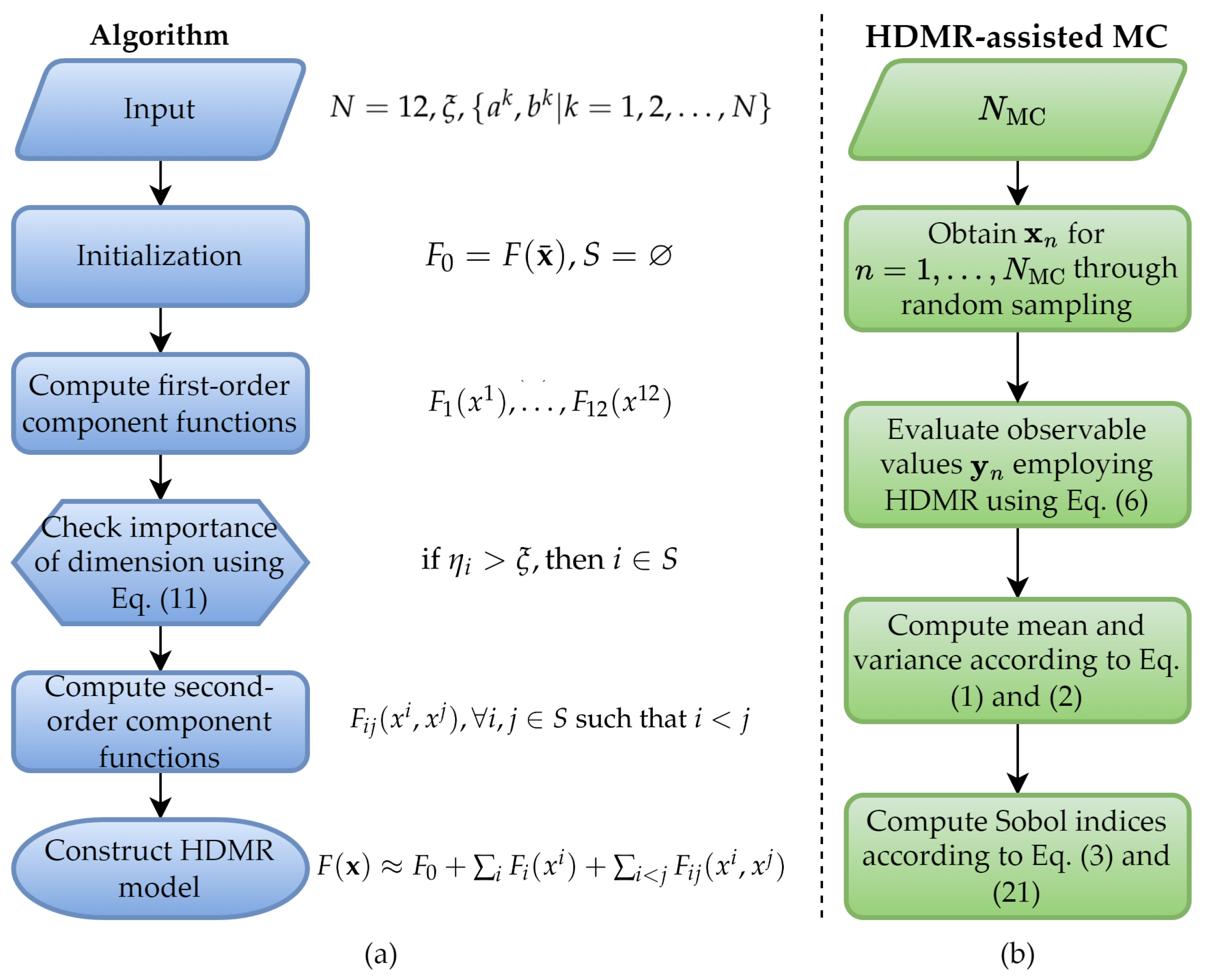
1. Introduction
Magnetic Resonance Imaging (MRI) stands as a cornerstone in medical diagnostics, offering unparalleled detail in imaging soft tissues without the risks associated with ionizing radiation [
1,
2]. During MRI scans, subjects are exposed to strong magnetic fields and radiofrequency (RF) pulses. These RF pulses cause hydrogen atoms in the body to resonate, emitting fields captured by the RF coil to produce high-resolution images of tissues and organs [
2]. However, this process results in energy deposition in the body, quantified via the specific absorption rate (SAR), which measures the RF energy absorbed per unit mass during the scans [
3]. High SAR values indicate elevated tissue temperatures, potentially causing tissue damage and burns. Consequently, ensuring SAR levels remain within safe limits is paramount for subject safety during an MRI scan [
4]. Nonetheless, this task becomes challenging with the increasing frequency of the RF pulses in the latest ultra-high-field (UHF) MRI systems, where standing waves form at a shortened wavelength [
5,
6].
The UHF MRI systems, particularly those operating at 7 T, have become available for clinical applications. The first 7 T MRI system was introduced to the market in 2017 [
7,
8]. As of 2023, over 100 7 T MRI systems are in operation globally [
9]. These machines utilize a stronger magnetic field than their predecessors, offering enhanced image clarity and resolution but presenting new challenges in assessing SAR values and safety considerations. The increased magnetic field corresponds to an increased frequency of RF pulses, resulting in non-uniform electric fields (E-fields) and SAR distributions within the body [
7,
8]. Given these advancements, understanding the SAR distributions in the context of 7 T MRI systems has become important. Furthermore, the variability in SAR distributions is influenced by various factors beyond just the non-uniformity of E-fields. Among these factors, the dielectric properties of the tissues have a significant impact on the amount of RF energy absorbed [
5,
6,
10]. Values of the dielectric properties, permittivity and conductivity, are not fixed but exhibit
variability around their nominal values due to the change in physiological parameters, e.g., oxygen levels [
11,
12,
13,
14,
15,
16], reflecting the natural heterogeneity found within biological tissues. Such variability (or uncertainty) in dielectric properties can directly lead to variations in SAR values, which significantly impact image quality and patient safety. These variations in the SAR can introduce artifacts and reduce image clarity, thus compromising diagnostic accuracy and potentially necessitating repeat scans [
17]. Additionally, such variations may cause tissue heating and thermal injuries in patients. This variability can also introduce significant discrepancies between the actual and computed E-field and SAR distributions [
18,
19]. Therefore, to lower such discrepancies and ensure the SAR values comply with high-tier SAR limit standards [
20,
21], there is a need for computational frameworks that can accurately quantify and assess the impact of uncertainties in tissue dielectric properties on the induced SAR values.
Current tools for computing SAR distributions rely on deterministic electromagnetic (EM) simulators [
22,
23]. However, these simulators do not account for the effects of uncertainties in tissue dielectric properties while generating the simulation results. In addressing this need, the traditional Monte Carlo (MC) methods [
24,
25] can be applied in conjunction with these simulators. The traditional MC methods require the execution of the deterministic simulator for numerous randomly selected realizations of tissue dielectric properties, yielding statistical measures, such as the mean and standard deviation, of the SAR. Despite their straightforward implementation, MC methods are inefficient as they require a large number of deterministic simulations, each of which is computationally expensive for EM characterization in MRI scenarios [
24]. To overcome these challenges, surrogate model-assisted MC presents itself as a viable alternative [
26]. This approach leverages surrogate models to approximate the outcomes of deterministic simulators, thereby significantly reducing the computational burden associated with traditional MC methods [
26]. By integrating surrogate models, the surrogate model-assisted MC method retains the versatility of traditional MC approaches while acquiring statistics more efficiently.
Previously, researchers have successfully utilized surrogate modeling techniques and surrogate model-assisted MC methods to quantify the effects of uncertainties in various bioelectromagnetic problems, including transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and cellphone radiation safety assessment studies. For TMS, non-intrusive generalized polynomial chaos (gPC) [
19] and high-dimensional model representation (HDMR) techniques [
18] have been applied in uncertainty quantification. Similarly, in tDCS, various techniques, including the non-intrusive stochastic collocation method (SCM) [
27] and gPC [
19], have been implemented to achieve the same objective. Furthermore, both gPC and SCM [
28,
29] have been employed to quantify the uncertainties in the SAR induced due to cellphone radiation. These applications have yielded promising results in terms of accuracy and efficiency, and shown the necessity and effectiveness of the surrogate model-assisted MC methods for uncertainty quantification in bioelectromagnetic problems. However, to our knowledge, no study has performed uncertainty quantification in the SAR induced by UHF MRI RF coils and necessarily employed these surrogate model-assisted MC methods.
This paper proposes a surrogate model-assisted MC framework to quantify the effects of uncertain tissue dielectric properties on the SAR induced by UHF MRI RF coils. The framework initially generates surrogate models of E-fields by using the outcomes of a small number of deterministic simulations performed by MARIE [
23], an open-source MRI EM analysis software. Then, the SAR’s mean, standard deviation, and sensitivity indices are obtained via MC using the surrogate models instead of the deterministic EM simulator. Specifically, the proposed framework constructs surrogate models by leveraging the HDMR technique [
30], combined with the gPC method, which requires significantly fewer deterministic simulations than the traditional MC method’s brute-force application [
30,
31,
32]. The HDMR technique allows approximation of the multivariate MRI observables (E-field and SAR) via univariate and bivariate component functions. Doing so effectively tackles the ‘curse of dimensionality’ arising during surrogate model generation of multivariate functions. While the technique iteratively includes the most significant component functions (of most influential tissue dielectric parameters) in the HDMR expansion, it approximates each component function via the gPC method [
31,
32].
The numerical results (in
Section 3) show that the proposed HDMR-based technique demonstrated superior performance over other surrogate modeling methods, including random vector functional link (RVFL) [
33,
34], extreme learning machine (ELM) [
35,
36], single-layer neural network (NN) [
36], Gaussian process (GP) [
37], and least square-based gPC [
38]. Among all these techniques, the HDMR required the minimum number of simulations while providing the statistics with the highest accuracy, thanks to its capability of selectively incorporating the most significant component functions (with combined effects of dielectric properties).
The contributions of this study are threefold:
This is the first and foremost study performing uncertainty quantification of the SAR induced by UHF MRI RF coils. It demonstrates the significance of uncertainties in the dielectric properties of human head tissues, which can cause up to 30% fluctuations in SAR values within specific head regions, as demonstrated in the numerical results section.
This study proposes an HDMR-based surrogate modeling technique, which emerges as the best among various tested surrogate modeling methods for approximating E-fields and SAR induced by UHF MRI RF coils. The technique obtains the surrogate models with a mean relative error of 0.28% by only 289 deterministic simulations, surpassing the accuracy and efficiency of other surrogate modeling methods, as shown in the numerical results section.
Finally, this study conducts statistical and sensitivity analyses on SAR values. The statistical analysis presents theoretical maximum 1g-SAR and 10g-SAR values after incorporating the uncertainties in tissue dielectric properties, which underscores their importance in MRI safety assessment. Furthermore, the sensitivity analysis shows the uncertainties in which tissues’ dielectric properties affect the SAR values more in certain regions of the brain.
The rest of this paper is organized as follows.
Section 2 presents the preliminary concepts, the formulation of the proposed gPC-based HDMR technique, and the general information on the deterministic simulator MARIE used in this study.
Section 3 provides numerical results and analysis, focusing on the accuracy and efficiency of the proposed framework and presenting statistical and sensitivity analyses. Finally,
Section 4 presents the conclusion, summarizing the study’s key findings.
3. Numerical Results and Discussion
This section demonstrates the proposed computational framework’s accuracy, efficiency, and applicability in obtaining the statistics of the UHF MRI RF coil-induced SAR on an MRI-derived head model. To construct the surrogate models, the tolerance for HDMR component function selection,
, is set to
, while the number of GL quadrature points along each dimension,
, is set to 3, 5, or 7. As alluded in
Section 2, the relative permittivities and conductivities of six types of head tissues are uniformly distributed in the ranges provided in
Table 1, while the observables are the SAR values. To evaluate the accuracy of the surrogate models, the relative error of the observable on each voxel,
,
, is computed via
where
is the number of testing points, randomly selected according to the distributions shown in
Table 1, while
and
represent the observable value on
voxel obtained by the deterministic simulator and surrogate model, respectively. Once the accuracy of each surrogate model of each voxel is assessed via its associated relative error, the overall accuracy of all surrogate models is evaluated via
Here, the maximum relative error, , gives insight into worst-case scenarios or outlier behavior, whereas the average relative error, , presents a more generalized view of the accuracy across the entire head model.
3.1. Numerical Settings
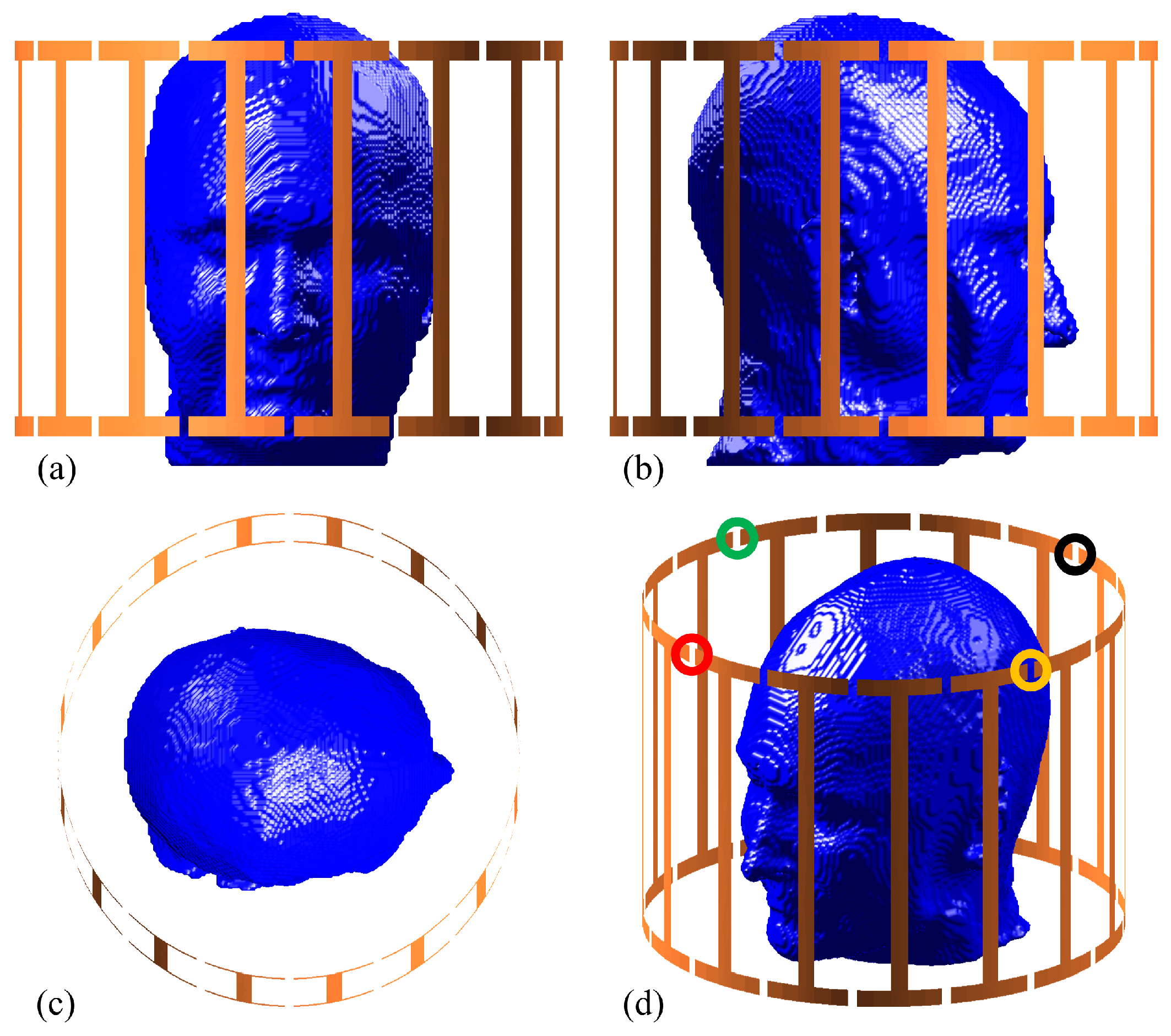
In the considered MRI scenario, a birdcage RF coil provided in MARIE [
23] is used to stimulate a human head model, as depicted in
Figure 2. This 32-port birdcage coil with a 140 mm radius operates at
for 7 T MRI scans and is initially excited from the port 1 [
Figure 2d]. Thereafter, ports 5, 9, and 13, demonstrated in
Figure 2d, are also activated to examine the accuracy of the constructed surrogate models, as discussed in subsequent subsections. The human head model is derived from an MR image selected from the OASIS2 dataset [
45]. Initially, the MR image is transformed into a tetrahedral mesh using the headreco function [
46] of SimNIBS [
47]. This process yields a segmented head model with tissues, including white matter, grey matter, cerebrospinal fluid (CSF), bone, scalp, and eye humor. The conductivities and relative permittivities of these tissues are sequentially encapsulated in
and set randomly or according to the collocation point for each deterministic simulation. For compatibility with MARIE, the segmented head model is then converted to a voxel head model situated in a computational domain of
with a voxel size of
, where 889,850 voxels occupy tissues. The head model is positioned at the center of the computational domain so that the head model coincides with the birdcage coil’s center [
Figure 2].
3.2. Accuracy
The accuracy of the surrogate models generated by the proposed method is examined. To this end, the surrogate models of the SAR for all voxels in the head model are obtained by two approaches. In the first approach, called the
direct approach, the input–output relation is directly formed between the input vector of the head tissues’ dielectric properties,
, and the output vector storing all SAR values on all 889,850 tissue voxels. In the second approach, called the
indirect approach, the input vector remains unchanged, while the components of E-fields,
, are considered as the output and stored in an output vector of a size of 2,669,550; each entry of the vector is a complex number. In the indirect approach, after the construction of the surrogate models of E-fields, those are used to compute the SAR values on each tissue voxel
i,
, via
where
are the components of E-fields on each voxel
i. The density,
, stands consistent for each tissue type throughout the study, as shown in
Table 1. The accuracy of the surrogate models obtained using direct and indirect approaches is assessed using the SAR values as observables in (
18) and (
19). To test the accuracy with different parameters, first, the HDMR expansion is truncated right after the first component functions, and
is set to 3, obtaining gPC coefficients in (
16). Furthermore, to achieve better accuracy, the number of component functions or GL points is increased in subsequent computations. This increase ensures a balanced approach between computational efficiency and the accuracy of the outcomes in the surrogate model assessments. Specifically, the number of GL points is augmented to 7 to improve approximation of component functions, or the HDMR is extended to encompass second-order component functions, which describe the combined effects between entries of the input vector. The results are shown in
Table 2 for the direct approach and
Table 3 for the indirect approach.
A straightforward comparison between
Table 2 and
Table 3 reveals that, generally, the results obtained through the indirect approach surpass those from the direct approach. This observation can be linked to the relationship between SAR values and the E-field, as shown in (
20). The added complexity and interactions introduced by the squaring operations might necessitate a larger number of collocation points and the incorporation of higher-order terms to achieve a similar level of accuracy as that achieved when modeling indirectly.
Moreover, based on the data presented in the tables, it is noticeable that, when including solely the first-order component functions, there exists no direct correlation between an increment in the number of collocation points and the enhancement of accuracy in both scenarios. This suggests that the augmentation of collocation points does not significantly contribute to improving the accuracy of the surrogate modeling technique. Conversely, upon incorporating the second-order component functions, a notable enhancement in accuracy is observed, as evidenced by the substantial reduction in both mean and maximum relative errors. The optimal results are obtained in the second scenario, where the mean error is noted to be and the maximum error is , ensuring the confined error range for each voxel. In light of this, one should notice that the optimal accuracy in the study is attained in a scenario necessitating 1105 simulations. Considering the time-intensive nature of the deterministic simulations by MARIE, this approach might not be pragmatically viable. However, employing a mere 289 simulations yields results that, although slightly inferior to the 1105-simulation case in accuracy, offer a more feasible balance between accuracy and efficiency. Therefore, the subsequent analysis will be performed upon the 289-simulation case, utilizing a total order of 2 for component functions and three GL quadrature points along each dimension.
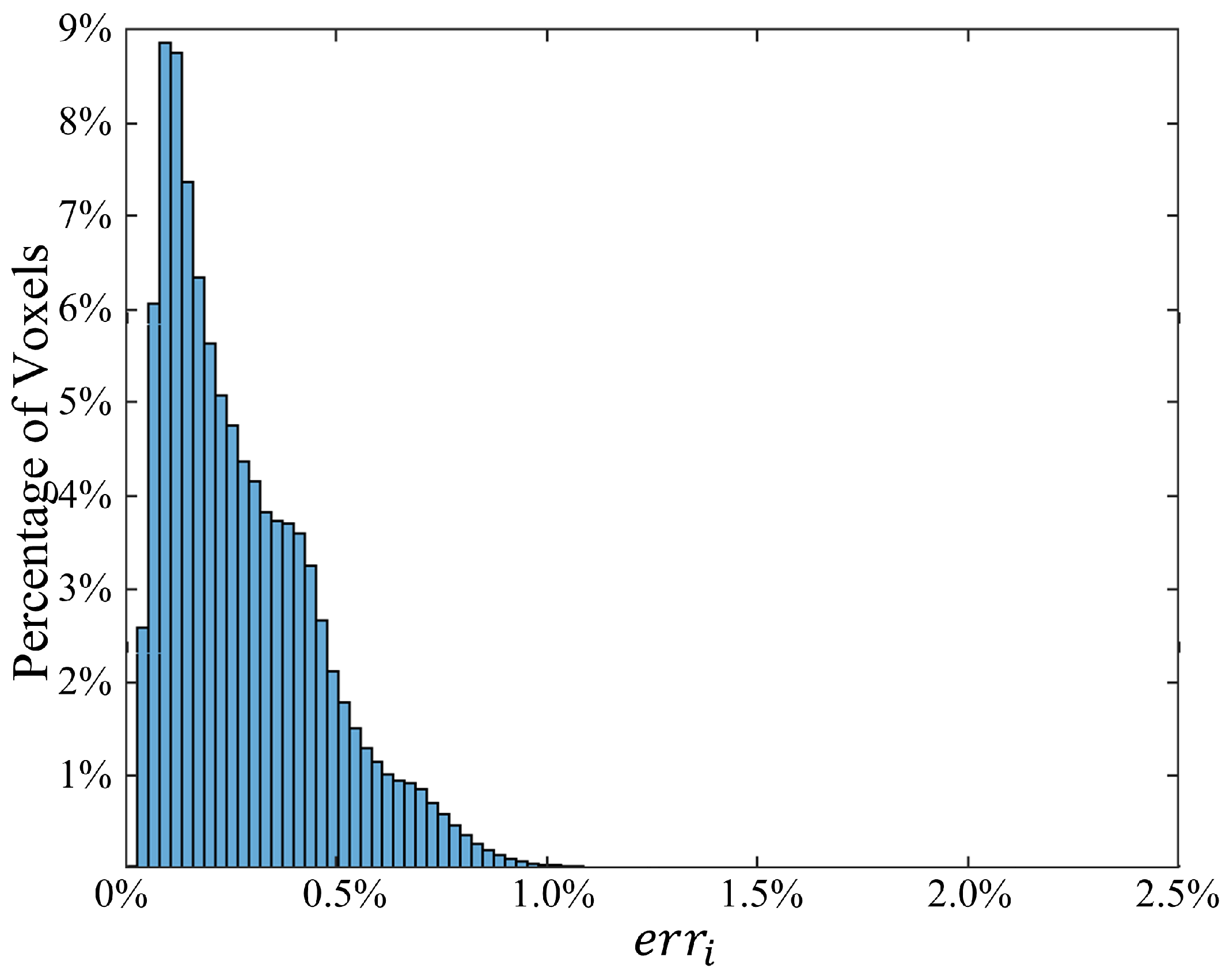
The distribution of voxel-based relative errors is shown in
Figure 3. Indeed, most of the voxels exhibit an error of less than
, with only
of voxels surpassing this value. In
Figure 4, selected slices are presented to highlight the accuracy of the proposed method under the second scenario. The left column depicts the ground truth SAR values obtained from the deterministic simulator MARIE, while the middle column displays values approximated by the HDMR-generated surrogate models. The right column illustrates the logarithm of the relative difference. With a logarithmic error spanning 2 to 5 digits, these illustrations underscore the high accuracy achieved by the proposed method.
The accuracy of the HDMR technique is assessed by comparing it with other surrogate modeling techniques such as RVFL [
33,
34], ELM [
34,
35], single-layer NN [
36], GP [
37], and least square-based gPC [
38]. Each of these techniques is applied within the framework of two scenarios (direct and indirect) and their performances are assessed in terms of SAR values utilizing the same error metrics. To ensure a fair comparison, a training set of 300 points is generated through Latin Hypercube Sampling; the testing set remains the same, with 100 testing points. The results are shown in
Table 4 and
Table 5. Note that RVFL, ELM, and single-layer NN possess associated hyper-parameters. Therefore, multiple test cases are conducted to fine-tune these parameters, and the most optimal results are presented in the tables to ensure a fair and comprehensive comparison. As the tables illustrate, the proposed HDMR significantly outperforms all other surrogate modeling techniques.
The proposed technique is further examined under various conditions by individually activating other ports (Port 5, Port 9, and Port 13), demonstrating the reliability of the approach. All results, obtained under the second (‘indirect’) scenario, incorporating a total of second-order component functions and three GL points, are presented in
Table 6. One can see that the mean relative errors for all conditions exhibit a consistent behavior, fluctuating within a narrow range. This indicates a stable performance of the proposed method, irrespective of the port activated. However, while the mean relative error demonstrates satisfactory performances, reflecting the method’s general effectiveness, the maximum relative error of port 9 is notably higher than that in all other conditions. The discrepancy in this case could be attributed to the spatial location of the voxels within an unexcited region. Given their substantial distance from the activation port, as shown in
Figure 2d, the voxels near the nose region experienced less field excitation, resulting in comparatively lower SAR values. Thus, inconsequential deviations in prediction within this region can result in amplified maximum relative errors, which is expected.
3.3. Statistical Analysis
After establishing the accuracy of the proposed HDMR in the preceding section, it is important to assess the crucial statistical metrics obtained from the surrogate model. Employing the HDMR-assisted MC method with a sample size of 10,000 random points, the computed means and variances provide crucial insights into the performance and reliability of the proposed surrogate modeling technique. These statistical metrics are compared with the results derived from the traditional (brute-force) MC method, using simulations on MARIE with a sample size of 5000 randomly selected points. It is important to note that all points for the MC simulations are selected randomly, following the uniform distributions illustrated in
Table 1. This comparative analysis is essential in corroborating the robustness and validity of the HDMR as a credible and efficient alternative to the traditional and computationally intensive MC methods for obtaining reliable statistical metrics.
Figure 5 illustrates the convergence trends for the mean and variances of two selected voxels determined by the traditional MC method with increasing sample size. These metrics are juxtaposed and compared with the corresponding estimations from the proposed framework’s indirect approach. It becomes evident that the proposed method achieves a similar level of accuracy with a significantly lower number of deterministic simulations, underscoring its efficiency.
Furthermore, the maximum SAR values are also examined, given their paramount significance related to MRI safety regulations. This inquiry aims to discern the impacts of variations in tissue properties on the SAR values, which is vital for ensuring the safety of RF exposure. The nominal SAR values, obtained under nominal conditions of relative permittivities and conductivities, serve as a benchmark for comparison. The SAR values are assumed to follow normal distributions, which allow the estimation of maximum SAR values, calculated as the mean plus three times the standard deviation. These mean and standard deviation values are computed using HDMR-assisted MC methods, based on a sample of 10,000 random points.
In keeping with regulatory standards, voxel SAR values undergo conversion to 1g-SAR and 10g-SAR using a standard method. This process involves averaging the SAR values in a progressively expanding region of tissue-containing voxels surrounding a central voxel. The expansion continues one voxel at a time until the specified tissue mass, either 1 g or 10 g, is achieved [
48]. This procedure is executed using the algorithm detailed in [
49]. The analysis of the maximum SAR further underscores the importance of the uncertainties of input parameters, which inevitably contribute to the variances observed in SAR value distributions.
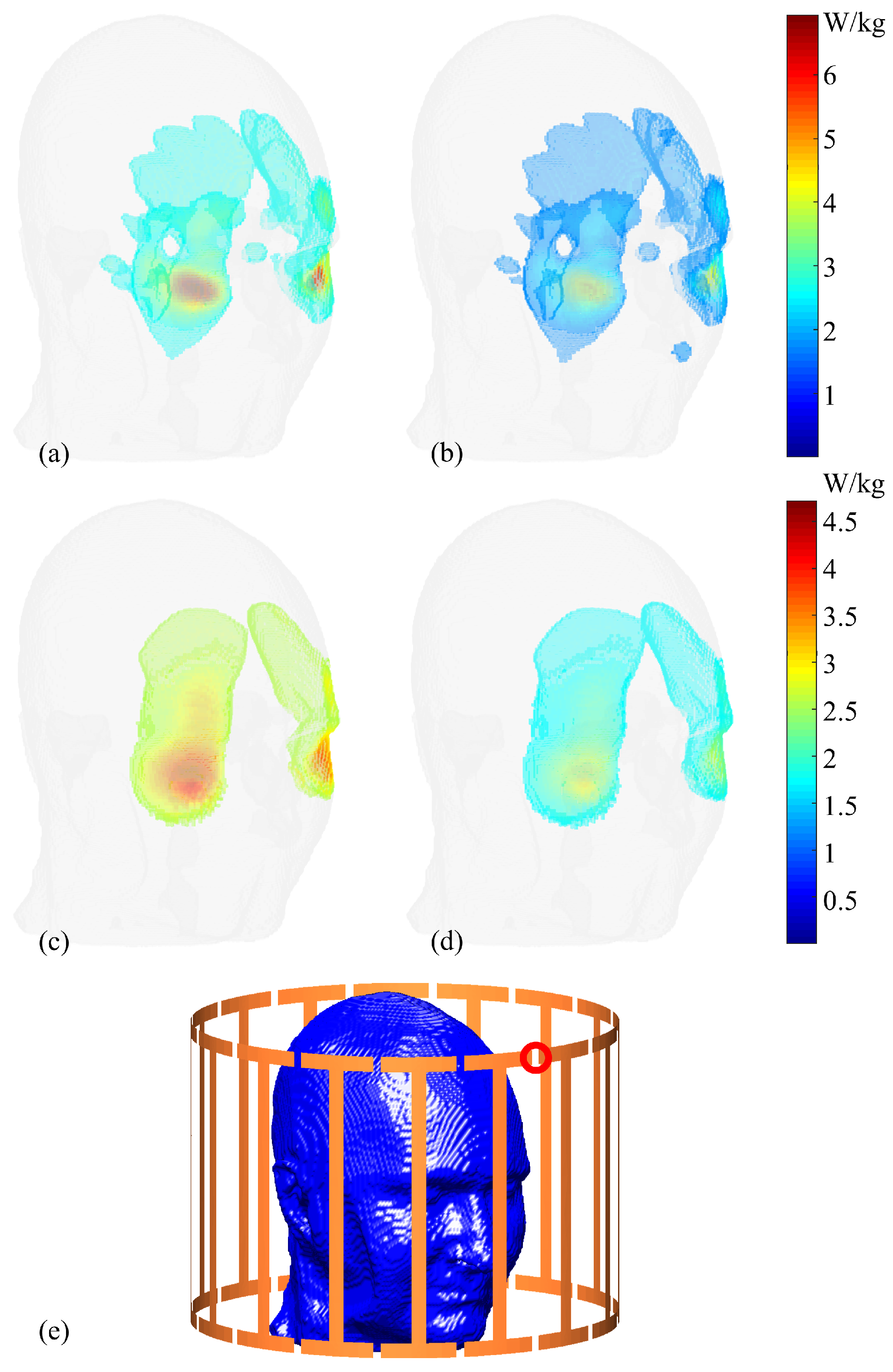
Figure 6 presents a comparison between maximum and nominal 1g-SAR values. Note that only the top
of voxels with the highest values are selected for plotting to optimize visualization. Utilizing consistent colormaps, the voxels representing maximum values for the 1g-SAR exceed 6 W/kg, while those reflecting the nominal values for the same case are approximately 3.5 W/kg. For the 10g-SAR scenario, the maximum SAR values reach up to 4.5 W/kg, while voxels in the nominal values center around 2.5 W/kg.
Selected sagittal slices (Slice 72) of both 1g-SAR and 10g-SAR are depicted in
Figure 7 for a comparative analysis between maximum and nominal SAR values, accompanied by their respective differences. For the 1g-SAR, there are noticeable increments of approximately
and
in the forehead and nose regions, respectively. Similarly, the 10g-SAR reveals an approximate
elevation in the forehead area.
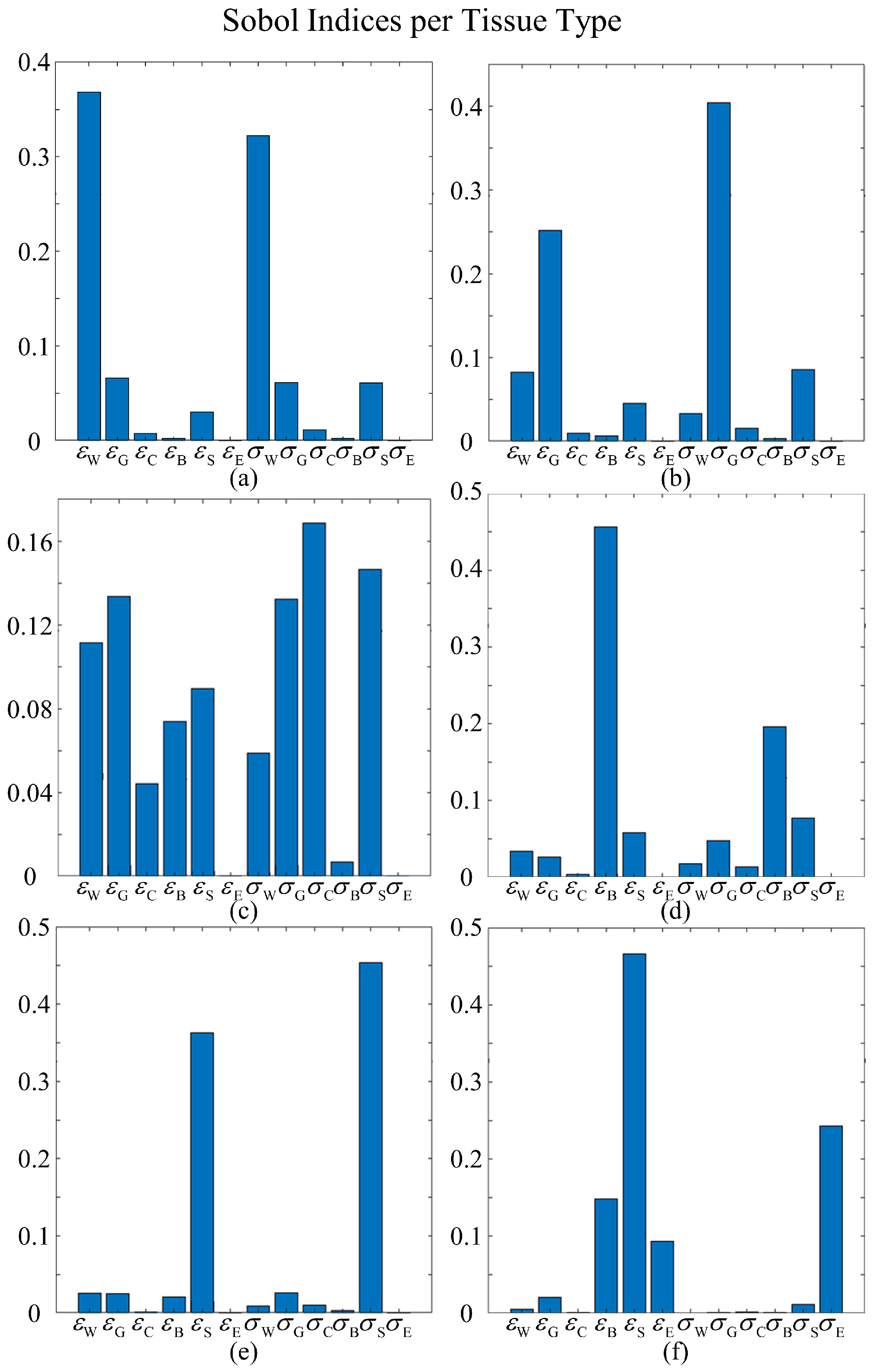
3.4. Sobol Indices
For voxel
i, its Sobol index with respect to
,
, can be computed as in (
3) and then classified based on the type of tissue it represents. The average Sobol indices for a given tissue type
t, denoted by
, is calculated by averaging
across all voxels of the same tissue type, such that
where
indicates total number of voxels of tissue type
t.
Figure 8 displays the first-order Sobol indices of each tissue type
t with respect to each input dimension. For most tissues, their own relative permittivity and conductivity have the greatest influence, followed closely by the relative permittivity and conductivity of neighboring tissue types. For the white matter, the primary influencers are its inherent relative permittivity and conductivity, with grey matter’s electrical properties coming next. In the case of grey matter, in addition to its own relative permittivity and conductivity, the relative permittivity of white matter and conductivity of the scalp also play significant roles. For CSF, while its own conductivity is the predominant contributor, other inputs also significantly influence the variances. This phenomenon can be attributed to the circumstance that CSF is a common neighbor to most tissues, resulting in its SAR values being impacted by the changes in the properties of adjacent tissues as well. This suggests a complex interaction between the CSF and its surrounding environment, emphasizing the necessity of incorporating second-order component functions in the HDMR framework. These functions are crucial for capturing the inputs’ combined effects, offering more accurate and detailed models. Sobol indices for bone and scalp tissues display similar patterns, with their respective relative permittivities and conductivities exerting the most impacts. The results for the eye require further analysis, given that the relative permittivity of the scalp appears to be the most influential dimension.