Muscle Synergy Analysis as a Tool for Assessing the Effectiveness of Gait Rehabilitation Therapies: A Methodological Review and Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility: Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
3. Results
3.1. Selected Studies
3.2. Study Characteristics
3.2.1. Patients
3.2.2. Task
3.2.3. Training and Clinical Evaluation
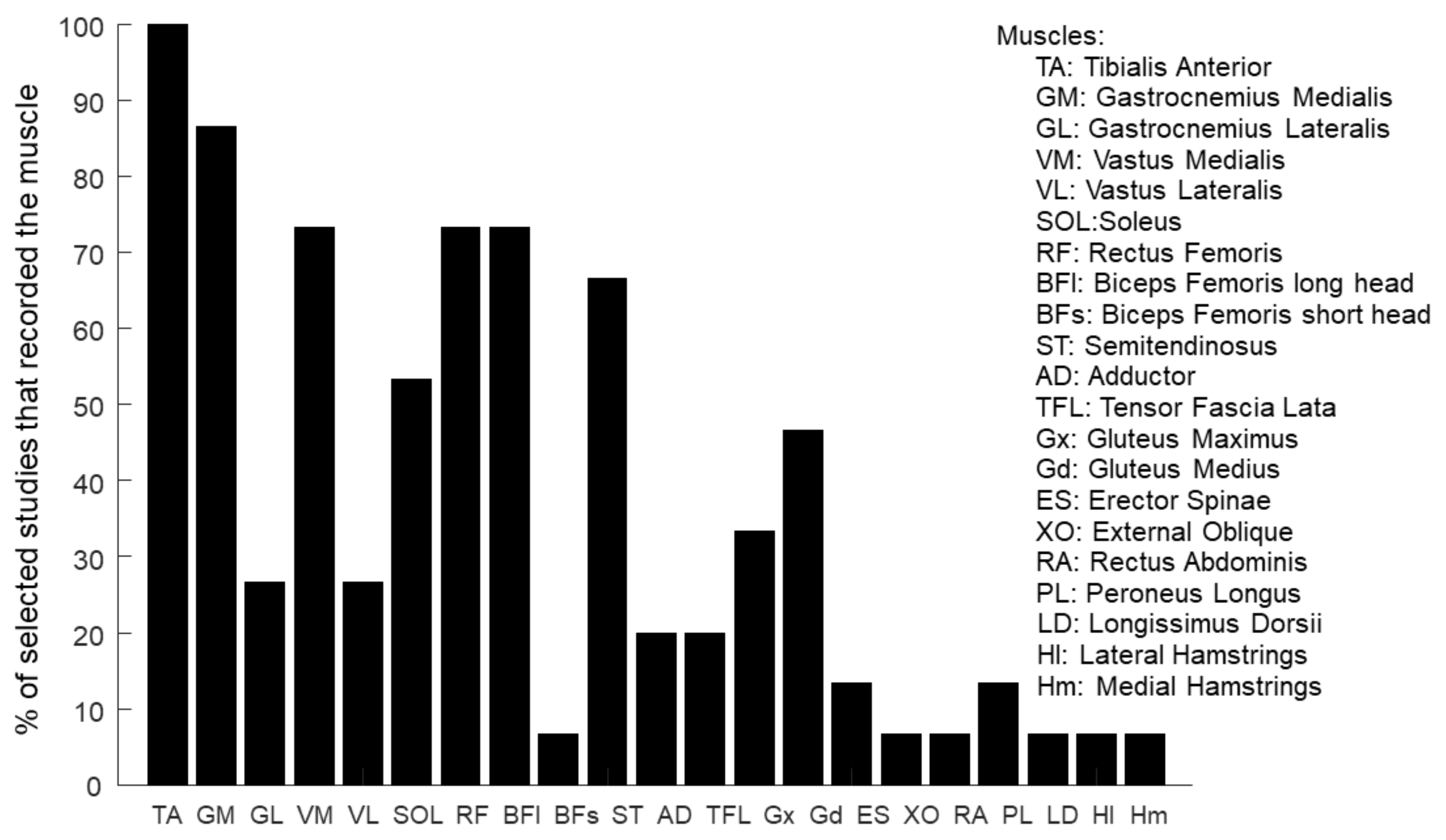
3.2.4. Muscles
| Muscles | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | TA | GM | GL | VM | VL | SOL | RF | BFl | BFs | ST | AD | TFL | Gx | Gd | ES | XO | RA | PL | LD | Hl | Hm | Number |
| Allen et al., 2017 [102] | X | X | X | X | X | X | X | X | X | X | X | X | X | 13 U | ||||||||
| Ambrosini et al., 2020 [93] | X | X | X | X | X | X | X | X | X | 9 B | ||||||||||||
| Conner et al., 2021 [104] | X | X | X | X | 4 U | |||||||||||||||||
| Ferrante et al., 2016 [94] | X | X | X | X | X | X | X | 8 U | ||||||||||||||
| Ghislieri et al., 2023 [103] | X | X | X | X | X | X | X | X | X | X | X | X | 12U + 1B | |||||||||
| Jonsdottir et al., 2020 [105] | X | X | X | X | X | X | X | X | 8 U | |||||||||||||
| Kadone et al., 2020 [106] | X | X | X | X | X | 5 B | ||||||||||||||||
| Kinugawa et al., 2022 [107] | X | X | X | X | 4 B | |||||||||||||||||
| Lim et al., 2021 [95] | X | X | X | X | X | X | X | X | 8 U | |||||||||||||
| Routson et al., 2013 [96] | X | X | X | X | X | X | X | X | 8 B | |||||||||||||
| Srivastava et al., 2016 [97] | X | X | X | X | X | X | X | X | X | X | 10 U | |||||||||||
| Tan et al., 2020 [99] | X | X | X | X | X | X | 6 B | |||||||||||||||
| Tan et al., 2018 [98] | X | X | X | X | X | X | 6 B | |||||||||||||||
| Van Criekinge et al., 2021 [100] | X | X | X | X | X | X | X | 6 U + 1 B | ||||||||||||||
| Zhu et al., 2021 [101] | X | X | X | X | X | X | X | X | 8 B | |||||||||||||
3.2.5. Muscle Synergies Extraction
3.2.6. Muscle Synergy Analysis and Improvement-Related Metrics
- Clinical evaluation
- Number of synergies
- Spatial and temporal organization
4. Discussion
- -
- M1—a knee-hip extensor module, activated during early stance, serving as body support and weight acceptance
- -
- M2—a calf plantar-flexor muscle module, activated during late stance, with forward propulsion, body support, and swing preparation function
- -
- M3—an ankle dorsiflexion module, activated during early swing, contributing to the ground clearance of the foot
- -
- M4—a knee flexor module, activated during late swing, to decelerate the leg an prepare heel strike
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knutsson, E.; Richards, C. Different Types of Disturbed Motor Control in Gait of Hemiparetic Patients. Brain 1979, 102, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Willerslev-Olsen, M.; Conway, B.A.; Nielsen, J.B. The Motor Cortex Drives the Muscles during Walking in Human Subjects. J. Physiol. 2012, 590, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Curt, A.; Jensen, L.; Dietz, V. Corticospinal Input in Human Gait: Modulation of Magnetically Evoked Motor Responses. Exp. Brain Res. 1997, 115, 234–246. [Google Scholar] [CrossRef]
- Winter, D.A.; MacKinnon, C.D.; Ruder, G.K.; Wieman, C. Chapter 32 An Integrated EMG/Biomechanical Model of Upper Body Balance and Posture during Human Gait. In Progress in Brain Research; Natural and Artificial Control of Hearing and Balance; Allum, J.H.J., Allum-Mecklenburg, D.J., Harris, F.P., Probst, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 97, pp. 359–367. [Google Scholar]
- Stolze, H.; Klebe, S.; Zechlin, C.; Baecker, C.; Friege, L.; Deuschl, G. Falls in Frequent Neurologicaldiseases. J. Neurol. 2004, 251, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ebersbach, G.; Sojer, M.; Valldeoriola, F.; Wissel, J.; Müller, J.; Tolosa, E.; Poewe, W. Comparative Analysis of Gait in Parkinson’s Disease, Cerebellar Ataxia and Subcortical Arteriosclerotic Encephalopathy. Brain 1999, 122, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Cárdenas, S.L.; Lozano-Guzmán, A.A.; Ramirez-Bautista, J.A.; Hernández-Zavala, A. A Review in Gait Rehabilitation Devices and Applied Control Techniques. Disabil. Rehabil. Assist. Technol. 2018, 13, 819–834. [Google Scholar] [CrossRef]
- Vitiello, N.; Oddo, C.M.; Lenzi, T.; Roccella, S.; Beccai, L.; Vecchi, F.; Carrozza, M.C.; Dario, P. Neuro-Robotics Paradigm for Intelligent Assistive Technologies. In Intelligent Assistive Robots: Recent Advances in Assistive Robotics for Everyday Activities; Springer Tracts in Advanced Robotics; Mohammed, S., Moreno, J.C., Kong, K., Amirat, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–40. ISBN 978-3-319-12922-8. [Google Scholar]
- Senanayake, C.; Senanayake, S.M.N.A. Emerging Robotics Devices for Therapeutic Rehabilitation of the Lower Extremity. In Proceedings of the 2009 IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Singapore, 14–17 July 2009; pp. 1142–1147. [Google Scholar]
- Díaz, I.; Gil, J.J.; Sánchez, E. Lower-Limb Robotic Rehabilitation: Literature Review and Challenges. J. Robot. 2011, 2011, e759764. [Google Scholar] [CrossRef]
- Balaji, E.; Brindha, D.; Balakrishnan, R. Supervised Machine Learning Based Gait Classification System for Early Detection and Stage Classification of Parkinson’s Disease. Appl. Soft Comput. 2020, 94, 106494. [Google Scholar] [CrossRef]
- Kalron, A.; Achiron, A.; Dvir, Z. Muscular and Gait Abnormalities in Persons With Early Onset Multiple Sclerosis. J. Neurol. Phys. Ther. 2011, 35, 164–169. [Google Scholar] [CrossRef]
- Pistacchi, M.; Gioulis, M.; Sanson, F.; De Giovannini, E.; Filippi, G.; Rossetto, F.; Marsala, S.Z. Gait Analysis and Clinical Correlations in Early Parkinson’s Disease. Funct. Neurol. 2017, 32, 28–34. [Google Scholar] [CrossRef]
- Alito, A.; Fenga, D.; Portaro, S.; Leonardi, G.; Borzelli, D.; Sanzarello, I.; Calabrò, R.; Milone, D.; Tisano, A.; Leonetti, D. Early Hip Fracture Surgery and Rehabilitation. How to Improve Functional Quality Outcomes. A Retrospective Study. Folia Med. 2023, 65, 879–884. [Google Scholar] [CrossRef]
- Borggraefe, I.; Meyer-Heim, A.; Kumar, A.; Schaefer, J.S.; Berweck, S.; Heinen, F. Improved Gait Parameters after Robotic-Assisted Locomotor Treadmill Therapy in a 6-Year-Old Child with Cerebral Palsy. Mov. Disord. 2008, 23, 280–283. [Google Scholar] [CrossRef]
- Gunning, E.; Uszynski, M.K. Effectiveness of the Proprioceptive Neuromuscular Facilitation Method on Gait Parameters in Patients With Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, 980–986. [Google Scholar] [CrossRef]
- Rocha, P.A.; Porfírio, G.M.; Ferraz, H.B.; Trevisani, V.F.M. Effects of External Cues on Gait Parameters of Parkinson’s Disease Patients: A Systematic Review. Clin. Neurol. Neurosurg. 2014, 124, 127–134. [Google Scholar] [CrossRef]
- Maggio, M.G.; Cezar, R.P.; Milardi, D.; Borzelli, D.; De Marchis, C.; D’avella, A.; Quartarone, A.; Calabrò, R.S. Do Patients with Neurological Disorders Benefit from Immersive Virtual Reality? A Scoping Review on the Emerging Use of the Computer-Assisted Rehabilitation Environment. Eur. J. Phys. Rehabil. Med. 2024, 60, 37–43. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, L.; Jimenez, A.R.; Garcia-Villamil, G.; Seco, F. Detecting Fall Risk and Frailty in Elders with Inertial Motion Sensors: A Survey of Significant Gait Parameters. Sensors 2021, 21, 6918. [Google Scholar] [CrossRef]
- Kondragunta, J.; Hirtz, G. Gait Parameter Estimation of Elderly People Using 3D Human Pose Estimation in Early Detection of Dementia. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5798–5801. [Google Scholar]
- Pfister, A.; West, A.M.; Bronner, S.; Noah, J.A. Comparative Abilities of Microsoft Kinect and Vicon 3D Motion Capture for Gait Analysis. J. Med. Eng. Technol. 2014, 38, 274–280. [Google Scholar] [CrossRef]
- Sigal, L.; Balan, A.O.; Black, M.J. HumanEva: Synchronized Video and Motion Capture Dataset and Baseline Algorithm for Evaluation of Articulated Human Motion. Int. J. Comput. Vis. 2010, 87, 4–27. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.K.; Strutzenberger, G.; Brown, M.; Selbie, W.S.; DePaul, V.; Scott, S.H.; Deluzio, K.J. Assessment of Spatiotemporal Gait Parameters Using a Deep Learning Algorithm-Based Markerless Motion Capture System. J. Biomech. 2021, 122, 110414. [Google Scholar] [CrossRef]
- Mathis, M.W.; Mathis, A. Deep Learning Tools for the Measurement of Animal Behavior in Neuroscience. Curr. Opin. Neurobiol. 2020, 60, 1–11. [Google Scholar] [CrossRef]
- Gu, C.; Lin, W.; He, X.; Zhang, L.; Zhang, M. IMU-Based Motion Capture System for Rehabilitation Applications: A Systematic Review. Biomim. Intell. Robot. 2023, 3, 100097. [Google Scholar] [CrossRef]
- Gujarathi, T.; Bhole, K. Gait Analysis Using Imu Sensor. In Proceedings of the 2019 10th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kanpur, India, 6–8 July 2019; pp. 1–5. [Google Scholar]
- Panero, E.; Digo, E.; Agostini, V.; Gastaldi, L. Comparison of Different Motion Capture Setups for Gait Analysis: Validation of Spatio-Temporal Parameters Estimation. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and Repeatability of Inertial Measurement Units for Measuring Gait Parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef]
- Belli, A.; Bui, P.; Berger, A.; Geyssant, A.; Lacour, J.-R. A Treadmill Ergometer for Three-Dimensional Ground Reaction Forces Measurement during Walking. J. Biomech. 2001, 34, 105–112. [Google Scholar] [CrossRef]
- Dierick, F.; Penta, M.; Renaut, D.; Detrembleur, C. A Force Measuring Treadmill in Clinical Gait Analysis. Gait Posture 2004, 20, 299–303. [Google Scholar] [CrossRef]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Stlberg, E.; Falck, B. The Role of Electromyography in Neurology. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 579–598. [Google Scholar] [CrossRef]
- Zwarts, M.J.; Drost, G.; Stegeman, D.F. Recent Progress in the Diagnostic Use of Surface EMG for Neurological Diseases. J. Electromyogr. Kinesiol. 2000, 10, 287–291. [Google Scholar] [CrossRef]
- Borzelli, D.; Cesqui, B.; Berger, D.J.; Burdet, E.; d’Avella, A. Muscle Patterns Underlying Voluntary Modulation of Co-Contraction. PLoS ONE 2018, 13, e0205911. [Google Scholar] [CrossRef]
- Borzelli, D.; Pastorelli, S.; Gastaldi, L. Determination of the Human Arm Stiffness Efficiency with a Two Antagonist Muscles Model. In Advances in Italian Mechanism Science: Proceedings of the First International Conference of IFToMM Italy, Vicenza, Italy, 1–2 December 2016; Boschetti, G., Gasparetto, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–78. [Google Scholar]
- Borzelli, D.; Burdet, E.; Pastorelli, S.; d’Avella, A.; Gastaldi, L. Identification of the Best Strategy to Command Variable Stiffness Using Electromyographic Signals. J. Neural Eng. 2020, 17, 016058. [Google Scholar] [CrossRef]
- Cimolato, A.; Driessen, J.J.M.; Mattos, L.S.; De Momi, E.; Laffranchi, M.; De Michieli, L. EMG-Driven Control in Lower Limb Prostheses: A Topic-Based Systematic Review. J. Neuroeng. Rehabil. 2022, 19, 43. [Google Scholar] [CrossRef]
- Manal, K.; Gonzalez, R.V.; Lloyd, D.G.; Buchanan, T.S. A Real-Time EMG-Driven Virtual Arm. Comput. Biol. Med. 2002, 32, 25–36. [Google Scholar] [CrossRef]
- Yang, H.; Wan, J.; Jin, Y.; Yu, X.; Fang, Y. EEG- and EMG-Driven Poststroke Rehabilitation: A Review. IEEE Sens. J. 2022, 22, 23649–23660. [Google Scholar] [CrossRef]
- Borzelli, D.; Pastorelli, S.; Gastaldi, L. Model of the Human Arm Stiffness Exerted by Two Antagonist Muscles. In Advances in Robot Design and Intelligent Control: Proceedings of the 25th Conference on Robotics in Alpe-Adria-Danube Region (RAAD16), Belgrade, Serbia, 30 June–2 July 2016; Rodić, A., Borangiu, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 285–292. [Google Scholar]
- Borzelli, D.; Pastorelli, S.; d’Avella, A.; Gastaldi, L. Virtual Stiffness: A Novel Biomechanical Approach to Estimate Limb Stiffness of a Multi-Muscle and Multi-Joint System. Sensors 2023, 23, 673. [Google Scholar] [CrossRef]
- Borzelli, D.; Gurgone, S.; De Pasquale, P.; Lotti, N.; d’Avella, A.; Gastaldi, L. Use of Surface Electromyography to Estimate End-Point Force in Redundant Systems: Comparison between Linear Approaches. Bioengineering 2023, 10, 234. [Google Scholar] [CrossRef]
- Borzelli, D.; Pastorelli, S.; Gastaldi, L. Elbow Musculoskeletal Model for Industrial Exoskeleton with Modulated Impedance Based on Operator’s Arm Stiffness. Int. J. Autom. Technol. 2017, 11, 442–449. [Google Scholar] [CrossRef]
- Berger, D.J.; Gentner, R.; Edmunds, T.; Pai, D.K.; d’Avella, A. Differences in Adaptation Rates after Virtual Surgeries Provide Direct Evidence for Modularity. J. Neurosci. 2013, 33, 12384–12394. [Google Scholar] [CrossRef]
- Berger, D.J.; Masciullo, M.; Molinari, M.; Lacquaniti, F.; d’Avella, A. Does the Cerebellum Shape the Spatiotemporal Organization of Muscle Patterns? Insights from Subjects with Cerebellar Ataxias. J. Neurophysiol. 2020, 123, 1691–1710. [Google Scholar] [CrossRef]
- Berger, D.J.; Borzelli, D.; d’Avella, A. Task Space Exploration Improves Adaptation after Incompatible Virtual Surgeries. J. Neurophysiol. 2022, 127, 1127–1146. [Google Scholar] [CrossRef]
- Borzelli, D.; Berger, D.; Pai, D.; D’avella, A. Effort Minimization and Synergistic Muscle Recruitment for Three-Dimensional Force Generation. Front. Comput. Neurosci. 2013, 7, 186. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of Muscle Synergies for Voluntary Actions after Cortical Stroke in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef]
- d’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of Fast-Reaching Movements by Muscle Synergy Combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef]
- De Marchis, C.; Schmid, M.; Bibbo, D.; Bernabucci, I.; Conforto, S. Inter-Individual Variability of Forces and Modular Muscle Coordination in Cycling: A Study on Untrained Subjects. Hum. Mov. Sci. 2013, 32, 1480–1494. [Google Scholar] [CrossRef]
- Dipietro, L.; Krebs, H.I.; Fasoli, S.E.; Volpe, B.T.; Stein, J.; Bever, C.; Hogan, N. Changing Motor Synergies in Chronic Stroke. J. Neurophysiol. 2007, 98, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Gentner, R.; Edmunds, T.; Pai, D.; D’avella, A. Robustness of Muscle Synergies during Visuomotor Adaptation. Front. Comput. Neurosci. 2013, 7, 120. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five Basic Muscle Activation Patterns Account for Muscle Activity during Human Locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in Upper Limb Muscle Synergy Structure in Chronic Stroke Survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Ting, L.H. Subject-Specific Muscle Synergies in Human Balance Control Are Consistent Across Different Biomechanical Contexts. J. Neurophysiol. 2010, 103, 3084–3098. [Google Scholar] [CrossRef]
- Borzelli, D.; Gurgone, S.; Mezzetti, M.; De Pasquale, P.; Berger, D.J.; Milardi, D.; Acri, G.; D’Avella, A. Adaptation to Virtual Surgeries Across Multiple Practice Sessions. In Converging Clinical and Engineering Research on Neurorehabilitation IV: Proceedings of the 5th International Conference on Neurorehabilitation (ICNR2020), Online, 13–16 October 2020; Torricelli, D., Akay, M., Pons, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 563–568. [Google Scholar]
- Borzelli, D.; Gurgone, S.; De Pasquale, P.; Berger, D.J.; d’Avella, A. Consistency of Myoelectric Control Across Multiple Sessions. In Converging Clinical and Engineering Research on Neurorehabilitation III: Proceedings of the 4th International Conference on NeuroRehabilitation (ICNR2018), Pisa, Italy, 16–20 October 2018; Masia, L., Micera, S., Akay, M., Pons, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1166–1170. [Google Scholar]
- Borzelli, D.; Vieira, T.M.M.; Botter, A.; Gazzoni, M.; Lacquaniti, F.; d’Avella, A. Synaptic Inputs to Motor Neurons Underlying Muscle Co-Activation for Functionally Different Tasks Have Different Spectral Characteristics. J. Neurophysiol. 2024, 131, 1126–1142. [Google Scholar] [CrossRef]
- Laine, C.M.; Martinez-Valdes, E.; Falla, D.; Mayer, F.; Farina, D. Motor Neuron Pools of Synergistic Thigh Muscles Share Most of Their Synaptic Input. J. Neurosci. 2015, 35, 12207–12216. [Google Scholar] [CrossRef]
- Levine, J.; Avrillon, S.; Farina, D.; Hug, F.; Pons, J.L. Two Motor Neuron Synergies, Invariant across Ankle Joint Angles, Activate the Triceps Surae during Plantarflexion. J. Physiol. 2023, 601, 4337–4354. [Google Scholar] [CrossRef]
- David, F.J.; Munoz, M.J.; Corcos, D.M. The Effect of STN DBS on Modulating Brain Oscillations: Consequences for Motor and Cognitive Behavior. Exp. Brain Res. 2020, 238, 1659–1676. [Google Scholar] [CrossRef]
- Leonardi, G.; Ciurleo, R.; Cucinotta, F.; Fonti, B.; Borzelli, D.; Costa, L.; Tisano, A.; Portaro, S.; Alito, A. The Role of Brain Oscillations in Post-Stroke Motor Recovery: An Overview. Front. Syst. Neurosci. 2022, 16, 947421. [Google Scholar] [CrossRef]
- MacKay, W.A. Synchronized Neuronal Oscillations and Their Role in Motor Processes. Trends Cogn. Sci. 1997, 1, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Basmajian, J.V. Muscles Alive. Their Functions Revealed by Electromyography. Acad. Med. 1962, 37, 802. [Google Scholar]
- De Luca, C.J. Physiology and Mathematics of Myoelectric Signals. IEEE Trans. Biomed. Eng. 1979, BME-26, 313–325. [Google Scholar] [CrossRef]
- Lugo, J.E.; Doti, R.; Faubert, J. Planckian Power Spectral Densities from Human Calves during Posture Maintenance and Controlled Isometric Contractions. PLoS ONE 2015, 10, e0131798. [Google Scholar] [CrossRef][Green Version]
- Lindstrom, L.H.; Magnusson, R.I. Interpretation of Myoelectric Power Spectra: A Model and Its Applications. Proc. IEEE 1977, 65, 653–662. [Google Scholar] [CrossRef]
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Non-Invasive Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-471-67580-8. [Google Scholar]
- Castronovo, A.M.; De Marchis, C.; Schmid, M.; Conforto, S.; Severini, G. Effect of Task Failure on Intermuscular Coherence Measures in Synergistic Muscles. Appl. Bionics Biomech. 2018, 2018, e4759232. [Google Scholar] [CrossRef]
- Yamada, H.; Okada, M.; Oda, T.; Nemoto, S.; Shiozaki, T.; Kizuka, T.; Kuno, S.; Masuda, T. Effects of Aging on Emg Variables During Fatiguing Isometric Contractions. J. Hum. Ergol. 2000, 29, 7–14. [Google Scholar] [CrossRef]
- Petrofsky, J.; Laymon, M. Muscle Temperature and EMG Amplitude and Frequency during Isometric Exercise. Aviat. Space Environ. Med. 2005, 76, 1024–1030. [Google Scholar]
- Farina, D.; Negro, F.; Dideriksen, J.L. The Effective Neural Drive to Muscles Is the Common Synaptic Input to Motor Neurons: Effective Neural Drive to Muscles. J. Physiol. 2014, 592, 3427–3441. [Google Scholar] [CrossRef]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Functional Significance of Cell Size in Spinal Motoneurons. J. Neurophysiol. 1965, 28, 560–580. [Google Scholar] [CrossRef]
- Henneman, E. Relation between Size of Neurons and Their Susceptibility to Discharge. Science 1957, 126, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Monster, A.W.; Chan, H. Isometric Force Production by Motor Units of Extensor Digitorum Communis Muscle in Man. J. Neurophysiol. 1977, 40, 1432–1443. [Google Scholar] [CrossRef]
- Hug, F.; Avrillon, S.; Ibáñez, J.; Farina, D. Common Synaptic Input, Synergies and Size Principle: Control of Spinal Motor Neurons for Movement Generation. J. Physiol. 2023, 601, 11–20. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.J.; LeFever, R.S.; McCue, M.P.; Xenakis, A.P. Behaviour of Human Motor Units in Different Muscles during Linearly Varying Contractions. J. Physiol. 1982, 329, 113–128. [Google Scholar] [CrossRef]
- Chvatal, S.A.; Ting, L.H. Voluntary and Reactive Recruitment of Locomotor Muscle Synergies during Perturbed Walking. J. Neurosci. 2012, 32, 12237–12250. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- De Marchis, C.; Ranaldi, S.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Lacquaniti, F.; Conforto, S. Modular Motor Control of the Sound Limb in Gait of People with Trans-Femoral Amputation. J. NeuroEng. Rehabil. 2019, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Clark, D.J.; Kautz, S.A. Modular Control of Human Walking: A Simulation Study. J. Biomech. 2009, 42, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Rimini, D.; Agostini, V.; Knaflitz, M. Intra-Subject Consistency during Locomotion: Similarity in Shared and Subject-Specific Muscle Synergies. Front. Hum. Neurosci. 2017, 11, 292485. [Google Scholar] [CrossRef]
- Safavynia, S.; Torres-Oviedo, G.; Ting, L. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Wen, H.; Liu, B.; Li, J.; d’Avella, A.; Scano, A. Muscle Synergies for Evaluating Upper Limb in Clinical Applications: A Systematic Review. Heliyon 2023, 9, e16202. [Google Scholar] [CrossRef]
- Scano, A.; Lanzani, V.; Brambilla, C.; d’Avella, A. Transferring Sensor-Based Assessments to Clinical Practice: The Case of Muscle Synergies. Sensors 2024, 24, 3934. [Google Scholar] [CrossRef]
- Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle Synergies in Parkinson’s Disease. Sensors 2020, 20, 3209. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.N.G.; Ballekere, A.N.; Fregly, B.J.; Roh, J. Are Muscle Synergies Useful for Stroke Rehabilitation? Curr. Opin. Biomed. Eng. 2021, 19, 100315. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Vermeulen, J.; Wagemans, K.; Schröder, J.; Embrechts, E.; Truijen, S.; Hallemans, A.; Saeys, W. Lower Limb Muscle Synergies during Walking after Stroke: A Systematic Review. Disabil. Rehabil. 2020, 42, 2836–2845. [Google Scholar] [CrossRef]
- Beltrame, G.; Scano, A.; Marino, G.; Peccati, A.; Molinari Tosatti, L.; Portinaro, N. Recent Developments in Muscle Synergy Analysis in Young People with Neurodevelopmental Diseases: A Systematic Review. Front. Bioeng. Biotechnol. 2023, 11, 1145937. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Farid, M.S.; Grzegorzek, M. Vision-Based Approaches towards Person Identification Using Gait. Comput. Sci. Rev. 2021, 42, 100432. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, E.; Parati, M.; Peri, E.; De Marchis, C.; Nava, C.; Pedrocchi, A.; Ferriero, G.; Ferrante, S. Changes in Leg Cycling Muscle Synergies after Training Augmented by Functional Electrical Stimulation in Subacute Stroke Survivors: A Pilot Study. J. NeuroEng. Rehabil. 2020, 17, 35. [Google Scholar] [CrossRef]
- Ferrante, S.; Chia Bejarano, N.; Ambrosini, E.; Nardone, A.; Turcato, A.M.; Monticone, M.; Ferrigno, G.; Pedrocchi, A. A Personalized Multi-Channel FES Controller Based on Muscle Synergies to Support Gait Rehabilitation after Stroke. Front. Neurosci. 2016, 10, 425. [Google Scholar] [CrossRef]
- Lim, J.; Lim, T.; Lee, J.; Sim, J.; Chang, H.; Yoon, B.; Jung, H. Patient-Specific Functional Electrical Stimulation Strategy Based on Muscle Synergy and Walking Posture Analysis for Gait Rehabilitation of Stroke Patients. J. Int. Med. Res. 2021, 49, 03000605211016782. [Google Scholar] [CrossRef]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The Influence of Locomotor Rehabilitation on Module Quality and Post-Stroke Hemiparetic Walking Performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kao, P.C.; Reisman, D.S.; Scholz, J.P.; Agrawal, S.K.; Higginson, J.S. Robotic Assist-As-Needed as an Alternative to Therapist-Assisted Gait Rehabilitation. Int. J. Phys. Med. Rehabil. 2016, 4, 370. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Kadone, H.; Watanabe, H.; Marushima, A.; Yamazaki, M.; Sankai, Y.; Suzuki, K. Lateral Symmetry of Synergies in Lower Limb Muscles of Acute Post-Stroke Patients After Robotic Intervention. Front. Neurosci. 2018, 12, 276. [Google Scholar] [CrossRef]
- Tan, C.K.; Kadone, H.; Watanabe, H.; Marushima, A.; Hada, Y.; Yamazaki, M.; Sankai, Y.; Matsumura, A.; Suzuki, K. Differences in Muscle Synergy Symmetry between Subacute Post-Stroke Patients with Bioelectrically-Controlled Exoskeleton Gait Training and Conventional Gait Training. Front. Bioeng. Biotechnol. 2020, 8, 770. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Hallemans, A.; Herssens, N.; Lafosse, C.; Claes, D.; De Hertogh, W.; Truijen, S.; Saeys, W. SWEAT2 Study: Effectiveness of Trunk Training on Gait and Trunk Kinematics After Stroke: A Randomized Controlled Trial. Phys. Ther. 2020, 100, 1568–1581. [Google Scholar] [CrossRef]
- Zhu, F.; Kern, M.; Fowkes, E.; Afzal, T.; Contreras-Vidal, J.-L.; Francisco, G.E.; Chang, S.-H. Effects of an Exoskeleton-Assisted Gait Training on Post-Stroke Lower-Limb Muscle Coordination. J. Neural Eng. 2021, 18, 046039. [Google Scholar] [CrossRef]
- Allen, J.L.; McKay, J.L.; Sawers, A.; Hackney, M.E.; Ting, L.H. Increased Neuromuscular Consistency in Gait and Balance after Partnered, Dance-Based Rehabilitation in Parkinson’s Disease. APSselect 2017, 4, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ghislieri, M.; Lanotte, M.; Knaflitz, M.; Rizzi, L.; Agostini, V. Muscle Synergies in Parkinson’s Disease before and after the Deep Brain Stimulation of the Bilateral Subthalamic Nucleus. Sci. Rep. 2023, 13, 6997. [Google Scholar] [CrossRef] [PubMed]
- Conner, B.C.; Schwartz, M.H.; Lerner, Z.F. Pilot Evaluation of Changes in Motor Control after Wearable Robotic Resistance Training in Children with Cerebral Palsy. J. Biomech. 2021, 126, 110601. [Google Scholar] [CrossRef]
- Jonsdottir, J.; Lencioni, T.; Gervasoni, E.; Crippa, A.; Anastasi, D.; Carpinella, I.; Rovaris, M.; Cattaneo, D.; Ferrarin, M. Improved Gait of Persons with Multiple Sclerosis after Rehabilitation: Effects on Lower Limb Muscle Synergies, Push-Off, and Toe-Clearance. Front. Neurol. 2020, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Kadone, H.; Kubota, S.; Abe, T.; Noguchi, H.; Miura, K.; Koda, M.; Shimizu, Y.; Hada, Y.; Sankai, Y.; Suzuki, K.; et al. Muscular Activity Modulation During Post-Operative Walking With Hybrid Assistive Limb (HAL) in a Patient With Thoracic Myelopathy Due to Ossification of Posterior Longitudinal Ligament: A Case Report. Front. Neurol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, K.; Mano, T.; Wada, H.; Ozaki, M.; Shirai, D.; Imura, T.; Kido, A. Improvement in Lower Extremity Hemiplegia in a Post-Operative Brain Tumor Patient by Applying an Integrated Volitional Control Electrical Stimulator. J. Phys. Ther. Sci. 2022, 34, 473–477. [Google Scholar] [CrossRef]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; de Sire, A. Efficacy of Robotic Exoskeleton for Gait Rehabilitation in Patients with Subacute Stroke: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2022, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chan, C.K.; Guo, Z.; Yu, H. A Review of Lower Extremity Assistive Robotic Exoskeletons in Rehabilitation Therapy. Crit. Rev. Biomed. Eng. 2013, 41, 343–363. [Google Scholar] [CrossRef]
- Kostov, A.; Stein, R.B.; Popović, D.; Armstrong, W.W. Improved Methods for Control of FES for Locomotion. IFAC Proc. Vol. 1994, 27, 445–450. [Google Scholar] [CrossRef]
- Peri, E.; Ambrosini, E.; Pedrocchi, A.; Ferrigno, G.; Nava, C.; Longoni, V.; Monticone, M.; Ferrante, S. Can FES-Augmented Active Cycling Training Improve Locomotion in Post-Acute Elderly Stroke Patients? Eur. J. Transl. Myol. 2016, 26, 6063. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-Weight–Supported Treadmill Rehabilitation after Stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef]
- Ivey, F.M.; Hafer-Macko, C.E.; Macko, R.F. Exercise Rehabilitation after Stroke. NeuroRX 2006, 3, 439–450. [Google Scholar] [CrossRef]
- Iaccarino, M.A.; Bhatnagar, S.; Zafonte, R. Chapter 26—Rehabilitation after Traumatic Brain Injury. In Handbook of Clinical Neurology; Grafman, J., Salazar, A.M., Eds.; Traumatic Brain Injury, Part I; Elsevier: Amsterdam, The Netherlands, 2015; Volume 127, pp. 411–422. [Google Scholar]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients After Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor Evaluation in Vascular Hemiplegia. Eur. Neurol. 2008, 19, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Norlin, V. The Post-Stroke Hemiplegic Patient. Scand. J. Rehabil. Med. 1975, 7, 73–83. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index: A Simple Index of Independence Useful in Scoring Improvement in the Rehabilitation of the Chronically Ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Wrisley, D.M.; Marchetti, G.F.; Kuharsky, D.K.; Whitney, S.L. Reliability, Internal Consistency, and Validity of Data Obtained with the Functional Gait Assessment. Phys. Ther. 2004, 84, 906–918. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability Assessment with Elderly Residents and Patients with an Acute Stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using Psychometric Techniques to Improve the Balance Evaluation System’s Test: The Mini-BESTest. J. Rehabil. Med. Off. J. UEMS Eur. Board Phys. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef]
- Klein, P.J.; Fiedler, R.C.; Rose, D.J. Rasch Analysis of the Fullerton Advanced Balance (FAB) Scale. Physiother. Can. 2011, 63, 115–125. [Google Scholar] [CrossRef]
- Whitney, S.L.; Hudak, M.T.; Marchetti, G.F. The Dynamic Gait Index Relates to Self-Reported Fall History in Individuals with Vestibular Dysfunction. J. Vestib. Res. 2000, 10, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Butland, R.J.; Pang, J.; Gross, E.R.; Woodcock, A.A.; Geddes, D.M. Two-, Six-, and 12-Minute Walking Tests in Respiratory Disease. Br. Med. J. Clin. Res. Ed 1982, 284, 1607–1608. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the Reliability and Validity of a Shorter Walk Test Compared With the 10-Meter Walk Test for Measurements of Gait Speed in Healthy, Older Adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Brunnstrom, S. Motor Testing Procedures in Hemiplegia: Based on Sequential Recovery Stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D. Assessing Motor Impairment after Stroke: A Pilot Reliability Study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A New Tool to Measure Motor Impairment of the Trunk after Stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and Reliability of a System to Classify Gross Motor Function in Children with Cerebral Palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Meder, K.G.; LoJacono, C.T.; Rhea, C.K. A Systematic Review of Non-Pharmacological Interventions to Improve Gait Asymmetries in Neurological Populations. Symmetry 2022, 14, 281. [Google Scholar] [CrossRef]
- Bernstein, N.A. The Co-Ordination and Regulation of Movements, 1st ed.; Pergamon Press: Oxford, NY, USA, 1967. [Google Scholar]
- Latash, M.L. There Is No Motor Redundancy in Human Movements. There Is Motor Abundance. Motor Control 2000, 4, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Learning the Parts of Objects by Non-Negative Matrix Factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef]
- Li, Y.; Ngom, A. The Non-Negative Matrix Factorization Toolbox for Biological Data Mining. Source Code Biol. Med. 2013, 8, 10. [Google Scholar] [CrossRef]
- Tresch, M.C.; Cheung, V.C.K.; d’Avella, A. Matrix Factorization Algorithms for the Identification of Muscle Synergies: Evaluation on Simulated and Experimental Data Sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Olney, S.J.; Richards, C. Hemiparetic Gait Following Stroke. Part I: Characteristics. Gait Posture 1996, 4, 136–148. [Google Scholar] [CrossRef]
- Coscia, M.; Monaco, V.; Martelloni, C.; Rossi, B.; Chisari, C.; Micera, S. Muscle Synergies and Spinal Maps Are Sensitive to the Asymmetry Induced by a Unilateral Stroke. J. NeuroEng. Rehabil. 2015, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, E.; Cheung, V.C.K.; d’Avella, A.; Saltiel, P.; Tresch, M. Combining Modules for Movement. Brain Res. Rev. 2008, 57, 125–133. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of Muscle Synergies in the Construction of a Natural Motor Behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Tresch, M.C.; Saltiel, P.; Bizzi, E. The Construction of Movement by the Spinal Cord. Nat. Neurosci. 1999, 2, 162–167. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle Synergy Patterns as Physiological Markers of Motor Cortical Damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Evidence for Altered Upper Extremity Muscle Synergies in Chronic Stroke Survivors with Mild and Moderate Impairment. Front. Hum. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; Rozumalski, A.; Schwartz, M.H. Muscle Synergies and Complexity of Neuromuscular Control during Gait in Cerebral Palsy. Dev. Med. Child Neurol. 2015, 57, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, F.; Cao, S.; Zhang, X.; Wu, D.; Chen, X. Muscle Synergy Analysis in Children with Cerebral Palsy. J. Neural Eng. 2015, 12, 046017. [Google Scholar] [CrossRef]
- Singh, R.E.; Iqbal, K.; White, G.; Hutchinson, T.E. A Systematic Review on Muscle Synergies: From Building Blocks of Motor Behavior to a Neurorehabilitation Tool. Appl. Bionics Biomech. 2018, 2018, e3615368. [Google Scholar] [CrossRef] [PubMed]
- Zhvansky, D.S.; Sylos-Labini, F.; Dewolf, A.; Cappellini, G.; d’Avella, A.; Lacquaniti, F.; Ivanenko, Y. Evaluation of Spatiotemporal Patterns of the Spinal Muscle Coordination Output during Walking in the Exoskeleton. Sensors 2022, 22, 5708. [Google Scholar] [CrossRef]

| Search Query (PubMed, Scopus, WoS) | (“gait”[Title/Abstract] OR “walk*”[Title/Abstract]) AND (“therapy”[Title/Abstract] OR “rehabilit*”[Title/Abstract] OR “neurorehabilit*”[Title/Abstract] OR “training”[Title/Abstract]) AND (“muscle synerg*”[Title/Abstract] OR “synergies”[Title/Abstract] OR “muscle coordination”[Title/Abstract] OR “motor module*”[Title/Abstract] OR “primitive*”[Title/Abstract]) |
| Clinical Scales | |||
|---|---|---|---|
| Improved | Not Improved | Not Altered | |
| Allen et al., 2017 [102] | UPDRS-III, BBS, FAB, DGI, TUG, 6MWT | ||
| Ambrosini et al., 2020 [93] | MI, TCT, BBS, FIMM | ||
| Conner et al., 2021 [104] | |||
| Ferrante et al., 2016 [94] | mini best test, Fugl-Meyer motor | ||
| Ghislieri et al., 2023 [103] | UPDRS-III, FAB | MMSE | |
| Jonsdottir et al., 2020 [105] | 2MWT, 10MWT | BBS | |
| Kadone et al., 2020 [106] | FIM motor, Barthel, FAC | 10MWT | |
| Kinugawa et al., 2022 [107] | 10MWT | FMA, BRS | |
| Lim et al., 2021 [95] | 10MWT | BBS | |
| Routson et al., 2013 [96] | FMA | DGI | |
| Srivastava et al., 2016 [97] | FMA, FGA, 6MWT, TUG | ||
| Tan et al., 2020 [99] | FIM motor, FIM locomotion, FMA lower ex | ||
| Tan et al., 2018 [98] | FIM motor, FIM locomotion, FMA lower ex | ||
| Van Criekinge et al., 2021 [100] | FAC, TIS, POMA Tinetti, | Barthel | |
| Zhu et al., 2021 [101] | 10MWT, 6MWT | TUG | |
| Type of Therapy |
Number of Synergies | Spatial Synergies | Temporal Activations | Coordination Symmetry | |
|---|---|---|---|---|---|
| Allen et al., 2017 [102] | O | − | + | n/a | n/a |
| Ambrosini et al., 2020 [93] | F | − | + | + | − |
| Conner et al., 2021 [104] | R | + | n/a | n/a | n/a |
| Ferrante et al., 2016 [94] | F | + | n/a | n/a | n/a |
| Ghislieri et al., 2023 [103] | O | + | + | + | n/a |
| Jonsdottir et al., 2020 [105] | O | − | − | + | n/a |
| Kadone et al., 2020 [106] | R | − | n/a | n/a | n/a |
| Kinugawa et al., 2022 [107] | F | + | n/a | n/a | n/a |
| Lim et al., 2021 [95] | F | n/a | + | + | n/a |
| Routson et al., 2013 [96] | O | + | + | + | n/a |
| Srivastava et al., 2016 [97] | R | n/a | + | − | n/a |
| Tan et al., 2020 [99] | R | + | n/a | n/a | + |
| Tan et al., 2018 [98] | R | − | n/a | n/a | + |
| Van Criekinge et al., 2021 [100] | O | − | + | n/a | n/a |
| Zhu et al., 2021 [101] | R | − | − | + | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzelli, D.; De Marchis, C.; Quercia, A.; De Pasquale, P.; Casile, A.; Quartarone, A.; Calabrò, R.S.; d’Avella, A. Muscle Synergy Analysis as a Tool for Assessing the Effectiveness of Gait Rehabilitation Therapies: A Methodological Review and Perspective. Bioengineering 2024, 11, 793. https://doi.org/10.3390/bioengineering11080793
Borzelli D, De Marchis C, Quercia A, De Pasquale P, Casile A, Quartarone A, Calabrò RS, d’Avella A. Muscle Synergy Analysis as a Tool for Assessing the Effectiveness of Gait Rehabilitation Therapies: A Methodological Review and Perspective. Bioengineering. 2024; 11(8):793. https://doi.org/10.3390/bioengineering11080793
Chicago/Turabian StyleBorzelli, Daniele, Cristiano De Marchis, Angelica Quercia, Paolo De Pasquale, Antonino Casile, Angelo Quartarone, Rocco Salvatore Calabrò, and Andrea d’Avella. 2024. "Muscle Synergy Analysis as a Tool for Assessing the Effectiveness of Gait Rehabilitation Therapies: A Methodological Review and Perspective" Bioengineering 11, no. 8: 793. https://doi.org/10.3390/bioengineering11080793
APA StyleBorzelli, D., De Marchis, C., Quercia, A., De Pasquale, P., Casile, A., Quartarone, A., Calabrò, R. S., & d’Avella, A. (2024). Muscle Synergy Analysis as a Tool for Assessing the Effectiveness of Gait Rehabilitation Therapies: A Methodological Review and Perspective. Bioengineering, 11(8), 793. https://doi.org/10.3390/bioengineering11080793







