Heating Induced Nanoparticle Migration and Enhanced Delivery in Tumor Treatment Using Nanotechnology
Abstract
1. Introduction
2. Development of Nanotechnology in Medicine
3. Nanoparticle Distribution in Tumors in Magnetic Nanoparticle Hyperthermia
3.1. Heating Mechanisms of Magnetic Nanoparticle Hyperthermia
3.2. Quantification of Heat Generation Rate Induced by Magnetic Nanoparticles
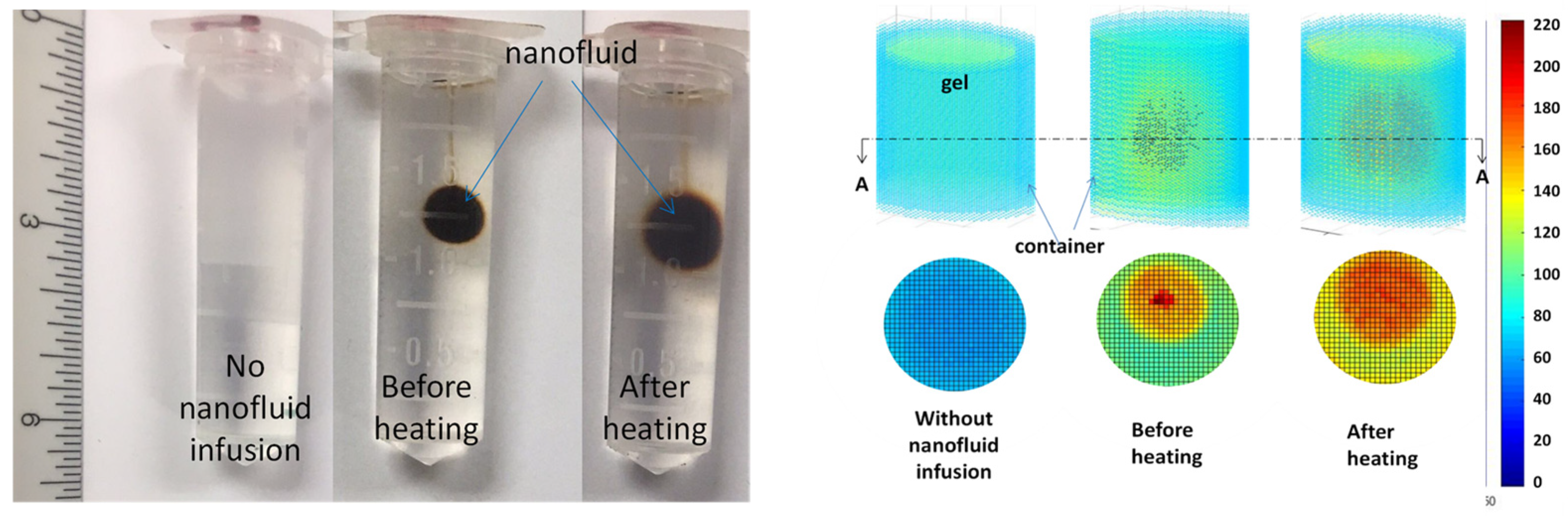
3.3. Experimental Studies in Magnetic Nanoparticle Migration during Hyperthermia
3.4. Theoretical Simulations to Understand Possible Mechanisms of Nanoparticle Migration during Heating
4. Nanoparticle Delivery in Tumors Enhanced by Mild Heating
4.1. Nanoparticles as Drug Carriers
4.2. Challenges in Drug Delivery
4.3. Mild Heating in Enhancing Systemic Drug Delivery in Tumors
4.3.1. Local or Whole-Body Heating on Blood Perfusion and IFPs in Tumors
4.3.2. Possible Mechanisms of Heating on Tumor Microenvironment
5. Conclusions Remarks
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures. 2024. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html (accessed on 30 August 2024).
- Shuichi Miyamoto, S.; Shimono, K. Molecular modeling to estimate the diffusioncoefficients of drugs and other small molecules. Molecules 2020, 25, 5340. [Google Scholar] [CrossRef]
- Zhang, A.; Mi, X.; Yang, G.; Xu, L.X. Numerical study of thermally targeted liposomal drug delivery in tumor. J. Heat Transf. 2009, 131, 043209. [Google Scholar] [CrossRef]
- Bala, V.-M.; Lampropoulou, D.I.; Grammatikaki, S.; Kouloulias, V.; Lagopati, N.; Aravantinos, G.; Gazouli, M. Nanoparticle-mediated hyperthermia and cytotoxicity mechanisms in cancer. Int. J. Mol. Sci. 2024, 25, 296. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.; Huang, X.; Elsayed, M. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; and El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–111. [Google Scholar] [CrossRef]
- LeBrun, A.; Ma, R.; Zhu, L. MicroCT Image based simulation to design heating protocols in magnetic nanoparticle hyperthermia for cancer treatment. J. Therm. Biol. 2016, 62, 129–137. [Google Scholar] [CrossRef]
- LeBrun, A.; Joglekar, T.; Bieberich, C.; Ma, R.; Zhu, L. Treatment efficacy for validating microCT-based theoretical simulation approach in magnetic nanoparticle hyperthermia for cancer treatment. J. Heat Transf. 2017, 139, 051101. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Tavangari, Z.; Parsaei, M.H.; Sargazi, S.; Sheervalilou, R.; Shirvaliloo, M.; Ghaznavi, H.; Khoei, S. The future opportunities and remaining challenges in the application of nanoparticle-mediated hyperthermia combined with chemo-radiotherapy in cancer. WIREs Nanomed. Nanotechnol. 2023, 15, e1922. [Google Scholar] [CrossRef] [PubMed]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Chen, Q.; Li, K.; Wen, S.; Liu, H.; Peng, C.; Cai, H.; Shen, M.; Zhang, G.; Shi, X. Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2013, 34, 5200–5209. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Zhang, X.; Huo, S.; Jin, S.; An, F.-F.; Wang, X.; Xue, X.; Okeke, C.I.; Duan, G.; et al. In vivo tumor-targeted dual-modal fluorescence/CT imaging using a nanoprobe co-loaded with an aggregation-induced emission dye and gold nanoparticles. Biomaterials 2015, 42, 103–111. [Google Scholar] [CrossRef]
- Yin, B.; Ho, W.K.H.H.; Xia, X.; Chan, C.K.W.C.; Zhang, Q.; Ng, Y.M.; Lam, C.Y.K.; Cheung, J.C.W.; Wang, J.; Yong, M.; et al. A multilayered mesoporous gold nanoarchitecture for ultraeffective near-infrared light-controlled chemo/photothermal therapy for cancer guided by SERS imaging. Small 2023, 19, 2206762. [Google Scholar] [CrossRef]
- Gobin, A.M.; Moon, J.J.; West, J.L. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int. J. Nanomed. 2008, 3, 351–358. [Google Scholar]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef]
- Yang, H.-W.; Liu, H.-L.; Li, M.-L.; His, I.W.; Fan, C.-T.; Huang, C.-Y.; Lu, Y.-J.; Hua, M.-Y.; Chou, H.-Y.; Liaw, J.-W.; et al. Magnetic gold-nanorod/PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials 2013, 34, 5651–5660. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of iron oxide nanoparticles in cancer therapy: Amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, J.; Mazur, M.; Osterberg, B.; Song, A.; Gladstone, J.; Steinmetz, F.; Fiering, N. Effect of intra-tumoral magnetic nanoparticle hyperthermia and viral nanoparticle immunogenicity on primary and metastatic cancer. Energy-Based Treat. Tissue Assess. IX 2017, 10066, 100660G. [Google Scholar]
- LeBrun, A.; Zhu, L. Magnetic Nanoparticle Hyperthermia in Cancer Treatment: History, Mechanism, Imaging-Assisted Protocol Design, and Challenges. In Theory and Applications of Heat Transfer in Humans; Shrivastava, D., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 631–667. [Google Scholar]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2010, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Petryk, A.; Misra, A.; Mazur, C.M.; Petryk, J.D.; Hoopes, P.J. Magnetic nanoparticle hyperthermia cancer treatment efficacy dependence on cellular and tissue level particle concentration and particle heating properties. In Energy-Based Treatment of Tissue and Assessment VIII; SPIE: Bellingham, WA, USA, 2015; Volume 9326. [Google Scholar] [CrossRef]
- Davarakonda, S.B.; Myers, M.R.; Lanier, M.; Dumoulin, C.; Banerjee, R.K. Assessment of gold nanoparticle-mediated-enhanced hyperthermia using MR-guided high-intensity focused ultrasound ablation procedure. Nano Lett. 2017, 17, 2532–2538. [Google Scholar] [CrossRef]
- Devarakonda, S.B.; Stringer, K.; Rao, M.; Myers, M.; Banerjee, R. Assessment of enhanced thermal effect due to gold nanoparticles during mr-guided high-intensity focused ultrasound (hifu) procedures using a mouse-tumor model. ACS Biomater. Sci. Eng. 2019, 5, 4102–4111. [Google Scholar] [CrossRef]
- Knanal, N.; Marciniak, M.; Daniel, M.-C.; Zhu, L.; Lanier, M.; Dumoulin, C.; Banerjee, R.K. Functionalized nanoparticles mediated high intensity focused ultrasound (HIFU) ablation in mice. In Proceedings of the Summer Biomechanics, Bioengineering and Biotransport Conference, Lake Geneva, WI, USA, 11–14 June 2024. [Google Scholar]
- Hosseinpour, A.; Soltani, M.; Souri, M. Improving tumor treatment through intratumoral injection of drug-loaded magnetic nanoparticles and low-intensity ultrasound. Sci. Rep. 2024, 14, 1452. [Google Scholar] [CrossRef]
- Souri, M.; Moradi Kashkooli, F.; Soltani, M. Analysis of magneto-hyperthermia duration in nano-sized drug delivery system to solid tumors using intravascular-triggered thermosensitive-liposome. Pharm. Res. 2022, 39, 753–765. [Google Scholar] [CrossRef]
- Zhan, W.; Gedroyc, W.; Xu, X.Y. Towards a multiphysics modelling framework for thermosensitive liposomal drug delivery to solid tumour combined with focused ultrasound hyperthermia. Biophys. Rep. 2019, 5, 43–59. [Google Scholar] [CrossRef]
- Dockery, L.; Zalesak-Kravec, S.; Kane, M.A.; Daniel, M.-C. Modular and efficient synthesis of a poly (propylene imine) (PPI) dendron applied to acid-sensitive doxorubicin conjugation. Tetrahedron 2022, 125, 133044. [Google Scholar] [CrossRef]
- Dockery, L.; Daniel, M.C. Dendronized systems for the delivery of chemotherapeutics. Adv. Cancer Res. 2018, 139, 85–120. [Google Scholar] [PubMed]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. Int. J. Rad. Onc. Biol. Phys. 2006, 32, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Salavati, H.; Debbaut, C.; Pullens, P.; Ceelen, W. Interstitial fluid pressure as an emerging biomarker in solid tumors. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188792. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Link, S.; Mohamed, M.B.; El-Sayed, M.A. Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J. Phys. Chem. B 1999, 103, 3073–3077. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Sofou, S. Radionuclide carriers for targeting of cancer. Int. J. Nanomed. 2008, 3, 181–199. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, Q.; Nan, Y.; Liu, M.; Xiao, Z.; Yang, Y.; Zhang, J.; Ying, X.; Long, X.; Wang, S.; et al. Nitric Oxide-based nanomedicines for conquering TME fortress: Say ”no” to insufficient tumor treatment. Adv. Funct. Mater. 2024, 34, 202312092. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.U.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging Research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 6315–6320. [Google Scholar] [CrossRef]
- Tong, R.; Cheng, J. Anticancer polymeric nanomedicines. Polym. Rev. 2007, 47, 345–381. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2006, 24, 1–16. [Google Scholar] [CrossRef]
- Jannin, V.; Musakhanian, J.; Marchaud, D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv. Drug Deliv. Rev. 2008, 60, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Giocondi, M.; Pacheco, L.; Milhiet, P.E.; Le Grimellec, C. Temperature dependence of the topology of supported dimirystoyl–distearoyl phosphatidylcholine bilayers. Ultramicroscopy 2001, 86, 151–157. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44–65. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Joseph, M.; Trinh, H.M.; Cholkar, K.; Pal, D.; Mitra, A.K. Recent perspectives on the delivery of biologics to back of the eye. Expert Opin. Drug Deliv. 2016, 14, 631–645. [Google Scholar] [CrossRef]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.; Laird, M.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef] [PubMed]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How can it be used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; De, S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burn. J. Int. Soc. Burn Inj. 2017, 43, 909–932. [Google Scholar] [CrossRef] [PubMed]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 01, 17–32. [Google Scholar] [CrossRef]
- Kolhatkar, A.; Jamison, A.; Litvinov, D.; Willson, R.; Lee, T. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- LeBrun, A.; Joglekar, T.; Bieberich, C.; Ma, R.; Zhu, L. Identification of infusion strategy for achieving repeatable nanoparticle distribution and quantification of thermal dosage using micro-CT Hounsfield unit in magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2016, 32, 132–143. [Google Scholar] [CrossRef][Green Version]
- Rytov, R.A.; Bautin, V.A.; Usov, N.A. Towards optimal thermal distribution in magnetic hyperthermia. Sci. Rep. 2022, 12, 3023. [Google Scholar] [CrossRef]
- Guibert, C.; Dupuis, V.; Peyre, V.; Fresnais, J. Hyperthermia of magnetic nanoparticles: Experimental study of the role of aggregation. J. Phys. Chem. C 2015, 119, 28148–28154. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Jordan, A.; Wust, P.; Fählin, H.; John, W.; Hinz, A.; Felix, R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperth. 1993, 9, 51–68. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Hurley, K.R.; Zhang, J.; Jeon, S.; Ring, H.L.; Hogan, C.; Haynes, C.; Garwood, M.; Bischof, J.C. Accounting for biological aggregation in heating and imaging of magnetic nanoparticles. Technology 2014, 02, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.; Manuchehrabadi, N.; Franklin, R.; Bischof, J. Superparamagnetic Iron Oxide Nanoparticle Heating. In Nanoparticle Heat Transfer and Fluid Flow; CRC Press: Boca Raton, FL, USA, 2016; pp. 97–122. [Google Scholar]
- Rosensweig, R. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Wust, P.; Nadobny, J.; Fähling, H.; Jordan, A.; Felix, R. Code Comparison and verification for patient-specific three-dimensional treatment planning in regional hyperthermia. In Tumor Response Monitoring and Treatment Planning; Breit, A., Heuck, A., Lukas, P., Kneschaurek, P., Mayr, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 747–751. [Google Scholar] [CrossRef]
- Wust, P.; Gneveckow, U.; Johannsen, M.; Böhmer, D.; Henkel, T.; Kahmann, F.; Sehouli, J.; Felix, R.; Ricke, J.; Jordan, A. Magnetic nanoparticles for interstitial thermotherapy—Feasibility, tolerance and achieved temperatures. Int. J. Hyperth. 2006, 22, 673–685. [Google Scholar] [CrossRef]
- Kalambur, V.S.; Longmire, E.K.; Bischof, J.C. Cellular level loading and heating of superparamagnetic iron oxide nanoparticles. Langmuir 2007, 23, 12329–12336. [Google Scholar] [CrossRef]
- Beola, L.; Grazu, V.; Fernandez-Afonso, Y.; Fratila, R.M.; de las Heras, M.; de la Fuente, J.M.; Gutierrez, L.; Asin, L. Critical parameters to improve pancreatic cancer treatment using magnetic hyperthermia: Field conditions, immune response, and particle biodistribution. ACS Appl. Mater. Interfaces 2021, 13, 12982–12996. [Google Scholar] [CrossRef]
- Vassallo, M.; Martella, D.; Barrera, G.; Celegato, F.; Coisson, M.; Ferrero, R.; Olivetti, E.S.; Troia, A.; Sozero, H.; Parmaggiani, C.; et al. Improvement of hyperthermia properties of iron oxide nanoparticles by surface coating. ACS Omega 2023, 8, 2143–2154. [Google Scholar] [CrossRef]
- Sheervalilou, R.; Khoei, S.; Khoee, S.; Shirvaliloo, M.; Sadri, E.; Shirvalilou, S.; Goudarzi, M. Magnetohyperthermia-synergistic glioma cancer therapy enabled by magnetic graphene oxide nanoheaters: Promising nanostructure for in vitro and in vivo applications. Cancer Nano 2023, 14, 44. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Kim, Y.; Kim, S.K. Ultra-high rate of temperature increment from superparamagnetic nanoparticles for highly efficient hyperthermia. Sci. Rep. 2021, 11, 4969. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.; Platt, S. Magnetic nanoparticle-based hyperthermia for head and neck cancer in mouse models. Theranostics 2012, 2, 113–121. [Google Scholar] [CrossRef]
- He, M.; Cao, X.C.; He, G.C.; Sheng, X.F.; Ai, X.H.; Wu, Y.H. Casticin inhibits epithelial-mesenchymal transition of liver cancer stem cells of the SMMC-7721 cell line through downregulating Twist. Oncol. Lett. 2014, 7, 1625–1631. [Google Scholar] [CrossRef][Green Version]
- Attaluri, A.; Ma, R.; Qiu, Y.; Li, W.; Zhu, L. Nanoparticle distribution and temperature elevations in prostate tumors in mice during magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2011, 27, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Kandala, S.K.; Zhou, H.; Wabler, M.; DeWeese, T.L.; Ivkov, R. Magnetic nanoparticle hyperthermia for treating locally advanced unresectable and borderline resectable pancreatic cancers: The role of tumor size and eddy-current heating. Int. J. Hyperth. 2020, 37, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Qian, Z.; Yang, Y. Evaluation of tumor treatment of magnetic nanoparticles driven by extremely low frequency magnetic field. Sci. Rep. 2017, 7, 46287. [Google Scholar] [CrossRef]
- Gu, Q.; Min Zaw, M.; Munuhe, T.; Ma, R.; Zhu, L. Nanoparticle re-distribution in tissue-equivalent gels induced by magnetic nanoparticle hyperthermia. In Proceedings of the Summer Biomechanics, Bioengineering, & Biotransport Conference, Tucson, AZ, USA, 21–24 June 2017. Paper number 17-A-713-SB3C. [Google Scholar]
- Gu, Q. Heating Induced Nanoparticle Redistribution in PC3 Tumors: In Vivo Experiments and MicroCT Imaging Analyses. Ph.D. Thesis, University of Maryland Baltimore County, Baltimore, MD, USA, 2019. [Google Scholar]
- Gu, Q.; Joglekar, T.; Bieberich, C.; Ma, R.; Zhu, L. Nanoparticle redistribution in PC3 tumors induced by local heating in magnetic nanoparticle hyperthermia: In vivo experimental study. J. Heat Transf. 2019, 141, 032402. [Google Scholar] [CrossRef]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef]
- Gunakala, S.R.; Job, V.M.; Murthy, P.V.S.N.; Sibanda, P.; Raju, C.S.K. Mathematical model of magnetic hyperthermia therapy for breast tumour via intratumoural injection of iron–platinum nanoparticles. Case Stud. Therm. Eng. 2024, 53, 103876. [Google Scholar] [CrossRef]
- Xu, C.; Miranda-Nieves, D.; Ankrum, J.A.; Matthiesen, M.E.; Phillips, J.A.; Roes, I.; Wojtkiewicz, G.; Juneja, V.; Kultima, J.; Zhao, W.; et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett. 2012, 12, 4131–4139. [Google Scholar] [CrossRef]
- Comerford, S.; Huang, Z.; Du, X.; Wang, Y.; Cai, L.; Witkiewicz, A.; Walters, H.; Tantawy, M.; Fu, A.; Manning, H.; et al. Acetate dependence of tumors. Cell 2014, 159, 1591–1602. [Google Scholar] [CrossRef]
- Costello, J.T.; Culligan, K.; Selfe, J.; Donnelly, A.E. Muscle, skin and core temperature after −110 °C cold air and 8 °C water treatment. PLoS ONE 2012, 7, e48190. [Google Scholar] [CrossRef]
- Singh, M.; Ma, R.; Zhu, L. Quantitative evaluation of effects of coupled temperature elevation, thermal damage, and enlarged porosity on nanoparticle migration in tumors during magnetic nanoparticle hyperthermia. Int. Commun. Heat Mass Transf. 2021, 126, 105393. [Google Scholar] [CrossRef]
- El-Kareh, A.W.; Secomb, T.W. Effect of increasing vascular hydraulic conductivity on delivery of macromolecular drugs to tumor cells. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, N.; Singh, N.; Talukdar, P. A mathematical model of intratumoral infusion, particle distribution and heat transfer in cancer tumors: In-silico investigation of magnetic nanoparticle hyperthermia. Int. J. Therm. Sci. 2023, 187, 107887. [Google Scholar] [CrossRef]
- Truskey, G.A.; Yuan, F.; Katz, D.F. Transport Phenomena in Biological Systems, 2nd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- De, J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. ACS Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Oh, H.; Jun, D.W.; Saeed, W.K.; Nguyen, M.H. Non-alcoholic fatty liver diseases: Update on the challenge of diagnosis and treatment. Clin. Mol. Hepatol. 2016, 22, 327–335. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Lin, X.; Chen, C.; Luo, C.; Huang, Y. Targeted inhibition of tumor inflammation and tumor-platelet crosstalk by nanoparticle-mediated drug delivery mitigates cancer metastasis. ACS Nano 2022, 16, 50–67. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef]
- Scott, M.C.; Chen, C.C.; Mecklenburg, M.; Zhu, C.; Xu, R.; Ercius, P.; Dahmen, U.; Regan, B.C.; Miao, J. Electron tomography at 2.4-Angstrom resolution. Nature 2012, 483, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Gooya, J.; Mao, S.; Kinneer, K.; Xu, L.; Camara, M.; Fazenbaker, C.; Fleming, R.; Swamynathan, S.; Meyer, D.; et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 2008, 68, 9367–9374. [Google Scholar] [CrossRef] [PubMed]
- Rodzinski, A.; Guduru, R.; Liang, P.; Hadjikhani, A.; Stewart, T.; Stimphil, E.; Runowica, C.; Cote, E.; Altman, N.; Datar, R.; et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci. Rep. 2016, 6, 20867. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Martin, J.D.; Chauhan, V.P.; Jain, R.K.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 4607–4612. [Google Scholar] [CrossRef]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26–58. [Google Scholar] [CrossRef]
- Margaris, K.N.; Black, R.A. Modelling the lymphatic system: Challenges and opportunities. J. R. Soc. Interface 2012, 9, 601–612. [Google Scholar] [CrossRef]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. Magnetic nanoparticle transport within flowing blood and into surrounding tissue. Nanomedicine 2010, 5, 1459–1466. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS Pharm. Sci. Technol. 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Woo, J.R.; Liss, M.A.; Muldong, M.T.; Palazzi, K.; Strasner, A.; Ammirante, M.; Varki, N.; Shabaik, A.; Howell, S.; Kane, C.; et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J. Transl. Med. 2014, 12, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Duffy, H.S. Fibroblasts and myofibroblasts: What are we talking about? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 14, 321–346. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: From mathematical modeling to bench to bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44, 4721s–4730s. [Google Scholar]
- Hauck, M.L.; Dewhirst, M.W.; Bigner, D.D.; Zalutsky, M.R. Local hyperthermia improves uptake of a chimeric monoclonal antibody in a subcutaneous xenograft model. Clin. Cancer Res. 1997, 3, 63–70. [Google Scholar]
- Lammers, T.; Peschke, P.; Kühnlein, R.; Subr, V.; Ulbrich, K.; Debus, J.; Huber, P.; Hennink, W.; Storm, G. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery system. J. Control. Release 2007, 117, 333–341. [Google Scholar] [CrossRef]
- Gu, Q.; Liu, S.; Ray, A.S.; Florinas, S.; Christie, R.J.; Daniel, M.-C.; Bieberich, C.; Ma, R.; Zhu, L. Mild whole body hyperthermia induced interstitial fluid pressure (IFP) reduction and enhanced nanoparticle delivery to PC3 tumours: In vivo studies and microCT analyses. ASME J. Therm. Sci. Eng. Appl. 2020, 12, 061001. [Google Scholar] [CrossRef]
- Fan, F.; Xie, B.; Yang, L. Promoting nanoparticle delivery efficiency to tumors by locally increasing blood flow there. ACS Appl. Bio Mater. 2021, 4, 7615–7625. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Gao, B.; Wang, H.; Huang, J.; Zhou, J.; Yang, R.; Yan, F.; Peng, Y. Synergistic cascade strategy based on modifying tumor microenvironment for enhanced breast cancer therapy. Front. Pharmacol. 2021, 12, 750847. [Google Scholar] [CrossRef]
- Leunig, M.; Goetz, A.E.; Dellian, M.; Zetterer, G.; Gamarra, F.; Jain, R.K.; Messmer, K. Interstitial fluid pressure in solid tumors following hyperthermia: Possible correlation with therapeutic response. Cancer Res. 1992, 52, 487–490. [Google Scholar] [PubMed]
- Stepleton, S.; Dunne, M.; Milosevic, M.; Tran, C.W.; Gold, M.J.; Vedadi, A.; Mckee, T.D.; Ohashi, P.S.; Allen, C.; Jaffray, D.A. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamic. ACS Nano 2018, 12, 7583–7600. [Google Scholar] [CrossRef]
- Hauck, M.L.; Coffin, D.O.; Dodge, R.K.; Dewhirst, M.W.; Mitchell, J.B.; Zalutsky, M.R. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumor interstitial fluid pressure. Int. J. Hyperth. 1997, 13, 307–316. [Google Scholar] [CrossRef]
- Sen, A.; Capitano, M.; Spernyak, J.A.; Schueckler, J.; Thomas, S.; Singh, A.; Evans, S.S.; Hylander, B.L.; Repasky, E.A. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia, and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011, 71, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Winslow, T.B.; Eranki, A.; Ullas, S.; Singh, A.K.; Repasky, E.A.; Sen, A. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: Analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int. J. Hyperth. 2015, 31, 693–701. [Google Scholar] [CrossRef]
- Koning, G.A.; Eggermont, A.M.M.; Lindner, L.H.; ten Hagen, T.L.M. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm. Res. 2010, 27, 1750–1754. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.M.; Bolkestein, M.; Gasselhuber, A.; Yatvin, J.; van Rhoon, G.C.; Eggermont, A.M.M.; Haemmerich, D.; Koning, G.A. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control. Release 2013, 167, 130–137. [Google Scholar] [CrossRef]
- Schiffelers, R.M.; Koning, G.A.; ten Hagen, T.L.M.; Fens, M.H.A.M.; Schraa, A.J.; Janssen, A.P.C.A.; Kok, R.J.; Molema, G.; Storm, G. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J. Control. Release 2003, 9, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Ahmed, M.; Goldberg, S.N. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int. J. Hyperth. 2004, 20, 781–802. [Google Scholar] [CrossRef]
- Ahmed, M.; Liu, Z.; Horkan, C.; Tochilin, V.P.; Lukyanov, A.N.; Goldberg, S.N. Raido-frequency tumor ablation combined with intravenous liposomal doxorubicin increases tumor coagulation and intratumoral drug accumulation in a large animal tumor model. Radiology 2003, 229, 457. [Google Scholar]
- Monsky, W.L.; Kruskal, J.B.; Lukyanov, A.N.; Girnun, G.D.; Ahmed, M.; Gazelle, G.S.; Huertas, J.C.; Stuart, K.E.; Torchilin, V.P.; Goldberg, S.N. Radio-frequency ablation increases intratumoral liposomal doxorubicin accumulation in a rat breast tumor model. Radiology 2002, 224, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Minamiya, Y.; Kitamura, M.; Matsuzaki, I.; Hashimoto, M.; Suzuki, H.; Abo, S. Direct measurement of doxorubicin concentration in the intact, living single cancer cell during hyperthermia. Cancer 1997, 79, 214–219. [Google Scholar] [CrossRef]
- Merlin, J.L.; Marchal, S.; Ramacci, C.; Notter, D.; Vigneron, C. Antiproliferative activity of thermosensitive liposome-encapsulated doxorubicin combined with 43 °C hyperthermia in sensitive and multidrug-resistant MCF-7 cells. Eur. J. Cancer 1993, 29, 2264–2268. [Google Scholar] [CrossRef]
- Osborne, E.J.; MacKillop, W.J. The effect of exposure to elevated temperatures on membrane permeability to adriamycin in Chinese hamster ovary cells in vitro. Cancer Lett. 1987, 37, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug Deliv. Rev. 2020, 163, 98–124. [Google Scholar] [CrossRef]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef]
- Toffoli, G.; Bevilacqua, C.; Franceschin, A.; Boiocchi, M. Effect of hyperthermia on intracellular drug accumulation and chemosensitivity in drug-sensitive and drug-resistant P388 leukaemia cell lines. Int. J. Hyperth. 1989, 5, 163–172. [Google Scholar] [CrossRef]
- Manpreet, S.; Ma, R.; Zhu, L. Theoretical evaluation of enhanced gold nanoparticle delivery to PC3 tumors due to increased hydraulic conductivity or recovered lymphatic function after mild whole body hyperthermia. Med. Biol. Eng. Comput. 2021, 59, 301–313. [Google Scholar]
- Mariana, V.F.; Maria de Fátima, G.G.; Maria, P. The effect of mechanical lymph drainage accompanied with heat on lymphedema. J. Res. Med. Sci. 2011, 16, 1448–1451. [Google Scholar] [PubMed]
- Allabashi, R.; Stach, W.; de la Escosura-Muñiz, A.; Liste Calleja, L.; Merkoçi, A. ICP-MS: A powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J. Nanoparticle Res. 2008, 11, 2003–2011. [Google Scholar] [CrossRef]
- Leu, A.J.; Berk, D.A.; Lymboussaki, A.; Alitalo, K.; Jain, R.K. Absence of functional lymphatics within a murine sarcoma: A molecular and functional evaluation. Cancer Res. 2000, 60, 4324–4327. [Google Scholar]
- Baxter, L.T.; Jain, R.K. Vascular permeability and interstitial diffusion in superfused tissue: A two-dimensional model. Microvasc. Res. 1988, 36, 108–115. [Google Scholar] [CrossRef]
- Baxter, L.T.; Jain, R.K. Transport of fluid and macromolecules in tumors I. role of interstitial pressure and convection. Microvasc. Res. 1989, 37, 77–104. [Google Scholar] [CrossRef]
- Su, D.; Ma, R.; Salloum, M.; Zhu, L. Multi-scale study of nanoparticle transport and deposition in tissues during an injection process. Med. Biol. Eng. Comput. 2010, 48, 853–863. [Google Scholar] [CrossRef]
- Stapleton, S.; Milosevic, M.; Allen, C.; Zheng, J.; Dunne, M.; Yeung, I.; Jaffray, D.A. A mathematical model of the enhanced permeability and retention effect for liposome transport in solid tumors. PLoS ONE 2013, 8, e81157. [Google Scholar] [CrossRef]
- Kockelmann, F.; Giger-Pabst, U.; Ouaissi, M.; Bucur, P.; Barbey, S.; von Ardenne, A.; Zieren, J. First clinical safety and feasibility data of whole-body hyperthermia pressurized intraperitoneal aerosol chemotherapy (wbh-pipac) for peritoneal surface malignancies. Anticancer Res. 2024, 44, 3043–3050. [Google Scholar] [CrossRef]
- Chia, D.K.A.; Demuytere, J.; Ernst, S.; Salavati, H.; Ceelen, W. Effects of hyperthermia and hyperthermic intraperitoneal chemoperfusion on the peritoneal and tumor immune contexture. Cancers 2023, 15, 4314. [Google Scholar] [CrossRef]
- Moghaddam, F.F.; Bakhshandeh, M.; Mofid, B.; Sahinbas, H.; Faeghi, F.; Mirzaei, H.; Rakhsha, A.; Kashi, A.S.Y.; Sadeghi, R.; Mahdavi, A. Clinical effectiveness of combined whole body hyperthermia and external beam radiation therapy (EBRT) versus EBRT alone in patients with painful bony metastases: A phase III clinical trial study. J. Therm. Biol. 2024, 120, 103804. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Zhu, L. Heating Induced Nanoparticle Migration and Enhanced Delivery in Tumor Treatment Using Nanotechnology. Bioengineering 2024, 11, 900. https://doi.org/10.3390/bioengineering11090900
Gu Q, Zhu L. Heating Induced Nanoparticle Migration and Enhanced Delivery in Tumor Treatment Using Nanotechnology. Bioengineering. 2024; 11(9):900. https://doi.org/10.3390/bioengineering11090900
Chicago/Turabian StyleGu, Qimei, and Liang Zhu. 2024. "Heating Induced Nanoparticle Migration and Enhanced Delivery in Tumor Treatment Using Nanotechnology" Bioengineering 11, no. 9: 900. https://doi.org/10.3390/bioengineering11090900
APA StyleGu, Q., & Zhu, L. (2024). Heating Induced Nanoparticle Migration and Enhanced Delivery in Tumor Treatment Using Nanotechnology. Bioengineering, 11(9), 900. https://doi.org/10.3390/bioengineering11090900




