1. Introduction
Cervical cancer (CCa) poses significant challenges for women, with increasing incidence and mortality rates in recent years. It is currently the fourth-most prevalent cancer in women worldwide with over 660,000 newly diagnosed cases and roughly 350,000 deaths in 2022 mainly impacting those in developing and low-income nations owing to the shortage of early detection methods [
1]. Various techniques, including visual inspection, Papanicolaou (Pap) tests, histopathology tests, and human papillomavirus (HPV) testing, are employed for CCa detection [
2]. However, the availability of these tests may be limited in certain countries. For instance, although the HPV test is susceptible, it can yield false negatives, potentially overlooking early stage HPV infections. Furthermore, HPV infections are more common in younger women but are more likely to persist in older women. Therefore, it is crucial to consider screening methods that account for social, biological, and ethical factors, as early detection of CCa improves prognosis. Hence, the development of an accurate system capable of early symptom detection is vital [
3,
4,
5].
Medical imaging tools involving ultrasound, computed tomography (CT) scans, and magnetic resonance imaging (MRI) reveal extensive information about infected tissues and tumor features like dimensions, position, spread, and growth rate [
6,
7,
8]. However, the full benefit of these images is often not realized due to a shortage of professionals, which leads to diagnostic subjectivity. Therefore, there is a critical need to integrate advanced technology, such as artificial intelligence (AI), with medical images to develop robust and generalized models that can provide accurate objective diagnoses. Both of Deep learning (DL) and machine learning (ML) were substantially enhanced several sectors, including healthcare, finance, and even healthcare [
9].
When it comes to CCa, computer vision techniques, such as convolutional neural networks (CNNs), can learn abstract features from images, which can then be utilized to build medical Computer-Aided Diagnosis (CAD) systems [
10]. However, traditional CNN models have limitations that impact their performance, especially when dealing with complex data [
3]. For instance, CNNs tend to focus on local patterns and rely solely on weight sharing within the same layer, which restricts their ability to understand global contexts and capture spatial dependencies [
7]. This returns to several reasons including the following: (1) ViT can capture the global context of data in contrast to traditional CNN, which can process the local context of data; (2) ViT also can employ self-attention, which allows it to capture and understand the relations and the long-term dependencies; (3) ViT is a highly scalable model which makes it suitable for high resolution without the need for resizing or cropping; and (4) ViT provides strong transfer learning, which allows it to fine-tune and converge faster. Transfer learning provides a promising approach by leveraging pre-trained deep learning models, particularly CNNs. This approach allows for the transfer of previously learned representations to new tasks, thereby overcoming the limitations associated with traditional CNN models [
11,
12,
13]. Revolutionary approaches, such as Swin [
14] and ViT [
15] Transformers, have been developed. ViT Transformers, in particular, utilize self-attention mechanisms to extract global dependencies. By leveraging self-attention mechanisms, there is no longer a need for manual feature engineering. This advancement provides promising techniques for image processing and classification, contributing to the development of more accurate and efficient diagnostic models [
16,
17].
A population-based metaheuristic model named particle swarm optimization (PSO) emerged upon observing the behavior of swarms. The way this algorithm works is by repeatedly looking for an optimal output. In this work, PSO is used to determine the best feature subset. This phase not only helps to make the proper decision based on the optimal feature subset, but it also reduces model complexity because it is dependent on the most impacted feature subset.
This paper’s primary goal is to present an expansive, reliable, and accurate framework for CCa classification. In addition to offering promising results in terms of classification accuracy, this model can identify the features who have the greatest impact based on the ViT Transformer and PSO metaheuristic optimization. The following points convey an outline of this paper’s primary contributions:
Utilization of the ViT Transformer to capture both local and global contextual information from medical images, thereby improving the accuracy of image classification.
Implementation of the PSO model for selecting an optimal feature subset and reducing feature complexity.
Enhancement of the model’s performance by replacing the last layer with an SVM machine classifier, leveraging the strengths of both DL and SVM techniques.
Conducting thorough comparison of the proposed model with other models, such as the ViT Transformer and pre-trained CNN models, to assess and verify its superiority in terms of functionality and efficacy.
Creation of visual explanations for the proposed model predictions by superimposing the GradCAM heatmaps across the original cell images as an explainable AI tool.
Remaining parts of this research is structured into four sections:
Section 2 covers the literature review, whereas
Section 3 details the deployed methodology and dataset.
Section 4 assesses the proposed technique, model results, and evaluation measures before concluding in
Section 5.
2. Literature Reviews
Precancerous mutations usually give rise to cervical cancer over a period of 10 to 20 years. A cervical screening test is the only effective way to tell whether the cervix possesses abnormal cells which could lead to cervical cancer. Cervical cancer is often screened for through a Pap test. Because the Pap test can detect abnormal or precancerous alterations in the cervix’s cells, it is crucial for the early detection of cervical cancer. For this reason, Kyi et al. [
18] employed a computer-assisted screening system of Pap smear pictures to construct a computer-assisted cervical cancer screening system. Researchers relied on a shape-based method of iteration to define nuclei via cell segmentation, combined with a marker-control watershed method for distinguishing between overlapping cytoplasm. Then, intriguing aspects of the fragmented nuclei and cytoplasm have been identified using feature extraction algorithms. Using the SipakMed and Herlev datasets, five classifiers’ results were combined to form the bagging ensemble classifier, which achieved 98.27% accuracy in two-class classification and 94.09% in five-class classification.
Rather than utilizing a microscope to identify lesion images, Wong et al. [
19] searched for a method of intelligently analyzing samples. The authors set out to develop an AI image identification system that could utilize liquid-based Pap tests to recognize the extent of CCa lesions in accordance with the Bethesda classification of cancer. ResNet50V2 with ResNet101V2 represent two models developed by integrating DL with transfer learning methods. The assessment findings indicated that the ResNet50V2 model performed more effectively, for certain image categories classified with 98% precision and 97% accuracy.
Pallavi et al. [
20] proposed a method utilizing adaptive fuzzy k means clustering to extract the ROI from cells with cytoplasm and nucleus segments from aberrant Pap smear images, making it useful for early CCa detection.
In order to save time and reduce the possibility of error, Wanli et al. [
21] developed a DL-based framework for replacing the manual screening of cytopathology pictures for cervical cell classification tasks. In order to figure out the final classification based on CNN and Visual Transformer modules, the Multilayer Perceptron module is built to fuse the local and global data. The accuracy of the suggested framework, against combined CRIC and SipakMed datasets reached 91.72%. For the same reasons and via the same approach, two automatic CAD methods have been suggested by Maurya et al. [
22]. Regarding the objective of classifying cervical cell Pap smear images, the first one employed an ensemble of CNNs and ViT networks, while the other used transfer learning with an LSTM and CNN framework. On the “SipakMed” Pap Smear dataset, the proposed algorithms ViT-CNN and CNN-LSTM achieved 95.80% and 97.65% accuracy, respectively. However, the CNN-LSTM technique exceeded ViT-CNN with regard to of computing resource efficiency.
Gurram et al. [
23] suggest utilizing Pap smear images for identifying cervical cancers. For better feature extraction and classification, the proposed approach leveraged a CNN based on the ResNet50 architecture. Their technique obtained 97.5% accuracy on the SipakMed pap-smear image dataset, while the VGG 11 architecture achieved 92.2%.
Transformers impose minimum architectural assumptions regarding the size of the data being received. Because of this property, Bhaswati et al. [
24] provided a cross-attention-based transformer technique which can deal with extremely large-scale inputs for the accurate classification of CCa in Pap smear images.
Jarbas et al. [
25] suggested a discriminatory texture analysis method for Pap smear cell images classification based on the Papanicolaou tests. This method achieved 87.57% accuracy with an AUC around 0.8983 applying LDA and SVM, respectively. In [
4], the authors applied different pre-trained models, namely InceptionResNetV2, VGG19, DenseNet201, and Xception, for classifying cervical images within the SipakMed dataset.
Yaman et al. [
26] provided an exemplar pyramid deep feature extraction method for detecting CCa using cervical cells at Pap smear images. The proposed method used DarkNet19 and DarkNet53 transfer learning-based feature extraction based on Neighborhood Component Analysis (NCA) and SVM on SipakMed and Mendeley Liquid-Based Cytology (LBC) datasets, achieving 98.26% for accuracy.
Despite the promising diagnostic performance achieved by the aforementioned studies in the early detection of CCa, none of them investigated the integration of the ViT Transformer that captures both local and global contextual information from medical images with a PSO model that has the ability to select an optimal feature subset to reduce feature complexity and with an optimized SVM classification model thereby improving the diagnostic accuracy of image classification. As far as we are aware, the suggested method is the first of its type to combine ViT with PSO and SVM in order to integrate ViT with PSO and SVM to seek early and precise classification of CCa using two well-known datasets, namely SipakMed and Herlev.
3. Materials and Method
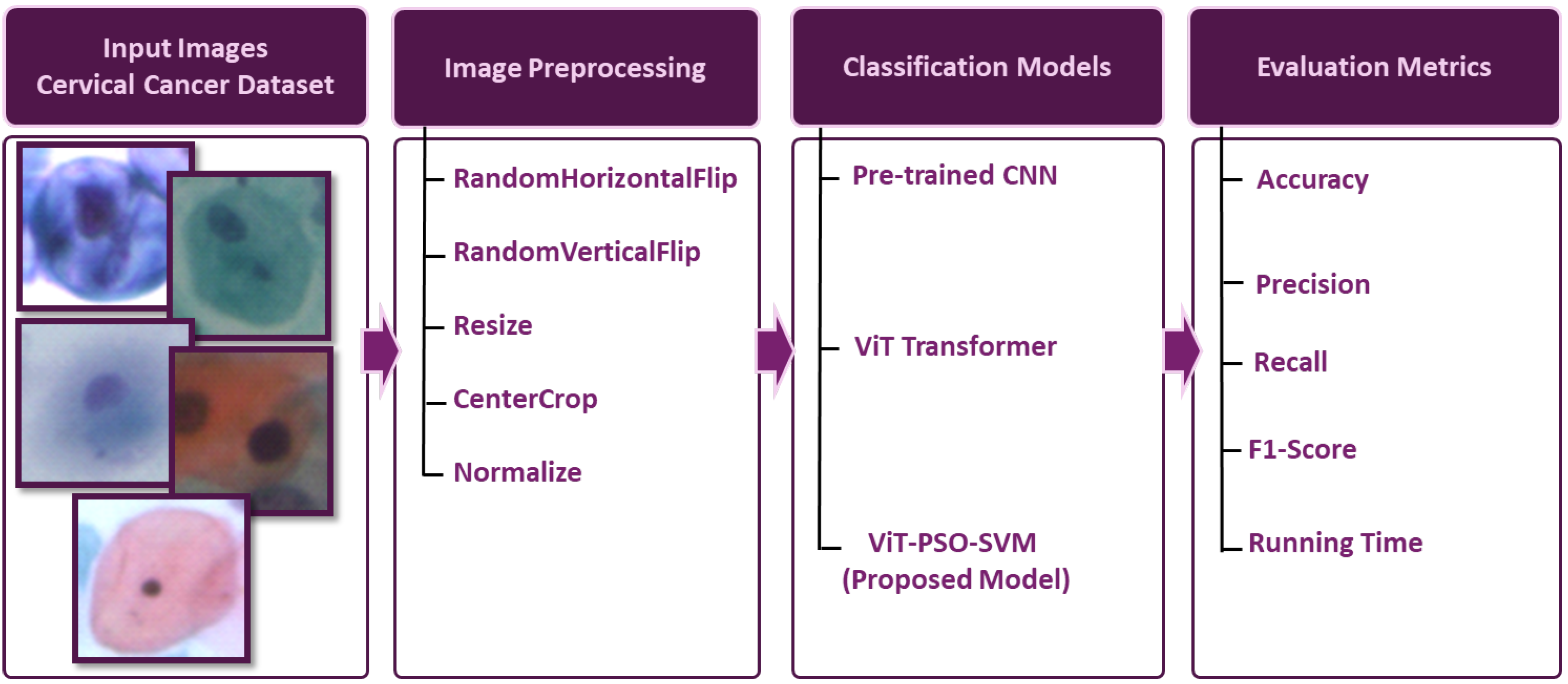
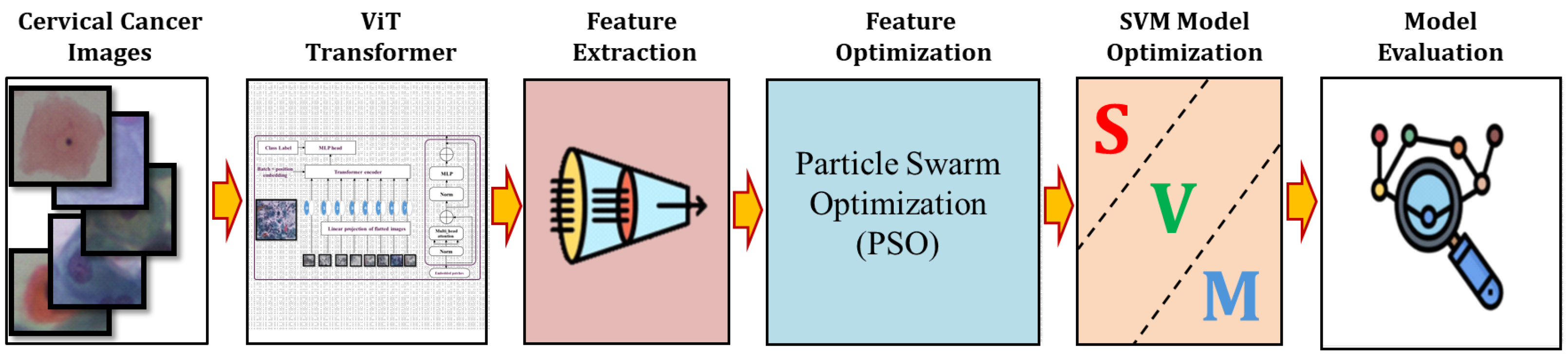
The primary steps involved in classifying cervical cells are depicted in
Figure 1. The main objective of this study is to propose a novel approach (ViT-PSO-SVM) to enhance the results obtained by ViT based on integrating PSO, and SVM. First, the proposed framework extracts features from the ViT. Then, PSO is used to reduce the complexity of extracted features and optimize feature representation. Finally, a softmax classification layer is replaced with an SVM classification model.
3.1. Database Description
We performed our experiments using two cervical cell image datasets:
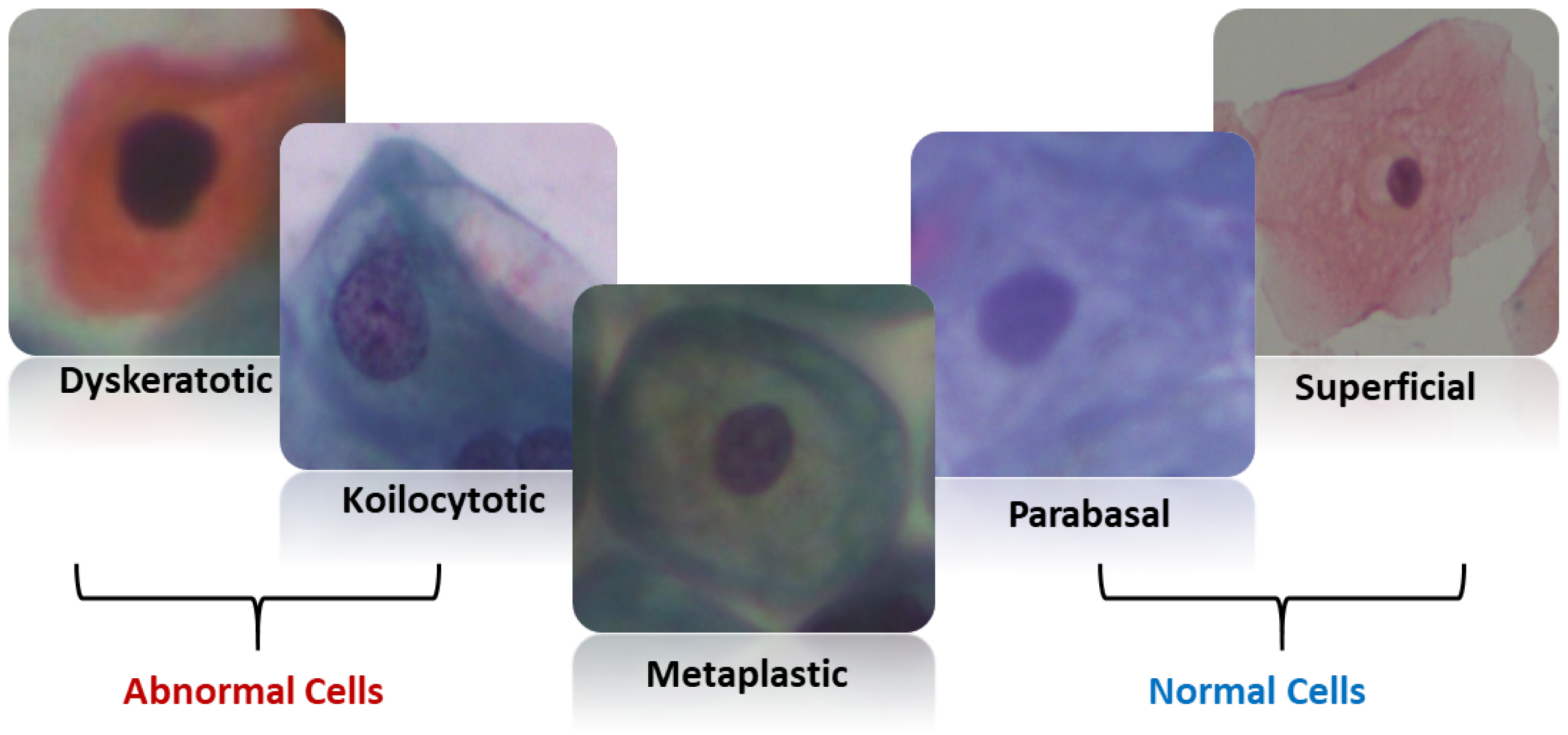
SipakMed consists of 4049 images of cervical cells provided by Plissiti et al. [
27], which is used to evaluate the proposed model. It is a balanced dataset and it includes five classes of Pap smear images which are superficial–intermediate, parabasal, koilocytes, dyskeratotic, and metaplastic. A sample image for each class is shown in
Figure 2.
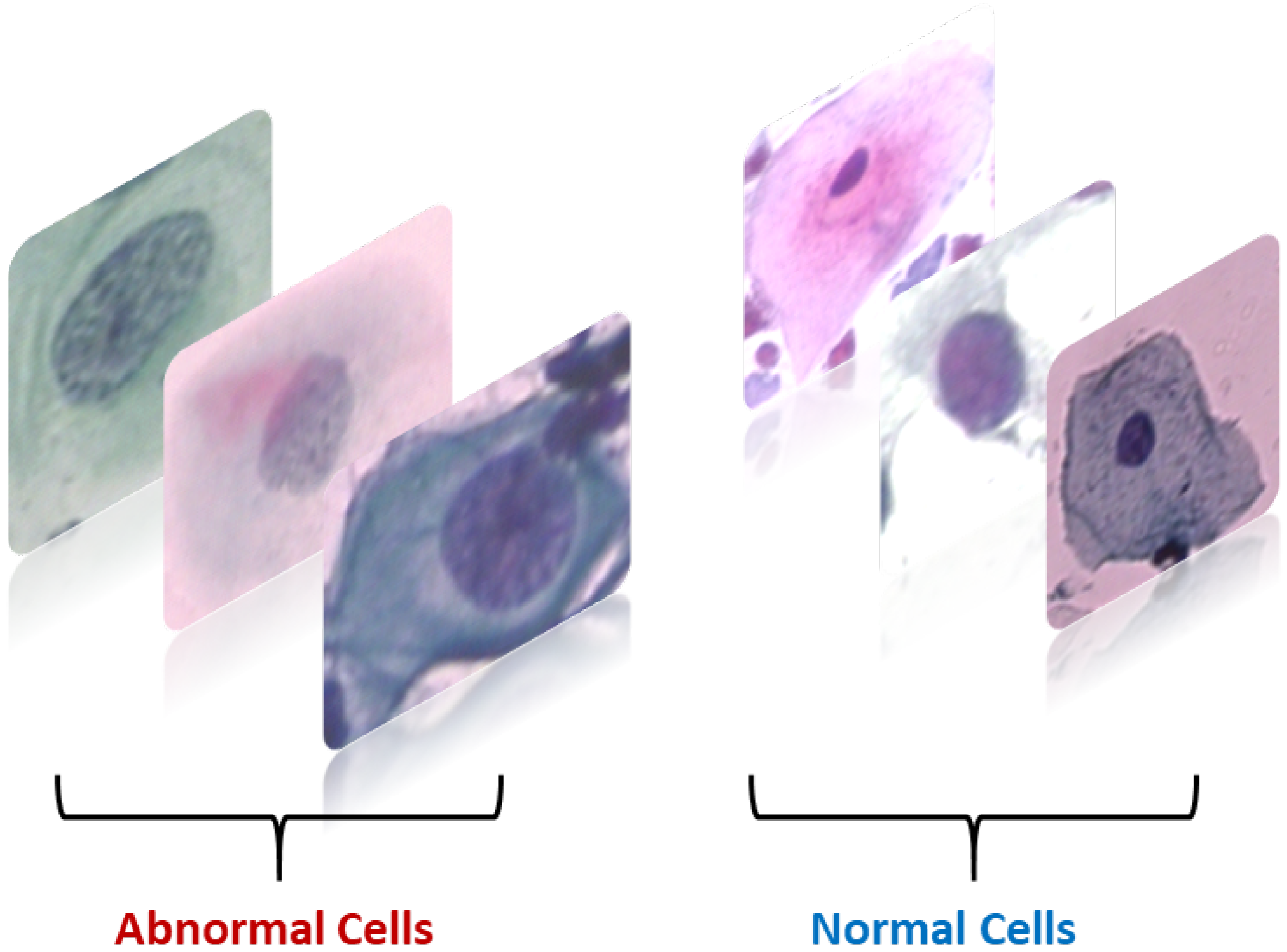
A total of 917 images of cervical cells located by Herlev [
28]. The images have been classified into regular and abnormal categories.
Figure 3 displays several sample images from each class.
3.2. Image Preprocessing/Augmentation
Image augmentation involves making changes to an image in terms of color and position. Positional manipulation is achieved by altering the position of pixels, while color manipulation involves changing the pixel values. It includes techniques such as flipping, cropping, resizing, and noise injection [
29]. These techniques contribute to improving the overall generalization performance of the model by exposing it to a wide variety of images during the training process.
Flipping comprises a horizontal flip that reverses the image’s left–right orientation. This can help the model develop the ability to recognize items independent of their left–right orientation, and vertical flip which reverses the image’s top-bottom orientation. This can help the model learn to recognize items regardless of their orientation up or down [
30].
Resize: Standardizes the size of images in the dataset, making them consistent for the model input. This can help in the efficient batching and processing of images.
Randomly cropping the image can assist the model in learning to recognize objects independent of where they appear in the image [
31]. This can be valuable for tasks where the item of interest is not perfectly aligned in the image.
Normalization helps in speeding up the convergence of the training process by standardizing the input data.
3.3. State-of-the-Art Approaches
DenseNet is built upon the concept of Dense blocks, where each layer within a block is connected to all other layers within the same block. These connections are established through concatenation. Input for the current layer is a stack of the feature maps from the earlier layers, allowing for effective information flow. To downsample the feature maps, DenseNet incorporates transition layers that consist of a 1 × 1 normalization layer and an average pooling layer. These transition layers also help compress the information and increase the efficiency of the network. As a result, DenseNet demonstrates its capability to perform various computer vision tasks [
32,
33].
The residual connections in ResNet are built on the concept of skip connections, which allow for direct connections to earlier layers, bypassing certain layers and creating shortcuts in the gradient flow. This approach highlights the distinction between residual mapping and identity mapping [
34]. By learning the residuals, the network can focus on refining the mapping rather than relying solely on a complete transformation from scratch. The ResNet architecture consists of a series of residual blocks, each containing multiple convolutional layers with varying structures [
35,
36]. These differences in structure reduce the computational complexity of the models and restore the feature dimension. This enables the network to learn both shallow and deep features effectively.
AlexNet leverages convolutional layers to derive spatial characteristics from input images. The convolution operation entails rolling a set of learnable filters across the input image or feature map and conducting element-wise multiplications followed by summations [
37]. The output feature map is created by stacking these activations across spatial dimensions. AlexNet captures several degrees of image information through convolutional layers with varying filter sizes and channel counts. AlexNet employs the rectified linear unit (ReLU) activation function after each convolutional and fully connected layer to cope with the vanishing gradient problem [
37]. AlexNet uses max-pooling layers to minimize the spatial dimensions of feature maps, lowering computational complexity while providing some translation invariance. AlexNet uses fully connected layers at the network’s end to carry out categorization based on previously learned features.
VGG-16 architecture is a deep CNN built for image classification problems. It stacks a succession of small 3 × 3 convolutional layers having a fixed receptive field, before adding max-pooling layers that minimize spatial dimensions. The VGG-16 design typically has 16 layers, including 13 convolutional layers and three fully connected layers [
38].
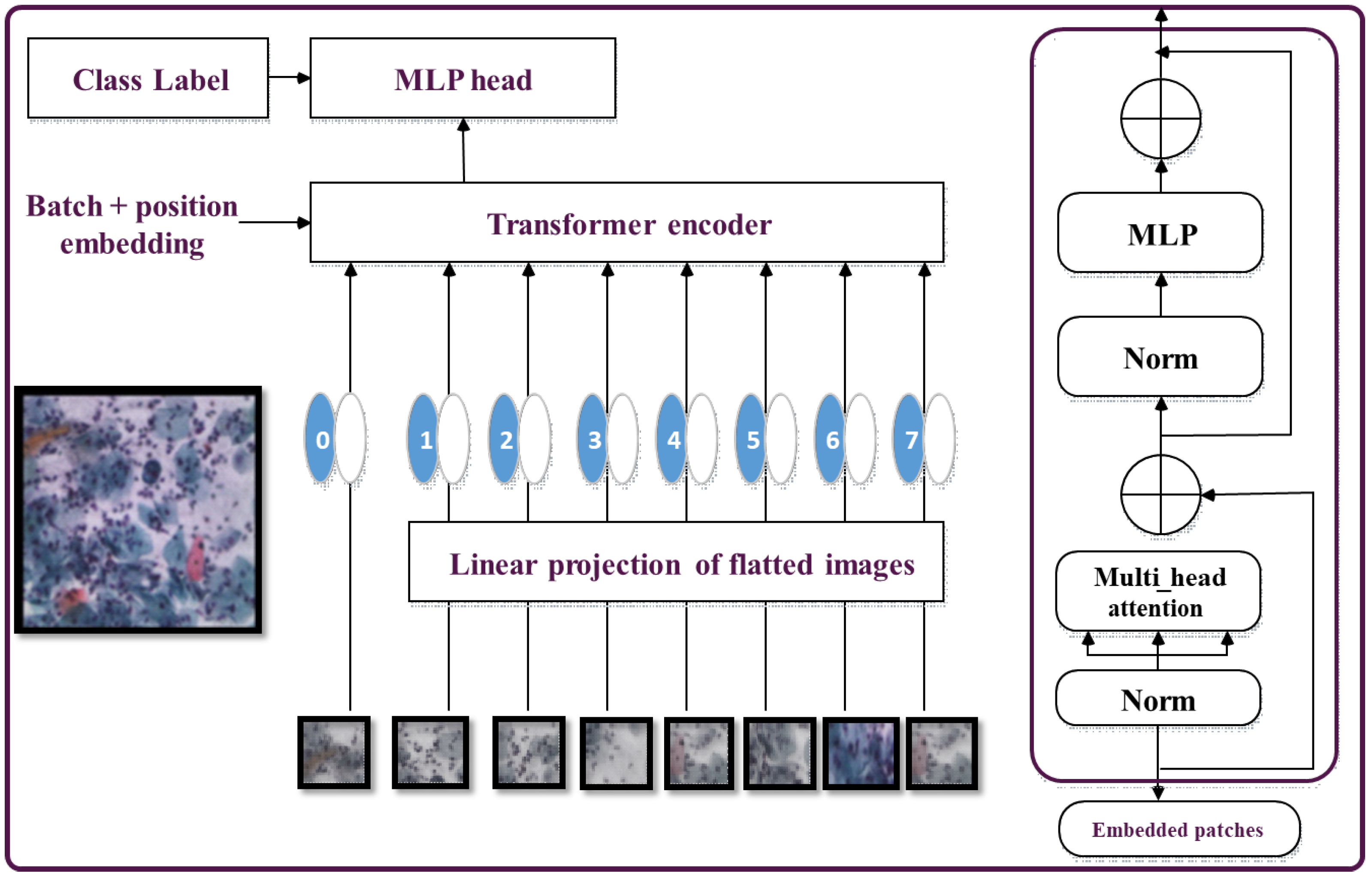
3.4. ViT Transformer
The Vision Transformer (ViT) is a Transformer-based architecture specifically designed for image classification tasks. The self-attention mechanism employed in ViT enables tokens to compare and capture dependencies and relationships among different regions within an image. The feed-forward network processes these tokens to extract high-level features [
39].
In the ViT architecture (shown in
Figure 4), the Transformer encoder takes in the token representations as input and produces encoded outputs. These outputs are then fed to the subsequent feed-forward network to further process the token representations into higher-level features. Finally, the Transformer encodes the generated tokens’ outputs, which are then passed through the classification head to predict the image label [
15].
One major advantage of ViT is its ability to capture image data without prior knowledge about spatial hierarchies or handcrafted features. However, ViT faces limitations when dealing with large-sized images [
40]. To address this challenge, techniques such as patch overlapping and hybrid architectures that combine CNNs and Transformers have been proposed to enhance ViT’s performance on larger images [
41].
The Vision Transformer (ViT) architecture replaces the convolutional layers typically with a self-attention system. The input image is divided into a grid of non-overlapping patches, each of which is linearly projected into a lower-dimensional representation known as a token [
42].
If the image dimensions are H × W and the patch size is P × P, the number of tokens is
. The augmented tokens are then linearly projected into a higher-dimensional space, enabling them to preserve convoluted visual features. To capture global connections and dependencies between tokens, self-attention is paired with a feed-forward neural network technique applied to token embeddings. Given the input token embeddings X, the self-attention (SA) approach generates attention weights and employs them to generate context-aware representations [
43]. It is composed of three linear transformations: query (Q), key (K), and value (V).
That
and
denote the projected token embedding where D is the dimensional of the projected space and weighted sum computed for all values vs. in the sequence. The attention weights
depend on the pairwise resemblance of the two components of the sequence, and their respective query
and key
representations are computed as a softmax of the scaled dot-product between queries and keys.
and this process is carried out individually by multiple attention heads, with the results concatenated and linearly projected to provide the final attention output [
43].
Multihead self-attention (MSA) is an extension of SA in which k self-attention procedures known as “heads” run concurrently and project their concatenated results.
The final Transformer layer’s output is fed into a classification head, which consists of a softmax activation followed by a linear transformation. The final class probabilities for the image are accordingly generated [
44].
3.5. Practical Swarm Optimization (PSO)
Swarm optimization is a meta-heuristic technique inspired by the collective behavior observed in colonies of organisms such as bees and ants [
45]. It leverages the collaboration and information exchange within these colonies to explore solution spaces and find optimal solutions [
46]. The algorithm consists of a population of individuals referred to as agents, which iteratively explore the problem space and update their positions based on their experiences. Swarm intelligence is founded on the concept that intelligent behavior at the group level emerges from the collective behavior of agents at the individual level. Each agent maintains its velocity and position, optimizing them based on the best solution found so far. This is achieved through iterative position updates aimed at searching the solution space for the optimal solution. Swarm intelligence offers several advantages for handling complex optimization problems. Its inherent nature allows for parallel exploration of the solution space, facilitating escape from local optima and enabling the discovery of global solution spaces. Furthermore, swarm optimization exhibits robustness, as it is capable of handling noise in uncertain environments [
47].
Our paper utilizes PSO [
48] for optimizing feature extraction from ViT. In PSO, each particle (agent) represents a point in the feature space. Let’s assume there are n parameters that require optimization, thus the position of a particle can be represented as:
The population size is denoted as m, which represents the number of candidate solutions:
During the exploration of the search space to reach the optimal solution, each particle defines its trajectory. It iteratively updates its solution based on the following equation
Here, t represents the iteration number of the algorithm, and V_i is the velocity component that aggregates along the M dimensions. This vector controls the movement of the particle throughout the search space. The movement is determined by three aspects:
Inertia: It prevents the particle from changing its direction drastically by preserving the previous direction.
Cognitive component: It influences the particle to return to its previous solution.
Social component: It determines the propensity of each particle towards the best solution. Considering the above parameters, the velocity of a particle can be defined as:
Here, P_i represents the best solution obtained so far (local best), and g refers to the overall best solution obtained (global best). The acceleration coefficients, C_1 and C_2, are real values ranging from 0 to 4. R_1 and R_2 are two diagonal matrices randomly generated from numbers between 0 and 1. Consequently, the trajectory followed by the particles can be visualized as
Initialization step
for each particle N, initialize the practice position
initialize the best solution according to the initial position
calculate fitness function for each agent (practical)
if intimate the global best as
repeat this step until reaching the stopping criteria (Update step)
updates the agent velocity according to the following equation (Equation (4))
Update the garnet position according to the following equation (Equation (3))
Evaluate the fitness function for each practice
if make update, make the best equal
if update the global best
At the end of an iterative process, the optimized salutation is g
3.6. Proposed Model
The proposed model (ViT-PSO-SVM) for image classification integrates the use of ViT Transformer, swarm optimization, and SVM to enhance feature extraction and classification as shown in
Figure 5. This is accomplished through the following steps as shown in Algorithm 1.
First, the ViT Transformer serves as the backbone of the model and is responsible for extracting local and global features from the images. With its powerful ability to capture contextual information and semantics, ViT divides the images into small patches and utilizes self-attention mechanisms to effectively learn meaningful features and understand local and global dependencies.
Second, to further enhance the feature selection process, PSO is utilized as an optimization technique. PSO collaboratively explores the search space, allowing the model to search for optimal features. PSO is capable of finding the most informative and discriminative features, thereby enhancing the overall classification performance.
Third, an SVM model replaced the role of the softmax activation function within the last layer. SVM was chosen due to its efficacy in multi-class classification problems and flexibility to handle high-dimensional data. By utilizing SVM, the model achieves robust and accurate classification.
| Algorithm 1: Proposed work |
![Bioengineering 11 00729 i001]() |
3.7. Explainable Artificial Intelligence (XAI)
Gradient-weighted Class Activation Mapping, or GradCAM, and XAI are both key concepts in the field of DL. XAI refers to the broader goal of boosting the transparency and understandability of AI systems for users. This includes methods that attempt to clarify how AI models make decisions, such as how they determine which predictions or classifications to make [
49]. XAI aims to increase user comprehension and validation of the model’s behavior, improve the interpretability of AI systems, and promote a sense of confidence in systems. GradCAM is a particular XAI method that assists in the explanation of the model’s decision-making [
50]. It achieves this by highlighting the regions of an input image that were responsible for the most influence on the prediction generated by the model. GradCAM does this by estimating the target class score’s gradients concerning the feature mappings [
50]. The key areas of the input image are then highlighted in a heatmap made using these gradients. GradCAM facilitates human comprehension and interpretation of the model’s behavior by providing an animated illustration of the decision-making process. GradCAM indicates the input regions that the model depends on, which might assist in identifying possible shortcomings or biases in the model [
50].
3.8. Model Evaluation
Where the correctly categorized positive class is termed as true positive (TP), the correctly classified negative class named true negative (TN), the wrongly classified positive class is labeled false positive (FP), and the incorrectly classified negative class is called false negative (FN).
5. Conclusions
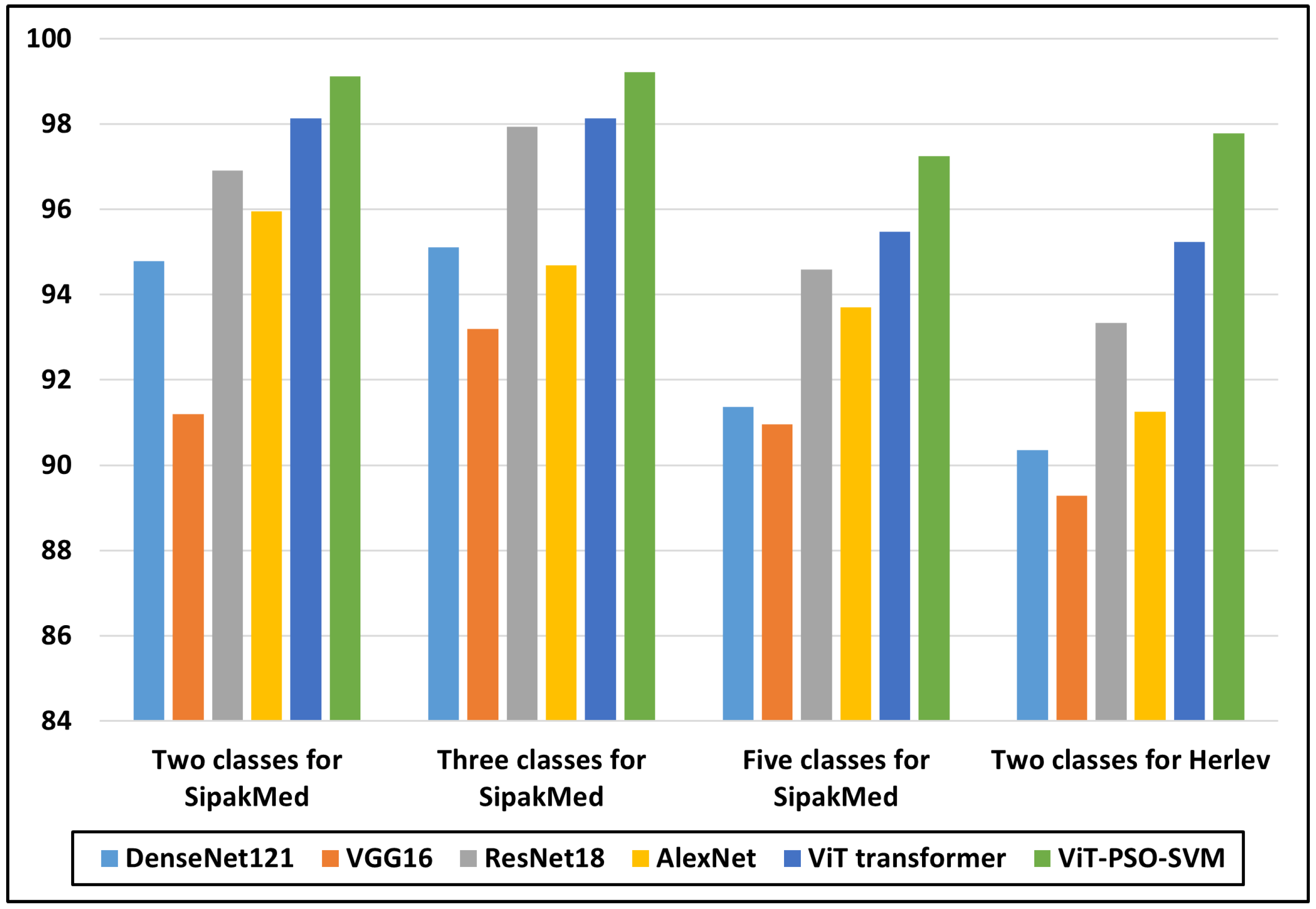
A key factor in lessening the number of fatal cases of cervical cancer remains the early identification. This research introduces a novel approach named ViT-PSO-SVM, which combines the ViT Transformer, PSO feature selection, and SVM to enhance the classification performance. ViT Transformer is used for getting global and local pertinent data from images, making dependency detection simpler. The retrieved features are then processed by PSO for effective feature selection, allowing the model to focus on the most significant characteristics. SVM classifier is used in place of the SoftMax classifier with the objective of boosting classification accuracy and generalization capacity. Efficacy of ViT-PSO-SVM is compared with various models, including the ViT Transformer, CNN, and pre-trained CNN models, in extensive tests. The outcomes show exactly how significantly superior the suggested model is than the present versions. Metrics including accuracy, precision, recall, and F1-score are used to evaluate the model. It is important to note that the detection and classification are performed at the cell level, which may not fully capture the complexity of cancerous lesions at the tissue or organ level. ViT-PSO-SVM recorded the highest accuracy for two, three, and five classes on the SipakMed dataset as 99.112, 99.211, and 97.247, respectively, and 97.778 accuracy on the Herlev dataset. Finally, GradCAM heatmaps were generated as XAI criteria to visualize and understand the regions of an image that are important for the proposed model prediction. Future work should focus on expanding the dataset, exploring additional optimization techniques for the ViT-PSO-SVM model, conducting further comparative analyses, improving dataset collection and preprocessing techniques, and optimizing the model for real-world deployment.