Feasibility and Reproducibility of T2 Mapping Compared with Diffusion-Weighted Imaging in Solid Renal Masses
Abstract
1. Introduction
2. Materials and Methods
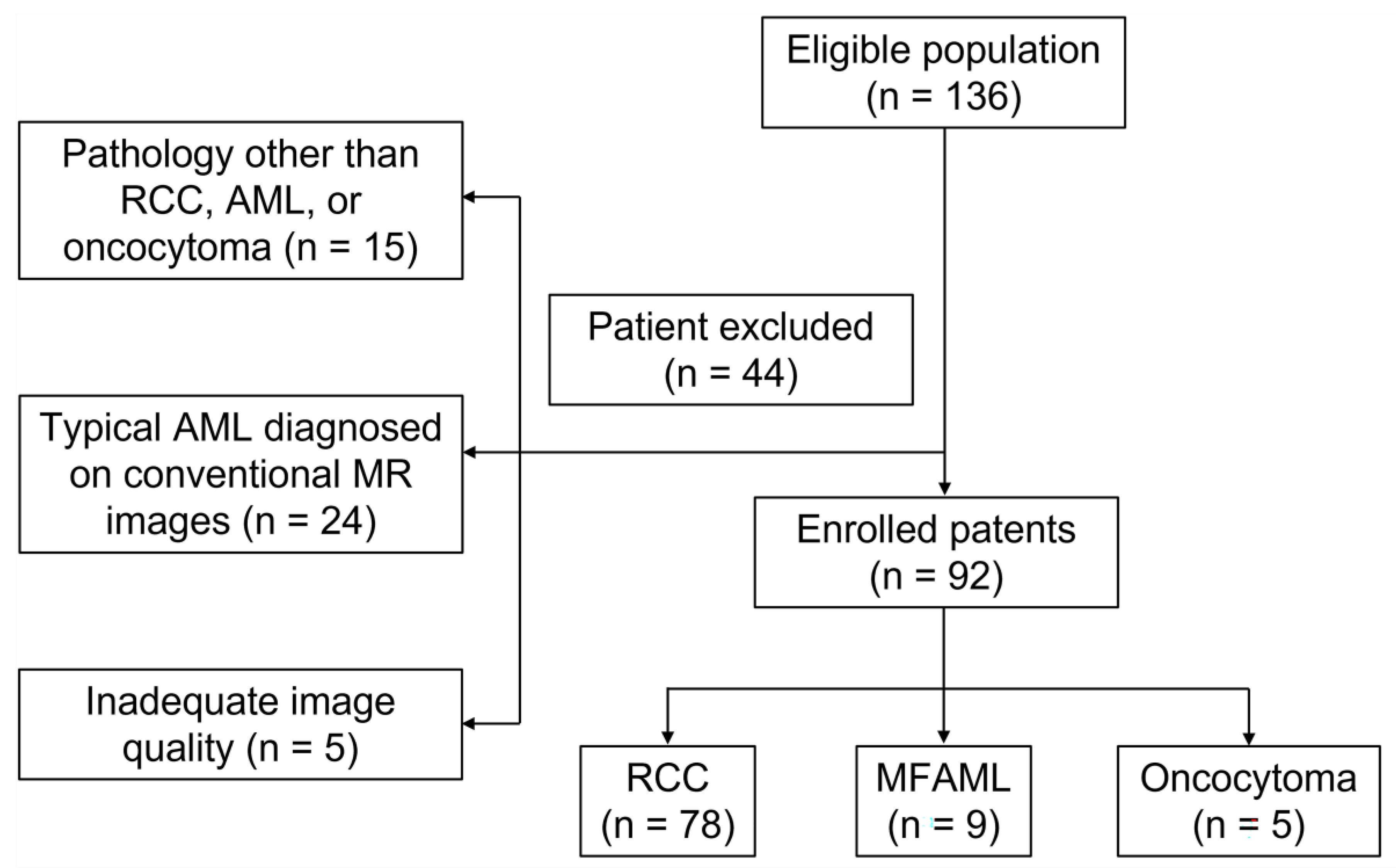
2.1. Patients
2.2. Imaging Acquisition
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Histopathological Findings
3.2. Intra- and Interobserver Agreement
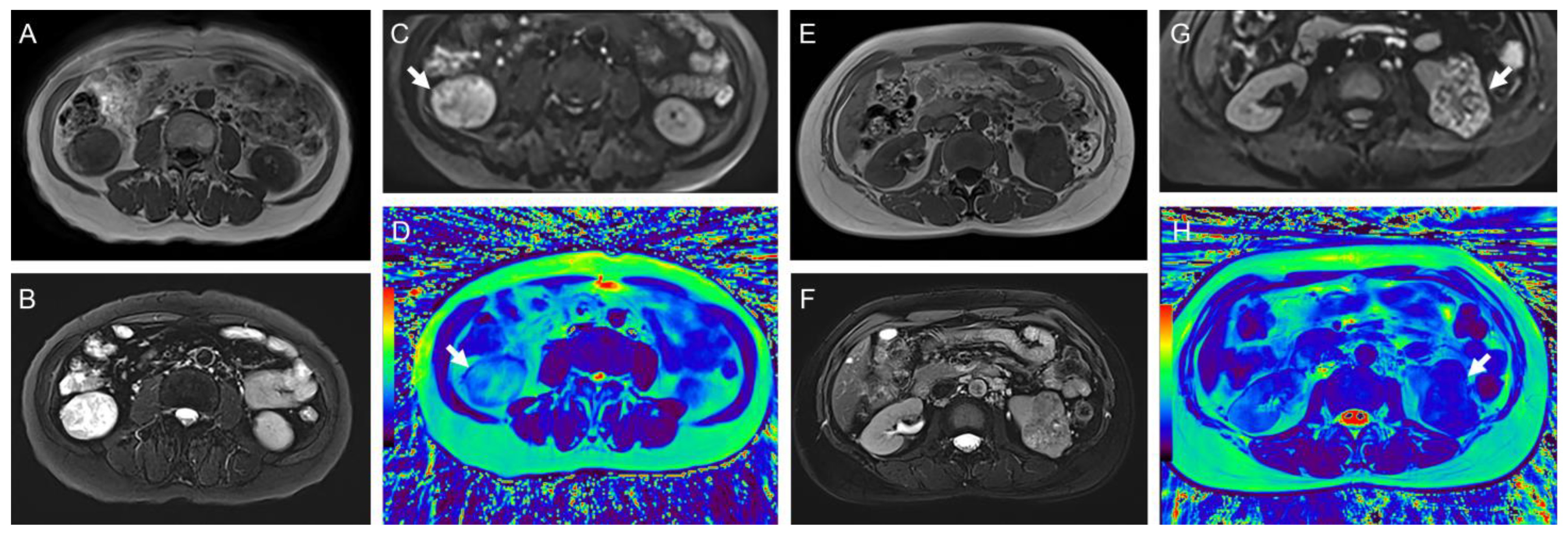
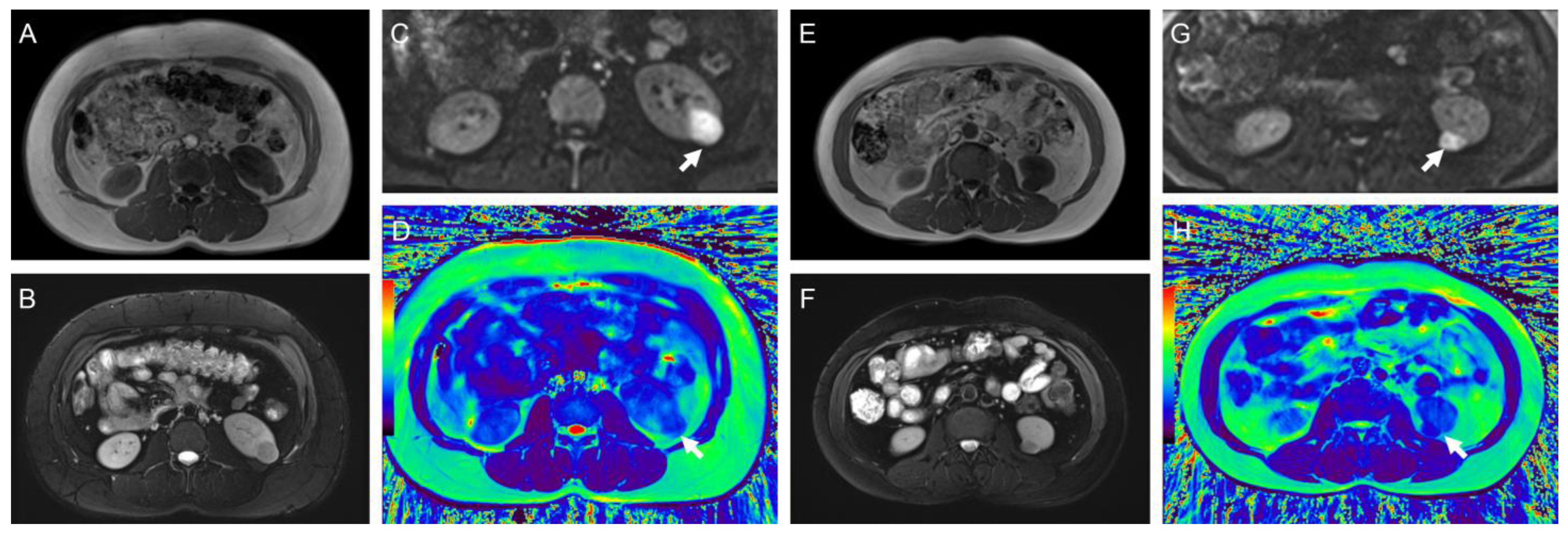
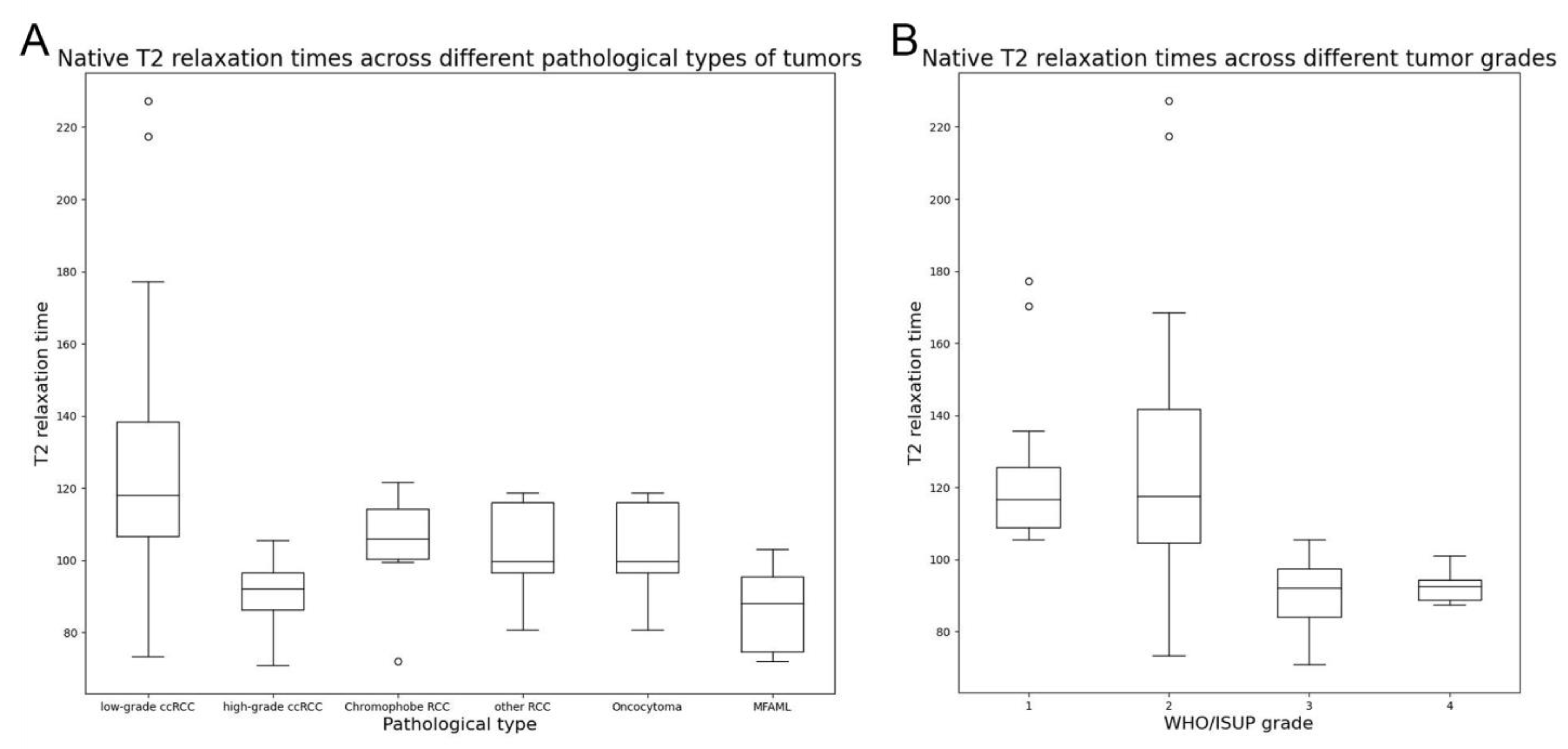
3.3. T2 Mapping and DWI Results of Different Tumor Pathological Types and Grades
3.4. Diagnostic Efficiency and Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schieda, N.; Davenport, M.S.; Silverman, S.G.; Bagga, B.; Barkmeier, D.; Blank, Z.; Curci, N.E.; Doshi, A.M.; Downey, R.T.; Edney, E.; et al. Multicenter Evaluation of Multiparametric MRI Clear Cell Likelihood Scores in Solid Indeterminate Small Renal Masses. Radiology 2023, 306, e239001. [Google Scholar] [CrossRef]
- Wilson, M.P.; Patel, D.; Murad, M.H.; McInnes, M.D.F.; Katlariwala, P.; Low, G. Diagnostic Performance of MRI in the Detection of Renal Lipid-Poor Angiomyolipomas: A Systematic Review and Meta-Analysis. Radiology 2020, 296, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Bektas, C.T.; Kocak, B.; Yardimci, A.H.; Turkcanoglu, M.H.; Yucetas, U.; Koca, S.B.; Erdim, C.; Kilickesmez, O. Clear Cell Renal Cell Carcinoma: Machine Learning-Based Quantitative Computed Tomography Texture Analysis for Prediction of Fuhrman Nuclear Grade. Eur. Radiol. 2019, 29, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, L.; Li, A.; Hu, Y.; Hu, D.; Li, Z.; Kamel, I.R. Monoexponential, biexponential, and stretched exponential diffusion-weighted imaging models: Quantitative biomarkers for differentiating renal clear cell carcinoma and minimal fat angiomyolipoma. J. Magn. Reson. Imaging 2017, 46, 240–247. [Google Scholar] [CrossRef]
- Delahunt, B.; Eble, J.N.; Egevad, L.; Samaratunga, H. Grading of renal cell carcinoma. Histopathology 2019, 74, 4–17. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.J.; Guru, K.; Fuchs, G.J.; Kim, H.L. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology 2010, 76, 610–613. [Google Scholar] [CrossRef]
- de Silva, S.; Lockhart, K.R.; Aslan, P.; Nash, P.; Hutton, A.; Malouf, D.; Lee, D.; Cozzi, P.; MacLean, F.; Thompson, J. Differentiation of renal masses with multi-parametric MRI: The de Silva St George classification scheme. BMC Urol. 2022, 22, 141. [Google Scholar] [CrossRef]
- Ding, Y.; Tan, Q.; Mao, W.; Dai, C.; Hu, X.; Hou, J.; Zeng, M.; Zhou, J. Differentiating between malignant and benign renal tumors: Do IVIM and diffusion kurtosis imaging perform better than DWI? Eur. Radiol. 2019, 29, 6930–6939. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic Performance of DWI for Differentiating High- From Low-Grade Clear Cell Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2017, 209, W374–W381. [Google Scholar] [CrossRef]
- Verhaert, D.; Thavendiranathan, P.; Giri, S.; Mihai, G.; Rajagopalan, S.; Simonetti, O.P.; Raman, S.V. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc. Imaging 2011, 4, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Peng, Q.; Sperling, K. Biochemical magnetic resonance imaging of knee articular cartilage: T1rho and T2 mapping as cartilage degeneration biomarkers. Ann. N. Y. Acad. Sci. 2016, 1383, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Liu, J.; Zhang, F.; Yang, M.; Lu, H.; Zhang, Y.; Han, F.; Cheng, J.; Zhu, J. The feasibility of a radial turbo-spin-echo T2 mapping for preoperative prediction of the histological grade and lymphovascular space invasion of cervical squamous cell carcinoma. Eur. J. Radiol. 2021, 139, 109684. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Abubrig, M.; Lehmann, T.; Hilbert, T.; Weiland, E.; Grimm, M.O.; Teichgräber, U.; Franiel, T. T2 Mapping in Prostate Cancer. Investig. Radiol. 2019, 54, 146–152. [Google Scholar] [CrossRef]
- Adams, L.C.; Bressem, K.K.; Jurmeister, P.; Fahlenkamp, U.L.; Ralla, B.; Engel, G.; Hamm, B.; Busch, J.; Makowski, M.R. Use of quantitative T2 mapping for the assessment of renal cell carcinomas: First results. Cancer Imaging 2019, 19, 35. [Google Scholar] [CrossRef]
- Horvat-Menih, I.; Li, H.; Priest, A.N.; Li, S.; Gill, A.B.; Mendichovszky, I.A.; Francis, S.T.; Warren, A.Y.; O’Carrigan, B.; Welsh, S.J.; et al. High-resolution and highly accelerated MRI T2 mapping as a tool to characterise renal tumour subtypes and grades. Eur. Radiol. Exp. 2024, 8, 76. [Google Scholar] [CrossRef]
- Altbach, M.I.; Bilgin, A.; Li, Z.; Clarkson, E.W.; Trouard, T.P.; Gmitro, A.F. Processing of radial fast spin-echo data for obtaining T2 estimates from a single k-space data set. Magn. Reson. Med. 2005, 54, 549–559. [Google Scholar] [CrossRef]
- Feng, L. Golden-Angle Radial MRI: Basics, Advances, and Applications. J. Magn. Reson. Imaging 2022, 56, 45–62. [Google Scholar] [CrossRef]
- Campbell, N.; Rosenkrantz, A.B.; Pedrosa, I. MRI phenotype in renal cancer: Is it clinically relevant? Top. Magn. Reson. Imaging 2014, 23, 95–115. [Google Scholar] [CrossRef]
- Li, S.; He, K.; Yuan, G.; Yong, X.; Meng, X.; Feng, C.; Zhang, Y.; Kamel, I.R.; Li, Z. WHO/ISUP grade and pathological T stage of clear cell renal cell carcinoma: Value of ZOOMit diffusion kurtosis imaging and chemical exchange saturation transfer imaging. Eur. Radiol. 2023, 33, 4429–4439. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Buy, X.; Han, F.; Toupin, S.; Kind, M. Assessment of Repeatability, Reproducibility, and Performances of T2 Mapping-Based Radiomics Features: A Comparative Study. J. Magn. Reson. Imaging 2021, 54, 537–548. [Google Scholar] [CrossRef]
- Meng, T.; He, N.; He, H.; Liu, K.; Ke, L.; Liu, H.; Zhong, L.; Huang, C.; Yang, A.; Zhou, C.; et al. The diagnostic performance of quantitative mapping in breast cancer patients: A preliminary study using synthetic MRI. Cancer Imaging 2020, 20, 88. [Google Scholar] [CrossRef]
- Mamisch, T.C.; Trattnig, S.; Quirbach, S.; Marlovits, S.; White, L.M.; Welsch, G.H. Quantitative T2 mapping of knee cartilage: Differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—Initial results. Radiology 2010, 254, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Andreisek, G.; Weiger, M. T2* mapping of articular cartilage: Current status of research and first clinical applications. Investig. Radiol. 2014, 49, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.A.; Lamb, H.J. Clinical application and technical considerations of T1 & T2(*) mapping in cardiac, liver, and renal imaging. Br. J. Radiol. 2018, 91, 20170825. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Klein, S.; Träber, F.; Schmeel, F.C.; Sprinkart, A.M.; Kuetting, D.L.R.; Block, W.; Uschner, F.E.; Schierwagen, R.; Hittatiya, K.; et al. Quantification of Liver Fibrosis at T1 and T2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018, 288, 748–754. [Google Scholar] [CrossRef]
- de Boer, A.; Harteveld, A.A.; Stemkens, B.; Blankestijn, P.J.; Bos, C.; Franklin, S.L.; Froeling, M.; Joles, J.A.; Verhaar, M.C.; van den Berg, N.; et al. Multiparametric Renal MRI: An Intrasubject Test-Retest Repeatability Study. J. Magn. Reson. Imaging 2021, 53, 859–873. [Google Scholar] [CrossRef]
- Gu, W.; Fang, S.; Hou, X.; Ma, D.; Li, S. Exploring diagnostic performance of T2 mapping in diffuse glioma grading. Quant. Imaging Med. Surg. 2021, 11, 2943–2954. [Google Scholar] [CrossRef]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Plotkin, A.; Trachtenberg, J.; Wilson, B.C.; Haider, M.A. Prostate tissue composition and MR measurements: Investigating the relationships between ADC, T2, Ktrans, ve, and corresponding histologic features. Radiology 2010, 255, 485–494. [Google Scholar] [CrossRef]
- O’Brien, A.T.; Gil, K.E.; Varghese, J.; Simonetti, O.P.; Zareba, K.M. T2 mapping in myocardial disease: A comprehensive review. J. Cardiovasc. Magn. Reson. 2022, 24, 33. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Sun, L.; Yaffe, M.J.; Trachtenberg, J.; Haider, M.A. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2—Sparse versus dense cancers. Radiology 2008, 249, 900–908. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujii, Y.; Tanaka, H.; Ishioka, J.; Matsuoka, Y.; Saito, K.; Uehara, S.; Numao, N.; Yuasa, T.; Yamamoto, S.; et al. Stepwise algorithm using computed tomography and magnetic resonance imaging for diagnosis of fat-poor angiomyolipoma in small renal masses: Development and external validation. Int. J. Urol. 2017, 24, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Eble, J.N.; Samaratunga, H.; Thunders, M.; Yaxley, J.W.; Egevad, L. Staging of renal cell carcinoma: Current progress and potential advances. Pathology 2021, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]


| Morphological T2w FSE | DWI | T2 Mapping | |
|---|---|---|---|
| Sequence | Spin-echo | EPI | Radio TSE |
| Repetition time (ms) | 4630 | 7700 | 2100 |
| Echo time (ms) | 77 | 71 | 7.7 |
| Slice thickness (mm) | 4 | 4 | 5 |
| Acquisition matrix | 320 × 320 | 128 × 128 | 256 × 256 |
| Field of view (mm) | 380 × 380 | 125 × 288 | 300 × 300 |
| No. averages | 1 | - | 1 |
| Bandwidth (Hz/Px) | 781 | 1666 | 488 |
| Radial views | - | - | 320 |
| b-values (s/mm2) | - | 50/800 | - |
| Phase encoding direction | R >> L | A >> P | A >> P |
| Acquisition duration (min:s) | - | 2:30 | 1:30 |
| Parameter | Data |
|---|---|
| Age (y) * | 54.4 ± 11.9 |
| Gender | |
| male | 59 |
| female | 33 |
| Mean tumor diameter (cm) * | 4.45 ± 2.27 |
| Pathological type | |
| Clear cell RCC | 67 |
| Minimal fat angiomyolipoma | 9 |
| Chromophobe RCC | 6 |
| Oncocytoma | 5 |
| Papillary RCC | 1 |
| MiT family translocation RCC | 1 |
| Mucinous tubular and spindle cell RCC | 1 |
| Unclassified RCC | 2 |
| WHO/ISUP grade | |
| I | 12 |
| II | 36 |
| III | 9 |
| IV | 6 |
| Pathological T stage | |
| T1a | 30 |
| T1b | 19 |
| T2a | 1 |
| T3a | 10 |
| T3b | 2 |
| T4 | 1 |
| Groups | T2 Values (ms) | ADC Values (×10−3 mm2/s) |
|---|---|---|
| Tissue type | ||
| Malignant (n = 78) | 115.00 ± 30.91 (96.91–125.79) | 1.35 ± 0.41 (1.05–1.66) |
| Benign (n = 14) | 92.13 ± 14.88 (79.27–100.51) | 1.11 ± 0.45 (0.83–1.22) |
| p-value | 0.002 | 0.016 |
| Tissue type | ||
| ccRCC (n = 67) | 116.02 ± 29.59 (97.52–126.41) | 1.39 ± 0.37 (1.08–1.68) |
| MFAML (n = 9) | 86.46 ± 11.78 (73.44–97.34) | 0.91 ± 0.11 (0.83–1.01) |
| p-value | <0.001 | <0.001 |
| WHO/ISUP grade | ||
| High-grade (n = 15) | 90.04 ± 9.20 (84.15–97.52) | 1.10 ± 0.22 (0.96–1.26) |
| Low-grade (n = 48) | 125.10 ± 29.82 (106.54–140.41) | 1.51 ± 0.35 (1.28–1.74) |
| p-value | <0.001 | <0.001 |
| Pathological T stage | ||
| High-stage (n = 13) | 98.25 ± 23.35 (82.33–110.17) | 1.22 ± 0.30 (1.05–1.55) |
| Low-stage (n = 50) | 121.56 ± 30.26 (104.03–136.08) | 1.46 ± 0.37 (1.22–1.72) |
| p-value | 0.002 | 0.056 |
| Category | Threshold | AUC (95% CI) | Sensitivity | Specificity | p for Comparison |
|---|---|---|---|---|---|
| Malignant vs. benign | |||||
| T2 value (ms) | 99.79 | 0.761 (0.639–0.883) | 69.23% | 78.57% | 0.003 |
| ADC (×10−3 mm2/s) | 1.05 | 0.702 (0.530–0.875) | 75.64% | 78.57% | 0.004 |
| Combination (age + size + T2 value) | NA | 0.875 (0.770–0.979) | 91.00% | 78.60% | Ref |
| ccRCC vs. MFAML | |||||
| T2 value (ms) | 103.54 | 0.862 (0.766–0.959) | 64.18% | 100.00% | 0.123 |
| ADC (×10−3 mm2/s) | 1.05 | 0.900 (0.832–0.969) | 83.58% | 100.00% | 0.084 |
| Combination (age + ADC) | NA | 0.949 (0.899–0.999) | 83.60% | 100.00% | Ref |
| WHO/ISUP grade | |||||
| T2 value (ms) | 105.56 | 0.936 (0.877–0.995) | 100.00% | 79.17% | 1.000 |
| ADC (×10−3 mm2/s) | 1.39 | 0.837 (0.741–0.934) | 100.00% | 70.83% | 0.027 |
| Combined (T2 value) | NA | 0.936 (0.877–0.995) | 100.00% | 79.17% | Ref |
| Pathological T stage | |||||
| T2 value (ms) | 101.98 | 0.775 (0.608–0.943) | 76.92% | 78.00% | 0.531 |
| ADC (×10−3 mm2/s) | - | - | - | - | - |
| Combination (size + T2 value) | NA | 0.848 (0.704–0.991) | 76.90% | 88.00% | Ref |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Gao, M.; He, K.; Yuan, G.; Yin, T.; Hu, D.; Li, Z. Feasibility and Reproducibility of T2 Mapping Compared with Diffusion-Weighted Imaging in Solid Renal Masses. Bioengineering 2024, 11, 901. https://doi.org/10.3390/bioengineering11090901
Li S, Gao M, He K, Yuan G, Yin T, Hu D, Li Z. Feasibility and Reproducibility of T2 Mapping Compared with Diffusion-Weighted Imaging in Solid Renal Masses. Bioengineering. 2024; 11(9):901. https://doi.org/10.3390/bioengineering11090901
Chicago/Turabian StyleLi, Shichao, Mengmeng Gao, Kangwen He, Guanjie Yuan, Ting Yin, Daoyu Hu, and Zhen Li. 2024. "Feasibility and Reproducibility of T2 Mapping Compared with Diffusion-Weighted Imaging in Solid Renal Masses" Bioengineering 11, no. 9: 901. https://doi.org/10.3390/bioengineering11090901
APA StyleLi, S., Gao, M., He, K., Yuan, G., Yin, T., Hu, D., & Li, Z. (2024). Feasibility and Reproducibility of T2 Mapping Compared with Diffusion-Weighted Imaging in Solid Renal Masses. Bioengineering, 11(9), 901. https://doi.org/10.3390/bioengineering11090901







