A Review of Bioelectrochemical Strategies for Enhanced Polyhydroxyalkanoate Production
Abstract
1. Introduction
2. Polyhydroxyalkanoates (PHAs): Characteristics and Production
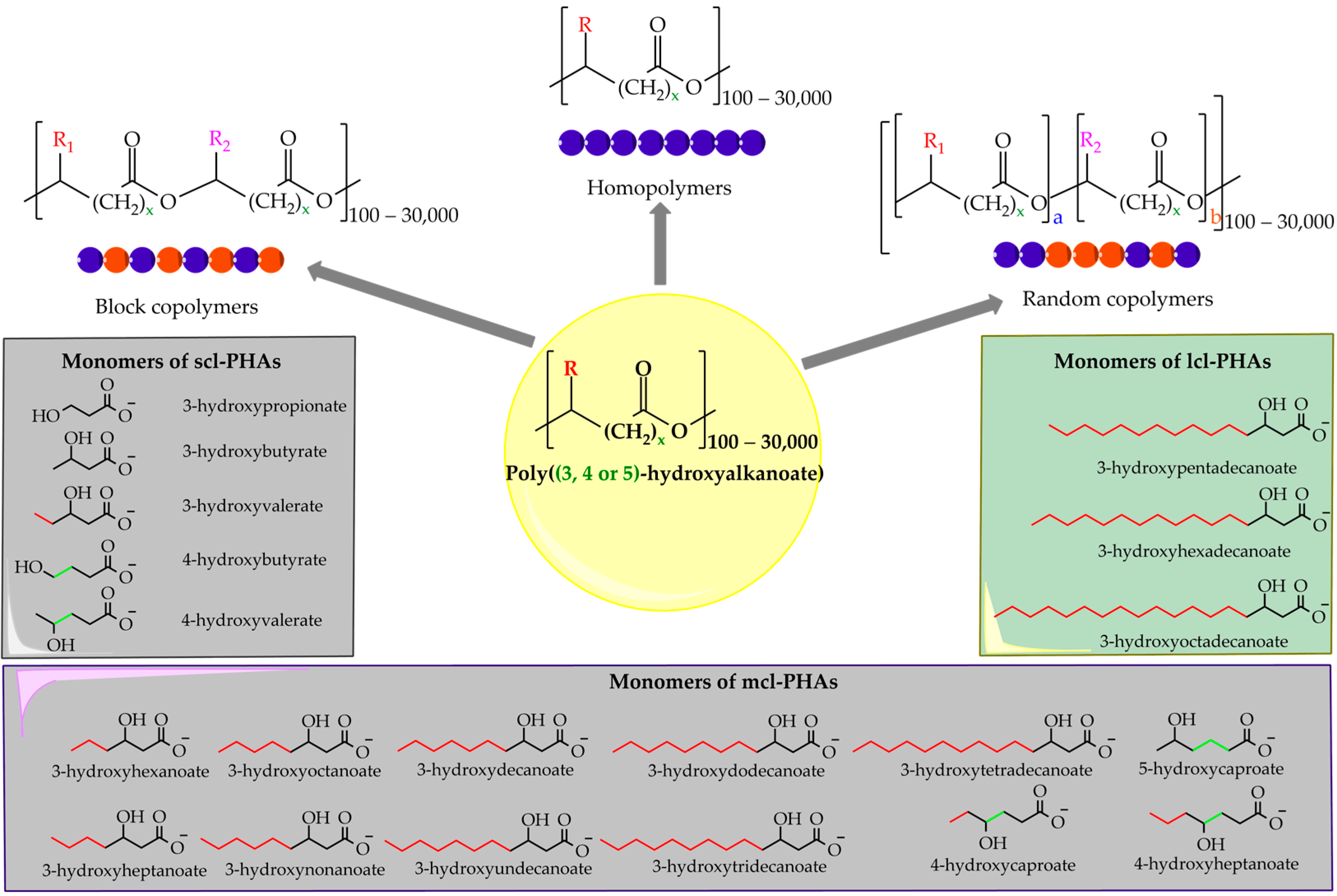
2.1. Types of PHA
2.2. Microorganisms Involved in PHA Production
2.3. Growth Conditions and Carbon Sources
3. Bioelectrochemistry Applied to PHA Production
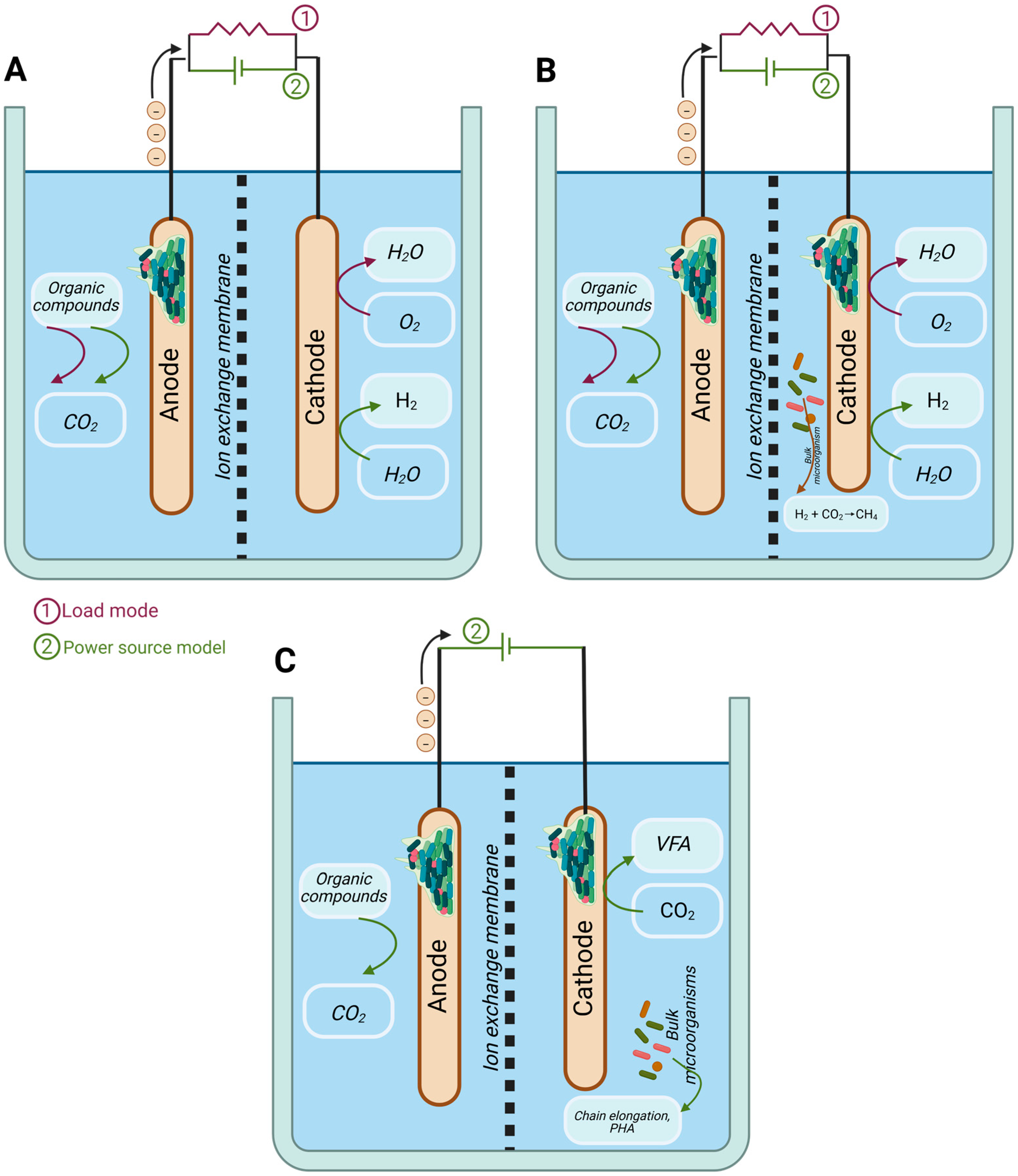
3.1. Principles of Bioelectrochemical Systems (BES)
3.2. Reactor Configurations and Limitations in Microbial Electrosynthesis for Value-Added Chemicals
4. Enhancing PHA Production Through Bioelectrochemical Technologies
4.1. Metabolic Optimization via Electrical Stimulation
4.2. Alternative Carbon Sources and Their Bioelectrochemical Conversion
4.2.1. Agricultural Residues
4.2.2. Industrial Byproducts
4.2.3. CO2 Fixation
4.3. Electrodes as Electron Acceptors and Donors
5. State of the Art and Recent Advances
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taguchi, S.; Matsumoto, K. Evolution of Polyhydroxyalkanoate Synthesizing Systems Toward a Sustainable Plastic Industry. Polym. J. 2021, 53, 67–79. [Google Scholar] [CrossRef]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to Bioplastics: How Close Are We to Sustainable Polyhydroxyalkanoates Production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef]
- Getino, L.; Martín, J.L.; Chamizo-Ampudia, A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms 2024, 12, 2028. [Google Scholar] [CrossRef]
- Alvarez Chavez, B.; Raghavan, V.; Tartakovsky, B. A Comparative Analysis of Biopolymer Production by Microbial and Bioelectrochemical Technologies. RSC Adv. 2022, 12, 16105–16118. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Pandya, R.S.; Kaur, T.; Bhattacharya, R.; Bose, D.; Saraf, D. Harnessing Microorganisms for Bioenergy with Microbial Fuel Cells: Powering the Future. Water-Energy Nexus 2024, 7, 1–12. [Google Scholar] [CrossRef]
- Nastro, R.A.; Kuppam, C.; Toscanesi, M.; Trifuoggi, M.; Pietrelli, A.; Pasquale, V.; Avignone-Rossa, C. Bio-Electrosynthesis of Polyhydroxybutyrate and Surfactants in Microbial Fuel Cells: A Preliminary Study. Front. Microbiol. 2025, 16, 1372302. [Google Scholar] [CrossRef]
- Chaijak, P.; Rakkan, T.; Paichaid, N.; Thipraksa, J.; Michu, P.; Sangkharak, K. Exploring Potential Aspect of Microbial Fuel Cell (MFC) for Simultaneous Energy, Polyhydroxyalkanoate (PHA) Production and Textile Wastewater (TW) Treatment. J. Polym. Environ. 2024, 32, 3104–3118. [Google Scholar] [CrossRef]
- Tsipa, A.; Varnava, C.K.; Nastro, R.A.; Ieropoulos, I. Biosurfactants, Polyhydroxyalkanoates, and Other Added-Value Products from Wastewater Electro-Bioremediation: A New Biorefinery Concept. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Aghaali, Z.; Naghavi, M.R. Biotechnological Approaches for Enhancing Polyhydroxyalkanoates (PHAs) Production: Current and Future Perspectives. Curr. Microbiol. 2023, 80, 345. [Google Scholar] [CrossRef]
- Khan, A.K.; Anjum, I.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview on Feasible Production of Bioplastic Polyhydroxyalkanoate (PHA) in Transgenic Plants. In Bioplastics for Sustainable Development; Springer: Singapore, 2021; pp. 555–579. [Google Scholar]
- Kim, G.B.; Choi, S.Y.; Cho, I.J.; Ahn, D.-H.; Lee, S.Y. Metabolic Engineering for Sustainability and Health. Trends Biotechnol. 2023, 41, 425–451. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Pathak, L.; Vyas, P. Biobased Polymers of Plant and Microbial Origin and Their Applications—A Review. Biotechnol. Sustain. Mater. 2024, 1, 13. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and Physical Properties of Polyhydroxyalkanoate (PHA)-Based Block Copolymers: A Review. Int. J. Biol. Macromol. 2024, 263, 130204. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Singh, R.; Selvaraj, M.; Malik, T.; Al Tawaha, A.R.M. Natural Biopolymers in Edible Coatings: Applications in Food Preservation. Food Chem. X 2025, 25, 102171. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, K.; Aversa, C.; Nofar, M.; Barletta, M.; Gisario, A.; Salehiyan, R.; Goksu, Y.A. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBH): Synthesis, Properties, and Applications—A Review. Eur. Polym. J. 2022, 167, 111044. [Google Scholar] [CrossRef]
- Zainuddin, M.Z.; Abu Bakar, A.A.; Adam, A.N.; Abdullah, S.M.; Tamchek, N.; Alauddin, M.S.; Mahat, M.M.; Wiwatcharagoses, N.; Alforidi, A.; Ghazali, M.I.M. Mechanical and Structural Properties of Polyhydroxybutyrate as Additive in Blend Material in Additive Manufacturing for Medical Applications. Polymers 2023, 15, 1849. [Google Scholar] [CrossRef]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Râpă, M.; Stefan, L.M.; Seciu-Grama, A.-M.; Gaspar-Pintiliescu, A.; Matei, E.; Zaharia, C.; Stănescu, P.O.; Predescu, C. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (P(3HB-Co-3HV))/Bacterial Cellulose (BC) Biocomposites for Potential Use in Biomedical Applications. Polymers 2022, 14, 5544. [Google Scholar] [CrossRef]
- Abbasi, M.; Pokhrel, D.; Coats, E.R.; Guho, N.M.; McDonald, A.G. Effect of 3-Hydroxyvalerate Content on Thermal, Mechanical, and Rheological Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Biopolymers Produced from Fermented Dairy Manure. Polymers 2022, 14, 4140. [Google Scholar] [CrossRef]
- Katagi, V.; Vytla, R.M.; Somashekara, D. Integrated Production of Microbial Biopolymer (PHA) with Other Value-Added Bioproducts: An Innovative Approach for Sustainable Production. Green Chem. Lett. Rev. 2024, 17, 2289983. [Google Scholar] [CrossRef]
- Volova, T.G.; Uspenskaya, M.V.; Kiselev, E.G.; Sukovatyi, A.G.; Zhila, N.O.; Vasiliev, A.D.; Shishatskaya, E.I. Effect of Monomers of 3-Hydroxyhexanoate on Properties of Copolymers Poly(3-Hydroxybutyrate-Co 3-Hydroxyhexanoate). Polymers 2023, 15, 2890. [Google Scholar] [CrossRef]
- Thiele, I.; Santolin, L.; Meyer, K.; Machatschek, R.; Bölz, U.; Tarazona, N.A.; Riedel, S.L. Microbially Synthesized Poly(Hydroxybutyrate-Co-Hydroxyhexanoate) with Low to Moderate Hydroxyhexanoate Content: Properties and Applications. Int. J. Biol. Macromol. 2024, 263, 130188. [Google Scholar] [CrossRef] [PubMed]
- Getino, L.; García, I.; Cornejo, A.; Mateos, R.; Ariza-Carmona, L.M.; Sánchez-Castro, N.; Moran, J.F.; Olivera, E.R.; Chamizo-Ampudia, A. The Effectiveness of Polyhydroxyalkanoate (PHA) Extraction Methods in Gram-Negative Pseudomonas putida U. Polymers 2025, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Alzate, M.O.; Leonhardt, S.; Tamang, P.; Zibek, S. Current Trends in Medium-chain-length Polyhydroxyalkanoates: Microbial Production, Purification, and Characterization. Eng. Life Sci. 2024, 24, e2300211. [Google Scholar] [CrossRef]
- Panaksri, A.; Tanadchangsaeng, N. Evaluation of 3D-Printing Scaffold Fabrication on Biosynthetic Medium-Chain-Length Polyhydroxyalkanoate Terpolyester as Biomaterial-Ink. Polymers 2021, 13, 2222. [Google Scholar] [CrossRef]
- Pospisilova, A.; Vodicka, J.; Trudicova, M.; Juglova, Z.; Smilek, J.; Mencik, P.; Masilko, J.; Slaninova, E.; Melcova, V.; Kalina, M.; et al. Effects of Differing Monomer Compositions on Properties of P(3HB-Co-4HB) Synthesized by Aneurinibacillus Sp. H1 for Various Applications. Polymers 2022, 14, 2007. [Google Scholar] [CrossRef]
- Huong, K.-H.; Sevakumaran, V.; Amirul, A.A. P(3HB-4HB) as High Value Polyhydroxyalkanoate: Its Development over Recent Decades and Current Advances. Crit. Rev. Biotechnol. 2021, 41, 474–490. [Google Scholar] [CrossRef]
- Min Song, H.; Chan Joo, J.; Hyun Lim, S.; Jin Lim, H.; Lee, S.; Jae Park, S. Production of Polyhydroxyalkanoates Containing Monomers Conferring Amorphous and Elastomeric Properties from Renewable Resources: Current Status and Future Perspectives. Bioresour. Technol. 2022, 366, 128114. [Google Scholar] [CrossRef]
- Derippe, G.; Philip, L.; Lemechko, P.; Eyheraguibel, B.; Meistertzheim, A.-L.; Pujo-Pay, M.; Conan, P.; Barbe, V.; Bruzaud, S.; Ghiglione, J.-F. Marine Biodegradation of Tailor-Made Polyhydroxyalkanoates (PHA) Influenced by the Chemical Structure and Associated Bacterial Communities. J. Hazard. Mater. 2024, 462, 132782. [Google Scholar] [CrossRef]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) Synthesis and Degradation by Microbes and Applications towards a Circular Economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Hadri, S.H.; Tareen, N.; Hassan, A.; Naseer, M.; Ali, K.; Javed, H. Alternatives to Conventional Plastics: Polyhydroxyalkanoates (PHA) from Microbial Sources and Recent Approaches—A Review. Process Saf. Environ. Prot. 2025, 195, 106809. [Google Scholar] [CrossRef]
- Ramanaiah, S.V.; Chandrasekhar, K.; Cordas, C.M.; Potoroko, I. Bioelectrochemical Systems (BESs) for Agro-Food Waste and Wastewater Treatment, and Sustainable Bioenergy-A Review. Environ. Pollut. 2023, 325, 121432. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Manoli, M.-T.; Nogales, J.; Prieto, A. Synthetic Control of Metabolic States in Pseudomonas Putida by Tuning Polyhydroxyalkanoate Cycle. mBio 2022, 13, e0179421. [Google Scholar] [CrossRef]
- Santolin, L.; Riedel, S.L.; Brigham, C.J. Synthetic Biology Toolkit of Ralstonia Eutropha (Cupriavidus Necator). Appl. Microbiol. Biotechnol. 2024, 108, 450. [Google Scholar] [CrossRef] [PubMed]
- Tanikkul, P.; Sullivan, G.L.; Sarp, S.; Pisutpaisal, N. Biosynthesis of Medium Chain Length Polyhydroxyalkanoates (Mcl-PHAs) from Palm Oil. Case Stud. Chem. Environ. Eng. 2020, 2, 100045. [Google Scholar] [CrossRef]
- Olivera, E.R.; Luengo, J.M. Engineering Strategies for Efficient and Sustainable Production of Medium-Chain Length Polyhydroxyalkanoates in Pseudomonads. In Bioplastics for Sustainable Development; Springer: Singapore, 2021; pp. 581–660. [Google Scholar]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering Native and Synthetic Pathways in Pseudomonas Putida for the Production of Tailored Polyhydroxyalkanoates. Biotechnol. J. 2021, 16, 2000165. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Sánchez, C.; Espín, G.; Peña, C.; Segura, D. The Modification of Regulatory Circuits Involved in the Control of Polyhydroxyalkanoates Metabolism to Improve Their Production. Front. Bioeng. Biotechnol. 2020, 8, 386. [Google Scholar] [CrossRef]
- García, A.; Segura, D.; Espín, G.; Galindo, E.; Castillo, T.; Peña, C. High Production of Poly-β-Hydroxybutyrate (PHB) by an Azotobacter Vinelandii Mutant Altered in PHB Regulation Using a Fed-Batch Fermentation Process. Biochem. Eng. J. 2014, 82, 117–123. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Koller, M.; Miranda-de Sousa Dias, M.; Calafell-Monfort, M.; Braunegg, G.; Marqués-Calvo, M.S. High Production of Poly(3-Hydroxybutyrate) from a Wild Bacillus Megaterium Bolivian Strain. J. Appl. Microbiol. 2013, 114, 1378–1387. [Google Scholar] [CrossRef]
- Velloso, C.C.V.; Camargo, B.C.P.; Sousa, M.D.B.; Buffo, M.M.; de Oliveira Paiva, C.A.; Farinas, C.S.; Badino, A.C. High Yield of Heat-Resistant Spores of Bacillus Megaterium in Bioreactors. Biochem. Eng. J. 2023, 198, 109030. [Google Scholar] [CrossRef]
- Wang, B.; Sharma-Shivappa, R.R.; Olson, J.W.; Khan, S.A. Production of Polyhydroxybutyrate (PHB) by Alcaligenes Latus Using Sugarbeet Juice. Ind. Crops. Prod. 2013, 43, 802–811. [Google Scholar] [CrossRef]
- Paduvari, R.; Somashekara, D.M. Advancements in Genetic Engineering for Enhanced Polyhydroxyalkanoates (PHA) Production: A Comprehensive Review of Metabolic Pathway Manipulation and Gene Deletion Strategies. Bioengineered 2025, 16, 2458363. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current Developments on Polyhydroxyalkanoates Synthesis by Using Halophiles as a Promising Cell Factory. Microb. Cell Fact. 2020, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dzakpasu, M.; Wang, X.; Zhang, L.; Ngo, H.H.; Guo, W.; Zhao, Y. Molecular Characterization of Long-Term Impacts of Macrophytes Harvest Management in Constructed Wetlands. Bioresour. Technol. 2018, 268, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Matsuoka, T.; Hosomi, K.; Park, J.; Nishimura, M.; Murakami, H.; Konishi, K.; Miyachi, M.; Kawashima, H.; Mizuguchi, K.; et al. Characteristic Gut Bacteria in High Barley Consuming Japanese Individuals without Hypertension. Microorganisms 2023, 11, 1246. [Google Scholar] [CrossRef]
- Ng, C.A.; Pernica, M.; Litvanova, K.; Kolouchova, I.; Branyik, T. Biocontrol Using Pythium Oligandrum during Malting of Fusarium-Contaminated Barley. Fermentation 2023, 9, 257. [Google Scholar] [CrossRef]
- Chin, J.H.-C.; Samian, M.R.; Normi, Y.M. Characterization of Polyhydroxyalkanoate Production Capacity, Composition and Weight Synthesized by Burkholderia Cepacia JC-1 from Various Carbon Sources. Heliyon 2022, 8, e09174. [Google Scholar] [CrossRef]
- Rogiers, T.; Merroun, M.L.; Williamson, A.; Leys, N.; Van Houdt, R.; Boon, N.; Mijnendonckx, K. Cupriavidus Metallidurans NA4 Actively Forms Polyhydroxybutyrate-Associated Uranium-Phosphate Precipitates. J. Hazard. Mater. 2022, 421, 126737. [Google Scholar] [CrossRef]
- Flüchter, S.; Follonier, S.; Schiel-Bengelsdorf, B.; Bengelsdorf, F.R.; Zinn, M.; Dürre, P. Anaerobic Production of Poly(3-Hydroxybutyrate) and Its Precursor 3-Hydroxybutyrate from Synthesis Gas by Autotrophic Clostridia. Biomacromolecules 2019, 20, 3271–3282. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A Review on Biological Synthesis of the Biodegradable Polymers Polyhydroxyalkanoates and the Development of Multiple Applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Mohanrasu, K.; Guru Raj Rao, R.; Dinesh, G.H.; Zhang, K.; Sudhakar, M.; Pugazhendhi, A.; Jeyakanthan, J.; Ponnuchamy, K.; Govarthanan, M.; Arun, A. Production and Characterization of Biodegradable Polyhydroxybutyrate by Micrococcus Luteus Isolated from Marine Environment. Int. J. Biol. Macromol. 2021, 186, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jamil, K.B. Synthesis of Environmental Bioplastic Polyhydroxyalkanoate (PHA) from Waste Glycerol, Palm Oil and Different Concentrations of Glucose by A New Strain Propionibacterium Sp. Iraqi J. Ind. Res. 2022, 9, 175–186. [Google Scholar] [CrossRef]
- Oyewole, O.A.; Abdulmalik, S.U.; Abubakar, A.O.; Chimbekujwo, K.I.; Obafemi, Y.D.; Oyegbile, B.; Abioye, O.P.; Adeniyi, O.D.; Egwim, E.C. Production of Polyhydroxyalkanoate (Pha) by Pseudomonas Aeruginosa (Ol405443) Using Agrowastes as Carbon Source. Clean. Mater. 2024, 11, 100217. [Google Scholar] [CrossRef]
- Song, H.M.; Jo, S.Y.; Lee, H.; Jeon, S.; Yun, D.; Kim, C.; Son, J.; Sohn, Y.J.; Choi, J.-I.; Park, S.J. Recent Advances on the Systems Metabolically Engineered Pseudomonas Species as Versatile Biosynthetic Platforms for the Production of Polyhydroxyalkanoates. Syst. Microbiol. Biomanuf. 2024, 4, 473–499. [Google Scholar] [CrossRef]
- Ranaivoarisoa, T.O.; Bai, W.; Karthikeyan, R.; Steele, H.; Silberman, M.; Olabode, J.; Conners, E.; Gallagher, B.; Bose, A. Overexpression of RuBisCO Form I and II Genes in Rhodopseudomonas Palustris TIE-1 Augments Polyhydroxyalkanoate Production Heterotrophically and Autotrophically. Appl. Environ. Microbiol. 2024, 90, e01438-24. [Google Scholar] [CrossRef]
- González-Resendiz, L.; Sánchez-García, L.; Hernández-Martínez, I.; Vigueras-Ramírez, G.; Jiménez-García, L.F.; Lara-Martínez, R.; Morales-Ibarría, M. Photoautotrophic Poly(3-Hydroxybutyrate) Production by a Wild-Type Synechococcus Elongatus Isolated from an Extreme Environment. Bioresour. Technol. 2021, 337, 125508. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aravena, A.C.; Yunus, K.; Zhang, L.; Norling, B.; Fisher, A.C. Tapping into Cyanobacteria Electron Transfer for Higher Exoelectrogenic Activity by Imposing Iron Limited Growth. RSC Adv. 2018, 8, 20263–20274. [Google Scholar] [CrossRef]
- Zhila, N.O.; Kiselev, E.G.; Shishatskaya, E.I.; Ghorabe, F.D.E.; Kazachenko, A.S.; Volova, T.G. Comparative Study of the Synthesis of Polyhydroxyalkanoates by Cyanobacteria Spirulina Platensis and Green Microalga Chlorella Vulgaris. Algal Res. 2025, 85, 103826. [Google Scholar] [CrossRef]
- Longtin, N.; Oliveira, D.; Mahadevan, A.; Gejji, V.; Gomes, C.; Fernando, S. Analysis of Spirulina Platensis Microalgal Fuel Cell. J. Power Sources 2021, 486, 229290. [Google Scholar] [CrossRef]
- Elma Karakaş, D.; Akdemir, M.; Atabani, A.E.; Kaya, M. A Dual Functional Material: Spirulina Platensis Waste-Supported Pd-Co Catalyst as a Novel Promising Supercapacitor Electrode. Fuel 2021, 304, 121334. [Google Scholar] [CrossRef]
- De Caprariis, B.; De Filippis, P.; Di Battista, A.; Di Palma, L.; Scarsella, M. Exoelectrogenic Activity of a Green Microalgae, Chlorella Vulgaris, in a Bio-Photovoltaic Cells (BPVs). Chem. Eng. Trans. 2014, 38, 523–528. [Google Scholar]
- Song, X.; Wang, W.; Cao, X.; Wang, Y.; Zou, L.; Ge, X.; Zhao, Y.; Si, Z.; Wang, Y. Chlorella Vulgaris on the Cathode Promoted the Performance of Sediment Microbial Fuel Cells for Electrogenesis and Pollutant Removal. Sci. Total Environ. 2020, 728, 138011. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent Developments in Polyhydroxyalkanoates (PHAs) Production—A Review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef]
- Kumar Sachan, R.S.; Devgon, I.; Mohammad Said Al-Tawaha, A.R.; Karnwal, A. Optimizing Polyhydroxyalkanoate Production Using a Novel Bacillus Paranthracis Isolate: A Response Surface Methodology Approach. Heliyon 2024, 10, e35398. [Google Scholar] [CrossRef]
- Ibrahim, R.; Aranjani, J.M.; Prasanna, N.; Biswas, A.; Gayam, P.K.R. Production, Isolation, Optimization, and Characterization of Microbial PHA from Bacillus Australimaris. Sci. Rep. 2025, 15, 8395. [Google Scholar] [CrossRef]
- Abd-El-Haleem, D.A.M.; Elkatory, M.R.; Abu-Elreesh, G.M. Uncovering Novel Polyhydroxyalkanoate Biosynthesis Genes and Unique Pathway in Yeast Hanseniaspora Valbyensis for Sustainable Bioplastic Production. Sci. Rep. 2024, 14, 27162. [Google Scholar] [CrossRef]
- Chien Bong, C.P.; Alam, M.N.H.Z.; Samsudin, S.A.; Jamaluddin, J.; Adrus, N.; Mohd Yusof, A.H.; Muis, Z.A.; Hashim, H.; Salleh, M.M.; Abdullah, A.R.; et al. A Review on the Potential of Polyhydroxyalkanoates Production from Oil-Based Substrates. J. Environ. Manag. 2021, 298, 113461. [Google Scholar] [CrossRef]
- Carvalho, A.C.F.; Ghosh, S.; Hoffmann, T.G.; Prudêncio, E.S.; de Souza, C.K.; Roy, S. Valuing Agro-Industrial Waste in the Development of Sustainable Food Packaging Based on the System of a Circular Bioeconomy: A Review. Clean. Waste Syst. 2025, 11, 100275. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Sabita, A.; Putri, N.A.; Azliza, N.; Illiyanasafa, N.; Darmokoesoemo, H.; Amenaghawon, A.N.; Kurniawan, T.A. Waste to Wealth: Polyhydroxyalkanoates (PHA) Production from Food Waste for a Sustainable Packaging Paradigm. Food Chem. Mol. Sci. 2024, 9, 100225. [Google Scholar] [CrossRef]
- Alaghemandi, M. Sustainable Solutions Through Innovative Plastic Waste Recycling Technologies. Sustainability 2024, 16, 10401. [Google Scholar] [CrossRef]
- Zong, Z.; Rao, C.; Du, C.; Lu, R.; Upham, D.C. Polyhydroxyalkanoates (PHA) Production in a Circular CO2 Economy: It’s Role in Mitigating Global CO2 Emissions. Resour. Conserv. Recycl. 2025, 219, 108303. [Google Scholar] [CrossRef]
- Yadav, B.S.; Yadav, R.B.; Kumari, M.; Khatkar, B.S. Studies on Suitability of Wheat Flour Blends with Sweet Potato, Colocasia and Water Chestnut Flours for Noodle Making. LWT-Food Sci. Technol. 2014, 57, 352–358. [Google Scholar] [CrossRef]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Production of Polyhydroxyalkanoates (PHAs) from Waste Materials and by-Products by Submerged and Solid-State Fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A Review on Polyhydroxyalkanoate Production from Agricultural Waste Biomass: Development, Advances, Circular Approach, and Challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biodiesel Industry Waste: A Potential Source of Bioenergy and Biopolymers. Indian J. Microbiol. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- Islam, M.; Xayachak, T.; Haque, N.; Lau, D.; Bhuiyan, M.; Kumar Pramanik, B. Impact of Bioplastics on Environment from Its Production to End-of-Life. Process Saf. Environ. Prot. 2024, 188, 155–166. [Google Scholar] [CrossRef]
- Guo, R.; Cen, X.; Ni, B.-J.; Zheng, M. Bioplastic Polyhydroxyalkanoate Conversion in Waste Activated Sludge. J. Environ. Manag. 2024, 370, 122866. [Google Scholar] [CrossRef]
- Madhusoodanan, G.; Chandrashekar Hariharapura, R.; Somashekara, D. Dissolved Oxygen as a Propulsive Parameter for Polyhydroxyalkanoate Production Using Bacillus Endophyticus Cultures. Environ. Dev. Sustain. 2021, 24, 4641–4658. [Google Scholar] [CrossRef]
- Muigano, M.N.; Mauti, G.O.; Anami, S.E.; Onguso, J.M. Advances and Challenges in Polyhydroxyalkanoates (PHA) Production Using Halomonas Species: A Review. Int. J. Biol. Macromol. 2025, 309, 142850. [Google Scholar] [CrossRef]
- Tang, R.; Weng, C.; Peng, X.; Han, Y. Metabolic Engineering of Cupriavidus Necator H16 for Improved Chemoautotrophic Growth and PHB Production under Oxygen-Limiting Conditions. Metab. Eng. 2020, 61, 11–23. [Google Scholar] [CrossRef]
- Li, D.; Yan, X.; Li, Y.; Ma, X.; Li, J. Achieving Polyhydroxyalkanoate Production from Rubber Wood Waste Using Mixed Microbial Cultures and Anaerobic-Aerobic Feeding Regime. Int. J. Biol. Macromol. 2022, 199, 162–171. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, J.; Chen, G.-Q. Polyhydroxyalkanoates, Challenges and Opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef]
- Koller, M. Polyhydroxyalkanoate Biosynthesis at the Edge of Water Activity-Haloarchaea as Biopolyester Factories. Bioengineering 2019, 6, 34. [Google Scholar] [CrossRef]
- Drakonaki, A.; Mathioudaki, E.; Geladas, E.D.; Konsolaki, E.; Vitsaxakis, N.; Chaniotakis, N.; Xie, H.; Tsiotis, G. Production of Polyhydroxybutyrate by Genetically Modified Pseudomonas Sp. PhDV1: A Comparative Study of Utilizing Wine Industry Waste as a Carbon Source. Microorganisms 2023, 11, 1592. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Cui, R.; Xu, Y.; Song, Z. Genetic Engineering Strategies for Sustainable Polyhydroxyalkanoate (PHA) Production from Carbon-Rich Wastes. Environ. Technol. Innov. 2023, 30, 103069. [Google Scholar] [CrossRef]
- Chacón, M.; Wongsirichot, P.; Winterburn, J.; Dixon, N. Genetic and Process Engineering for Polyhydroxyalkanoate Production from Pre- and Post-Consumer Food Waste. Curr. Opin. Biotechnol. 2024, 85, 103024. [Google Scholar] [CrossRef]
- Morlino, M.S.; Serna García, R.; Savio, F.; Zampieri, G.; Morosinotto, T.; Treu, L.; Campanaro, S. Cupriavidus Necator as a Platform for Polyhydroxyalkanoate Production: An Overview of Strains, Metabolism, and Modeling Approaches. Biotechnol. Adv. 2023, 69, 108264. [Google Scholar] [CrossRef]
- Salvachúa, D.; Rydzak, T.; Auwae, R.; De Capite, A.; Black, B.A.; Bouvier, J.T.; Cleveland, N.S.; Elmore, J.R.; Furches, A.; Huenemann, J.D.; et al. Metabolic Engineering of Pseudomonas Putida for Increased Polyhydroxyalkanoate Production from Lignin. Microb. Biotechnol. 2020, 13, 290–298. [Google Scholar] [CrossRef]
- Hur, D.H.; Lee, J.; Park, S.J.; Jeong, K.J. Engineering of Pseudomonas Putida to Produce Medium-Chain-Length Polyhydroxyalkanoate from Crude Glycerol. Int. J. Biol. Macromol. 2024, 281, 136411. [Google Scholar] [CrossRef]
- Jaffur, B.N.; Kumar, G.; Khadoo, P. Production and Functionalization Strategies for Superior Polyhydroxybutyrate Blend Performance. Int. J. Biol. Macromol. 2024, 278, 134907. [Google Scholar] [CrossRef] [PubMed]
- Abbate, E.; Andrion, J.; Apel, A.; Biggs, M.; Chaves, J.; Cheung, K.; Ciesla, A.; Clark-ElSayed, A.; Clay, M.; Contridas, R.; et al. Optimizing the Strain Engineering Process for Industrial-Scale Production of Bio-Based Molecules. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad025. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kim, M.; Kong, D.S.; Min, K.; Wu, G.; Cui, M.; Kim, C.; Oh, Y.-K.; Kim, S.; Lee, S.Y.; et al. Electron Uptake from Solid Electrodes Promotes the More Efficient Conversion of CO2 to Polyhydroxybutyrate by Using Rhodobacter Sphaeroides. Chem. Eng. J. 2023, 469, 143785. [Google Scholar] [CrossRef]
- Pilania, P.; Bhushan, K.; Phutela, U.G. Electro-Fermentation for Biofuel and Biochemical Production. Fermentation 2025, 11, 219. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, M.; Lyu, C.; Meng, L.; Liu, J.; Wang, B. Polyhydroxyalkanoate Production during Electroactive Biofilm Formation and Stabilization in Wetland Microbial Fuel Cells for Petroleum Hydrocarbon Bioconversion. Synth. Syst. Biotechnol. 2025, 10, 474–483. [Google Scholar] [CrossRef]
- Langsdorf, A.; Schütz, J.P.; Ulber, R.; Stöckl, M.; Holtmann, D. Production of Polyhydroxybutyrate from Industrial Flue Gas by Microbial Electrosynthesis. J. CO2 Util. 2024, 83, 102800. [Google Scholar] [CrossRef]
- Le, G.T.H.; Omar Mohamed, H.; Kim, H.; Yoo, K.; Eisa, T.; Jadhav, D.A.; Nguyen, H.T.T.; Eam, H.; Myung, J.; Castaño, P.; et al. Microbial Symbiotic Electrobioconversion of Carbon Dioxide to Biopolymer (Poly (3-Hydroxybutyrate)) via Single-Step Microbial Electrosynthesis Cell. Chem. Eng. J. 2024, 500, 156635. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Clauwaert, P.; Verstraete, W. Microbial Fuel Cells for Wastewater Treatment. Water Sci. Technol. 2006, 54, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Boto, S.T.; Cristiani, L.; Rosenbaum, M.A. Biochemical Production with Microbial Bioelectrochemical Systems. Curr. Opin. Biotechnol. 2025, 93, 103291. [Google Scholar] [CrossRef]
- Mateos, R.; Sotres, A.; Alonso, R.M.; Escapa, A.; Morán, A. Impact of the Start-up Process on the Microbial Communities in Biocathodes for Electrosynthesis. Bioelectrochemistry 2018, 121, 27–37. [Google Scholar] [CrossRef]
- Cabau-Peinado, O.; Winkelhorst, M.; Stroek, R.; de Kat Angelino, R.; Straathof, A.J.J.; Masania, K.; Daran, J.M.; Jourdin, L. Microbial Electrosynthesis from CO2 Reaches Productivity of Syngas and Chain Elongation Fermentations. Trends Biotechnol. 2024, 42, 1503–1522. [Google Scholar] [CrossRef] [PubMed]
- Paquete, C.M.; Rosenbaum, M.A.; Bañeras, L.; Rotaru, A.-E.; Puig, S. Let’s Chat: Communication between Electroactive Microorganisms. Bioresour. Technol. 2022, 347, 126705. [Google Scholar] [CrossRef]
- Rosenbaum, M.A.; Henrich, A.W. Engineering Microbial Electrocatalysis for Chemical and Fuel Production. Curr. Opin. Biotechnol. 2014, 29, 93–98. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Callander, G.; Spormann, A.M. Improved Reactor Design Enables Productivity of Microbial Electrosynthesis on Par with Classical Biotechnology. Bioresour. Technol. 2025, 416, 131733. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Spormann, A.M. High Acetate Titer Obtained from CO2 by Thermophilic Microbial Electrosynthesis with Thermoanaerobacter Kivui. Bioresour. Technol. Rep. 2024, 25, 101740. [Google Scholar] [CrossRef]
- Ale Enriquez, F.; Ahring, B.K. Strategies to Overcome Mass Transfer Limitations of Hydrogen during Anaerobic Gaseous Fermentations: A Comprehensive Review. Bioresour. Technol. 2023, 377, 128948. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A Review of the Substrates Used in Microbial Fuel Cells (MFCs) for Sustainable Energy Production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Rabaey, K.; Yuan, Z.; Keller, J. Electron and Carbon Balances in Microbial Fuel Cells Reveal Temporary Bacterial Storage Behavior During Electricity Generation. Environ. Sci. Technol. 2007, 41, 2915–2921. [Google Scholar] [CrossRef]
- Schröder, U. Anodic Electron Transfer Mechanisms in Microbial Fuel Cells and Their Energy Efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Sun, L.; Chen, X.; An, X.; Yin, C.; Cao, Y.; Wu, H.; Song, H. Modular Engineering Intracellular NADH Regeneration Boosts Extracellular Electron Transfer of Shewanella Oneidensis MR-1. ACS Synth. Biol. 2018, 7, 885–895. [Google Scholar] [CrossRef]
- Yadav, R.; Chattopadhyay, B.; Kiran, R.; Yadav, A.; Bachhawat, A.K.; Patil, S.A. Microbial Electrosynthesis from Carbon Dioxide Feedstock Linked to Yeast Growth for the Production of High-Value Isoprenoids. Bioresour. Technol. 2022, 363, 127906. [Google Scholar] [CrossRef] [PubMed]
- Alkotaini, B.; Abdellaoui, S.; Hasan, K.; Grattieri, M.; Quah, T.; Cai, R.; Yuan, M.; Minteer, S.D. Sustainable Bioelectrosynthesis of the Bioplastic Polyhydroxybutyrate: Overcoming Substrate Requirement for NADH Regeneration. ACS Sustain. Chem. Eng. 2018, 6, 4909–4915. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Dinesh Kumar, M.; Kavitha, S.; Rajesh Banu, J.; Kumar Tyagi, V.; Rajaguru, P.; Kumar, G. Production and Recovery of Polyhydroxyalkanoates (PHA) from Waste Streams—A Review. Bioresour. Technol. 2022, 366, 128203. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Son, J.; Lim, H.J.; Lim, S.H.; Park, S.J. Valorization of Lignocellulosic Biomass for Polyhydroxyalkanoate Production: Status and Perspectives. Bioresour. Technol. 2022, 360, 127575. [Google Scholar] [CrossRef]
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.; Ramarao, B.V.; Misra, M. A Review on Polyhydroxyalkanoate (PHA) Production through the Use of Lignocellulosic Biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- Renju; Singh, R. (Bio)Electrochemical System: A Systematic Approach from Agricultural Waste to Sewage Wastewater Treatment with Nutrients and Hydrogen Recovery. J. Clean. Prod. 2024, 457, 142387. [Google Scholar] [CrossRef]
- Koller, M.; Obruča, S. Biotechnological Production of Polyhydroxyalkanoates from Glycerol: A Review. Biocatal. Agric. Biotechnol. 2022, 42, 102333. [Google Scholar] [CrossRef]
- Zhou, M.; Freguia, S.; Dennis, P.G.; Keller, J.; Rabaey, K. Development of Bioelectrocatalytic Activity Stimulates Mixed-culture Reduction of Glycerol in a Bioelectrochemical System. Microb. Biotechnol. 2015, 8, 483–489. [Google Scholar] [CrossRef]
- Dennis, P.G.; Harnisch, F.; Yeoh, Y.K.; Tyson, G.W.; Rabaey, K. Dynamics of Cathode-Associated Microbial Communities and Metabolite Profiles in a Glycerol-Fed Bioelectrochemical System. Appl. Environ. Microbiol. 2013, 79, 4008–4014. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Show, P.L.; Lan, J.C.-W.; Loh, H.-S.; Lam, H.L.; Ling, T.C. Economic and Environmental Analysis of PHAs Production Process. Clean. Technol. Environ. Policy 2017, 19, 1941–1953. [Google Scholar] [CrossRef]
- Al Rowaihi, I.S.; Paillier, A.; Rasul, S.; Karan, R.; Grötzinger, S.W.; Takanabe, K.; Eppinger, J. Poly(3-Hydroxybutyrate) Production in an Integrated Electromicrobial Setup: Investigation under Stress-Inducing Conditions. PLoS ONE 2018, 13, e0196079. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Leininger, A.M.; Zhang, W.; Liang, Y.; Ren, Z.J. Co-Valorization of Food Waste and CO2 to Produce Volatile Fatty Acids Using Liter-Scale Tubular Microbial Electrosynthesis Cells. ACS ES&T Eng. 2024, 4, 2243–2251. [Google Scholar] [CrossRef]
- Bian, B.; Bajracharya, S.; Xu, J.; Pant, D.; Saikaly, P.E. Microbial Electrosynthesis from CO2: Challenges, Opportunities and Perspectives in the Context of Circular Bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.E.; Kim, C.; Li, S.; Baek, J.; Seol, E.; Park, C.; Na, J.-G.; Lee, J.; Oh, Y.-K.; Kim, J.R. Supply of Proton Enhances CO Electrosynthesis for Acetate and Volatile Fatty Acid Productions. Bioresour. Technol. 2021, 320, 124245. [Google Scholar] [CrossRef]
- Humphreys, C.M.; Minton, N.P. Advances in Metabolic Engineering in the Microbial Production of Fuels and Chemicals from C1 Gas. Curr. Opin. Biotechnol. 2018, 50, 174–181. [Google Scholar] [CrossRef] [PubMed]
- ter Heijne, A.; Pereira, M.A.; Pereira, J.; Sleutels, T. Electron Storage in Electroactive Biofilms. Trends. Biotechnol. 2021, 39, 34–42. [Google Scholar] [CrossRef]
- Srikanth, S.; Venkateswar Reddy, M.; Venkata Mohan, S. Microaerophilic Microenvironment at Biocathode Enhances Electrogenesis with Simultaneous Synthesis of Polyhydroxyalkanoates (PHA) in Bioelectrochemical System (BES). Bioresour. Technol. 2012, 125, 291–299. [Google Scholar] [CrossRef]
- Rengasamy, K.; Ranaivoarisoa, T.O.; Singh, R.; Bose, A. Improving microbial electrosynthesis of polyhydroxybutyrate (PHB) from CO2 by Rhodopseudomonas palustris TIE-1 using an immobilized iron complex modified cathode. bioRxiv 2017, 214577. [Google Scholar] [CrossRef]
- Pepè Sciarria, T.; Batlle-Vilanova, P.; Colombo, B.; Scaglia, B.; Balaguer, M.D.; Colprim, J.; Puig, S.; Adani, F. Bio-Electrorecycling of Carbon Dioxide into Bioplastics. Green Chem. 2018, 20, 4058–4066. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Song, T.; Xie, J. Bioplastic Production from the Microbial Electrosynthesis of Acetate through CO2 Reduction. Energy Fuels 2021, 35, 15978–15986. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Moon, Y.-M.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Jeon, J.-M.; Yoon, J.-J.; Kim, Y.-G.; et al. One-Pot Exploitation of Chitin Biomass for Simultaneous Production of Electricity, n-Acetylglucosamine and Polyhydroxyalkanoates in Microbial Fuel Cell Using Novel Marine Bacterium Arenibacter Palladensis YHY2. J. Clean. Prod. 2019, 209, 324–332. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Jadhav, D.A.; Eisa, T.; Nguyen, H.Y.; Le, G.T.H.; Le, T.T.Q.; Jae, M.-R.; Khoo, K.S.; Yang, E.; Chae, K.-J. Sustainable Conversion of Carbon Dioxide to High-Value Antioxidant Astaxanthin through Microbial Electrosynthesis-Assisted Microalgae Cultivation. Process Saf. Environ. Prot. 2024, 190, 212–225. [Google Scholar] [CrossRef]
- Díaz-Rullo Edreira, S.; Vasiliadou, I.A.; Prado, A.; Espada, J.J.; Wattiez, R.; Leroy, B.; Martínez, F.; Puyol, D. Elucidating Metabolic Tuning of Mixed Purple Phototrophic Bacteria Biofilms in Photoheterotrophic Conditions through Microbial Photo-Electrosynthesis. Commun. Biol. 2024, 7, 1526. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Leicester, D.D.; Heidrich, E.S.; Alonso, R.M.; Mateos, R.; Escapa, A. Bioelectrochemical Systems for Energy Valorization of Waste Streams. In Energy Systems and Environment; Intechopen: London, UK, 2018. [Google Scholar]
- Patel, D.; Bapodra, S.L.; Madamwar, D.; Desai, C. Electroactive Bacterial Community Augmentation Enhances the Performance of a Pilot Scale Constructed Wetland Microbial Fuel Cell for Treatment of Textile Dye Wastewater. Bioresour. Technol. 2021, 332, 125088. [Google Scholar] [CrossRef]
- Li, S.; Song, Y.E.; Baek, J.; Im, H.S.; Sakuntala, M.; Kim, M.; Park, C.; Min, B.; Kim, J.R. Bioelectrosynthetic Conversion of CO2 Using Different Redox Mediators: Electron and Carbon Balances in a Bioelectrochemical System. Energies 2020, 13, 2572. [Google Scholar] [CrossRef]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of Membraneless Microbial Electrolysis Cells (MECs) for Domestic Wastewater Treatment: Bottlenecks and Limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef]
- Alonso, R.M.; San-Martín, M.I.; Sotres, A.; Escapa, A. Graphene Oxide Electrodeposited Electrode Enhances Start-up and Selective Enrichment of Exoelectrogens in Bioelectrochemical Systems. Sci. Rep. 2017, 7, 13726. [Google Scholar] [CrossRef]
| Domain | Microorganism | PHAs * | Bioelectrochemical Behavior | References |
|---|---|---|---|---|
| Bacteria (Prokaryotes) | ||||
| Burkholderiaceae | Burkholderia cepacia | scl- and mcl-PHAs | Electrotrophic | [5,51] |
| Cupriavidus metallidurans | scl-PHAs | Electrotrophic | [5,52] | |
| Cupriavidus necator | scl-PHAs | Electrotrophic | [3,5] | |
| Clostridiaceae | Clostridium butyricum | scl-PHAs | Electrotrophic | [5,53] |
| Clostridium pasteurianum | scl-PHAs | Exoelectrogenic | [5,53] | |
| Comamonadaceae | Comamonas testosteroni | scl-PHAs | Exoelectrogenic | [5,54] |
| Micrococcaceae | Micrococcus luteus | scl-PHAs | Electrotrophic | [5,55] |
| Propionibacteriaceae | Propionibacterium spp. | scl-PHAs | Electrotrophic | [5,56] |
| Pseudomonadaceae | Pseudomonas aeruginosa | mcl-PHAs | Exoelectrogenic | [5,57] |
| Pseudomonas alcaliphila | mcl-PHAs | Exoelectrogenic | [5,58] | |
| Rhodobacteraceae | Rhodopseudomonas palustris | scl-PHAs | Exoelectrogenic | [5,59] |
| Cyanobacteria (Prokaryote) | ||||
| Synechococcaceae | Synechococcus elongatus | PHB | Exoelectrogenic | [60,61] |
| Spirulinaceae | Spirulina platensis | PHB | Exoelectrogenic | [62,63,64] |
| Algae (Eukaryotes) | ||||
| Chlorellaceae | Chlorella vulgaris | PHB | Exoelectrogenic and Electrotrophic | [62,65,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamizo-Ampudia, A.; Alonso, R.M.; Ariza-Carmona, L.; Sanchiz, Á.; San-Martín, M.I. A Review of Bioelectrochemical Strategies for Enhanced Polyhydroxyalkanoate Production. Bioengineering 2025, 12, 616. https://doi.org/10.3390/bioengineering12060616
Chamizo-Ampudia A, Alonso RM, Ariza-Carmona L, Sanchiz Á, San-Martín MI. A Review of Bioelectrochemical Strategies for Enhanced Polyhydroxyalkanoate Production. Bioengineering. 2025; 12(6):616. https://doi.org/10.3390/bioengineering12060616
Chicago/Turabian StyleChamizo-Ampudia, Alejandro, Raúl. M. Alonso, Luisa Ariza-Carmona, África Sanchiz, and María Isabel San-Martín. 2025. "A Review of Bioelectrochemical Strategies for Enhanced Polyhydroxyalkanoate Production" Bioengineering 12, no. 6: 616. https://doi.org/10.3390/bioengineering12060616
APA StyleChamizo-Ampudia, A., Alonso, R. M., Ariza-Carmona, L., Sanchiz, Á., & San-Martín, M. I. (2025). A Review of Bioelectrochemical Strategies for Enhanced Polyhydroxyalkanoate Production. Bioengineering, 12(6), 616. https://doi.org/10.3390/bioengineering12060616



_Li.png)






