Electric Potential of Chlorella sp. Microalgae Biomass in Microbial Fuel Cells (MFCs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Culture Medium for Chlorella sp.
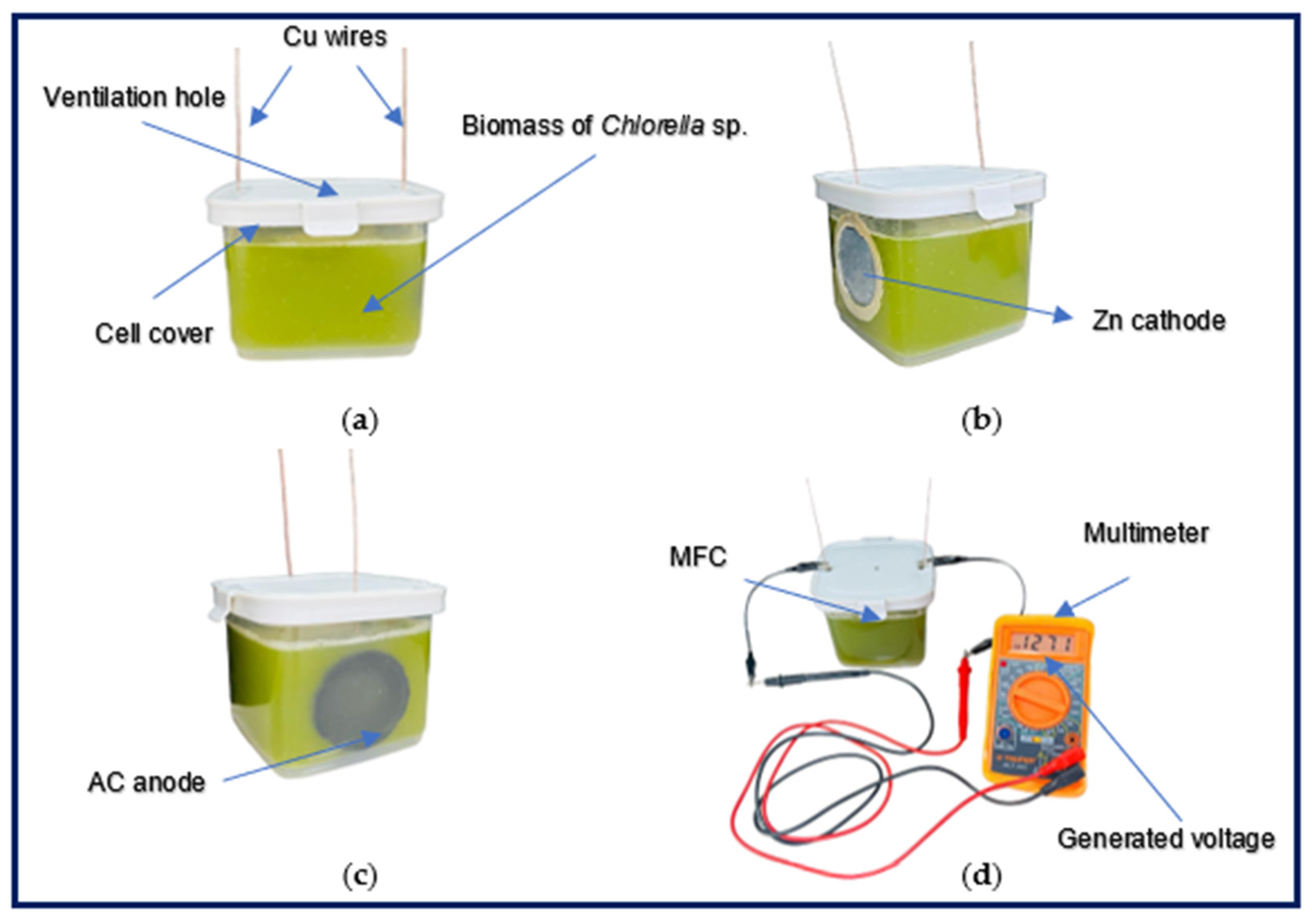
2.2. Construction of MFC
2.3. Inoculation of Chlorella sp. and Experimental Configuration of the MFC
2.4. Monitoring of Physicochemical Parameters of Chlorella sp. Biomass
2.5. Monitoring of Electrical Production of the MFC
2.6. Measurement of Optical Density of Chlorella sp. Biomass
2.7. Determination of Cell Density of Chlorella sp.
2.8. Physicochemical Characterization Analysis of Anodic Electrodes in MFC
3. Results and Discussion
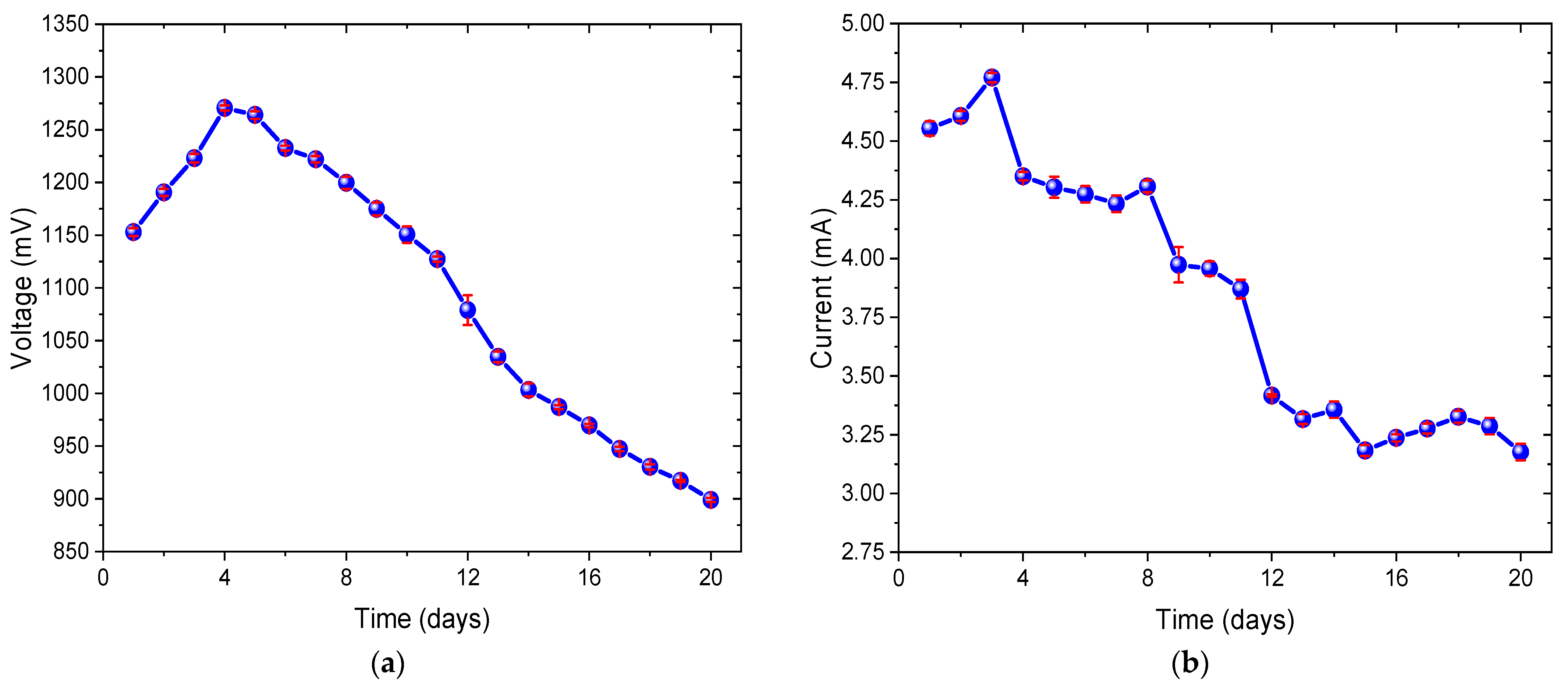
3.1. Electricity Production with Chlorella sp. in MFCs
3.2. Monitoring of pH, Electrical Conductivity (EC), and Oxidation–Reduction Potential (ORP) of Chlorella sp. Biomass
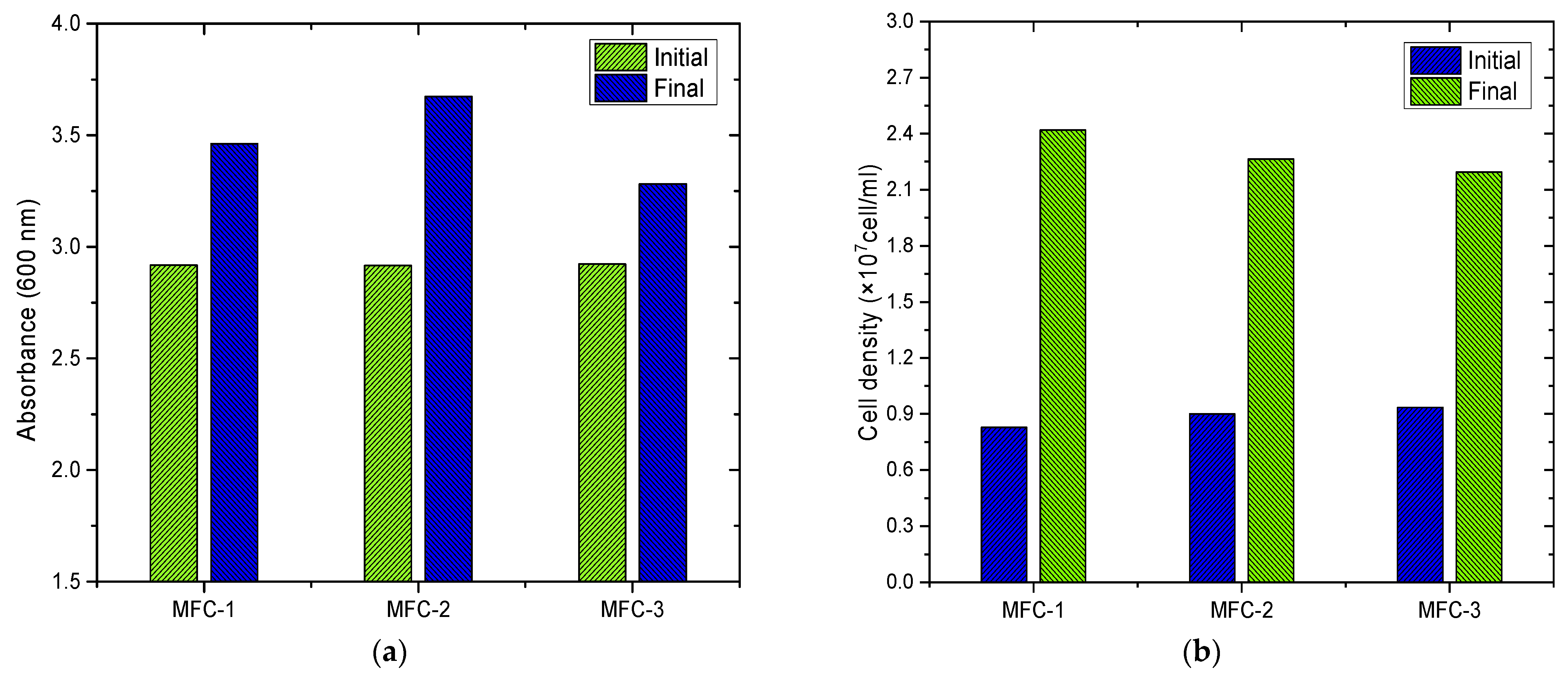
3.3. Evaluation of Optical Density and Cell Density of Chlorella sp. Biomass
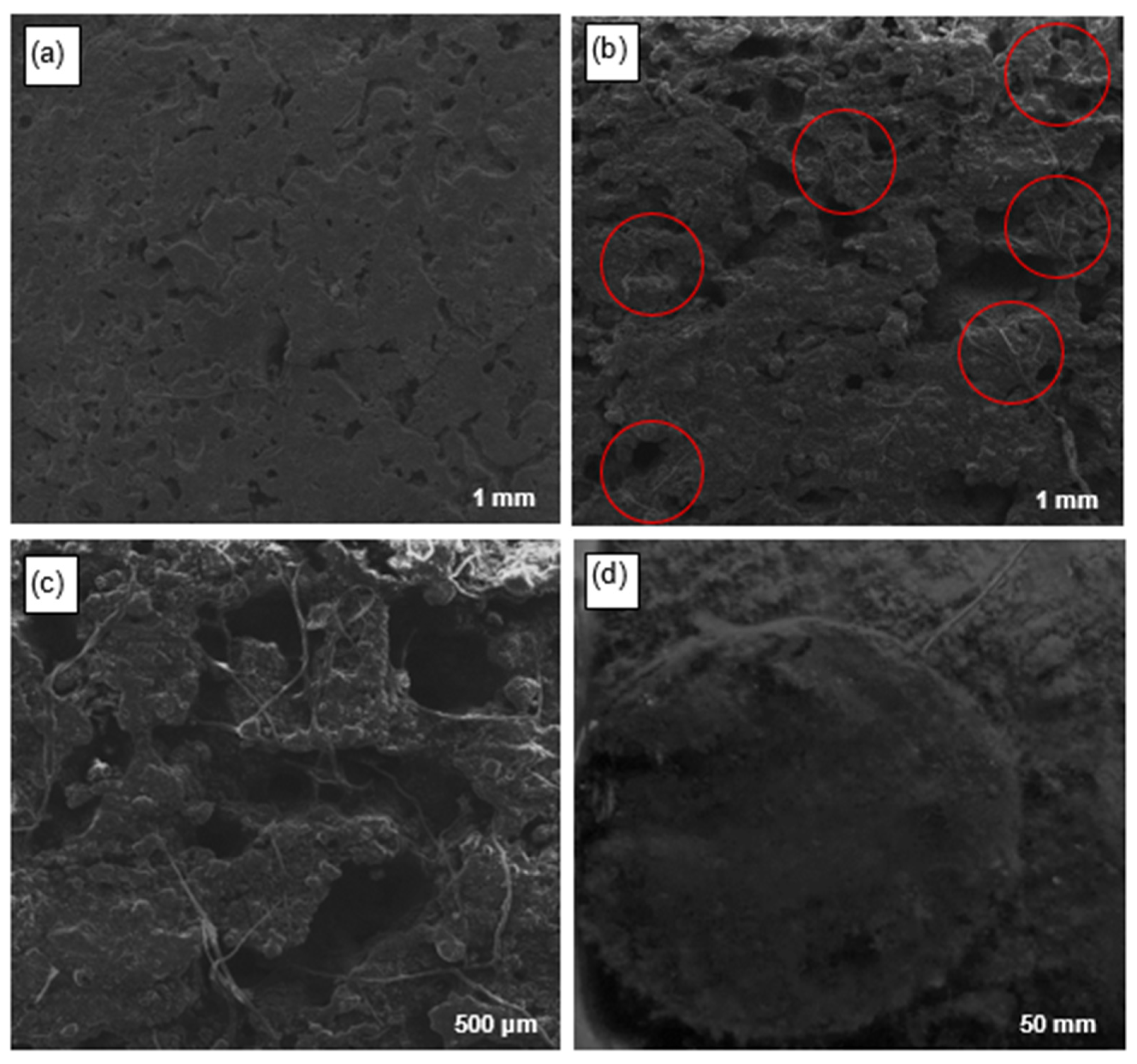
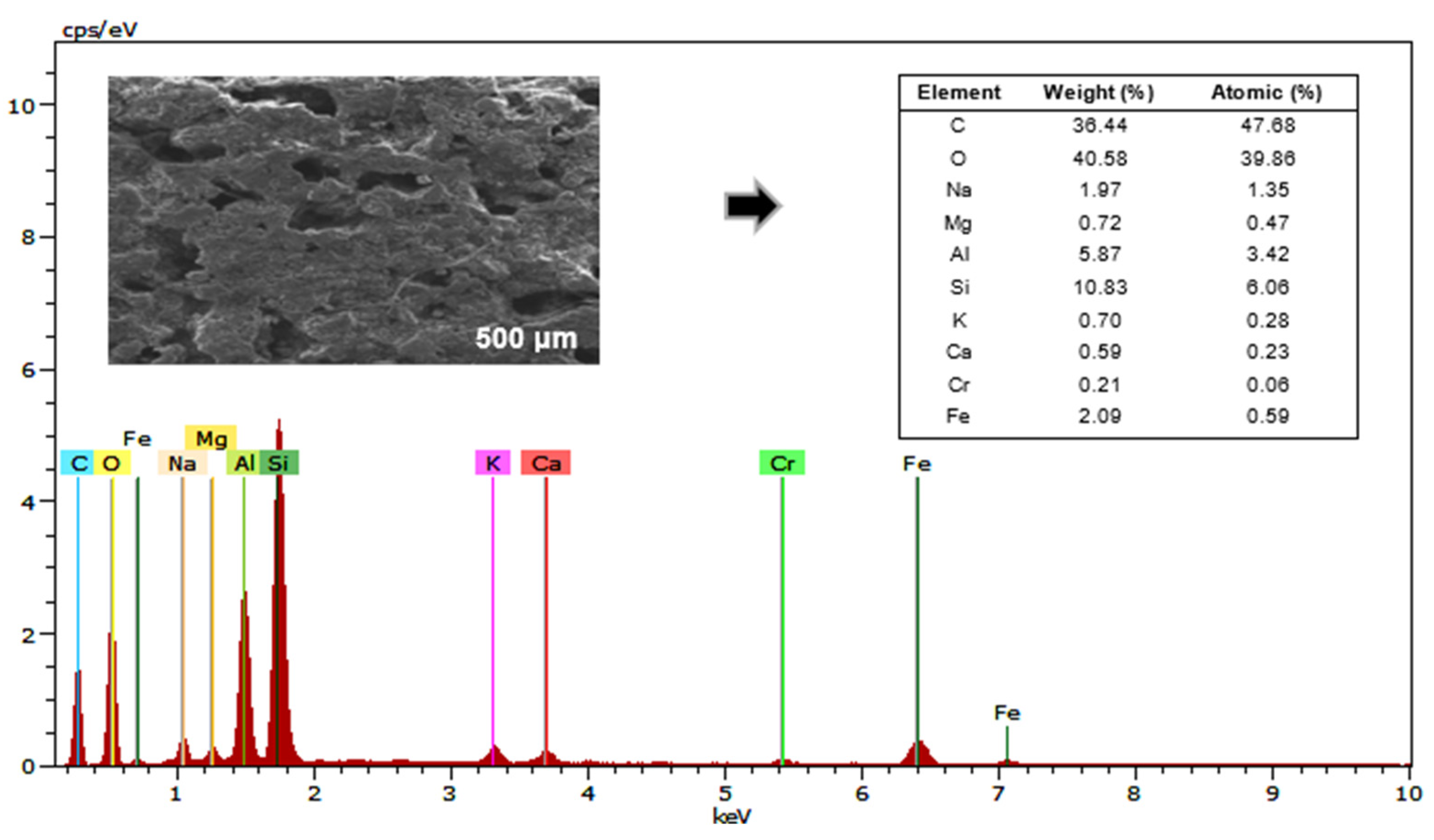
3.4. Physicochemical Characterization of the Anodic Electrodes of MFCs Using SEM and EDS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoang, A.T.; Nižetić, S.; Ng, K.H.; Papadopoulos, A.M.; Le, A.T.; Kumar, S.; Hadiyanto, H.; Pham, V.V. Microbial fuel cells for bioelectricity production from waste as sustainable prospect of future energy sector. Chemosphere 2022, 287, 132285. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Manna, S.; Sil, A.K. A new electro-active bacterium, Paraclostridium sp. AKS46, converts waste efficiently into electricity in microbial fuel cell. Chem. Eng. J. 2023, 475, 145626. [Google Scholar] [CrossRef]
- Sevda, S.; Garlapati, V.K.; Sharma, S.; Bhattacharya, S.; Mishra, S.; Sreekrishnan, T.R.; Pant, D. Microalgae at niches of bioelectrochemical systems: A new platform for sustainable energy production coupled industrial effluent treatment. Bioresour. Technol. Rep. 2019, 7, 100290. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.M.; Cheng, X.L.; Jiang, Y.Q.; Tian, R.R.; Fu, H.; Ji, Y.X.; Zhou, J.; Ji, G.S.; Yong, X.Y. Electricity generation and energy storage in microbial fuel cells with manganese dioxide capacitive electrode. J. Power Sources 2024, 598, 234192. [Google Scholar] [CrossRef]
- Verma, P.; Daverey, A.; Kumar, A.; Arunachalam, K. Microbial Fuel Cell—A Sustainable Approach for Simultaneous Wastewater Treatment and Energy Recovery. J. Water Proc. Eng. 2021, 40, 101768. [Google Scholar] [CrossRef]
- Yu, J.; Park, Y.; Widyaningsih, E.; Kim, S.; Kim, Y.; Lee, T. Microbial fuel cells: Devices for real wastewater treatment, rather than electricity production. Sci. Total Environ. 2021, 775, 145904. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Q.; Wider, W.; Zhang, S.; Fauzi, M.A.; Jiang, L.; Udang, L.N.; An, Z. Research landscape of energy transition and green finance: A bibliometric analysis. Heliyon 2024, 10, 24783. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Y.; Zhang, S.E.; Bourdeau, J.E.; Chipangamate, N.S.; Rose, D.H.; Valodia, I.; Nwaila, G.T. The strategic role of lithium in the green energy transition: Towards an OPEC-style framework for green energy-mineral exporting countries (GEMEC). Resour. Policy 2024, 90, 104737. [Google Scholar] [CrossRef]
- Cuicui, L.; Liang, B.; Zhong, M.; Li, K.; Qi, Y. Activated carbon-supported multi-doped graphene as high-efficient catalyst to modify air cathode in microbial fuel cells. Electrochim. Acta 2019, 304, 360–369. [Google Scholar] [CrossRef]
- Sharma, M.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Xue, W.; Thakur, N.; Salama, E.-S.; Li, X. Microalgal cycling in the cathode of microbial fuel cells (MFCs) induced oxygen reduction reaction (ORR) and electricity: A biocatalytic process for clean energy. Chem. Eng. J. 2024, 479, 147431. [Google Scholar] [CrossRef]
- Wu, J.; Liu, R.; Dong, P.; Li, N.; He, W.; Feng, Y.; Liu, J. Enhanced electricity generation and storage by nitrogen-doped hierarchically porous carbon modification of the capacitive bioanode in microbial fuel cells. Sci. Total Environ. 2023, 858, 159688. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Sathyamurthy, S.; Kumar, S.S.; Huynh, G.B.; Saravanan, R.; Rajasimman, M. Amplifying power generation in microbial fuel cells with cathode catalyst of graphite-based nanomaterials. Int. J. Hydrog. Energy 2022, 52, 257–267. [Google Scholar] [CrossRef]
- Qi, X.; Cai, H.; Wang, X.; Liu, R.; Cai, T.; Wang, S.; Liu, X.; Wang, X. Electricity generation by Pseudomonas putida B6-2 in microbial fuel cells using carboxylates and carbohydrate as substrates. Eng. Microbiol. 2024, 4, 100148. [Google Scholar] [CrossRef]
- Javanmard, A.; Zuki, F.M.; Patah, M.F.A.; Daud, W.M.A.W. Revolutionizing Microbial Fuel Cells: Biochar’s Energy Conversion Odyssey. Process Saf. Environ. Prot. 2024, 187, 26–58. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Farooq, M.; Hussain, I.; Ali, M.; Mujtaba, M.A.; Sultan, M.; Yang, B. Recent innovations for scaling up microbial fuel cell systems: Significance of physicochemical factors for electrodes and membranes materials. J. Taiwan Inst. Chem. Eng. 2021, 129, 207–226. [Google Scholar] [CrossRef]
- Bhaduri, S.; Behera, M. From single-chamber to multi-anodic microbial fuel cells: A review. J. Environ. Manag. 2024, 355, 120465. [Google Scholar] [CrossRef] [PubMed]
- Silveira, G.; Neto, S.-A.; Schneedorf, J.M. Development, characterization and application of a low-cost single chamber microbial fuel cell based on hydraulic couplers. Energy 2020, 208, 118395. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Mahadevan, M.; Pandey, A.; Greener, J. Recent progress in microbial fuel cells using substrates from diverse sources. Heliyon 2022, 8, 12353. [Google Scholar] [CrossRef] [PubMed]
- Mahurede, T.P.; Chihobo, C.H.; Utete, B.; Taru, P. A review of microbial fuel cell prototypes, their efficacy in wastewater treatment and the contextual situation for Zimbabwe. Fuel Commun. 2023, 17, 100094. [Google Scholar] [CrossRef]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N.L. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Han, J.-I. Recycling and reuse of spent microalgal biomass for sustainable biofuels. Biochem. Eng. J. 2013, 75, 101–107. [Google Scholar] [CrossRef]
- Cao, T.N.-D.; Mukhtar, H.; Le, L.T.; Tran, D.P.-H.; Ngo, M.T.T.; Pham, M.-D.-T.; Nguyen, T.B.; Vo, T.-K.-Q.; Bui, X.-T. Roles of microalgae-based biofertilizer in sustainability of green agriculture and food-water-energy security nexus. Sci. Total Environ. 2023, 870, 161927. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Patil, S.A. 10-Microbial fuel cell coupled with microalgae cultivation for wastewater treatment and energy recovery. Integr. Microb. Fuel Cells Wastewater Treat. 2020, 2020, 213–227. [Google Scholar] [CrossRef]
- Haris, N.; Manan, H.; Jusoh, M.; Khatoon, H.; Katayama, T.; Kasan, N.A. Effect of different salinity on the growth performance and proximate composition of isolated indigenous microalgae species. Aquac. Rep. 2022, 22, 100925. [Google Scholar] [CrossRef]
- Gomes, A.C.S.; De La Cruz, L.T.; Garcia, Y.; Bastos, R.B.; Lopes, R.M. Frame Rhythm: A New Cost-Effective Approach for Semi-Automatic Microalgal Imaging and Enumeration. Algal Res. 2022, 64, 102659. [Google Scholar] [CrossRef]
- Bo, Y.; Chu, R.; Sun, D.; Deng, X.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. Mixotrophic culture of bait microalgae for biomass and nutrients accumulation and their synergistic carbon metabolism. Bioresour. Technol. 2023, 367, 128301. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Salama, E.-S.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; Li, X. Microalgae-assisted microbial fuel cells for electricity generation coupled with wastewater treatment: Biotechnological perspective. J. Water Process Eng. 2022, 49, 102966. [Google Scholar] [CrossRef]
- Deng, Z.; Cheng, Y.; Zhu, J.; Yang, L.; Zhang, Z.; Wu, L. Electricity generation and enhanced thiacloprid biodegradation in microbial fuel cells using microalgae biocathode. Process Saf. Environ. Prot. 2022, 166, 393–401. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Prasad, S.; Singh, H.; Das, D. Improvement of bioelectricity generation and microalgal productivity with concomitant wastewater treatment in flat-plate microbial carbon capture cell. Fuel 2020, 263, 116696. [Google Scholar] [CrossRef]
- Christwardana, M.; Hadiyanto, H.; Motto, S.A.; Sudarno, S.; Haryani, K. Performance evaluation of yeast-assisted microalgal microbial fuel cells on bioremediation of cafeteria wastewater for electricity generation and microalgae biomass production. Biomass Bioenergy 2020, 139, 105617. [Google Scholar] [CrossRef]
- Cui, Y.; Rashid, N.; Hu, N.; Rehman, M.S.U.; Han, J.-I. Electricity generation and microalgae cultivation in microbial fuel cell using microalgae-enriched anode and bio-cathode. Energy Convers. Manag. 2014, 79, 674–680. [Google Scholar] [CrossRef]
- De La Cruz-Noriega, M.; Rojas-Flores, S.; Quiroz-De la Cruz, J.; Carranza-Vigo, C.; Zavaleta-Portilla, S.; Cabanillas-Chirinos, L.; Angelats-Silva, L. Generación de energía eléctrica mediante un fotobiorreactor con microalgas “Chlorella sp.”. In Proceedings of the 19th LACCEI International Multi-Conference for Engineering, Education, and Technology, Virtual, 19–23 July 2021; Volume 19. [Google Scholar] [CrossRef]
- Agüero-Quiñones, R.; Ávila-Sánchez, Z.; Rojas-Flores, S.; Cabanillas-Chirinos, L.; Cruz-Noriega, M.D.L.; Cruz-Monzón, J.; Nazario-Naveda, R. Cadmium and COD Removal from Municipal Wastewater Using Chlorella sp. Biomass in Microbial FuelCells. Sustainability 2023, 15, 14513. [Google Scholar] [CrossRef]
- Agüero-Quiñones, R.; Ávila-Sánchez, Z.; Rojas-Flores, S.; Cabanillas-Chirinos, L.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Rojas-Villacorta, W. Activated Carbon Electrodes for Bioenergy Production in Microbial Fuel Cells Using Synthetic Wastewater as Substrate. Sustainability 2023, 15, 13767. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. The influential role of external electrical load in microbial fuel cells and related improvement strategies: A review. Bioelectrochemistry 2021, 140, 107749. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Benites, S.M.; Delfín-Narciso, D.; Luis, A.S.; Díaz, F.; Luis, C.C.; Moises, G.C. Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells. Molecules 2022, 27, 5198. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, T.; Zhao, J.; Wang, C.; Wei, Q.; Ma, X.; Yang, G. Unraveling non-linear dynamics of biomass, photosynthesis efficiency, and lipid accumulation in Chlorella vulgaris under mixotrophic cultivation. J. Clean. Prod. 2024, 448, 141692. [Google Scholar] [CrossRef]
- Abate, R.; Oon, Y.-S.; Oon, Y.-L.; Bi, Y. Microalgae-bacteria nexus for environmental remediation and renewable energy resources: Advances, mechanisms and biotechnological applications. Heliyon 2024, 10, 31170. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Rezk, H.; Abdelkareem, M.A.; Olabi, A.G. Artificial neural network based modelling and optimization of microalgae microbial fuel cell. Int. J. Hydrog. Energy 2024, 52, 1015–1025. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent insights into microalgae-assisted microbial fuel cells for generating sustainable bioelectricity. Int. J. Hydrog. Energy 2021, 46, e3135–e3159. [Google Scholar] [CrossRef]
- Kannan, N.; Donnellan, P. Algae-assisted microbial fuel cells: A practical overview. Bioresour. Technol. Rep. 2021, 15, 100747. [Google Scholar] [CrossRef]
- Deka, R.; Shreya, S.; Mourya, M.; Sirotiya, V.; Rai, A.; Khan, M.J.; Ahirwar, A.; Schoefs, B.; Bilal, M.; Saratale, G.D.; et al. A techno-economic approach for eliminating dye pollutants from industrial effluent employing microalgae through microbial fuel cells: Barriers and perspectives. Environ. Res. 2022, 212, 113454. [Google Scholar] [CrossRef] [PubMed]
- Mekuto, L.; Olowolafe, A.V.A.; Pandit, S.; Dyantyi, N.; Nomngongo, P.; Huberts, R. Microalgae as a biocathode and feedstock in anode chamber for a self-sustainable microbial fuel cell technology: A review. S. Afr. J. Chem. Eng. 2020, 31, 7–16. [Google Scholar] [CrossRef]
- Qin, L.; Qin, Y.; Cui, N.; Han, X.; Lu, H.; Yang, T.; Liang, W. Photosynthetic microalgae microbial fuel cells for bioelectricity generation and microalgae lipid recovery using Gd-Co@N-CSs/NF as cathode. Chem. Eng. J. 2024, 490, 151647. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Y.; Qin, Y.; Liu, C.; Lu, H.; Yang, T.; Liang, W. Gd-Co nanosheet arrays coated on N-doped carbon spheres as cathode catalyst in photosynthetic microalgae microbial fuel cells. Sci. Total Environ. 2022, 849, 157711. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dong, Y.; Li, J.; Fu, Q.; Zhang, L. Templating synthesis of hierarchically meso/macroporous N-doped microalgae derived biocarbon as oxygen reduction reaction catalyst for microbial fuel cells. Int. J. Hydrog. Energy 2021, 46, 2530–2542. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Li, M.-J.; Hung, T.C. The study on coupled CO2 fixation and power generation in microalgae-microbial fuel cells embedded with oxygen-consuming biofilms. Fuel 2024, 367, 131410. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Sustainable electricity generation and ammonium removal by microbial fuel cell with a microalgae assisted cathode at various environmental conditions. Bioresour. Technol. 2019, 284, 161–167. [Google Scholar] [CrossRef]
- Sharma, A.; Chhabra, M.; Kumar, S. Performance evaluation of genetically modified microalgae in photosynthetic microbial fuel cells for carotenoids and power generation. J. Environ. Chem. Eng. 2024, 12, 112751. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Kirah, K.; Gomaa, O.M. Light-induced aquaculture wastewater valorization, nutrient recovery, and microalgae biomass production in a biocathode-assisted microbial fuel cell. Bioresour. Technol. Rep. 2024, 25, 101733. [Google Scholar] [CrossRef]
- Gao, S.; Edmundson, S.; Huesemann, M. Oxygen stress mitigation for microalgal biomass productivity improvement in outdoor raceway ponds. Algal Res. 2022, 68, 102901. [Google Scholar] [CrossRef]
- Nadzri, N.A.A.; Yasin, N.H.M.; Bakar, M.H.A.; Thanakodi, S.; Salehmin, M.N.I.; Takriff, M.S.; Aznan, M.F.N.; Maeda, T. Photosynthetic microbial desalination cell (PhMDC) using Chlamydomonas sp. (UKM6) and Scenedesmus sp. (UKM9) as biocatalysts for electricity production and water treatment. Int. J. Hydrog. Energy 2023, 48, 11860–11873. [Google Scholar] [CrossRef]
- Lan, L.; Li, J.; Feng, Q.; Zhang, L.; Fu, Q.; Zhu, X.; Liao, Q. Enhanced current production of the anode modified by microalgae derived nitrogen-rich biocarbon for microbial fuel cells. Int. J. Hydrog. Energy 2020, 45, 3833–3839. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Cabanillas-Chirinos, L.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Delfin-Narciso, D.; Rojas-Villacorta, W. Use of Tangerine Waste as Fuel for the Generation of Electric Current. Sustainability 2023, 15, 3559. [Google Scholar] [CrossRef]
- Commault, A.S.; Laczka, O.; Siboni, N.; Tamburic, B.; Crosswell, J.R.; Seymour, J.R.; Ralph, P.J. Electricity and biomass production in a bacteria-Chlorella based microbial fuel cell treating wastewater. J. Power Sources 2017, 356, 299–309. [Google Scholar] [CrossRef]
- Angioni, S.; Millia, L.; Mustarelli, P.; Doria, E.; Temporiti, M.E.; Mannucci, B.; Corana, F.; Quartarone, E. Photosynthetic microbial fuel cell with polybenzimidazole membrane: Synergy between bacteria and algae for wastewater removal and biorefinery. Heliyon 2018, 4, 00560. [Google Scholar] [CrossRef]
- Rojas-Villacorta, W.; Rojas-Flores, S.; Benites, S.M.; Delfín-Narciso, D.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Rodríguez-Serin, H.; Rebaza-Araujo, S. Potential use of pepper waste and microalgae Spirulina sp. for bioelectricity generation. Energy Rep. 2023, 9, 253–261. [Google Scholar] [CrossRef]
- Yadav, G.; Sharma, I.; Ghangrekar, M.; Sen, R. A live bio-cathode to enhance power output steered by bacteria-microalgae synergistic metabolism in microbial fuel cell. J. Power Sources 2020, 449, 227560. [Google Scholar] [CrossRef]
- Silva-Palacios, F.; Salvador-Salinas, A.; Quezada-Alvarez, M.A.; Rodriguez-Yupanqui, M.; Rojas-Flores, S.; Nazario-Naveda, R.; Cabanillas-Chirinos, L. Bioelectricity generation through Microbial Fuel Cells using Serratia fonticola bacteria and Rhodotorula glutinis yeast. Energy Rep. 2023, 9, 295–301. [Google Scholar] [CrossRef]
- Du, H.; Shao, Z. Synergistic effects between solid potato waste and waste activated sludge for waste-to-power conversion in microbial fuel cells. Appl. Energy 2022, 314, 118994. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Dynamic evolution of anodic biofilm when maturing under different external resistive loads in microbial fuel cells. Electrochemical perspective. J. Power Sources 2018, 400, 392–401. [Google Scholar] [CrossRef]
- Li, S.; Jiang, J.; Ho, S.-H.; Zhang, S.; Zeng, W.; Li, F. Sustainable conversion of antibiotic wastewater using microbial fuel cells: Energy harvesting and resistance mechanism analysis. Chemosphere 2023, 313, 137584. [Google Scholar] [CrossRef] [PubMed]
- Bazina, N.; Ahmed, T.G.; Almdaaf, M.; Jibia, S.; Sarker, M. Power generation from wastewater using microbial fuel cells: A review. J. Biotechnol. 2023, 374, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Benites, S.M.; Nazario-Naveda, R.; Delfín-Narciso, D.; Gallozzo-Cardemas, M.; Díaz, F.; Murga-Torres, E.; Rojas-Villacorta, W. Use of Kiwi waste as fuel in MFC and its potential for use as renewable energy. Fermentation 2023, 9, 446. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-Based Microbial-Fuel-Cell Electrodes: From Conductive Supports to Active Catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef] [PubMed]
- Sallam, E.R.; Khairy, H.M.; Elnouby, M.S.; Fetouh, H.A. Sustainable electricity production from seawater using Spirulina platensis microbial fuel cell catalyzed by silver nanoparticles-activated carbon composite prepared by a new modified photolysis method. Biomass Bioenergy 2021, 148, 106038. [Google Scholar] [CrossRef]
- Wang, C.-T.; Huang, Y.-S.; Sangeetha, T.; Chen, Y.-M.; Chong, W.-T.; Ong, H.-C.; Zhao, F.; Yan, W.-M. Novel bufferless photosynthetic microbial fuel cell (PMFCs) for enhanced electrochemical performance. Bioresour. Technol. 2018, 255, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Periyasamy, S.; Zhen, G.; Koók, L.; Bakonyi, P.; Nemestóthy, N.; Kumar, G. Bioelectrochemical systems using microalgae—A concise research update. Chemosphere 2017, 177, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hadiyanto, H.; Christwardana, M.; Pratiwi, W.Z.; Purwanto, P.; Sudarno, S.; Haryani, K.; Hoang, A.T. Response surface optimization of microalgae microbial fuel cell (MMFC) enhanced by yeast immobilization for bioelectricity production. Chemosphere 2022, 287, 132275. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Kakarla, R.; Min, B. Algae cathode microbial fuel cells for electricity generation and nutrient removal from landfill leachate wastewater. Int. J. Hydrog. Energy 2017, 42, 29433–29442. [Google Scholar] [CrossRef]
- Vilas-Boas, J.; Marcon, L.R.C.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Performance evaluation of a single-chamber microbial fuel cell with Zygosaccharomyces bailii. Bioresour. Technol. Rep. 2023, 23, 101547. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Delgado-Osório, H.D.; Ulrich, C.A.; Rizzetti, T.M.; Sanchez-Barrios, A.; Schneider, R.; Brittes-Benitez, L. The use of microalgae-microbial fuel cells in wastewater bioremediation and bioelectricity generation. J. Water Process Eng. 2022, 48, 102882. [Google Scholar] [CrossRef]
- Nookwam, K.; Cheirsilp, B.; Maneechote, W.; Boonsawang, P.; Sukkasem, C. Microbial fuel cells with Photosynthetic-Cathodic chamber in vertical cascade for integrated Bioelectricity, biodiesel feedstock production and wastewater treatment. Bioresour. Technol. 2022, 346, 126559. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Rocha, J.C.; Guajardo-Barbosa, C.; Barceló-Quintal, I.D.; Reyna-Martínez, G.; Fariz-Salinas, E.; Ramírez-Castillo, A.; Rodríguez-Fuentes, H.; López-Chuken, U.J. Effect of natural increase of pH and microalgae cyclical re-cultivation on biomass production and polishing of municipal secondary effluent. Desalination Water Treat. 2024, 317, 100103. [Google Scholar] [CrossRef]
- Kokabian, B.; Smith, R.; Brooks, J.P.; Gude, V.G. Bioelectricity production in photosynthetic microbial desalination cells under different flow configurations. J. Ind. Eng. Chem. 2018, 58, 131–139. [Google Scholar] [CrossRef]
- Gadkari, S.; Fontmorin, J.M.; Yu, E.; Sadhukhan, J. Influence of temperature and other system parameters on microbial fuel cell performance: Numerical and experimental investigation. Chem. Eng. J. 2020, 388, 124176. [Google Scholar] [CrossRef]
- Khazraee-Zamanpour, M.; Kariminia, H.-R.; Vosoughi, M. Electricity generation, desalination and microalgae cultivation in a biocathode-microbial desalination cell. J. Environ. Chem. Eng. 2017, 5, 843–848. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.; Zhang, H.; Jia, H.; Zhang, Y.; Fang, H.; Gao, F.; Li, J. Fluctuation of electrode potential based on molecular regulation induced diversity of electrogenesis behavior in multiple equilibrium microbial fuel cell. Chemosphere 2019, 237, 124453. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Kumar, V.K.; Manmohan, K.; Gajalakshmi, S.; Pathak, B. Bioelectrogenesis from ceramic membrane-based algal-microbial fuel cells treating dairy industry wastewater. Sustain. Energy Technol. Assess. 2021, 48, 101653. [Google Scholar] [CrossRef]
- Alalawy, A.I.; Zidan, N.S.; Sakran, M.; Hazazi, A.Y.; Salama, E.-S.; Alotaibi, M.A. Enhancing bioelectricity generation in seaweed-derived microbial fuel cells using modified anodes with Fe2O3@AuNPs/PANI nanocomposites. Biomass Bioenergy 2024, 182, 107104. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Sharma, N.; Rautela, I.; Nanda, M.; Arora, N.; Singh, A.; Chauhan, P.K. Microalgae fuel cell for wastewater treatment: Recent advances and challenges. J. Water Process Eng. 2020, 38, 101549. [Google Scholar] [CrossRef]
- Xing, F.; Xi, H.; Yu, Y.; Zhou, Y. Anode biofilm influence on the toxic response of microbial fuel cells under different operating conditions. Sci. Total Environ. 2021, 775, 145048. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Singh, N.; Mishra, S.; Ahirwar, A.; Bast, F.; Varjani, S.; Schoefs, B.; Marchand, J.; Rajendran, K.; Banu, J.R.; et al. Impact of light on microalgal photosynthetic microbial fuel cells and removal of pollutants by nanoadsorbent biopolymers: Updates, challenges and innovations. Chemosphere 2022, 288, 132589. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qu, W.; Chang, H.; Li, J.; Ho, S.-H. Microalgae-driven swine wastewater biotreatment: Nutrient recovery, key microbial community and current challenges. J. Hazard. Mater. 2022, 440, 129785. [Google Scholar] [CrossRef] [PubMed]
- García-Galán, M.J.; Matamoros, V.; Uggetti, E.; Díez-Montero, R.; García, J. Removal and environmental risk assessment of contaminants of emerging concern from irrigation waters in a semi-closed microalgae photobioreactor. Environ. Res. 2021, 194, 110278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, L.; Nie, C.; Hou, Q.; Zhang, S.; Pei, H. Multiple anodic chambers sharing an algal raceway pond to establish a photosynthetic microbial fuel cell stack: Voltage boosting accompany wastewater treatment. Water Res. 2019, 164, 114955. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Vijay, A.; Dixit, A.; Chhabra, M. Microbial fuel cell powered by lipid extracted algae: A promising system for algal lipids and power generation. Bioresour. Technol. 2018, 247, 520–527. [Google Scholar] [CrossRef]
- Peter, A.P.; Koyande, A.K.; Chew, K.W.; Ho, S.-H.; Chen, W.H.; Chang, J.-S.; Krishnamoorthy, R.; Banat, F.; Show, P.L. Continuous cultivation of microalgae in photobioreactors as a source of renewable energy: Current status and future challenges. Renew. Sustain. Energy Rev. 2022, 154, 111852. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, J.; Huang, B.; Zhou, X.; Zhang, Y. Microalgae-based technologies for carbon neutralization and pollutant remediation: A comprehensive and systematic review. Renew. Sustain. Energy Rev. 2024, 202, 107323. [Google Scholar] [CrossRef]
- Mao, B.-D.; Vadiveloo, A.; Qiu, J.; Gao, F. Artificial photosynthesis: Promising approach for the efficient production of high-value bioproducts by microalgae. Bioresour. Technol. 2024, 401, 130718. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.K.; Ghosh, U.K. Post treatment of microalgae treated pharmaceutical wastewater in photosynthetic microbial fuel cell (PMFC) and biodiesel production. Biomass Bioenergy 2019, 131, 105415. [Google Scholar] [CrossRef]
- Ullah, Z.Z. Effect of catholyte on performance of photosynthetic microbial fuel cell for wastewater treatment and energy recovery. Renew. Energy 2024, 221, 119810. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Hu, L.; Zhang, Y.; Fu, Q.; Zhang, L.; Zhu, X.; Liao, Q. Microalgae hydrogel-derived monolithicfree-standing air cathode for microbial fuel cells: Tailoring the macroporous structure for enhanced bioelectricity generation. Renew. Sustain. Energy Rev. 2022, 153, 111773. [Google Scholar] [CrossRef]
- Sharma, M.; Jalalah, M.; Alsareii, A.A.; Harraz, F.A.; Almadiy, A.A.; Thakur, N.; Salama, E.-S.; Li, X. Fat, oil, and grease as new feedstock towards bioelectrogenesis in microbial fuel cells: Microbial diversity, metabolic pathways, and key enzymes. J. Energy Chem. 2023, 85, 418–429. [Google Scholar] [CrossRef]
- Tang, X.; Cui, Y.; Liu, L. Pyrolyzing pyrite and microalgae for enhanced anode performance in microbial fuel cells. Int. J. Hydrog. Energy 2021, 46, 37460–37468. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Ling, Y.; Wang, H.; Wang, H.; Yan, G.; Duan, L.; Dong, W.; Chang, Y. Novel slow-release carbon source improves anodic denitrification and electricity generation efficiency in microbial fuel cells. Environ. Res. 2023, 236, 116644. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Omine, K.; Zhang, Z.; Sivasankar, V.; Sano, H.; Chicas, S.D. Development of peat microbial fuel cells (Peat MFCs)—The green and sustainable generators of electricity. Energy Convers. Manag. 2023, 279, 116771. [Google Scholar] [CrossRef]
- Liu, F.-F.; Lu, T.; Zhang, Y.-X. Performance assessment of constructed wetland-microbial fuel cell for treatment of mariculture wastewater containing heavy metals. Process Saf. Environ. Prot. 2022, 168, 633–641. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüero-Quiñones, R.; De La Cruz-Noriega, M.; Rojas-Villacorta, W. Electric Potential of Chlorella sp. Microalgae Biomass in Microbial Fuel Cells (MFCs). Bioengineering 2025, 12, 635. https://doi.org/10.3390/bioengineering12060635
Agüero-Quiñones R, De La Cruz-Noriega M, Rojas-Villacorta W. Electric Potential of Chlorella sp. Microalgae Biomass in Microbial Fuel Cells (MFCs). Bioengineering. 2025; 12(6):635. https://doi.org/10.3390/bioengineering12060635
Chicago/Turabian StyleAgüero-Quiñones, Rickelmi, Magaly De La Cruz-Noriega, and Walter Rojas-Villacorta. 2025. "Electric Potential of Chlorella sp. Microalgae Biomass in Microbial Fuel Cells (MFCs)" Bioengineering 12, no. 6: 635. https://doi.org/10.3390/bioengineering12060635
APA StyleAgüero-Quiñones, R., De La Cruz-Noriega, M., & Rojas-Villacorta, W. (2025). Electric Potential of Chlorella sp. Microalgae Biomass in Microbial Fuel Cells (MFCs). Bioengineering, 12(6), 635. https://doi.org/10.3390/bioengineering12060635


_Li.png)



