Predictive Value of Optical Coherence Tomography Biomarkers in Patients with Persistent Diabetic Macular Edema Undergoing Cataract Surgery Combined with a Dexamethasone Intravitreal Implant
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedure
2.3. Data Collection
2.4. Statistical Analysis
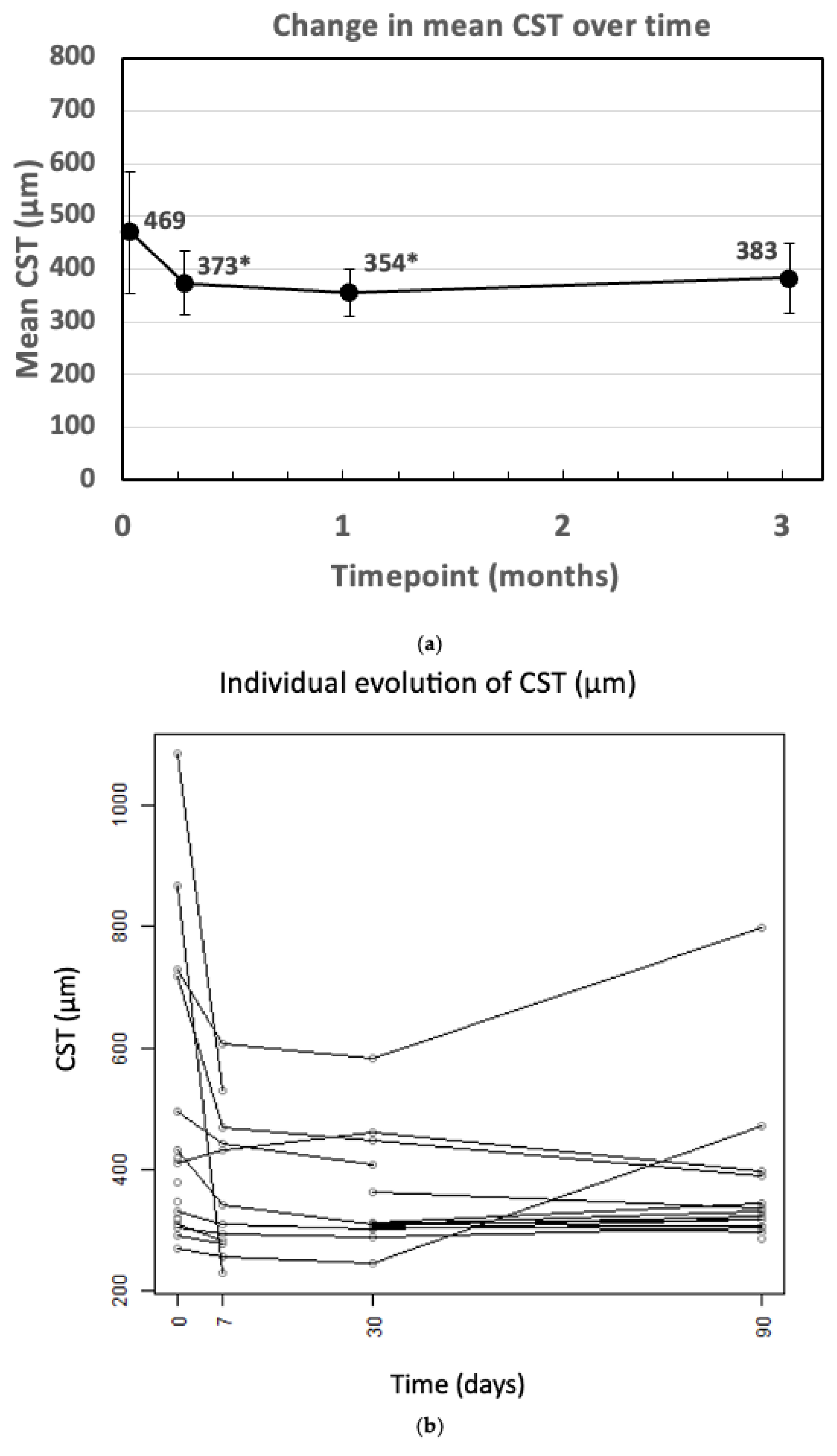
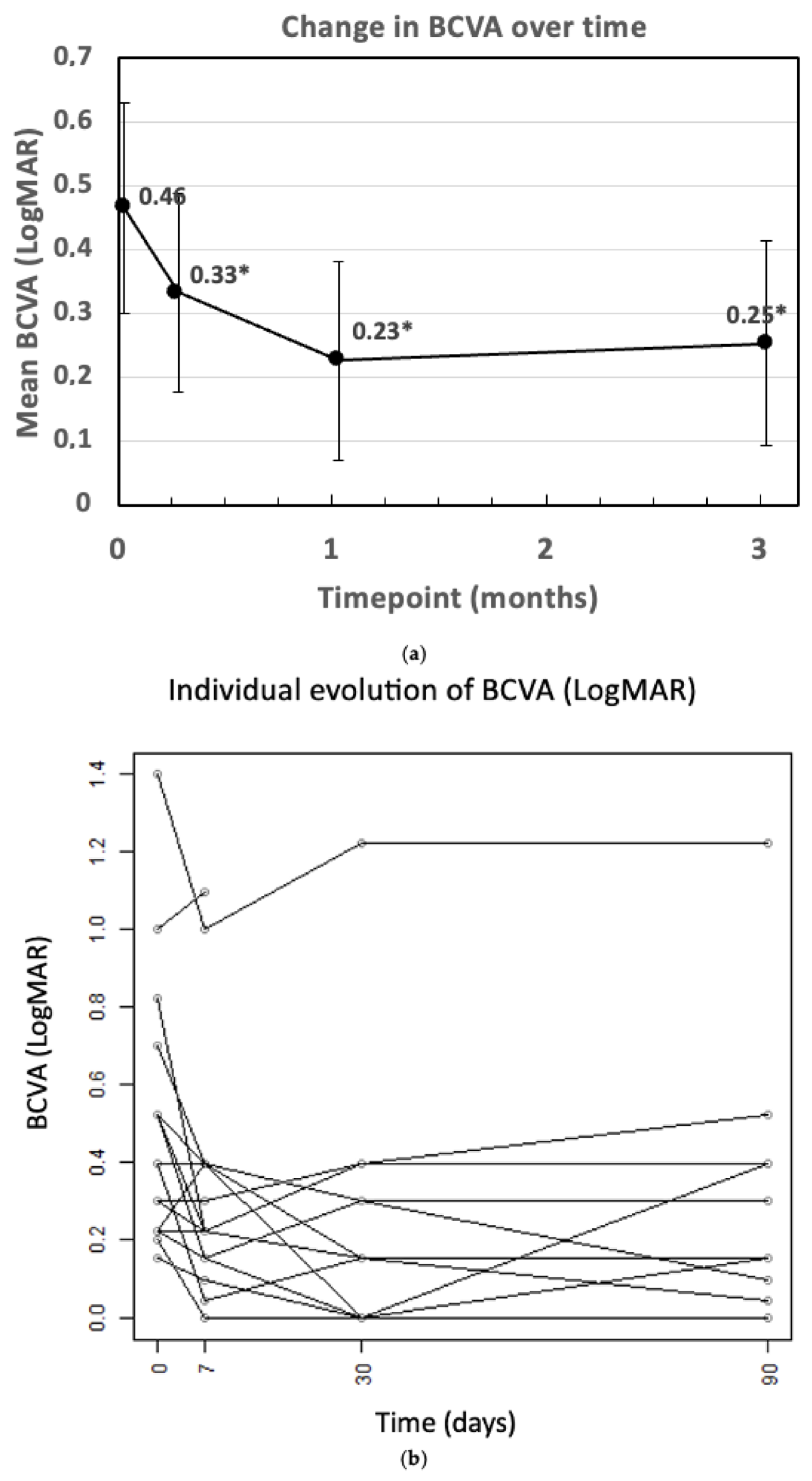
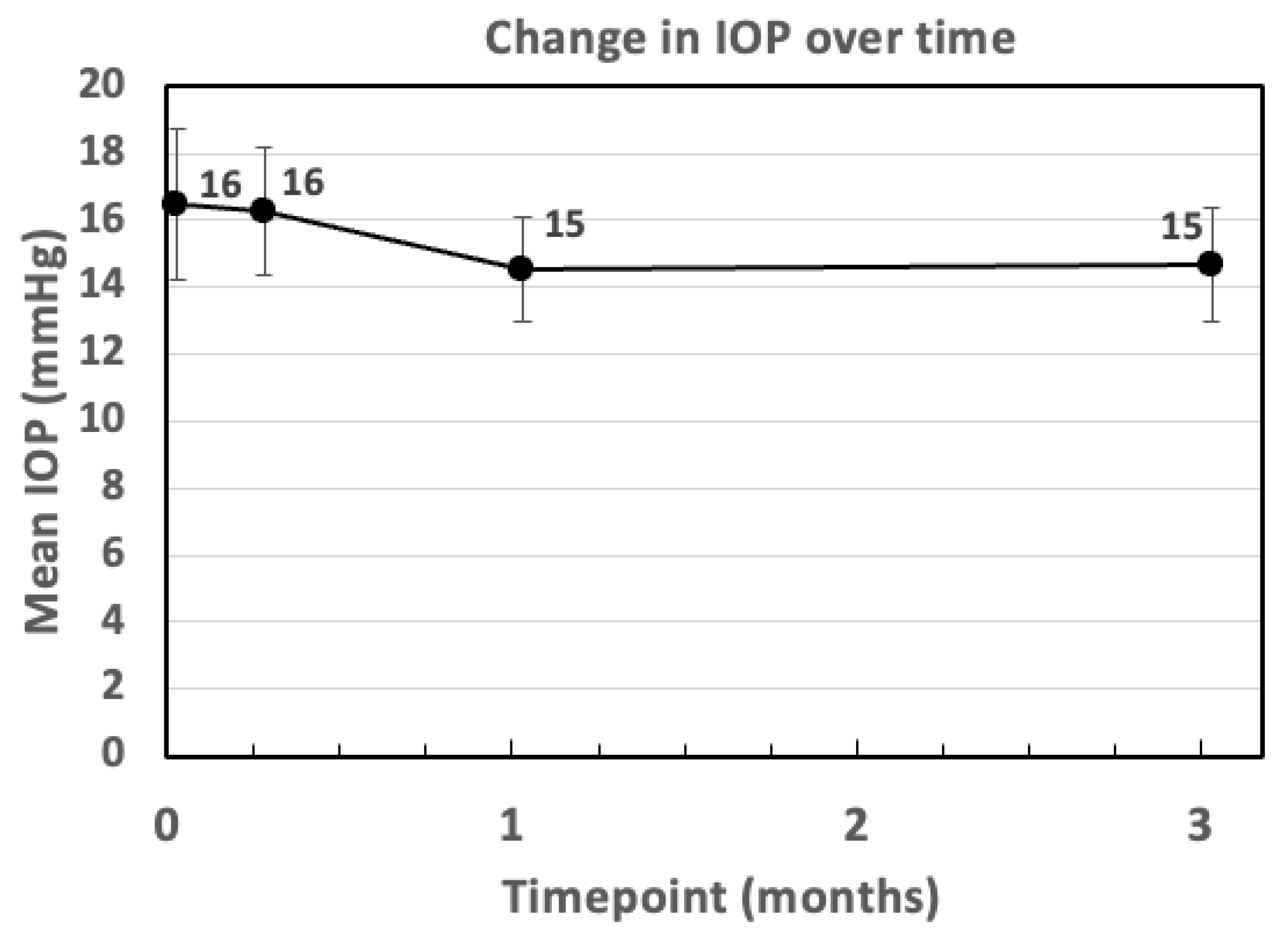
3. Results
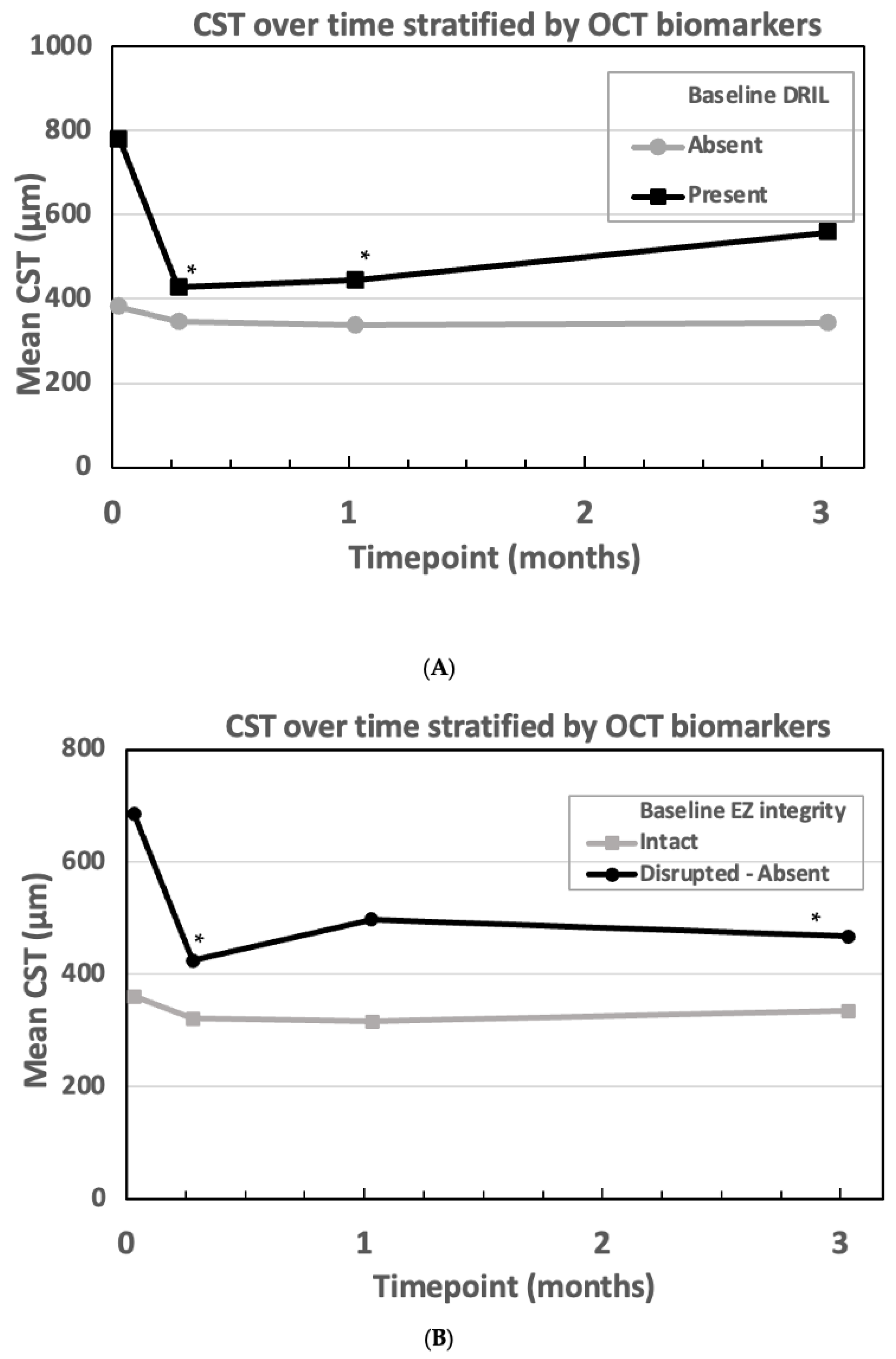
Correlation Between OCT Biomarkers and Treatment Response
4. Discussion
4.1. Comparing DEX-I with and Without Simultaneous Cataract Surgery
4.2. OCT Biomarkers and Their Role in Treatment Response
4.3. HbA1c and Treatment Response
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCVA | best-corrected visual acuity |
| CRT | central retinal thickness |
| CST | central subfoveal thickness |
| DDM | diabetes mellitus |
| DEX-I | dexamethasone intravitreal implant |
| DRIL | disorganization of the retinal inner layers |
| ELM | external limiting membrane |
| ETDRS | early treatment diabetic retinopathy sstudy |
| EZ | ellipsoid zone |
| HRF | hyperreflective foci |
| IOP | intraocular pressure |
| VEGF | vascular endothelial growth factor |
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Obrosova, I.G.; Chung, S.S.; Kador, P.F. Diabetic cataracts: Mechanisms and management. Diabetes Metab. Res. Rev. 2010, 26, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Musa, M.; Gagliano, C.; Zeppieri, M. The Treatment of Diabetic Retinal Edema with Intravitreal Steroids: How and When. J. Clin. Med. 2024, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.S.; Cheung, N.; Simó, R.; Cheung, G.C.; Wong, T.Y. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017, 5, 143–155. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Reibaldi, M.; Pizzo, A.; Russo, A.; Macchi, I.; Faro, G.; Avitabile, T.; Longo, A. Dexamethasone for unresponsive diabetic macular oedema: Optical coherence tomography biomarkers. Acta Ophthalmol. 2019, 97, e540–e544. [Google Scholar] [CrossRef]
- Taloni, A.; Coco, G.; Rastelli, D.; Buffon, G.; Scorcia, V.; Giannaccare, G. Safety and Efficacy of Dexamethasone Intravitreal Implant Given Either First-Line or Second-Line in Diabetic Macular Edema. Patient Prefer. Adher. 2023, 17, 3307–3329. [Google Scholar] [CrossRef]
- Sorour, O.A.; Levine, E.S.; Baumal, C.R.; Elnahry, A.G.; Braun, P.; Girgis, J.; Waheed, N.K. Persistent diabetic macular edema: Definition, incidence, biomarkers, and treatment methods. Surv. Ophthalmol. 2023, 68, 147–174. [Google Scholar] [CrossRef]
- Munk, M.R.; Somfai, G.M.; de Smet, M.D.; Donati, G.; Menke, M.N.; Garweg, J.G.; Ceklic, L. The Role of Intravitreal Corticosteroids in the Treatment of DME: Predictive OCT Biomarkers. Int. J. Mol. Sci. 2022, 23, 7585. [Google Scholar] [CrossRef]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic. Vis. Res. 2011, 6, 317–329. [Google Scholar]
- Bucolo, C.; Gozzo, L.; Longo, L.; Mansueto, S.; Vitale, D.C.; Drago, F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J. Pharmacol. Sci. 2018, 138, 219–232. [Google Scholar] [CrossRef]
- Furino, C.; Boscia, F.; Niro, A.; D’Addario, M.; Grassi, M.O.; Saglimbene, V.; Reibaldi, M.; Alessio, G. Diabetic macular edema and cataract surgery: Phacoemulsification Combined with Dexamethasone Intravitreal Implant Compared with Standard Phacoemulsification. RETINA 2021, 41, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Panozzo, G.; Cicinelli, M.V.; Augustin, A.J.; Battaglia Parodi, M.; Cunha-Vaz, J.; Guarnaccia, G.; Kodjikian, L.; Jampol, L.M.; Jünemann, A.; Lanzetta, P.; et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur. J. Ophthalmol. 2020, 30, 8–18. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M.; et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Lam, W.C.; Albiani, D.A.; Yoganathan, P.; Chen, J.C.; Kherani, A.; Maberley, D.A.; Oliver, A.; Rabinovitch, T.; Sheidow, T.G.; Tourville, E.; et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: The CHROME study. Clin. Ophthalmol. 2015, 9, 1255–1268. [Google Scholar] [CrossRef]
- Hernández Martínez, A.; Pereira Delgado, E.; Silva Silva, G.; Castellanos Mateos, L.; Lorente Pascual, J.; Lainez Villa, J.; García Vicente, P.; Almeida-González, C.V. Early versus late switch: How long should we extend the anti-vascular endothelial growth factor therapy in unresponsive diabetic macular edema patients? Eur. J. Ophthalmol. 2020, 30, 1091–1098. [Google Scholar] [CrossRef]
- Busch, C.; Zur, D.; Fraser-Bell, S.; Laíns, I.; Santos, A.R.; Lupidi, M.; Cagini, C.; Gabrielle, P.H.; Couturier, A.; Mané-Tauty, V.; et al. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018, 55, 789–796. [Google Scholar] [CrossRef]
- Lazic, R.; Lukic, M.; Boras, I.; Draca, N.; Vlasic, M.; Gabric, N.; Tomic, Z. Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. RETINA 2014, 34, 719–724. [Google Scholar] [CrossRef]
- Lobo, C. Pseudophakic cystoid macular edema. Ophthalmologica 2012, 227, 61–67. [Google Scholar] [CrossRef]
- Chawan-Saad, J.; Wu, M.; Wu, A.; Wu, L. Corticosteroids for Diabetic Macular Edema. Taiwan J. Ophthalmol. 2019, 9, 233–242. [Google Scholar] [CrossRef]
- Huang, H.; Jansonius, N.M.; Chen, H.; Los, L.I. Hyperreflective Dots on OCT as a Predictor of Treatment Outcome in Diabetic Macular Edema: A Systematic Review. Ophthalmol. Retina 2022, 6, 814–827. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Ma, Y.; Kwapong, W.R.; Ying, J.; Lu, J.; Ma, S.; Yan, Q.; Yi, Q.; Zhao, Y. Automated evaluation of retinal hyperreflective foci changes in diabetic macular edema patients before and after intravitreal injection. Front. Med. 2023, 10, 1280714. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.F.; Christensen, J.L.; Dahl, V.A.; Toosy, A.; Petzold, A.; Hanson, J.V.M.; Schippling, S.; Frederiksen, J.L.; Larsen, M. Automated detection of hyperreflective foci in the outer nuclear layer of the retina. Acta Ophthalmol. 2023, 101, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lim, J.; Lim, G.Y.S.; Ong, J.C.L.; Ke, Y.; Tan, T.F.; Tan, T.E.; Vujosevic, S.; Ting, D.S.W. Novel artificial intelligence algorithms for diabetic retinopathy and diabetic macular edema. Eye Vis. 2024, 11, 23. [Google Scholar] [CrossRef]
- Wahab Sait, A.R. A Lightweight Diabetic Retinopathy Detection Model Using a Deep-Learning Technique. Diagnostics 2023, 13, 3120. [Google Scholar] [CrossRef]
- Chou, T.H.; Wu, P.C.; Kuo, J.Z.; Lai, C.H.; Kuo, C.N. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye 2009, 23, 1360–1363. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Matsuda, S.; Tam, T.; Singh, R.P.; Kaiser, P.K.; Petkovsek, D.; Carneiro, G.; Zanella, M.T.; Ehlers, J.P. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J. Diabetes Complicat. 2014, 28, 166–170. [Google Scholar] [CrossRef]
- Ozturk, B.T.; Kerimoglu, H.; Adam, M.; Gunduz, K.; Okudan, S. Glucose regulation influences treatment outcome in ranibizumab treatment for diabetic macular edema. J. Diabetes Complicat. 2011, 25, 298–302. [Google Scholar] [CrossRef]
- Bansal, A.S.; Khurana, R.N.; Wieland, M.R.; Wang, P.W.; Van Everen, S.A.; Tuomi, L. Influence of Glycosylated Hemoglobin on the Efficacy of Ranibizumab for Diabetic Macular Edema: A Post Hoc Analysis of the RIDE/RISE Trials. Ophthalmology 2015, 122, 1573–1579. [Google Scholar] [CrossRef]
| Variables | Mean ± SD or N (%) |
|---|---|
| Age (years) | 62.7 ± 9.7 |
| Male Gender | 11 (100%) |
| History of diabetes (years) | 19.4 ± 9.1 |
| DM Type 1/Type 2 | 3 (17%)/15 (83%) |
| HbA1c (%) | 7.5 ± 1.1 |
| Systolic Blood Pressure (mmHg) | 127.6 ± 13.1 |
| Diastolic Blood Pressure (mmHg) | 71.0 ± 8.9 |
| Baseline | 1 Week | 1 Month | 3 Months | |
|---|---|---|---|---|
| CST (µm) | 469.44 ± 228.31 | 373.08 ± 120.33 | 354.36 ± 90.31 | 383.07 ± 132.60 |
| BCVA (LogMAR) | 0.46 ± 0.33 | 0.33 ± 0.31 | 0.23 ± 0.31 | 0.25 ± 0.32 |
| IOP (mmHg) | 16.47 ± 4.53 | 16.26 ± 3.61 | 14.53 ± 2.85 | 14.67 ± 3.09 |
| Baseline Variables | Mean (±SD) or N (%) |
|---|---|
| BCVA (LogMAR) | 0.46 (±0.33) |
| CST (µm) | 469.4 (±228.31) |
| EZ/ELM | |
| 12 (66.7%) |
| 6 (33.3%) |
| DRIL | |
| 14 (77.8%) |
| 4 (22.2%) |
| HRF (1 missing) | |
| 6 (33.3%) |
| 11 (61.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasolino, G.; Lazaar, M.; Della Rocca, D.G.; Oellerich, S.; Ní Dhubhghaill, S. Predictive Value of Optical Coherence Tomography Biomarkers in Patients with Persistent Diabetic Macular Edema Undergoing Cataract Surgery Combined with a Dexamethasone Intravitreal Implant. Bioengineering 2025, 12, 556. https://doi.org/10.3390/bioengineering12050556
Fasolino G, Lazaar M, Della Rocca DG, Oellerich S, Ní Dhubhghaill S. Predictive Value of Optical Coherence Tomography Biomarkers in Patients with Persistent Diabetic Macular Edema Undergoing Cataract Surgery Combined with a Dexamethasone Intravitreal Implant. Bioengineering. 2025; 12(5):556. https://doi.org/10.3390/bioengineering12050556
Chicago/Turabian StyleFasolino, Giuseppe, Maryam Lazaar, Domenico Giovanni Della Rocca, Silke Oellerich, and Sorcha Ní Dhubhghaill. 2025. "Predictive Value of Optical Coherence Tomography Biomarkers in Patients with Persistent Diabetic Macular Edema Undergoing Cataract Surgery Combined with a Dexamethasone Intravitreal Implant" Bioengineering 12, no. 5: 556. https://doi.org/10.3390/bioengineering12050556
APA StyleFasolino, G., Lazaar, M., Della Rocca, D. G., Oellerich, S., & Ní Dhubhghaill, S. (2025). Predictive Value of Optical Coherence Tomography Biomarkers in Patients with Persistent Diabetic Macular Edema Undergoing Cataract Surgery Combined with a Dexamethasone Intravitreal Implant. Bioengineering, 12(5), 556. https://doi.org/10.3390/bioengineering12050556






