Electrophysiological Approaches to Understanding Brain–Muscle Interactions During Gait: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- -
- Population (P): Studies involving participants of any age or health status. Both healthy and clinical populations were considered;
- -
- Intervention (I): Simultaneous use of EMG and EEG during a gait or stepping task on a stable, level surface;
- -
- Comparison (C): No explicit comparison was required, but studies could include different populations or experimental conditions;
- -
- Outcomes (O): Quantification of synchronization between EEG and EMG signals;
- -
- Study design (S): Full-text articles published in English, indexed in at least one of the screened databases, and excluding reviews, editorials, and conference abstracts.
2.3. Study Selection
2.4. Data Extraction and Synthesis
2.5. Risk of Bias Assessment
3. Results
3.1. Selected Papers
3.2. Risk of Bias Assessment
3.3. Study Objectives
3.4. Participants
3.5. Gait Tasks
3.6. EEG Set-Up and Processing
3.7. EMG Set-Up and Processing
3.8. NMC Methods
3.9. Neuromuscular Connectivity Modulations During the Gait Cycle
3.9.1. Tibialis Anterior (TA)
3.9.2. Gastrocnemius (GC) and Soleus (SOL)
4. Discussion
4.1. Neuromuscular Connectivity Modulations over the Gait Cycle
4.2. Inconsistency in Research Design and Methodology
4.3. Altered NMC in Aging and Motor Disorders
4.4. Task-Dependent Modulations of NMC
4.5. Neuromuscular Connectivity During Gait: Evidence for Cortical Control?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EEG | Electroencephalography |
| EMG | Electromyography |
| NMC | Neuromuscular connectivity |
| CMC | Corticomuscular coherence |
| PD | Parkinson’s disease |
| FOG | Freezing of gait |
| MS | Multiple sclerosis |
| IQR | Interquartile range |
| F | Female |
| TA | Tibialis anterior |
| GC | Gastrocnemius |
| GCM | Gastrocnemius medialis |
| GCL | Gastrocnemius lateralis |
| SOL | Soleus |
| VM | Vastus medialis |
| VL | Vastus lateralis |
| RF | Rectus femoris |
| BF | Biceps femoris |
| SM | Semimembranosus |
| ST | Semitendinosus |
| PL | Peroneus longus |
| HL | Hallucis longus |
| ICA | Independent component analysis |
| ASR | Artifact subspace reconstruction |
| HS | Heel strike |
| CP | Cerebral palsy |
| CNS | Central nervous system |
| fNIRS | Functional infrared spectroscopy |
| fMRI | Functional magnetic resonance imaging |
| TMS | Transcranial magnetic stimulation |
| ERS | Event-related synchronization |
| ERD | Event-related desynchronization |
References
- Paluch, A.E.; Bajpai, S.; Bassett, D.R.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Jefferis, B.J.; Kraus, W.E.; Lee, I.-M.; et al. Daily Steps and All-Cause Mortality: A Meta-Analysis of 15 International Cohorts. Lancet Public Health 2022, 7, e219–e228. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.G. On the Nature of the Fundamental Activity of the Nervous Centres; Together with an Analysis of the Conditioning of Rhythmic Activity in Progression, and a Theory of the Evolution of Function in the Nervous System. J. Physiol. 1914, 48, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Guertin, P.A. The Mammalian Central Pattern Generator for Locomotion. Brain Res. Rev. 2009, 62, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Wang, C.; Wai, Y.; Kuo, B.; Yeh, Y.-Y.; Wang, J. Cortical Control of Gait in Healthy Humans: An FMRI Study. J. Neural Transm. 2008, 115, 1149–1158. [Google Scholar] [CrossRef]
- Allali, G.; van der Meulen, M.; Beauchet, O.; Rieger, S.W.; Vuilleumier, P.; Assal, F. The Neural Basis of Age-Related Changes in Motor Imagery of Gait: An FMRI Study. J. Gerontol. Ser. A 2014, 69, 1389–1398. [Google Scholar] [CrossRef]
- Suzuki, M.; Miyai, I.; Ono, T.; Kubota, K. Activities in the Frontal Cortex and Gait Performance Are Modulated by Preparation. An FNIRS Study. Neuroimage 2008, 39, 600–607. [Google Scholar] [CrossRef]
- Suzuki, M.; Miyai, I.; Ono, T.; Oda, I.; Konishi, I.; Kochiyama, T.; Kubota, K. Prefrontal and Premotor Cortices Are Involved in Adapting Walking and Running Speed on the Treadmill: An Optical Imaging Study. Neuroimage 2004, 23, 1020–1026. [Google Scholar] [CrossRef]
- Miyai, I.; Tanabe, H.C.; Sase, I.; Eda, H.; Oda, I.; Konishi, I.; Tsunazawa, Y.; Suzuki, T.; Yanagida, T.; Kubota, K. Cortical Mapping of Gait in Humans: A Near-Infrared Spectroscopic Topography Study. Neuroimage 2001, 14, 1186–1192. [Google Scholar] [CrossRef]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-Time Variability and Sensorimotor Cortical Activation during Walking. Neuroimage 2012, 59, 1602–1607. [Google Scholar] [CrossRef]
- Schubert, M.; Curt, A.; Jensen, L.; Dietz, V. Corticospinal Input in Human Gait: Modulation of Magnetically Evoked Motor Responses. Exp. Brain Res. 1997, 115, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Christensen, L.O.D.; Nielsen, J. The Effect of Transcranial Magnetic Stimulation on the Soleus H Reflex during Human Walking. J. Physiol. 1998, 513, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Capaday, C.; Lavoie, B.A.; Barbeau, H.; Schneider, C.; Bonnard, M. Studies on the Corticospinal Control of Human Walking. I. Responses to Focal Transcranial Magnetic Stimulation of the Motor Cortex. J. Neurophysiol. 1999, 81, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, M.; Camus, M.; Coyle, T.; Pailhous, J. Task-Induced Modulation of Motor Evoked Potentials in Upper-Leg Muscles during Human Gait: A TMS Study. Eur. J. Neurosci. 2002, 16, 2225–2230. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic Principles of Transcranial Magnetic Stimulation (TMS) and Repetitive TMS (RTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Walsh, V.; Cowey, A. Transcranial Magnetic Stimulation and Cognitive Neuroscience. Nat. Rev. Neurosci. 2000, 1, 73–80. [Google Scholar] [CrossRef]
- Vitorio, R.; Stuart, S.; Rochester, L.; Alcock, L.; Pantall, A. FNIRS Response during Walking—Artefact or Cortical Activity? A Systematic Review. Neurosci. Biobehav. Rev. 2017, 83, 160–172. [Google Scholar] [CrossRef]
- Korivand, S.; Jalili, N.; Gong, J. Experiment Protocols for Brain-Body Imaging of Locomotion: A Systematic Review. Front. Neurosci. 2023, 17, 1051500. [Google Scholar] [CrossRef]
- Zhao, M.; Bonassi, G.; Guarnieri, R.; Pelosin, E.; Nieuwboer, A.; Avanzino, L.; Mantini, D. A Multi-Step Blind Source Separation Approach for the Attenuation of Artifacts in Mobile High-Density Electroencephalography Data. J. Neural Eng. 2021, 18, 066041. [Google Scholar] [CrossRef]
- Taberna, G.A.; Samogin, J.; Mantini, D. Automated Head Tissue Modelling Based on Structural Magnetic Resonance Images for Electroencephalographic Source Reconstruction. Neuroinformatics 2021, 19, 585–596. [Google Scholar] [CrossRef]
- Taberna, G.A.; Marino, M.; Ganzetti, M.; Mantini, D. Spatial Localization of EEG Electrodes Using 3D Scanning. J. Neural Eng. 2019, 16, 026020. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical Activity Is Coupled to Gait Cycle Phase during Treadmill Walking. Neuroimage 2011, 54, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Severens, M.; Nienhuis, B.; Desain, P.; Duysens, J. Feasibility of Measuring Event Related Desynchronization with Electroencephalography during Walking. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2764–2767. [Google Scholar]
- Fries, P. A Mechanism for Cognitive Dynamics: Neuronal Communication through Neuronal Coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheng, Y.; Liu, H. Corticomuscular Coherence and Its Applications: A Review. Front. Hum. Neurosci. 2019, 13, 100. [Google Scholar] [CrossRef]
- Conway, B.A.; Halliday, D.M.; Farmer, S.F.; Shahani, U.; Maas, P.; Weir, A.I.; Rosenberg, J.R. Synchronization between Motor Cortex and Spinal Motoneuronal Pool during the Performance of a Maintained Motor Task in Man. J. Physiol. 1995, 489, 917–924. [Google Scholar] [CrossRef]
- Gross, J.; Tass, P.A.; Salenius, S.; Hari, R.; Freund, H.-J.; Schnitzler, A. Cortico-muscular Synchronization during Isometric Muscle Contraction in Humans as Revealed by Magnetoencephalography. J. Physiol. 2000, 527, 623–631. [Google Scholar] [CrossRef]
- Halliday, D.M.; Conway, B.A.; Farmer, S.F.; Rosenberg, J.R. Using Electroencephalography to Study Functional Coupling between Cortical Activity and Electromyograms during Voluntary Contractions in Humans. Neurosci. Lett. 1998, 241, 5–8. [Google Scholar] [CrossRef]
- Salenius, S.; Portin, K.; Kajola, M.; Salmelin, R.; Hari, R. Cortical Control of Human Motoneuron Firing During Isometric Contraction. J. Neurophysiol. 1997, 77, 3401–3405. [Google Scholar] [CrossRef]
- Chakarov, V.; Naranjo, J.R.; Schulte-Mönting, J.; Omlor, W.; Huethe, F.; Kristeva, R. Beta-Range EEG-EMG Coherence With Isometric Compensation for Increasing Modulated Low-Level Forces. J. Neurophysiol. 2009, 102, 1115–1120. [Google Scholar] [CrossRef]
- Witte, M.; Patino, L.; Andrykiewicz, A.; Hepp-Reymond, M.-C.; Kristeva, R. Modulation of Human Corticomuscular Beta-Range Coherence with Low-Level Static Forces. Eur. J. Neurosci. 2007, 26, 3564–3570. [Google Scholar] [CrossRef]
- Baker, S.N.; Olivier, E.; Lemon, R.N. Coherent Oscillations in Monkey Motor Cortex and Hand Muscle EMG Show Task-Dependent Modulation. J. Physiol. 1997, 501, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.M.; Baker, S.N.; Salenius, S.; Hari, R.; Lemon, R.N. Human Cortical Muscle Coherence Is Directly Related to Specific Motor Parameters. J. Neurosci. 2000, 20, 8838–8845. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, M.; Chiaramonte, R.; De Sire, A.; Buccheri, E.; Finocchiaro, P.; Scaturro, D.; Letizia Mauro, G.; Cioni, M. Do Proprioceptive Training Strategies with Dual-Task Exercises Positively Influence Gait Parameters in Chronic Stroke? A Systematic Review. J. Rehabil. Med. 2024, 56, jrm18396. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Willerslev-Olsen, M.; Conway, B.A.; Nielsen, J.B. The Motor Cortex Drives the Muscles during Walking in Human Subjects. J. Physiol. 2012, 590, 2443–2452. [Google Scholar] [CrossRef]
- De Tommaso, M.; Vecchio, E.; Ricci, K.; Montemurno, A.; De Venuto, D.; Annese, V.F. Combined EEG/EMG Evaluation During a Novel Dual Task Paradigm for Gait Analysis. In Proceedings of the Proceedings—2015 6th IEEE International Workshop on Advances in Sensors and Interfaces, IWASI 2015, Gallipoli, Italy, 18–19 June 2015; pp. 181–186. [Google Scholar]
- Winslow, A.; Brantley, J.; Zhu, F.; Contreras-Vidal, J.; Huang, H. Corticomuscular Coherence Variation throughout the Gait Cycle during Overground Walking and Ramp Ascent: A Preliminary Investigation. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; ISBN 9781457702204. [Google Scholar]
- Brantley, J.; Phat Luu, T.; Ozdemir, R.; Zhu, F.; Winslow, A.; Huang, H.; Contreras-Vidal, J. Noninvasive EEG Correlates of Overground and Stair Walking. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Storzer, L.; Butz, M.; Hirschmann, J.; Abbasi, O.; Gratkowski, M.; Saupe, D.; Schnitzler, A.; Dalal, S.S. Bicycling and Walking Are Associated with Different Cortical Oscillatory Dynamics. Front. Hum. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef]
- Artoni, F.; Fanciullacci, C.; Bertolucci, F.; Panarese, A.; Makeig, S.; Micera, S.; Chisari, C. Unidirectional Brain to Muscle Connectivity Reveals Motor Cortex Control of Leg Muscles during Stereotyped Walking. Neuroimage 2017, 159, 403–416. [Google Scholar] [CrossRef]
- Roeder, L.; Boonstra, T.W.; Smith, S.S.; Graham, X.; Kerr, K. Dynamics of Corticospinal Motor Control during Overground and Treadmill Walking in Humans. J. Neurophysiol. 2018, 120, 1017–1031. [Google Scholar] [CrossRef]
- Jensen, P.; Jensen, N.J.; Terkildsen, C.U.; Choi, J.T.; Nielsen, J.B.; Geertsen, S.S. Increased Central Common Drive to Ankle Plantar Flexor and Dorsiflexor Muscles during Visually Guided Gait. Physiol. Rep. 2018, 6, e13598. [Google Scholar] [CrossRef]
- Jensen, P.; Frisk, R.; Spedden, M.E.; Geertsen, S.S.; Bouyer, L.J.; Halliday, D.M.; Nielsen, J.B. Using Corticomuscular and Intermuscular Coherence to Assess Cortical Contribution to Ankle Plantar Flexor Activity During Gait. J. Mot. Behav. 2019, 51, 668–680. [Google Scholar] [CrossRef]
- Günther, M.; Bartsch, R.P.; Miron-Shahar, Y.; Hassin-Baer, S.; Inzelberg, R.; Kurths, J.; Plotnik, M.; Kantelhardt, J.W. Coupling between Leg Muscle Activation and EEG during Normal Walking, Intentional Stops, and Freezing of Gait in Parkinson’s Disease. Front. Physiol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Li, J.; Dimitrakopoulos, G.N.; Thangavel, P.; Chen, G.; Sun, Y.; Guo, Z.; Yu, H.; Thakor, N.; Bezerianos, A. What Are Spectral and Spatial Distributions of EEG-EMG Correlations in Overground Walking? An Exploratory Study. IEEE Access 2019, 7, 143935–143946. [Google Scholar] [CrossRef]
- Spedden, M.E.; Choi, J.T.; Nielsen, J.B.; Geertsen, S.S. Corticospinal Control of Normal and Visually Guided Gait in Healthy Older and Younger Adults. Neurobiol. Aging 2019, 78, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, A.; Glassen, M.; DeLuca, J.; Kwasnica, M.; Yue, G.; Saleh, S. Difference in Cortical Modulation of Walking between Persons with Multiple Sclerosis and Healthy Controls: An EEG Pilot Study. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3010–3013. [Google Scholar]
- Chen, I.H.; Yang, Y.R.; Lu, C.F.; Wang, R.Y. Novel Gait Training Alters Functional Brain Connectivity during Walking in Chronic Stroke Patients: A Randomized Controlled Pilot Trial. J. Neuroeng. Rehabil. 2019, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Short, M.R.; Damiano, D.L.; Kim, Y.; Bulea, T.C. Children With Unilateral Cerebral Palsy Utilize More Cortical Resources for Similar Motor Output During Treadmill Gait. Front. Hum. Neurosci. 2020, 14, 36. [Google Scholar] [CrossRef]
- Roeder, L.; Boonstra, T.W.; Kerr, G.K. Corticomuscular Control of Walking in Older People and People with Parkinson’s Disease. Sci. Rep. 2020, 10, 2980. [Google Scholar] [CrossRef]
- Yokoyama, H.; Yoshida, T.; Zabjek, K.; Chen, R.; Masani, K. Defective Corticomuscular Connectivity during Walking in Patients with Parkinson’s Disease. J. Neurophysiol. 2020, 124, 1399–1414. [Google Scholar] [CrossRef]
- Gennaro, F.; de Bruin, E.D. A Pilot Study Assessing Reliability and Age-Related Differences in Corticomuscular and Intramuscular Coherence in Ankle Dorsiflexors during Walking. Physiol. Rep. 2020, 8, e14378. [Google Scholar] [CrossRef]
- Gennaro, F.; Maino, P.; Kaelin-lang, A.; De Bock, K.; de Bruin, E.D. Corticospinal Control of Human Locomotion as a New Determinant of Age-related Sarcopenia: An Exploratory Study. J. Clin. Med. 2020, 9, 720. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Liu, X.; Kong, W.; Xi, X. Analysis of Corticomuscular Connectivity during Walking Using Vine Copula. Math. Biosci. Eng. 2021, 18, 4341–4357. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, J.; Wang, B.; Hong, J. Surface Electromyography and Electroencephalogram-Based Gait Phase Recognition and Correlations Between Cortical and Locomotor Muscle in the Seven Gait Phases. Front. Neurosci. 2021, 15, 607905. [Google Scholar] [CrossRef]
- Manuel Mayor-Torres, J.; Korik, A.; Del Felice, A.; Coyle, D.; Murphy, S.; Lennon, O. Robotic-Assisted Gait for Lower-Limb Rehabilitation: Evidence of Altered Neural Mechanisms in Stroke. medRxiv 2022. [Google Scholar] [CrossRef]
- Caffi, L.; Boccia, S.; Longatelli, V.; Guanziroli, E.; Molteni, F.; Pedrocchi, A. Brain-Muscle Connectivity during Gait: Corticomuscular Coherence as Quantification of the Cognitive Reserve. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhao, M.; Bonassi, G.; Samogin, J.; Taberna, G.A.; Porcaro, C.; Pelosin, E.; Avanzino, L.; Mantini, D. Assessing Neurokinematic and Neuromuscular Connectivity During Walking Using Mobile Brain-Body Imaging. Front. Neurosci. 2022, 16, 912075. [Google Scholar] [CrossRef] [PubMed]
- Arunganesh, K.; Selvaraju, V.; Sivakumaran, N.; Kumaravel, S.; Karthick, P.A. Analysis of Corticomuscular Coherence between Motor Cortex Region and Tibialis Anterior Muscle Using Symbolic Transfer Entropy. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022. [Google Scholar]
- Roeder, L.; Breakspear, M.; Kerr, G.K.; Boonstra, T.W. Dynamic Brain-Muscle Networks during Gait Dynamics of Brain-Muscle Networks Reveal Effects of Age and Somatosensory Function on Gait. Iscience 2023, 27, 109162. [Google Scholar] [CrossRef]
- Spedden, M.E.; Beck, M.M.; West, T.O.; Farmer, S.F.; Nielsen, J.B.; Lundbye-Jensen, J. Dynamics of Cortical and Corticomuscular Connectivity during Planning and Execution of Visually Guided Steps in Humans. Cereb. Cortex 2023, 33, 258–277. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; Joanna Briggs Institute, University of Adelaide: Adelaide, Australia, 2024; Available online: https://synthesismanual.jbi.global (accessed on 20 March 2025). [CrossRef]
- O’Neill, G.C.; Barratt, E.L.; Hunt, B.A.E.; Tewarie, P.K.; Brookes, M.J. Measuring Electrophysiological Connectivity by Power Envelope Correlation: A Technical Review on MEG Methods. Phys. Med. Biol. 2015, 60, R271–R295. [Google Scholar] [CrossRef]
- Hipp, J.F.; Hawellek, D.J.; Corbetta, M.; Siegel, M.; Engel, A.K. Large-Scale Cortical Correlation Structure of Spontaneous Oscillatory Activity. Nat. Neurosci. 2012, 15, 884–890. [Google Scholar] [CrossRef]
- Lachaux, J.-P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring Phase Synchrony in Brain Signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Fraser, A.M.; Swinney, H.L. Independent Coordinates for Strange Attractors from Mutual Information. Phys. Rev. A 1986, 33, 1134–1140. [Google Scholar] [CrossRef]
- Pirker, W.; Katzenschlager, R. Gait Disorders in Adults and the Elderly. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef]
- Mima, T.; Hallett, M. Corticomuscular Coherence: A Review. J. Clin. Neurophysiol. 1999, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy-Mazure, A. Stéphane Armand Normal Gait. Orthop. Manag. Child. Cereb. Palsy 2015, 40, 567. [Google Scholar]
- Witham, C.L.; Riddle, C.N.; Baker, M.R.; Baker, S.N. Contributions of Descending and Ascending Pathways to Corticomuscular Coherence in Humans. J. Physiol. 2011, 589, 3789–3800. [Google Scholar] [CrossRef]
- Riddle, C.N.; Baker, S.N. Manipulation of Peripheral Neural Feedback Loops Alters Human Corticomuscular Coherence. J. Physiol. 2005, 566, 625–639. [Google Scholar] [CrossRef]
- Mima, T.; Steger, J.; Schulman, A.E.; Gerloff, C.; Hallett, M. Electroencephalographic Measurement of Motor Cortex Control of Muscle Activity in Humans. Clin. Neurophysiol. 2000, 111, 326–337. [Google Scholar] [CrossRef]
- Mehrkanoon, S.; Breakspear, M.; Boonstra, T.W. The Reorganization of Corticomuscular Coherence during a Transition between Sensorimotor States. Neuroimage 2014, 100, 692–702. [Google Scholar] [CrossRef]
- Almeida, Q.J.; Frank, J.S.; Roy, E.A.; Jenkins, M.E.; Spaulding, S.; Patla, A.E.; Jog, M.S. An Evaluation of Sensorimotor Integration during Locomotion toward a Target in Parkinson’s Disease. Neuroscience 2005, 134, 283–293. [Google Scholar] [CrossRef]
- Hansen, N.L.; Conway, B.A.; Halliday, D.M.; Hansen, S.; Pyndt, H.S.; Biering-Sørensen, F.; Nielsen, J.B. Reduction of Common Synaptic Drive to Ankle Dorsiflexor Motoneurons During Walking in Patients With Spinal Cord Lesion. J. Neurophysiol. 2005, 94, 934–942. [Google Scholar] [CrossRef]
- Norton, J.A.; Gorassini, M.A. Changes in Cortically Related Intermuscular Coherence Accompanying Improvements in Locomotor Skills in Incomplete Spinal Cord Injury. J. Neurophysiol. 2006, 95, 2580–2589. [Google Scholar] [CrossRef]
- Barthélemy, D.; Willerslev-Olsen, M.; Lundell, H.; Conway, B.A.; Knudsen, H.; Biering-Sørensen, F.; Nielsen, J.B. Impaired Transmission in the Corticospinal Tract and Gait Disability in Spinal Cord Injured Persons. J. Neurophysiol. 2010, 104, 1167–1176. [Google Scholar] [CrossRef]
- Halliday, D.M.; Conway, B.A.; Christensen, L.O.D.; Hansen, N.L.; Petersen, N.P.; Nielsen, J.B. Functional Coupling of Motor Units Is Modulated During Walking in Human Subjects. J. Neurophysiol. 2003, 89, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dewald, J.P.A.; van der Helm, F.C.T.; Schouten, A.C. Unveiling Neural Coupling within the Sensorimotor System: Directionality and Nonlinearity. Eur. J. Neurosci. 2018, 48, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The Aging Neuromuscular System and Motor Performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Bayram, M.B.; Siemionow, V.; Yue, G.H. Weakening of Corticomuscular Signal Coupling During Voluntary Motor Action in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1037–1043. [Google Scholar] [CrossRef]
- Ozdemir, R.A.; Contreras-Vidal, J.L.; Paloski, W.H. Cortical Control of Upright Stance in Elderly. Mech. Ageing Dev. 2018, 169, 19–31. [Google Scholar] [CrossRef]
- Yoshida, T.; Masani, K.; Zabjek, K.; Chen, R.; Popovic, M.R. Dynamic Cortical Participation during Bilateral, Cyclical Ankle Movements: Effects of Aging. Sci. Rep. 2017, 7, 44658. [Google Scholar] [CrossRef]
- Spedden, M.E.; Nielsen, J.B.; Geertsen, S.S. Oscillatory Corticospinal Activity during Static Contraction of Ankle Muscles Is Reduced in Healthy Old versus Young Adults. Neural Plast. 2018, 2018, 3432649. [Google Scholar] [CrossRef]
- Kamp, D.; Krause, V.; Butz, M.; Schnitzler, A.; Pollok, B. Changes of Cortico-Muscular Coherence: An Early Marker of Healthy Aging? Age 2013, 35, 49–58. [Google Scholar] [CrossRef]
- Johnson, A.N.; Shinohara, M. Corticomuscular Coherence with and without Additional Task in the Elderly. J. Appl. Physiol. 2012, 112, 970–981. [Google Scholar] [CrossRef]
- Salenius, S.; Avikainen, S.; Kaakkola, S.; Hari, R.; Brown, P. Defective Cortical Drive to Muscle in Parkinson’s Disease and Its Improvement with Levodopa. Brain 2002, 125, 491–500. [Google Scholar] [CrossRef]
- Mima, T.; Toma, K.; Koshy, B.; Hallett, M. Coherence Between Cortical and Muscular Activities After Subcortical Stroke. Stroke 2001, 32, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Krauth, R.; Schwertner, J.; Vogt, S.; Lindquist, S.; Sailer, M.; Sickert, A.; Lamprecht, J.; Perdikis, S.; Corbet, T.; Millán, J. del R.; et al. Cortico-Muscular Coherence Is Reduced Acutely Post-Stroke and Increases Bilaterally During Motor Recovery: A Pilot Study. Front. Neurol. 2019, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Daly, J.J.; Sun, J.; Hvorat, K.; Fredrickson, E.; Pundik, S.; Sahgal, V.; Yue, G.H. Functional Corticomuscular Connection during Reaching Is Weakened Following Stroke. Clin. Neurophysiol. 2009, 120, 994–1002. [Google Scholar] [CrossRef]
- Chen, X.; Xie, P.; Zhang, Y.; Chen, Y.; Yang, F.; Zhang, L.; Li, X. Multiscale Information Transfer in Functional Corticomuscular Coupling Estimation Following Stroke: A Pilot Study. Front. Neurol. 2018, 9, 287. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, Y.; Xu, G.; Li, L.; Wang, J. Using Corticomuscular Coherence to Reflect Function Recovery of Paretic Upper Limb after Stroke: A Case Study. Front. Neurol. 2018, 8, 728. [Google Scholar] [CrossRef]
- Omlor, W.; Patino, L.; Hepp-Reymond, M.-C.; Kristeva, R. Gamma-Range Corticomuscular Coherence during Dynamic Force Output. Neuroimage 2007, 34, 1191–1198. [Google Scholar] [CrossRef]
- Kilner, J.M.; Salenius, S.; Baker, S.N.; Jackson, A.; Hari, R.; Lemon, R.N. Task-Dependent Modulations of Cortical Oscillatory Activity in Human Subjects during a Bimanual Precision Grip Task. Neuroimage 2003, 18, 67–73. [Google Scholar] [CrossRef]
- Drew, T.; Marigold, D.S. Taking the next Step: Cortical Contributions to the Control of Locomotion. Curr. Opin. Neurobiol. 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Drew, T.; Jiang, W.; Kably, B.; Lavoie, S. Role of the Motor Cortex in the Control of Visually Triggered Gait Modifications. Can. J. Physiol. Pharmacol. 1996, 74, 426–442. [Google Scholar] [CrossRef]
- Armstrong, D.M. The Supraspinal Control of Mammalian Locomotion. J. Physiol. 1988, 405, 1–37. [Google Scholar] [CrossRef]
- Lee, S.J.; Hidler, J. Biomechanics of Overground vs. Treadmill Walking in Healthy Individuals. J. Appl. Physiol. 2008, 104, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Aye, N.; Hamacher, D.; Schega, L. Towards the Neuromotor Control Processes of Steady-State and Speed-Matched Treadmill and Overground Walking. Brain Topogr. 2019, 32, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; King, G.A. Dynamic Gait Stability of Treadmill versus Overground Walking in Young Adults. J. Electromyogr. Kinesiol. 2016, 31, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.O.; Paolini, G.; Della Croce, U.; Paylo, K.W.; Kerrigan, D.C. A Kinematic and Kinetic Comparison of Overground and Treadmill Walking in Healthy Subjects. Gait Posture 2007, 26, 17–24. [Google Scholar] [CrossRef]
- Kristeva-Feige, R.; Fritsch, C.; Timmer, J.; Lücking, C.-H. Effects of Attention and Precision of Exerted Force on Beta Range EEG-EMG Synchronization during a Maintained Motor Contraction Task. Clin. Neurophysiol. 2002, 113, 124–131. [Google Scholar] [CrossRef]
- Glories, D.; Duclay, J. Recurrent Inhibition Contribution to Corticomuscular Coherence Modulation between Contraction Types. Scand. J. Med. Sci. Sports 2023, 33, 597–608. [Google Scholar] [CrossRef]
- Matsuya, R.; Ushiyama, J.; Ushiba, J. Inhibitory Interneuron Circuits at Cortical and Spinal Levels Are Associated with Individual Differences in Corticomuscular Coherence during Isometric Voluntary Contraction. Sci. Rep. 2017, 7, 44417. [Google Scholar] [CrossRef]
- Kamiński, M.; Ding, M.; Truccolo, W.A.; Bressler, S.L. Evaluating Causal Relations in Neural Systems: Granger Causality, Directed Transfer Function and Statistical Assessment of Significance. Biol. Cybern. 2001, 85, 145–157. [Google Scholar] [CrossRef]
- Ushiyama, J.; Katsu, M.; Masakado, Y.; Kimura, A.; Liu, M.; Ushiba, J. Muscle Fatigue-Induced Enhancement of Corticomuscular Coherence Following Sustained Submaximal Isometric Contraction of the Tibialis Anterior Muscle. J. Appl. Physiol. 2011, 110, 1233–1240. [Google Scholar] [CrossRef]


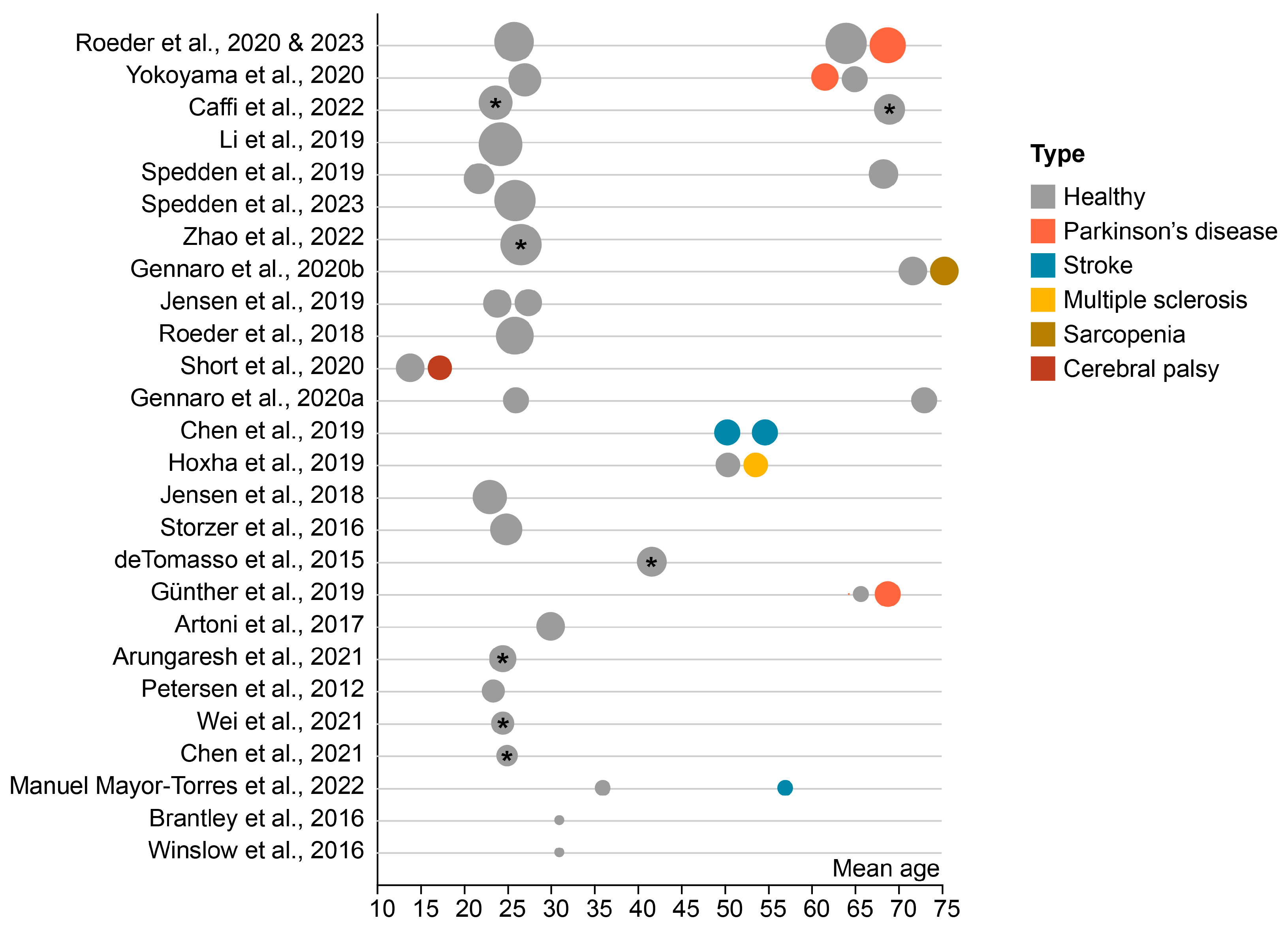

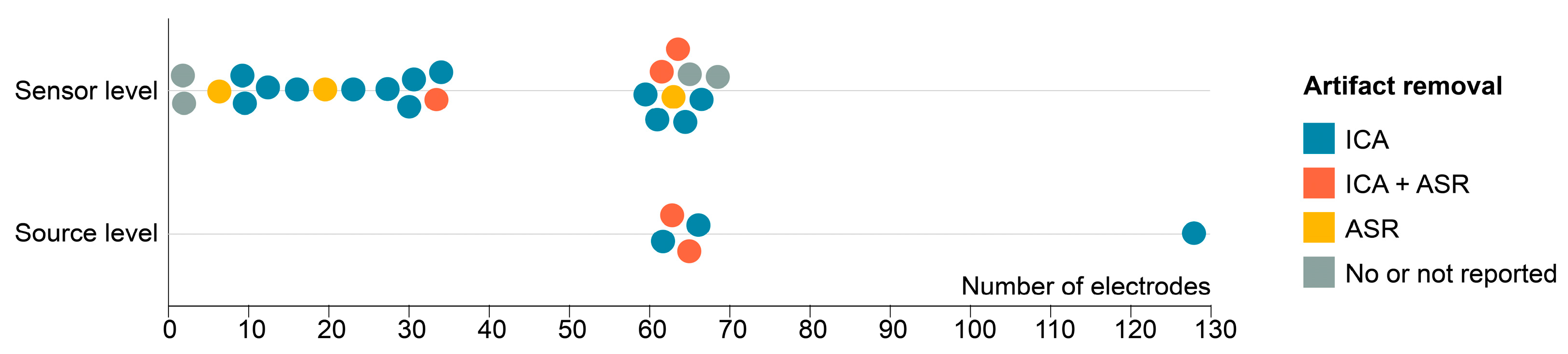
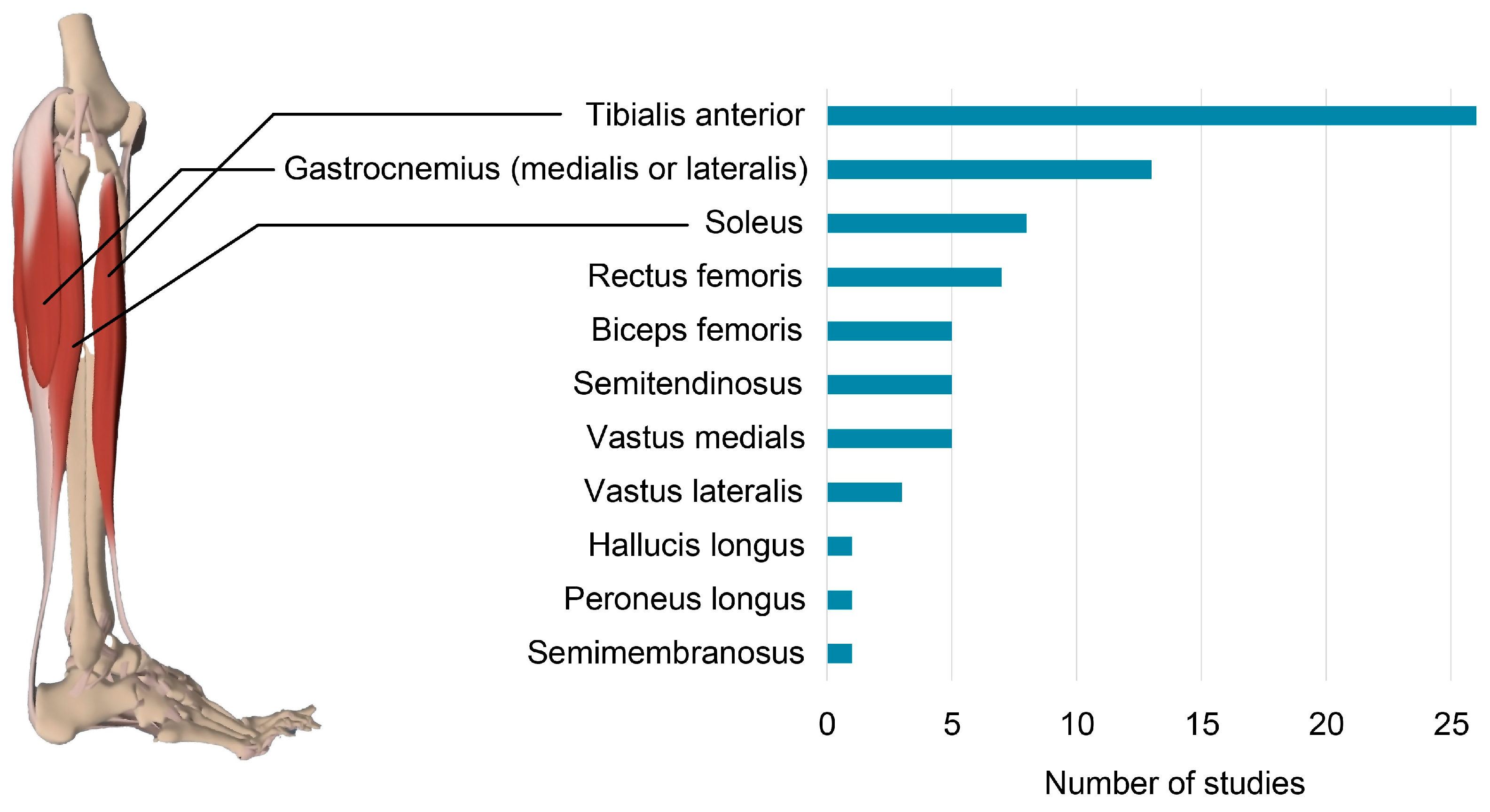

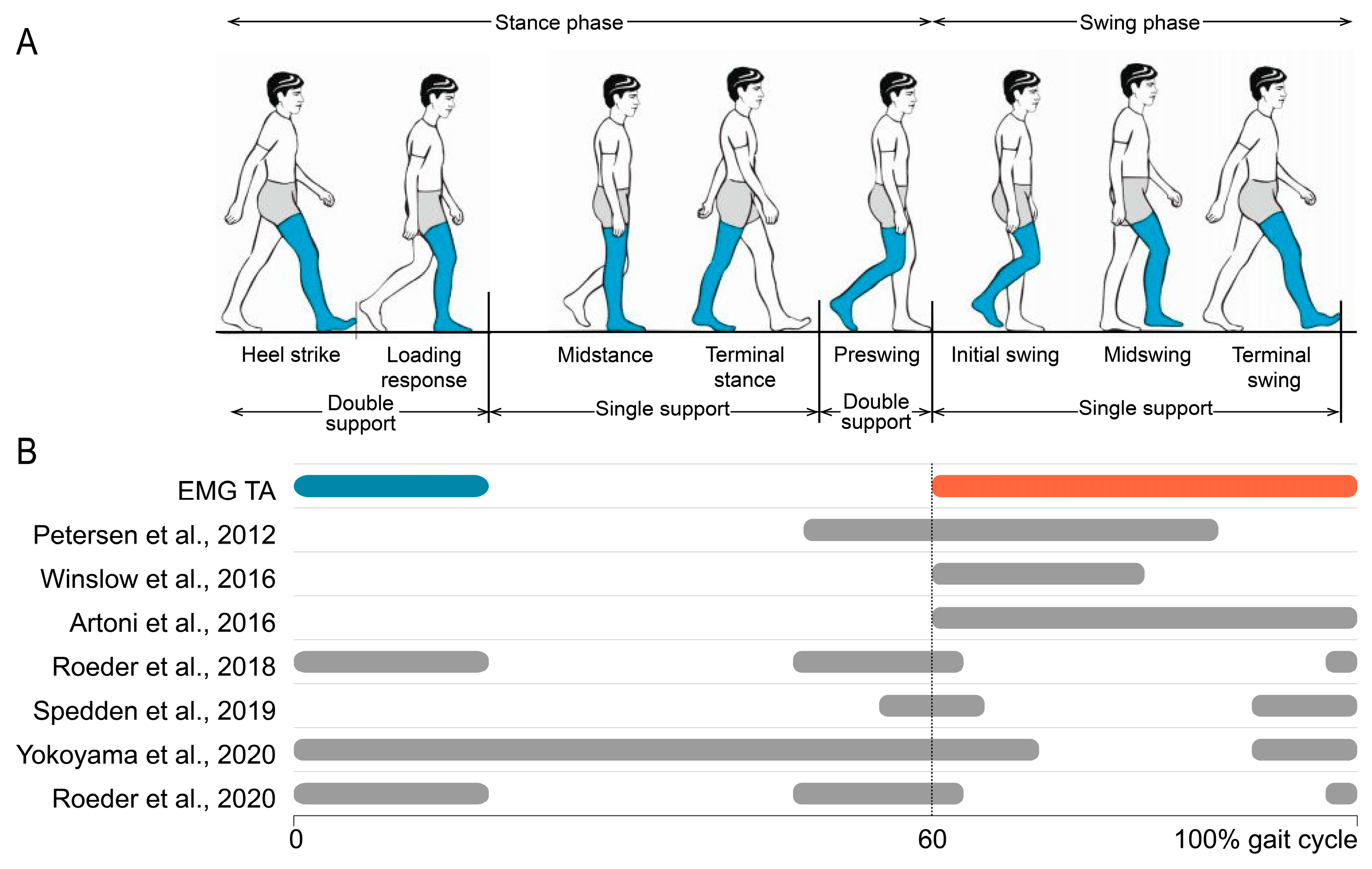
| Participants (Sample Size, Mean Age) | Gait Task | EMG Set-Up and Preprocessing | EEG Set-Up and Preprocessing | EMG–EEG Synchronization Measure, Frequency Ranges of Interest, and Gait Phase of Interest | Main Conclusions | |
|---|---|---|---|---|---|---|
| Petersen et al., 2012 [35] | Healthy young (9, F: 5, age: 23.4 ± 4.1) | Treadmill walking at 3.5–4 km/h and 1 km/h | Unilateral TA Freq. 1–1000 Hz Rectified signal | 28-channel montage Freq. 1–250 Hz ICA artifact removal Sensor-level analysis (Cz) | Coherence [8–13 Hz] [24–40 Hz] 800 ms before HS–200 ms after HS | Significant coherence at 24–40 Hz prior to heel strike during the swing phase. |
| de Tommaso et al., 2015 [36] | Healthy (17, F: 12, age range: 18–65) | Overground walking and cognitive dual-task walking at preferred speed | Bilateral TA, GCL Freq. 10–90 Hz Unrectified signal | 21-channel montage Freq. 7–30 Hz ICA artifact removal Sensor-level analysis | Coherence [8–13 Hz] [13–30 Hz] | Coherence between 7 and 12 Hz, which is reduced while performing a cognitive task. |
| Winslow et al., 2016 [37] | Healthy young (1, F: 0, age: 31) | Overground walking at preferred speed | Unilateral TA Freq. 1–450 Hz Unrectified signal | 64-channel montage Freq. 0.1–500 Hz ICA artifact removal Sensor-level analysis (Cz) | Coherence [20–40 Hz] Complete gait cycle | Coherence in low gamma band during swing phase. |
| Brantley et al., 2016 [38] | Healthy young (1, F: 0, age: 31) | Overground walking at preferred speed | Bilateral TA, GCM, RF, VL, ST Freq. 30–50 Hz Unrectified signal | 64-channel montage Freq. 0.1–6 Hz ASR artifact removal Sensor-level analysis (Cz, C1, C2) | Coherence [1–6 Hz] Complete gait cycle | Significant EEG-led coherence to TA in high delta (3–4 Hz) and low theta (4–5 Hz). |
| Storzer et al., 2016 [39] | Healthy young (15, F: 6, age: 24.9 ± 3) | Overground walking at 40 rpm at preferred speed | Bilateral TA, RF, BF Freq. 20–450 Hz Rectified signal | 18-channel montage Freq. 1–100 Hz ICA artifact removal Sensor-level analysis (Cz) | Power correlation [8–13 Hz] [24–40 Hz] Complete gait cycle | TA and BF activity are correlated with the cortical power envelope. |
| Artoni et al., 2017 [40] | Healthy young (11, age: 30 ± 4) | Treadmill walking at 3.5 km/h | Bilateral TA, VM, BF Freq. 2–500 Hz Unrectified signal | 64-channel montage Freq. 0–256 Hz ICA + ASR artifact removal Source-level analysis | Phase coupling [1–45 Hz] HS excluded | Brain-to-muscle connectivity is stronger than muscle-to-brain connectivity. Connectivity to TA and BF stronger in swing phase. |
| Roeder et al., 2018 [41] | Healthy young (24, F: 12, age: 25.9 ± 3.2) | Overground walking and treadmill walking at preferred speed (4.2 ± 0.4 km/h), barefoot | Bilateral TA Freq. 0–10 Hz Rectified signal | 10-channel montage Freq. 1–500 Hz ICA artifact removal Sensor-level analysis (C3–F3, C4–F4) | Coherence [4–8 Hz] [8–13 Hz] [13–30 Hz] [30–50 Hz] 800 ms before HS–200 ms after HS | Increased coherence (4–45 Hz) during double-support phase. Higher beta coherence (21–30 Hz) during overground compared with treadmill walking. EEG response precedes EMG response. |
| Jensen et al., 2018 [42] | Healthy young (16, F: 10, age: 23 ± 5) | Treadmill walking and visually guided walking at 2.2 km/h | Unilateral TA, SOL, GCM Rectified signal | 2-channel montage Freq. 0.5–70 Hz No artifact removal Sensor-level analysis (Cz) | Coherence [8–13 Hz] [13–30 Hz] [30–70 Hz] Swing phase (TA) and stance phase (SOL/GCM) HS excluded | Increase in coherence during visually guided walking; however, not significant. |
| Jensen et al., 2019a (main study) [43] | Healthy young (11, F: 6, age: 24.9 ± 2.8) | Treadmill walking at 3.6 km/h | SOL, GCM Rectified signal | 2-channel montage Freq. 5–500 Hz No artifact removal Sensor-level analysis (Cz) | Coherence [5–50 Hz] HS excluded | Coherence during stance phase. EEG activity leads EMG activity. |
| Jensen et al., 2019b (control experiment) [43] | Healthy young (10, F: 6, age: 26.3 ± 4.5) | Treadmill walking at 3.6 km/h | SOL, GCM Unrectified signal | 64-channel montage Freq. 0–1024 Hz No artifact removal Sensor-level analysis | Coherence [30–64 Hz] HS excluded | Peak of coherence in 30–64 Hz range over the Cz electrode. Coherence is larger for Cz than for any other position. |
| Günther et al., 2019 [44] | Healthy old (3, F: 1, age: 65.7 ± 14.2) PD-FOG (5, F: 2, age: 68.8 ± 9) PD + FOG (4, F: 0, age: 64.3 ± 8.2) | Overground figure-eight walking | Bilateral TA, GCM Freq. 0–10 Hz Unrectified signal | 32-channel montage Freq. 0.1–128 Hz ICA artifact removal Sensor-level analysis (C3–C4) | Power correlation [4–8 Hz] [8–13 Hz] [13–30 Hz] | Increase in coupling at the beginning of stop and FOG episodes, especially for PD-FOG and PD + FOG groups. |
| Li et al., 2019 [45] | Healthy young (30, F: 0, age: 24 ± 2.32) | Overground walking and walking with exoskeleton at preferred speed | Unilateral TA, GCL, RF, ST Freq. 2–125 Hz Unrectified signal | 62-channel montage Freq. 0.5–45 Hz ICA artifact removal Sensor-level analysis | Coherence [2–4 Hz] [4–8 Hz] [8–13 Hz] [13–30 Hz] [30–50 Hz] | Alpha and beta bands involved in increasing coherence; theta band involved in decreasing coherence. Increased assistive torque is associated with increased coherence in the alpha and beta bands and with decreased coherence in the theta band. |
| Spedden et al., 2019 [46] | Healthy young (15, F: 8, age: 22.1 ± 1.7) Healthy old (15, F: 8, age: 68.3 ± 2.7) | Treadmill walking and visually guided walking. Step length and speed normalized to leg length (healthy young: 2.13 ± 0.12 and old: 2.15 ± 0.16 km/h) | Unilateral TA Freq. 4–80 Hz Rectified signal | 64-channel montage Freq. 4–80 Hz ICA artifact removal Sensor-level analysis (Cz) | Coherence [13–30 Hz] [30–50 Hz] Swing phase, 650–50 ms before HS, HS excluded | Coherence is lower in older compared to younger participants for both tasks. Coherence is greater during VG walking than during normal walking. During late swing, older participants drive the observed task-related coherence increase. |
| Hoxha et al., 2019 [47] | Healthy (8, F: 6, age: 51 ± 6) MS (8, F:6, age: 53 ± 6) | Treadmill walking at preferred speed (healthy: 2.156 ± 0.3 km/h and MS: 1.787 ± 1.1 km/h) | Bilateral TA, SOL, GC | 64-channel montage Freq. 3–50 Hz ICA artifact removal Source-level analysis | Phase coupling [13–30 Hz] Complete gait cycle | Connectivity is higher in healthy controls. Higher connectivity is correlated with higher walking speed. |
| Chen et al., 2019 [48] | Stroke control group (9, F: 1, 50.33 ± 9.77) Stroke experimental group (9, F: 0, 54.67 ± 8.32) | Treadmill walking at preferred speed (range: 2.08–2.56 km/h) | Unilateral TA | 32-channel montage Freq. 1–50 Hz ICA artifact removal Sensor-level analysis (all channels) | Coherence [8–13 Hz] [30–50 Hz] Swing phase | Turning-based treadmill training resulted in larger increases in coherence compared to regular treadmill training. |
| Short et al., 2020 [49] | Healthy adolescents (12, F: 8, 14.8 ± 3) CP (9, F: 7, 16 ± 2.7) | Treadmill walking at preferred speed (3.2–3.6 km/h) | Bilateral TA, SOL, GCM, RF, VL, ST, PL, HL Freq. 5–35 Hz Rectified signal | 64-channel montage Freq. 1–500 Hz ICA + ASR artifact removal Source-level analysis | Coherence [2–4 Hz] [4–8 Hz] [8–13 Hz] [13–30 Hz] [30–50 Hz] Complete gait cycle | CP has larger bilateral coherence with the HL in the gamma band than healthy adolescents. |
| Roeder et al., 2020 [50] | Healthy young (24, F: 12, age: 25.9 ± 3.2) Healthy old (24, F: 12, age: 65.1 ± 7.8) PD (21, F: 8, age: 67.4 ± 7.3) | Overground and treadmill walking at preferred speed (healthy young: 4.16 ± 0.12, healthy old: 4.00 ± 0.11, PD: 3.97 ± 0.12), barefoot | Bilateral TA Freq. 20–45 Hz Rectified signal | 10-channel montage Freq. 1–500 Hz ICA artifact removal Sensor-level analysis (C3-F3, C4-F4) | Coherence [4–8 Hz] [8–13 Hz] [13–30 Hz] [30–50 Hz] 800 ms before HS–200 ms after HS | Coherence significantly lower in older and PD participants compared to younger participants. No difference between older and PD groups. |
| Yokoyama et al., 2020 [51] | Healthy young (15, F: 0, age: 26.7 ± 7.5) Healthy old (9, F: 0, age: 64.9 ± 6.3) PD: (10, F: 0, age: 61.6 ± 6.3) | Overground walking at preferred speed (4.104 ± 0.504 km/h for PD, 4.572 ± 0.504 km/h for healthy old, and 4.5 ± 0.468 km/h for healthy young), barefoot | Bilateral TA, GCM Freq. 0–64 Hz Rectified signal | 20-channel montage Freq. 2–200 Hz ASR artifact removal Sensor-level analysis (Cz) | Phase coupling [8–13 Hz] [13–30 Hz] [30–50 Hz] Swing phase (TA) and stance phase (GCM) | PD group shows smaller coherence in alpha band (TA and GCM) and beta band (TA) compared to healthy old group. No difference between older and younger groups. |
| Gennaro and de Bruin, 2020a [52] | Healthy young (9, F: 5, age: 26 ± 3) Healthy old (9, F: 3, age: 73 ± 6) | Overground figure eight at preferred speed | Bilateral TA Freq. 20–250 Hz Rectified signal | 64-channel montage Freq. 1.5–48 Hz ICA +ASR artifact removal Sensor-level analysis (Cz) | Coherence [13–30 Hz] [30–40 Hz] Swing phase, 650–50 ms before HS, HS excluded | Low test–retest reliability for coherence in both young and older adults. |
| Gennaro et al., 2020b [53] | Healthy old (11, F: 6, age: 72 ± 4) Sarcopenia (11, F: 9, age: 75 ± 7) | Overground figure eight at preferred speed (3.888 ± 0.756 km/h) | Bilateral TA, SOL, GCM, GCL, RF, VL, VM, BF Freq. 0–20 Hz Rectified signal | 64-channel montage Freq. 1.5–48 Hz ICA + ASR artifact removal Sensor-level analysis (Cz) | Coherence [13–30 Hz] [30–48 Hz] Swing phase, 650–50 ms before HS, HS excluded | Coherence to VM and BF shows high sensitivity, precision, and accuracy to discriminate between sarcopenic and non-sarcopenic older adults. |
| Chen et al., 2021 [54] | Healthy young (6, F: 3, age range: 24–26) | Overground slow walking at 60 bpm and fast walking at 120 bpm | Bilateral TA, VM, ST Freq. 0.5–50 Hz Unrectified signal | 64-channel montage Freq. 0.5–50 Hz ICA artifact removal Sensor-level analysis | Mutual information [4–8 Hz] [8–13 Hz] [13–30 Hz] [30–50 Hz] | A weakened connectivity for fast walking compared to slow walking, mainly in the alpha frequency range. |
| Wei et al., 2021 [55] | Healthy young (9, F: 3, age range: 23–26) | Treadmill slow walking at 1.4 km/h, normal walking at 2 km/h and fast walking at 2.6 km/h | Bilateral TA, VM, BF Freq. 30–450 Hz Unrectified signal | 32-channel montage Freq. 0.5–50 Hz ICA artifact removal Sensor-level analysis | Mutual information [13–30 Hz] Complete gait cycle | Differences in mutual information between pre-swing and terminal swing. No differences between loading response and mid-stance and between terminal stance and pre-swing. |
| Manuel Mayor-Torres et al., 2022 [56] | Healthy young (3, F: 2, age: 36 ± 12.12) Stroke (3, F: 1, age: 57 ± 8.71) | Overground walking and exoskeleton walking | Bilateral TA, SOL, RF, ST Freq. 5–100 Hz Unrectified signal | 32-channel montage Freq. 0.1–100 Hz ICA +ASR artifact removal Sensor-level analysis | Coherence Complete gait cycle | Stroke survivors show lower and non-focal beta coherence during overground gait both with and without exoskeleton. |
| Caffi et al., 2022 [57] | Healthy young (16, F: 9, age IQR: 24–25.25) Healthy old (13, F: 4, age IQR: 68–71) | Overground walking, cognitive dual-task walking and targeted walking at preferred speed | Bilateral TA, SOL, RF, SM Freq. 0–120 Hz Rectified signal | 8-channel montage Freq. 1–120 Hz ASR artifact removal Sensor-level analysis (C3, C4) | Coherence [15–30 Hz] [30–60 Hz] HS excluded, only single-support phases | Higher coherence during targeted walking compared to normal gait. For dual-task walking, coherence increased only in the elderly. |
| Zhao et al., 2022 [58] | Healthy young (24, F: 14, age range: 22–31) | Treadmill walking at preferred speed | TA, GC, VM, BF Freq. 1–100 Hz Unrectified signal | 128-channel montage Freq. 1–80 Hz ICA artifact removal Source-level analysis | Power correlation [8–13 Hz] [13–30 Hz] [30–50 Hz] Complete gait cycle | Connectivity to primary motor cortex but also premotor cortex, posterior parietal cortex, and cerebellum. More distinct connectivity in alpha and beta bands compared to gamma band. |
| Arunganesh et al., 2022 [59] | Healthy young (10, F: 5, age range: (18–31) | Overground walking at preferred speed | Bilateral TA | 64-channel montage Freq. 0.1–500 Hz Sensor-level analysis (Cz, C1, C2) | Mutual information [0.4–45 Hz] | Notable bidirectional coherence between brain and muscular system. |
| Roeder et al., 2023 [60] | Healthy young (24, F: 12, age: 25.9 ± 3.2) Healthy old (24, F: 12, age: 65.1 ± 7.8) PD (21, F: 8, age: 67.4 ± 7.3) | Overground walking at preferred speed (3.3–4.8 km/h), barefoot | Bilateral TA, SOL, GCM, GCL Freq. 0–20 Hz Rectified signal | 10-channel montage Freq. 0.5–70 Hz ICA artifact removal Sensor-level analysis (C3-F3, C4-F4) | Coherence 300 ms before HS–300 ms after HS | Identification of three brain–muscle networks: bilateral network, left-lateralized network active during left swing, and right-lateralized network active during right swing. Older adults show a reduction in connectivity. |
| Spedden et al., 2023 [61] | Healthy young (31, F: 9, age: 26 ± 4) | Overground visually guided steps and self-guided steps | TA Freq. 0–5 Hz Rectified signal | 64-channel montage Freq. 1–256 Hz ICA artifact removal Source-level analysis | Coherence [5–15 Hz] [25–45 Hz] Swing phase of single step | Absence of task-related modulations of coherence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seynaeve, M.; Mantini, D.; de Beukelaar, T.T. Electrophysiological Approaches to Understanding Brain–Muscle Interactions During Gait: A Systematic Review. Bioengineering 2025, 12, 471. https://doi.org/10.3390/bioengineering12050471
Seynaeve M, Mantini D, de Beukelaar TT. Electrophysiological Approaches to Understanding Brain–Muscle Interactions During Gait: A Systematic Review. Bioengineering. 2025; 12(5):471. https://doi.org/10.3390/bioengineering12050471
Chicago/Turabian StyleSeynaeve, Maura, Dante Mantini, and Toon T. de Beukelaar. 2025. "Electrophysiological Approaches to Understanding Brain–Muscle Interactions During Gait: A Systematic Review" Bioengineering 12, no. 5: 471. https://doi.org/10.3390/bioengineering12050471
APA StyleSeynaeve, M., Mantini, D., & de Beukelaar, T. T. (2025). Electrophysiological Approaches to Understanding Brain–Muscle Interactions During Gait: A Systematic Review. Bioengineering, 12(5), 471. https://doi.org/10.3390/bioengineering12050471









