M.; Tao, Y.K.; Jayaraman, V.; Figueiredo, M.; Huang, Q.; et al. Ultrahigh Speed Endoscopic Optical Coherence Tomography for Gastroenterology. Biomed. Opt. Express 2014, 5, 4387. [Google Scholar] [CrossRef]
- De Boer, J.F.; Milner, T.E.; Van Gemert, M.J.C.; Nelson, J.S. Two-Dimensional Birefringence Imaging in Biological Tissue by Polarization-Sensitive Optical Coherence Tomography. Opt. Lett. 1997, 22, 934. [Google Scholar] [CrossRef]
- Izatt, J.A.; Kulkarni, M.D.; Yazdanfar, S.; Barton, J.K.; Welch, A.J. In Vivo Bidirectional Color Doppler Flow Imaging of Picoliter Blood Volumes Using Optical Coherence Tomography. Opt. Lett. 1997, 22, 1439. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-Spectrum Amplitude-Decorrelation Angiography with Optical Coherence Tomography. Opt. Express 2012, 20, 4710. [Google Scholar] [CrossRef]
- Schmitt, J.M. OCT Elastography: Imaging Microscopic Deformation and Strain of Tissue. Opt. Express 1998, 3, 199. [Google Scholar] [CrossRef]
- Povazay, B.; Apolonski, A.A.; Unterhuber, A.; Hermann, B.; Bizheva, K.K.; Sattmann, H.; Russell, P.S.J.; Krausz, F.; Fercher, A.F.; Drexler, W. Visible Light Optical Coherence Tomography. In International Symposium on Biomedical Optics; Tuchin, V.V., Izatt, J.A., Fujimoto, J.G., Eds.; SPIE: San Jose, CA, USA, 2002; p. 90. [Google Scholar] [CrossRef]
- Song, S.; Hormel, T.T.; Jia, Y. Visible-Light Optical Coherence Tomography and Its Applications. Neurophotonics 2025, 12, 020601. [Google Scholar] [CrossRef]
- Povazay, B.; Bizheva, K.; Unterhuber, A.; Hermann, B.; Sattmann, H.; Fercher, A.F.; Drexler, W.; Apolonski, A.; Wadsworth, W.J.; Knight, J.C.; et al. Submicrometer Axial Resolution Optical Coherence Tomography. Opt. Lett. 2002, 27, 1800. [Google Scholar] [CrossRef]
- Yun, S.; Tearney, G.; De Boer, J.; Iftimia, N.; Bouma, B. High-Speed Optical Frequency-Domain Imaging. Opt. Express 2003, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Kuranov, R.; Miller, D.A.; Zhang, T.; Yeo, W.H.; Atkinson, R.; Zhang, P.; Sun, C.; Zhang, H.F. Swept-Source Visible-Light Optical Coherence Tomography. Opt. Lett. 2025, 50, 928. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fujimoto, J.G. (Eds.) Optical Coherence Tomography: Technology and Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Dubois, A.; Grieve, K.; Moneron, G.; Lecaque, R.; Vabre, L.; Boccara, C. Ultrahigh-Resolution Full-Field Optical Coherence Tomography. Appl. Opt. 2004, 43, 2874. [Google Scholar] [CrossRef] [PubMed]
- Scholler, J.; Groux, K.; Goureau, O.; Sahel, J.A.; Fink, M.; Reichman, S.; Boccara, C.; Grieve, K. Dynamic Full-Field Optical Coherence Tomography: 3D Live-Imaging of Retinal Organoids. Light. Sci. Appl. 2020, 9, 140. [Google Scholar] [CrossRef]
- Yin, Z.; He, B.; Ying, Y.; Zhang, S.; Yang, P.; Chen, Z.; Hu, Z.; Shi, Y.; Xue, R.; Wang, C.; et al. Fast and Label-Free 3D Virtual H&E Histology via Active Phase Modulation-Assisted Dynamic Full-Field OCT. NPJ Imaging 2025, 3, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Knighton, R.W.; Huang, X.R.; Puliafito, C.A.; Jiao, S. Dual-Band Spectral-Domain Optical Coherence Tomography for in Vivo Imaging the Spectral Contrasts of the Retinal Nerve Fiber Layer. Opt. Express 2011, 19, 19653. [Google Scholar] [CrossRef]
- Yi, J.; Wei, Q.; Liu, W.; Backman, V.; Zhang, H.F. Visible-Light Optical Coherence Tomography for Retinal Oximetry. Opt. Lett. 2013, 38, 1796. [Google Scholar] [CrossRef]
- Yi, J.; Chen, S.; Shu, X.; Fawzi, A.A.; Zhang, H.F. Human Retinal Imaging Using Visible-Light Optical Coherence Tomography Guided by Scanning Laser Ophthalmoscopy. Biomed. Opt. Express 2015, 6, 3701. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shu, X.; Nesper, P.L.; Liu, W.; Fawzi, A.A.; Zhang, H.F. Retinal Oximetry in Humans Using Visible-Light Optical Coherence Tomography [Invited]. Biomed. Opt. Express 2017, 8, 1415. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Hormel, T.T.; Wei, X.; Cepurna, W.; Morrison, J.C.; Jia, Y. Imaging Retinal Structures at Cellular-Level Resolution by Visible-Light Optical Coherence Tomography. Opt. Lett. 2020, 45, 2107. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; Grannonico, M.; Liu, M.; Kuranov, R.V.; Netland, P.A.; Liu, X.; Zhang, H.F. Visible-Light Optical Coherence Tomography Fibergraphy for Quantitative Imaging of Retinal Ganglion Cell Axon Bundles. Transl. Vis. Sci. Technol. 2020, 9, 11. [Google Scholar] [CrossRef]
- Wang, J.; Nolen, S.; Song, W.; Shao, W.; Yi, W.; Kashani, A.; Yi, J. A Dual-Channel Visible Light Optical Coherence Tomography System Enables Wide-Field, Full-Range, and Shot-Noise Limited Human Retinal Imaging. Commun. Eng. 2024, 3, 21. [Google Scholar] [CrossRef]
- Leitgeb, R.A.; Werkmeister, R.M.; Blatter, C.; Schmetterer, L. Doppler Optical Coherence Tomography. Prog. Retin. Eye Res. 2014, 41, 26–43. [Google Scholar] [CrossRef]
- Chong, S.P.; Bernucci, M.; Radhakrishnan, H.; Srinivasan, V.J. Structural and Functional Human Retinal Imaging with a Fiber-Based Visible Light OCT Ophthalmoscope. Biomed. Opt. Express 2017, 8, 323. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Soetikno, B.; Yi, J.; Zhang, K.; Chen, S.; Linsenmeier, R.A.; Sorenson, C.M.; Sheibani, N.; Zhang, H.F. Increased Retinal Oxygen Metabolism Precedes Microvascular Alterations in Type 1 Diabetic Mice. Investig. Opthalmology Vis. Sci. 2017, 58, 981. [Google Scholar] [CrossRef]
- Soetikno, B.T.; Yi, J.; Shah, R.; Liu, W.; Purta, P.; Zhang, H.F.; Fawzi, A.A. Inner Retinal Oxygen Metabolism in the 50/10 Oxygen-Induced Retinopathy Model. Sci. Rep. 2015, 5, 16752. [Google Scholar] [CrossRef]
- Chong, S.P.; Merkle, C.W.; Leahy, C.; Srinivasan, V.J. Cerebral Metabolic Rate of Oxygen (CMRO_2) Assessed by Combined Doppler and Spectroscopic OCT. Biomed. Opt. Express 2015, 6, 3941. [Google Scholar] [CrossRef]
- Liu, R.; Winkelmann, J.A.; Spicer, G.; Zhu, Y.; Eid, A.; Ameer, G.A.; Backman, V.; Yi, J. Single Capillary Oximetry and Tissue Ultrastructural Sensing by Dual-Band Dual-Scan Inverse Spectroscopic Optical Coherence Tomography. Light. Sci. Appl. 2018, 7, 57. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Duan, L.; Zhang, H.F. Spectroscopic Doppler Analysis for Visible-Light Optical Coherence Tomography. J. Biomed. Opt. 2017, 22, 1. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chen, Z. Advances in Doppler Optical Coherence Tomography and Angiography. Transl. Biophotonics 2019, 1, e201900005. [Google Scholar] [CrossRef]
- Gao, S.S.; Jia, Y.; Zhang, M.; Su, J.P.; Liu, G.; Hwang, T.S.; Bailey, S.T.; Huang, D. Optical Coherence Tomography Angiography. Investig. Opthalmology Vis. Sci. 2016, 57, OCT27. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Hormel, T.T.; Wei, X.; Cepurna, W.; Wang, B.; Morrison, J.C.; Jia, Y. Retinal Capillary Oximetry with Visible Light Optical Coherence Tomography. Proc. Natl. Acad. Sci. USA 2020, 117, 11658–11666. [Google Scholar] [CrossRef] [PubMed]
- Rubinoff, I.; Miller, D.A.; Kuranov, R.; Wang, Y.; Fang, R.; Volpe, N.J.; Zhang, H.F. High-Speed Balanced-Detection Visible-Light Optical Coherence Tomography in the Human Retina Using Subpixel Spectrometer Calibration. IEEE Trans. Med Imaging 2022, 41, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Winkelmann, J.A.; Eid, A.; Spicer, G.; Almassalha, L.M.; Nguyen, T.Q.; Backman, V. Spectral Contrast Optical Coherence Tomography Angiography Enables Single-Scan Vessel Imaging. Light. Sci. Appl. 2019, 8, 7. [Google Scholar] [CrossRef]
- Yi, J.; Radosevich, A.J.; Rogers, J.D.; Norris, S.C.; Çapoğlu, İ.R.; Taflove, A.; Backman, V. Can OCT Be Sensitive to Nanoscale Structural Alterations in Biological Tissue? Opt. Express 2013, 21, 9043. [Google Scholar] [CrossRef][Green Version]
- Chen, C.L.; Wang, R.K. Optical Coherence Tomography Based Angiography [Invited]. Biomed. Opt. Express 2017, 8, 1056. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Fingler, J.; Schwartz, D.; Yang, C.; Fraser, S.E. Mobility and Transverse Flow Visualization Using Phase Variance Contrast with Spectral Domain Optical Coherence Tomography. Opt. Express 2007, 15, 12636. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Camino, A.; Pi, S.; Cepurna, W.; Huang, D.; Morrison, J.C.; Jia, Y. Fast and Robust Standard-Deviation-Based Method for Bulk Motion Compensation in Phase-Based Functional OCT. Opt. Lett. 2018, 43, 2204. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.S.; Soetikno, B.T.; Yi, J.; Liu, W.; Skondra, D.; Zhang, H.F.; Fawzi, A.A. Visible-Light Optical Coherence Tomography Angiography for Monitoring Laser-Induced Choroidal Neovascularization in Mice. Investig. Opthalmology Vis. Sci. 2016, 57, OCT86. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Camino, A.; Zhang, M.; Cepurna, W.; Liu, G.; Huang, D.; Morrison, J.; Jia, Y. Angiographic and Structural Imaging Using High Axial Resolution Fiber-Based Visible-Light OCT. Biomed. Opt. Express 2017, 8, 4595. [Google Scholar] [CrossRef]
- Pi, S.; Camino, A.; Wei, X.; Simonett, J.; Cepurna, W.; Huang, D.; Morrison, J.C.; Jia, Y. Rodent Retinal Circulation Organization and Oxygen Metabolism Revealed by Visible-Light Optical Coherence Tomography. Biomed. Opt. Express 2018, 9, 5851. [Google Scholar] [CrossRef]
- Pi, S.; Hormel, T.T.; Wei, X.; Cepurna, W.; Camino, A.; Guo, Y.; Huang, D.; Morrison, J.; Jia, Y. Monitoring Retinal Responses to Acute Intraocular Pressure Elevation in Rats with Visible Light Optical Coherence Tomography. Neurophotonics 2019, 6, 1. [Google Scholar] [CrossRef]
- Wang, R.K. Optical Microangiography: A Label-Free 3-D Imaging Technology to Visualize and Quantify Blood Circulations Within Tissue Beds Vivo. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 545–554. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Thorell, M.R.; An, L.; Durbin, M.K.; Laron, M.; Sharma, U.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Swept-Source OCT Angiography of the Retinal Vasculature Using Intensity Differentiation-based Optical Microangiography Algorithms. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 382–389. [Google Scholar] [CrossRef]
- Yi, J.; Chen, S.; Backman, V.; Zhang, H.F. In Vivo Functional Microangiography by Visible-Light Optical Coherence Tomography. Biomed. Opt. Express 2014, 5, 3603. [Google Scholar] [CrossRef]
- Chen, S.; Yi, J.; Zhang, H.F. Measuring Oxygen Saturation in Retinal and Choroidal Circulations in Rats Using Visible Light Optical Coherence Tomography Angiography. Biomed. Opt. Express 2015, 6, 2840. [Google Scholar] [CrossRef]
- Nam, A.S.; Chico-Calero, I.; Vakoc, B.J. Complex Differential Variance Algorithm for Optical Coherence Tomography Angiography. Biomed. Opt. Express 2014, 5, 3822. [Google Scholar] [CrossRef]
- Shi, J.; Guo, Z.; Dong, C.; Xing, F.; Di, Y.; Gan, Z.; Liu, C.; Liu, J.; Zhu, H. High-Contrast Optical Coherence Tomography Angiography Based on Normalized Complex Decorrelation. IEEE Photonics Technol. Lett. 2024, 36, 799–802. [Google Scholar] [CrossRef]
- Wei, X.; Hormel, T.T.; Jia, Y. Phase-Stabilized Complex-Decorrelation Angiography. Biomed. Opt. Express 2021, 12, 2419. [Google Scholar] [CrossRef]
- Robles, F.E.; Chowdhury, S.; Wax, A. Assessing Hemoglobin Concentration Using Spectroscopic Optical Coherence Tomography for Feasibility of Tissue Diagnostics. Biomed. Opt. Express 2010, 1, 310. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Li, X. Estimation of Oxygen Saturation from Erythrocytes by High-Resolution Spectroscopic Optical Coherence Tomography. Opt. Lett. 2010, 35, 2094. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Camino, A.; Cepurna, W.; Wei, X.; Zhang, M.; Huang, D.; Morrison, J.; Jia, Y. Automated Spectroscopic Retinal Oximetry with Visible-Light Optical Coherence Tomography. Biomed. Opt. Express 2018, 9, 2056. [Google Scholar] [CrossRef]
- Yi, J.; Backman, V. Imaging a Full Set of Optical Scattering Properties of Biological Tissue by Inverse Spectroscopic Optical Coherence Tomography. Opt. Lett. 2012, 37, 4443. [Google Scholar] [CrossRef]
- Qiao, D.; Rubinoff, I.S.; Zhou, J.; Troy, J.B.; Zhang, H.F.; Tong, S.; Miao, P. Spectral Domain Isolation of Ballistic Component in Visible Light OCT Based on Random Matrix Description. APL Photonics 2023, 8, 046111. [Google Scholar] [CrossRef]
- Rubinoff, I.; Kuranov, R.V.; Fang, R.; Ghassabi, Z.; Wang, Y.; Beckmann, L.; Miller, D.A.; Wollstein, G.; Ishikawa, H.; Schuman, J.S.; et al. Adaptive Spectroscopic Visible-Light Optical Coherence Tomography for Clinical Retinal Oximetry. Commun. Med. 2023, 3, 57. [Google Scholar] [CrossRef]
- Chen, S.; Shu, X.; Yi, J.; Fawzi, A.; Zhang, H.F. Dual-Band Optical Coherence Tomography Using a Single Supercontinuum Laser Source. J. Biomed. Opt. 2016, 21, 066013. [Google Scholar] [CrossRef]
- Khan, S.; Neuhaus, K.; Thaware, O.; Ni, S.; Ju, M.J.; Redd, T.; Huang, D.; Jian, Y. Corneal Imaging with Blue-Light Optical Coherence Microscopy. Biomed. Opt. Express 2022, 13, 5004. [Google Scholar] [CrossRef]
- Lichtenegger, A.; Salas, M.; Sing, A.; Duelk, M.; Licandro, R.; Gesperger, J.; Baumann, B.; Drexler, W.; Leitgeb, R.A. Reconstruction of Visible Light Optical Coherence Tomography Images Retrieved from Discontinuous Spectral Data Using a Conditional Generative Adversarial Network. Biomed. Opt. Express 2021, 12, 6780. [Google Scholar] [CrossRef]
- Fercher, A.F.; Drexler, W.; Hitzenberger, C.K.; Lasser, T. Optical Coherence Tomography—Principles and Applications. Rep. Prog. Phys. 2003, 66, 239. [Google Scholar] [CrossRef]
- De Wit, J.; Glentis, G.O.; Kalkman, J. Computational 3D Resolution Enhancement for Optical Coherence Tomography with a Narrowband Visible Light Source. Biomed. Opt. Express 2023, 14, 3532. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Srinivasan, V.J.; Ko, T.H.; Fujimoto, J.G.; Kowalczyk, A.; Duker, J.S. Ultrahigh-Resolution, High-Speed, Fourier Domain Optical Coherence Tomography and Methods for Dispersion Compensation. Opt. Express 2004, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Hitzenberger, C.K.; Baumgartner, A.; Drexler, W.; Fercher, A.F. Dispersion Effects in Partial Coherence Interferometry: Implications for Intraocular Ranging. J. Biomed. Opt. 1999, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.P.; Zhang, T.; Kho, A.; Bernucci, M.T.; Dubra, A.; Srinivasan, V.J. Ultrahigh Resolution Retinal Imaging by Visible Light OCT with Longitudinal Achromatization. Biomed. Opt. Express 2018, 9, 1477. [Google Scholar] [CrossRef]
- Pan, L.; Wang, X.; Li, Z.; Zhang, X.; Bu, Y.; Nan, N.; Chen, Y.; Wang, X.; Dai, F. Depth-Dependent Dispersion Compensation for Full-Depth OCT Image. Opt. Express 2017, 25, 10345. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, S.; Soetikno, B.; Liu, W.; Tong, S.; Zhang, H.F. Monitoring Acute Stroke in Mouse Model Using Laser Speckle Imaging-Guided Visible-Light Optical Coherence Tomography. IEEE Trans. Biomed. Eng. 2018, 65, 2136–2142. [Google Scholar] [CrossRef]
- Lichtenegger, A.; Gesperger, J.; Kiesel, B.; Muck, M.; Eugui, P.; Harper, D.J.; Salas, M.; Augustin, M.; Merkle, C.W.; Hitzenberger, C.K.; et al. Revealing Brain Pathologies with Multimodal Visible Light Optical Coherence Microscopy and Fluorescence Imaging. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-Resolution Ophthalmic Optical Coherence Tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Bouma, B.E.; Fujimoto, J.G. High-Speed Phase- and Group-Delay Scanning with a Grating-Based Phase Control Delay Line. Opt. Lett. 1997, 22, 1811. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.C.; Rogers, J.; Podoleanu, A.G. Fast Scanning Transmissive Delay Line for Optical Coherence Tomography. Opt. Lett. 2005, 30, 3263. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Wang, Z.; Pan, Y. Dispersion Compensation in High-Speed Optical Coherence Tomography by Acousto-Optic Modulation. Appl. Opt. 2005, 44, 4272. [Google Scholar] [CrossRef]
- Iyer, S.; Coen, S.; Vanholsbeeck, F. Dual-Fiber Stretcher as a Tunable Dispersion Compensator for an All-Fiber Optical Coherence Tomography System. Opt. Lett. 2009, 34, 2903. [Google Scholar] [CrossRef]
- Kho, A.; Srinivasan, V.J. Compensating Spatially Dependent Dispersion in Visible Light OCT. Opt. Lett. 2019, 44, 775. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Zhu, S.; Chen, D.; Qiu, H.; Lam, A.K.N.; Leung, C.K.S.; Yuan, W. A Generic and Effective System Dispersion Compensation Method: Development and Validation in Visible-Light OCT. Photonics 2023, 10, 892. [Google Scholar] [CrossRef]
- Hu, Z.; Rollins, A.M. Fourier Domain Optical Coherence Tomography with a Linear-in-Wavenumber Spectrometer. Opt. Lett. 2007, 32, 3525. [Google Scholar] [CrossRef]
- Bouma, B.E.; Yun, S.H.; Vakoc, B.J.; Suter, M.J.; Tearney, G.J. Fourier-Domain Optical Coherence Tomography: Recent Advances toward Clinical Utility. Curr. Opin. Biotechnol. 2009, 20, 111–118. [Google Scholar] [CrossRef]
- Wang, R.K. In Vivo Full Range Complex Fourier Domain Optical Coherence Tomography. Appl. Phys. Lett. 2007, 90, 054103. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Kowalczyk, A.; Leitgeb, R.; Fercher, A.F. Full Range Complex Spectral Optical Coherence Tomography Technique in Eye Imaging. Opt. Lett. 2002, 27, 1415. [Google Scholar] [CrossRef]
- Hillmann, D.; Franke, G.; Hinkel, L.; Bonin, T.; Koch, P.; Hüttmann, G. Off-Axis Full-Field Swept-Source Optical Coherence Tomography Using Holographic Refocusing. In SPIE BiOS; Fujimoto, J.G., Izatt, J.A., Tuchin, V.V., Eds.; SPIE: San Francisco, CA, USA, 2013; p. 857104. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Yun, S.H.; Tearney, G.J.; Bouma, B.E. Elimination of Depth Degeneracy in Optical Frequency-Domain Imaging through Polarization-Based Optical Demodulation. Opt. Lett. 2006, 31, 362. [Google Scholar] [CrossRef] [PubMed]
- Sarunic, M.V.; Choma, M.A.; Yang, C.; Izatt, J.A. Instantaneous Complex Conjugate Resolved Spectral Domain and Swept-Source OCT Using 3x3 Fiber Couplers. Opt. Express 2005, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Sarunic, M.V.; Applegate, B.E.; Izatt, J.A. Real-Time Quadrature Projection Complex Conjugate Resolved Fourier Domain Optical Coherence Tomography. Opt. Lett. 2006, 31, 2426. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.K.; Zhao, M.; Izatt, J.A. High-Speed Complex Conjugate Resolved Retinal Spectral Domain Optical Coherence Tomography Using Sinusoidal Phase Modulation. Opt. Lett. 2007, 32, 2918. [Google Scholar] [CrossRef]
- Mao, Y.; Sherif, S.; Flueraru, C.; Chang, S. 3×3 Mach–Zehnder Interferometer with Unbalanced Differential Detection for Full-Range Swept-Source Optical Coherence Tomography. Appl. Opt. 2008, 47, 2004. [Google Scholar] [CrossRef]
- Niemeier, R.C.; Simmons, Z.J.; Rogers, J.D. Noise Reduction in Supercontinuum Sources for OCT by Single-Pulse Spectral Normalization. Appl. Opt. 2020, 59, 5521. [Google Scholar] [CrossRef]
- De Boer, J.F.; Leitgeb, R.; Wojtkowski, M. Twenty-Five Years of Optical Coherence Tomography: The Paradigm Shift in Sensitivity and Speed Provided by Fourier Domain OCT [Invited]. Biomed. Opt. Express 2017, 8, 3248. [Google Scholar] [CrossRef]
- Brown, W.J.; Kim, S.; Wax, A. Noise Characterization of Supercontinuum Sources for Low-Coherence Interferometry Applications. J. Opt. Soc. Am. A 2014, 31, 2703. [Google Scholar] [CrossRef]
- Jensen, M.; Gonzalo, I.B.; Engelsholm, R.D.; Maria, M.; Israelsen, N.M.; Podoleanu, A.; Bang, O. Noise of Supercontinuum Sources in Spectral Domain Optical Coherence Tomography. J. Opt. Soc. Am. B 2019, 36, A154. [Google Scholar] [CrossRef]
- Szkulmowski, M.; Wojtkowski, M.; Bajraszewski, T.; Gorczyńska, I.; Targowski, P.; Wasilewski, W.; Kowalczyk, A.; Radzewicz, C. Quality Improvement for High Resolution in Vivo Images by Spectral Domain Optical Coherence Tomography with Supercontinuum Source. Opt. Commun. 2005, 246, 569–578. [Google Scholar] [CrossRef]
- Dudley, J.M.; Genty, G.; Coen, S. Supercontinuum Generation in Photonic Crystal Fiber. Rev. Mod. Phys. 2006, 78, 1135–1184. [Google Scholar] [CrossRef]
- Rao D.S., S.; Jensen, M.; Grüner-Nielsen, L.; Olsen, J.T.; Heiduschka, P.; Kemper, B.; Schnekenburger, J.; Glud, M.; Mogensen, M.; Israelsen, N.M.; et al. Shot-Noise Limited, Supercontinuum-Based Optical Coherence Tomography. Light. Sci. Appl. 2021, 10, 133. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Lin, K.; Chen, X.; Xu, Z.; Lou, S.; Ge, X.; Ni, G.; Yu, X.; Mo, J.; et al. Multi-Channel Spectral-Domain Optical Coherence Tomography Using Single Spectrometer. Chin. Opt. Lett. 2023, 21, 051102. [Google Scholar] [CrossRef]
- Wan, M.; Liang, S.; Li, X.; Duan, Z.; Zou, J.; Chen, J.; Yuan, J.; Zhang, J. Balanced Detection Spectral-Domain Optical Coherence Tomography with a Single Line-Scan Camera. Opt. Express 2022, 30, 2578. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef]
- Jia, Y.; Morrison, J.C.; Tokayer, J.; Tan, O.; Lombardi, L.; Baumann, B.; Lu, C.D.; Choi, W.; Fujimoto, J.G.; Huang, D. Quantitative OCT Angiography of Optic Nerve Head Blood Flow. Biomed. Opt. Express 2012, 3, 3127. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 45. [Google Scholar] [CrossRef]
- Beckmann, L.; Cai, Z.; Cole, J.; Miller, D.A.; Liu, M.; Grannonico, M.; Zhang, X.; Ryu, H.J.; Netland, P.A.; Liu, X.; et al. In Vivo Imaging of the Inner Retinal Layer Structure in Mice after Eye-Opening Using Visible-Light Optical Coherence Tomography. Exp. Eye Res. 2021, 211, 108756. [Google Scholar] [CrossRef]
- Chauhan, P.; Kho, A.M.; Srinivasan, V.J. From Soma to Synapse: Imaging Age-Related Rod Photoreceptor Changes in the Mouse with Visible Light OCT. Ophthalmol. Sci. 2023, 3, 100321. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wen, R.; Lam, B.L.; Puliafito, C.A.; Jiao, S. Depth-Resolved Rhodopsin Molecular Contrast Imaging for Functional Assessment of Photoreceptors. Sci. Rep. 2015, 5, 13992. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xiu, W.; Wu, S.; Li, K.; Li, B.; Tang, Z.; Yan, C.; Wang, X.; He, C.; Lu, F.; et al. Visible Light Optical Coherence Tomography for Assessing Retinal Structure and Function in Diseased Mouse Models. Biomed. Opt. Express 2025, 16, 2077. [Google Scholar] [CrossRef]
- Zhang, X.; Beckmann, L.; Miller, D.A.; Shao, G.; Cai, Z.; Sun, C.; Sheibani, N.; Liu, X.; Schuman, J.; Johnson, M.; et al. In Vivo Imaging of Schlemm’s Canal and Limbal Vascular Network in Mouse Using Visible-Light OCT. Investig. Opthalmology Vis. Sci. 2020, 61, 23. [Google Scholar] [CrossRef]
- Fang, R.; Zhang, P.; Kim, D.; Kweon, J.; Sun, C.; Huang, A.S.; Zhang, H.F. Robotic Visible-Light Optical Coherence Tomography Visualizes Segmental Schlemm’s Canal Anatomy and Segmental Pilocarpine Response. Investig. Ophthalmol. Vis. Sci. 2025, 66, 47. [Google Scholar] [CrossRef]
- Grannonico, M.; Miller, D.A.; Liu, M.; Norat, P.; Deppmann, C.D.; Netland, P.A.; Zhang, H.F.; Liu, X. Global and Regional Damages in Retinal Ganglion Cell Axon Bundles Monitored Non-Invasively by Visible-Light Optical Coherence Tomography Fibergraphy. J. Neurosci. 2021, 41, 10179–10193. [Google Scholar] [CrossRef]
- Miller, D.A.; Grannonico, M.; Liu, M.; Savier, E.; McHaney, K.; Erisir, A.; Netland, P.A.; Cang, J.; Liu, X.; Zhang, H.F. Visible-Light Optical Coherence Tomography Fibergraphy of the Tree Shrew Retinal Ganglion Cell Axon Bundles. IEEE Trans. Med Imaging 2024, 43, 2769–2777. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Oxygen Distribution and Consumption within the Retina in Vascularised and Avascular Retinas and in Animal Models of Retinal Disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Zouache, M.A. Variability in Retinal Neuron Populations and Associated Variations in Mass Transport Systems of the Retina in Health and Aging. Front. Aging Neurosci. 2022, 14, 778404. [Google Scholar] [CrossRef]
- Hammer, M.; Vilser, W.; Riemer, T.; Mandecka, A.; Schweitzer, D.; Kühn, U.; Dawczynski, J.; Liemt, F.; Strobel, J. Diabetic Patients with Retinopathy Show Increased Retinal Venous Oxygen Saturation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1025–1030. [Google Scholar] [CrossRef]
- Hardarson, S.H.; Stefánsson, E. Retinal Oxygen Saturation Is Altered in Diabetic Retinopathy. Br. J. Ophthalmol. 2012, 96, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Gong, J.; Li, X. Analyzing Absorption and Scattering Spectra of Micro-Scale Structures with Spectroscopic Optical Coherence Tomography. Opt. Express 2009, 17, 13157. [Google Scholar] [CrossRef] [PubMed]
- Robles, F.E.; Zhu, Y.; Lee, J.; Sharma, S.; Wax, A. Detection of Early Colorectal Cancer Development in the Azoxymethane Rat Carcinogenesis Model with Fourier Domain Low Coherence Interferometry. Biomed. Opt. Express 2010, 1, 736. [Google Scholar] [CrossRef] [PubMed]
- Robles, F.E.; Wilson, C.; Grant, G.; Wax, A. Molecular Imaging True-Colour Spectroscopic Optical Coherence Tomography. Nat. Photonics 2011, 5, 744–747. [Google Scholar] [CrossRef]
- Yi, J.; Liu, W.; Chen, S.; Backman, V.; Sheibani, N.; Sorenson, C.M.; Fawzi, A.A.; Linsenmeier, R.A.; Zhang, H.F. Visible Light Optical Coherence Tomography Measures Retinal Oxygen Metabolic Response to Systemic Oxygenation. Light. Sci. Appl. 2015, 4, e334. [Google Scholar] [CrossRef]
- Pi, S.; Wang, B.; Gao, M.; Cepurna, W.; Lozano, D.C.; Morrison, J.C.; Jia, Y. Longitudinal Observation of Retinal Response to Optic Nerve Transection in Rats Using Visible Light Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2023, 64, 17. [Google Scholar] [CrossRef]
- Zhang, T.; Kho, A.M.; Yiu, G.; Srinivasan, V.J. Visible Light Optical Coherence Tomography (OCT) Quantifies Subcellular Contributions to Outer Retinal Band 4. Transl. Vis. Sci. Technol. 2021, 10, 30. [Google Scholar] [CrossRef]
- Rubinoff, I.; Beckmann, L.; Wang, Y.; Fawzi, A.A.; Liu, X.; Tauber, J.; Jones, K.; Ishikawa, H.; Schuman, J.S.; Kuranov, R.; et al. Speckle Reduction in Visible-Light Optical Coherence Tomography Using Scan Modulation. Neurophotonics 2019, 6, 1. [Google Scholar] [CrossRef]
- Song, W.; Shao, W.; Yi, W.; Liu, R.; Desai, M.; Ness, S.; Yi, J. Visible Light Optical Coherence Tomography Angiography (Vis-OCTA) Facilitates Local Microvascular Oximetry in the Human Retina. Biomed. Opt. Express 2020, 11, 4037. [Google Scholar] [CrossRef]
- Wang, J.; Baker, A.; Subramanian, M.L.; Siegel, N.H.; Chen, X.; Ness, S.; Yi, J. Simultaneous Visible Light Optical Coherence Tomography and near Infrared OCT Angiography in Retinal Pathologies: A Case Study. Exp. Biol. Med. 2022, 247, 377–384. [Google Scholar] [CrossRef]
- Soetikno, B.T.; Beckmann, L.; Zhang, X.; Fawzi, A.A.; Zhang, H.F. Visible-Light Optical Coherence Tomography Oximetry Based on Circumpapillary Scan and Graph-Search Segmentation. Biomed. Opt. Express 2018, 9, 3640. [Google Scholar] [CrossRef]
- Ghassabi, Z.; Kuranov, R.V.; Schuman, J.S.; Zambrano, R.; Wu, M.; Liu, M.; Tayebi, B.; Wang, Y.; Rubinoff, I.; Liu, X.; et al. In Vivo Sublayer Analysis of Human Retinal Inner Plexiform Layer Obtained by Visible-Light Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2022, 63, 18. [Google Scholar] [CrossRef]
- Wang, J.; Sadlak, N.; Fiorello, M.G.; Desai, M.; Yi, J. Macular Oxygen Saturation in Glaucoma Using Retinal Oximetry of Visible Light Optical Coherence Tomography: A Pilot Study. Transl. Vis. Sci. Technol. 2025, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Wang, J.; Alonzo, B.; Yi, J.; Kashani, A.H. Photoreceptor Outer Segment Reflectivity With Ultrahigh-Resolution Visible-Light Optical Coherence Tomography in Systemic Hydroxychloroquine Use. Transl. Vis. Sci. Technol. 2025, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Grannonico, M.; Miller, D.A.; Liu, M.; Krause, M.A.; Savier, E.; Erisir, A.; Netland, P.A.; Cang, J.; Zhang, H.F.; Liu, X. Comparative In Vivo Imaging of Retinal Structures in Tree Shrews, Humans, and Mice. eNeuro 2024, 11, ENEURO.0373-23.2024. [Google Scholar] [CrossRef]
- Chong, S.P.; Merkle, C.W.; Leahy, C.; Radhakrishnan, H.; Srinivasan, V.J. Quantitative Microvascular Hemoglobin Mapping Using Visible Light Spectroscopic Optical Coherence Tomography. Biomed. Opt. Express 2015, 6, 1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Q.; Shu, X.; Soetikno, B.; Tong, S.; Zhang, H.F. Imaging Hemodynamic Response after Ischemic Stroke in Mouse Cortex Using Visible-Light Optical Coherence Tomography. Biomed. Opt. Express 2016, 7, 3377. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, A.; Harper, D.J.; Augustin, M.; Eugui, P.; Muck, M.; Gesperger, J.; Hitzenberger, C.K.; Woehrer, A.; Baumann, B. Spectroscopic Imaging with Spectral Domain Visible Light Optical Coherence Microscopy in Alzheimer’s Disease Brain Samples. Biomed. Opt. Express 2017, 8, 4007. [Google Scholar] [CrossRef]
- Beckmann, L.; Zhang, X.; Nadkarni, N.A.; Cai, Z.; Batra, A.; Sullivan, D.P.; Muller, W.A.; Sun, C.; Kuranov, R.; Zhang, H.F. Longitudinal Deep-Brain Imaging in Mouse Using Visible-Light Optical Coherence Tomography through Chronic Microprism Cranial Window. Biomed. Opt. Express 2019, 10, 5235. [Google Scholar] [CrossRef]
- Marchand, P.J.; Szlag, D.; Bouwens, A.; Lasser, T. In Vivo High-Resolution Cortical Imaging with Extended-Focus Optical Coherence Microscopy in the Visible-NIR Wavelength Range. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Merkle, C.W.; Chong, S.P.; Kho, A.M.; Zhu, J.; Dubra, A.; Srinivasan, V.J. Visible Light Optical Coherence Microscopy of the Brain with Isotropic Femtoliter Resolution in Vivo. Opt. Lett. 2018, 43, 198. [Google Scholar] [CrossRef]
- Lichtenegger, A.; Muck, M.; Eugui, P.; Harper, D.J.; Augustin, M.; Leskovar, K.; Hitzenberger, C.K.; Woehrer, A.; Baumann, B. Assessment of Pathological Features in Alzheimer’s Disease Brain Tissue with a Large Field-of-View Visible-Light Optical Coherence Microscope. Neurophotonics 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Revin, D.G.; Byers, R.A.; Duan, M.Q.; Li, W.; Matcher, S.J. Visible-Light Optical Coherence Tomography Platform for the Characterization of the Skin Barrier. Biomed. Opt. Express 2023, 14, 3914. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; McRaven, M.D.; Liu, W.; Shu, X.; Hu, J.; Sun, C.; Veazey, R.S.; Hope, T.J.; Zhang, H.F. Colposcopic Imaging Using Visible-Light Optical Coherence Tomography. J. Biomed. Opt. 2017, 22, 056003. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, J.A.; Eid, A.; Nguyen, T.Q.; Bui, T.; Yi, J.; Backman, V. In Vivo Broadband Visible Light Optical Coherence Tomography Probe Enables Inverse Spectroscopic Analysis. Opt. Lett. 2018, 43, 619. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, T.; Liu, P.; Yuan, W. Miniature Ultrahigh-Resolution Visible-Light OCT Endoscopy. In Endoscopic Microscopy XIX; Suter, M.J., Tearney, G.J., Wang, T.D., Eds.; SPIE: San Francisco, CA, USA, 2024; p. 27. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, T.; Abbasi, S.A.; Liu, P.; Yan, B.P.; Ng, S.H.C.; Yuan, W. Ultrathin Visible-Light OCT Endomicroscopy for Vivo Ultrah.-Resolut. Neuroimaging Deep Brain. APL Photonics 2024, 9, 110804. [Google Scholar] [CrossRef]
- Dai, C.; Liu, X.; Jiao, S. Simultaneous Optical Coherence Tomography and Autofluorescence Microscopy with a Single Light Source. J. Biomed. Opt. 2012, 17, 080502. [Google Scholar] [CrossRef]
- Nafar, Z.; Jiang, M.; Wen, R.; Jiao, S. Visible-Light Optical Coherence Tomography-Based Multimodal Retinal Imaging for Improvement of Fluorescent Intensity Quantification. Biomed. Opt. Express 2016, 7, 3220. [Google Scholar] [CrossRef]
- Nafar, Z.; Wen, R.; Jiao, S. Visible-Light Optical Coherence Tomography-Based Multimodal System for Quantitative Fundus Autofluorescence Imaging. Exp. Biol. Med. 2018, 243, 1265–1274. [Google Scholar] [CrossRef]
- Meng, R.; Gupta, A.K.; Kho, A.; Srinivasan, V.J. Imaging Melanin-Associated Organelles in the Retinal Pigment Epithelium via Visible Light OCT Redshift. Opt. Lett. 2025, 50, 475. [Google Scholar] [CrossRef]
- Wahl, D.J.; Jian, Y.; Bonora, S.; Zawadzki, R.J.; Sarunic, M.V. Wavefront Sensorless Adaptive Optics Fluorescence Biomicroscope for in Vivo Retinal Imaging in Mice. Biomed. Opt. Express 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.J.; Ng, R.; Ju, M.J.; Jian, Y.; Sarunic, M.V. Sensorless Adaptive Optics Multimodal En-Face Small Animal Retinal Imaging. Biomed. Opt. Express 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.J.; Huang, C.; Wahl, D.J.; Jian, Y.; Sarunic, M.V. Visible Light Sensorless Adaptive Optics for Retinal Structure and Fluorescence Imaging. Opt. Lett. 2018, 43, 5162. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, B.; Black, A.J.; Heilbronner, S.R.; Akkin, T. Visible Light Polarization-Sensitive Optical Coherence Tomography with Balanced Detection. J. Biomed. Opt. 2025, 30, 036002. [Google Scholar] [CrossRef]
- Malone, J.D.; Hussain, I.; Bowden, A.K. SmartOCT: Smartphone-Integrated Optical Coherence Tomography. Biomed. Opt. Express 2023, 14, 3138. [Google Scholar] [CrossRef]
- Ye, T.; Wang, J.; Yi, J. Deep Learning Network for Parallel Self-Denoising and Segmentation in Visible Light Optical Coherence Tomography of the Human Retina. Biomed. Opt. Express 2023, 14, 6088. [Google Scholar] [CrossRef]
- Wang, L.; Sahel, J.A.; Pi, S. Sub2Full: Split Spectrum to Boost Optical Coherence Tomography Despeckling without Clean Data. Opt. Lett. 2024, 49, 3062. [Google Scholar] [CrossRef]
- Ganjee, R.; Wang, B.; Wang, L.; Zhao, C.; Sahel, J.A.; Pi, S. BreakNet: Discontinuity-Resilient Multi-Scale Transformer Segmentation of Retinal Layers. Biomed. Opt. Express 2024, 15, 6725. [Google Scholar] [CrossRef]
- Guo, Y.; Hormel, T.T.; Pi, S.; Wei, X.; Gao, M.; Morrison, J.C.; Jia, Y. An End-to-End Network for Segmenting the Vasculature of Three Retinal Capillary Plexuses from OCT Angiographic Volumes. Biomed. Opt. Express 2021, 12, 4889. [Google Scholar] [CrossRef]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical Damage of the Retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef]
- Asghari, H. Visible Wavelength Time-Stretch Optical Coherence Tomography. Opt. Express 2023, 31, 24085. [Google Scholar] [CrossRef]
- Blair, N.P.; Tan, M.R.; Felder, A.E.; Teng, P.y.; Wanek, J.; Shahidi, M. Retinal Tissue Oxygen Tension and Consumption during Light Flicker Stimulation in Rat. Exp. Eye Res. 2018, 175, 207–211. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Song, C.; Kang, J.U. Motion-Compensated Hand-Held Common-Path Fourier-domain Optical Coherence Tomography Probe for Image-Guided Intervention. Biomed. Opt. Express 2012, 3, 3105–3118. [Google Scholar] [CrossRef]
- Fang, W.; Li, Q.; Fan, J.; Tang, N.; Yu, J.; Xu, H.; Zong, Y.; Jiang, C.; Shi, G.; Sun, X. Microscope-Integrated Intraoperative Optical Coherence Tomography for Anterior Segment Surgical Maneuvers. Transl. Vis. Sci. Technol. 2020, 9, 18. [Google Scholar] [CrossRef]
- Tao, J.; Chen, H.; Yu, J.; Cheng, D.; Chen, Y.; Mao, J.; Fang, J.; Shen, L. Feasibility and Utility of Intraoperative Optical Coherence Tomography during Vitreoretinal Surgery: A 4-Year Report in Chinese Population. J. Innov. Opt. Health Sci. 2021, 14, 2140004. [Google Scholar] [CrossRef]
- Zhu, D.; Larin, K.V.; Luo, Q.; Tuchin, V.V. Recent Progress in Tissue Optical Clearing. Laser Photonics Rev. 2013, 7, 732–757. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wang, S.; Li, B.; Wang, Z. Visible Light Optical Coherence Tomography: Technology and Biomedical Applications. Bioengineering 2025, 12, 770. https://doi.org/10.3390/bioengineering12070770
Wu S, Wang S, Li B, Wang Z. Visible Light Optical Coherence Tomography: Technology and Biomedical Applications. Bioengineering. 2025; 12(7):770. https://doi.org/10.3390/bioengineering12070770
Chicago/Turabian StyleWu, Songzhi, Shuo Wang, Baihan Li, and Zhao Wang. 2025. "Visible Light Optical Coherence Tomography: Technology and Biomedical Applications" Bioengineering 12, no. 7: 770. https://doi.org/10.3390/bioengineering12070770
APA StyleWu, S., Wang, S., Li, B., & Wang, Z. (2025). Visible Light Optical Coherence Tomography: Technology and Biomedical Applications. Bioengineering, 12(7), 770. https://doi.org/10.3390/bioengineering12070770





