Deciphering Factors Contributing to Cost-Effective Medicine Using Machine Learning
Abstract
1. Introduction
- Novel CER metric: Introducing a metric that combines user ratings and prices to evaluate OTC medications, providing a comprehensive measure beneficial to consumers, manufacturers, and retailers.
- Comprehensive factor analysis: Evaluating factors such as FSA/HSA eligibility, symptom treatment range, safety warnings, special effects, active ingredients, and packaging size to identify key cost-effectiveness drivers across medication categories.
- Explainable ML/AI techniques: Assuring model accuracy and interpretability using SHAP values and logistic regression coefficients, aligned with the principles of explainable AI and the scope of the journal.
- Cross-domain impact: Bridging biomedical research and practical applications in the pharmaceutical industry by analyzing real-world data from Amazon, providing actionable insights for consumers, manufacturers, and retailers.
- ○
- Consumers: Simplifying the decision-making process, enabling informed choices about medication purchases, and balancing efficacy and cost, leading to enhanced satisfaction.
- ○
- Retailers: Improving the shopping experience for retailers like Amazon Pharmacy by optimizing website layouts and recommendation systems [36], aiding in inventory management and targeted marketing strategies.
- ○
- Manufacturers: Guiding product development by understanding consumer values such as efficacy, side effects, cost, and convencience [37], leading to more effective, user-friendly medicines, and better market positioning.
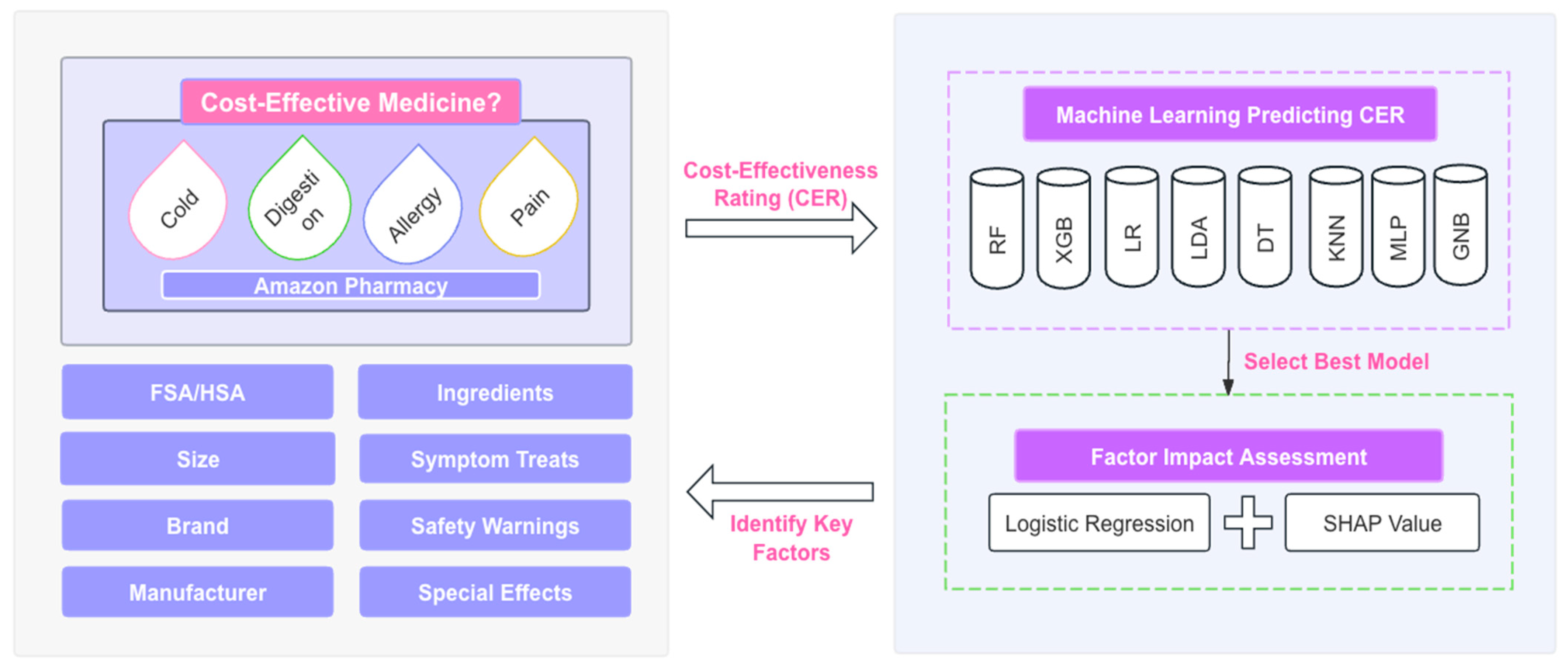
2. Methodology
2.1. Dataset
2.2. Data Preprocessing
2.3. Data Exploration and Feature Engineering
2.3.1. FSA or HSA Eligible
2.3.2. Size
- Counts per pack: Refers to the number of items per pack.
- Weight: Represents the weight of the item.
- Inches: Indicates the dimensions of the item.
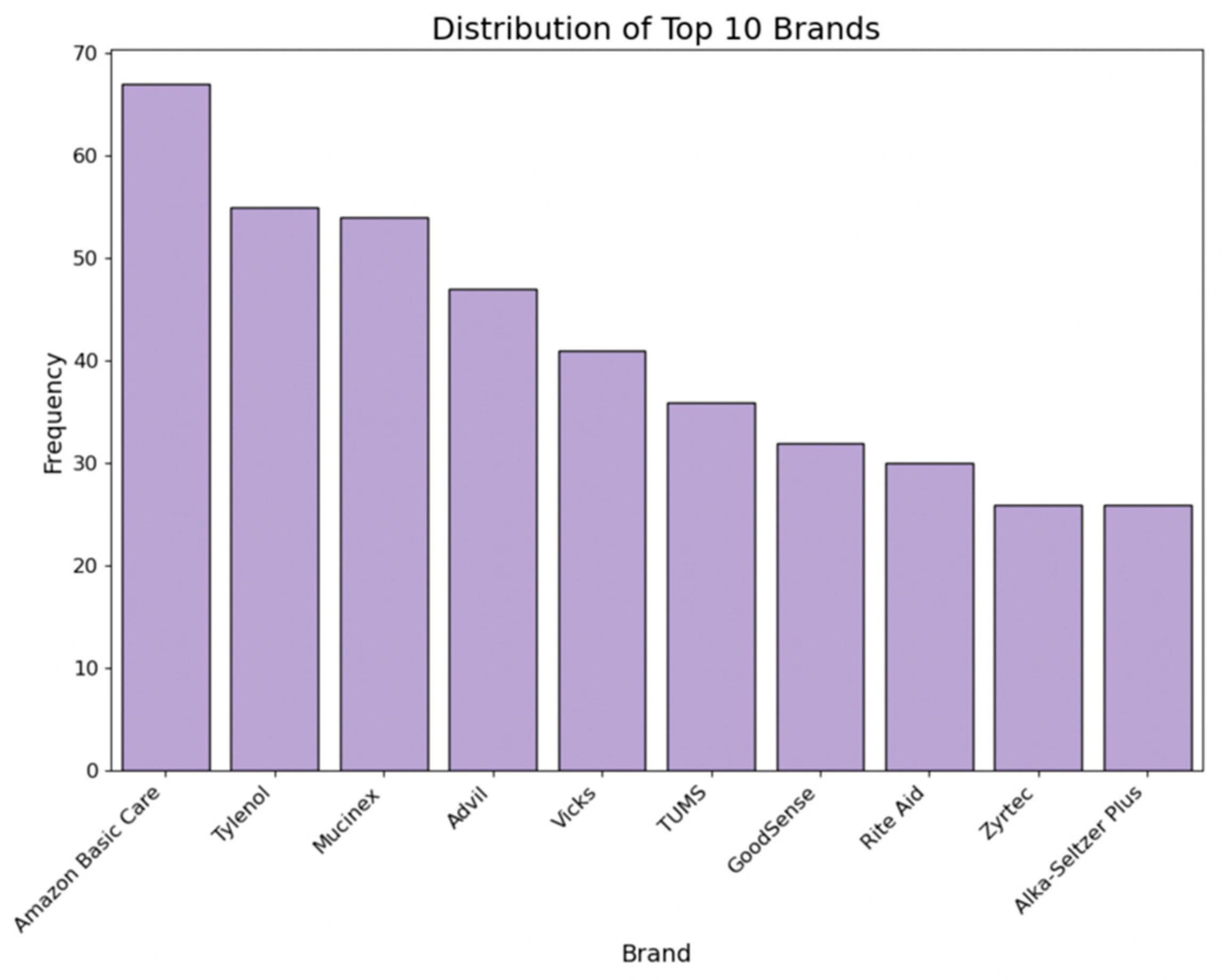
2.3.3. Brand
2.3.4. Manufacturer
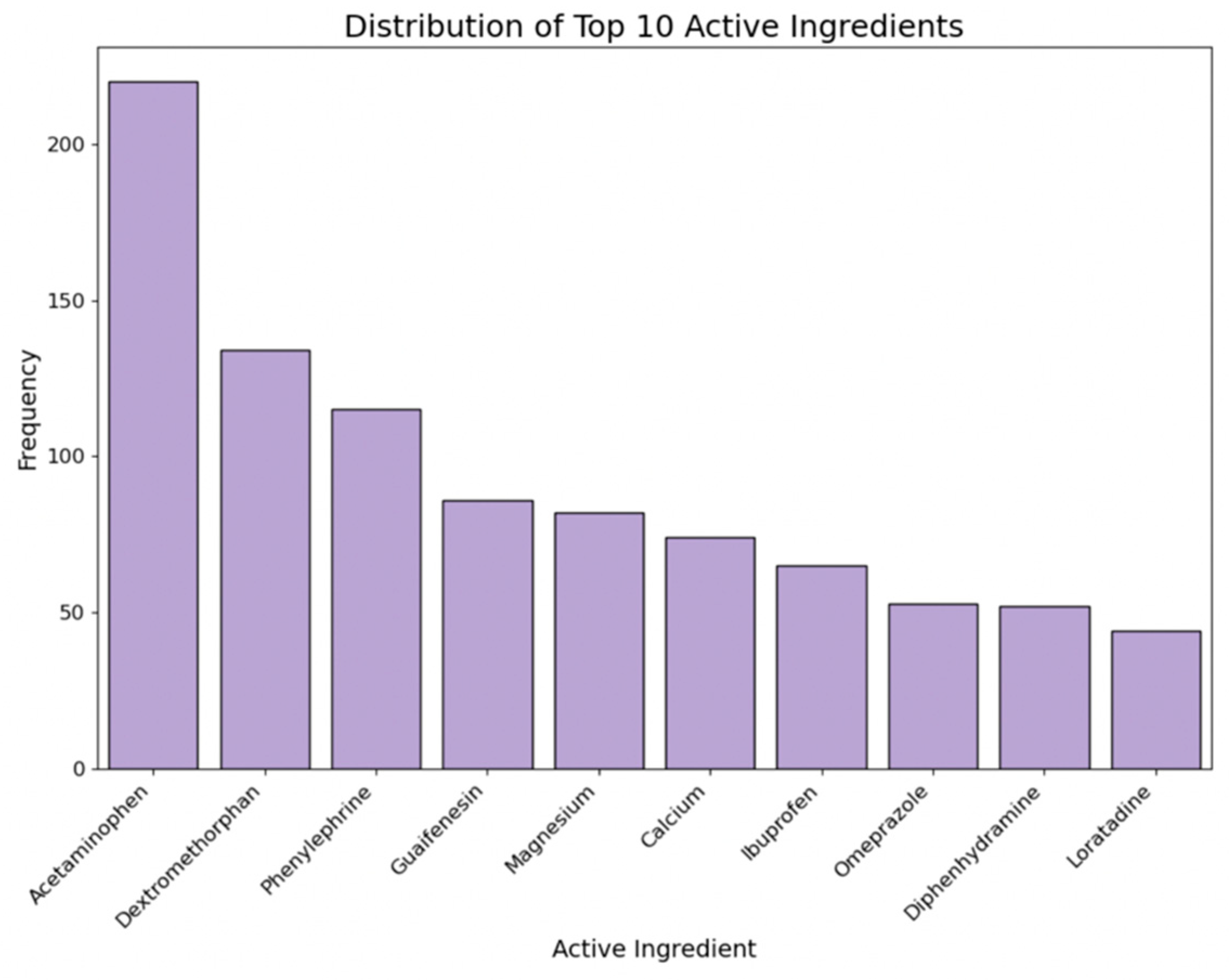
2.3.5. Active Ingredients
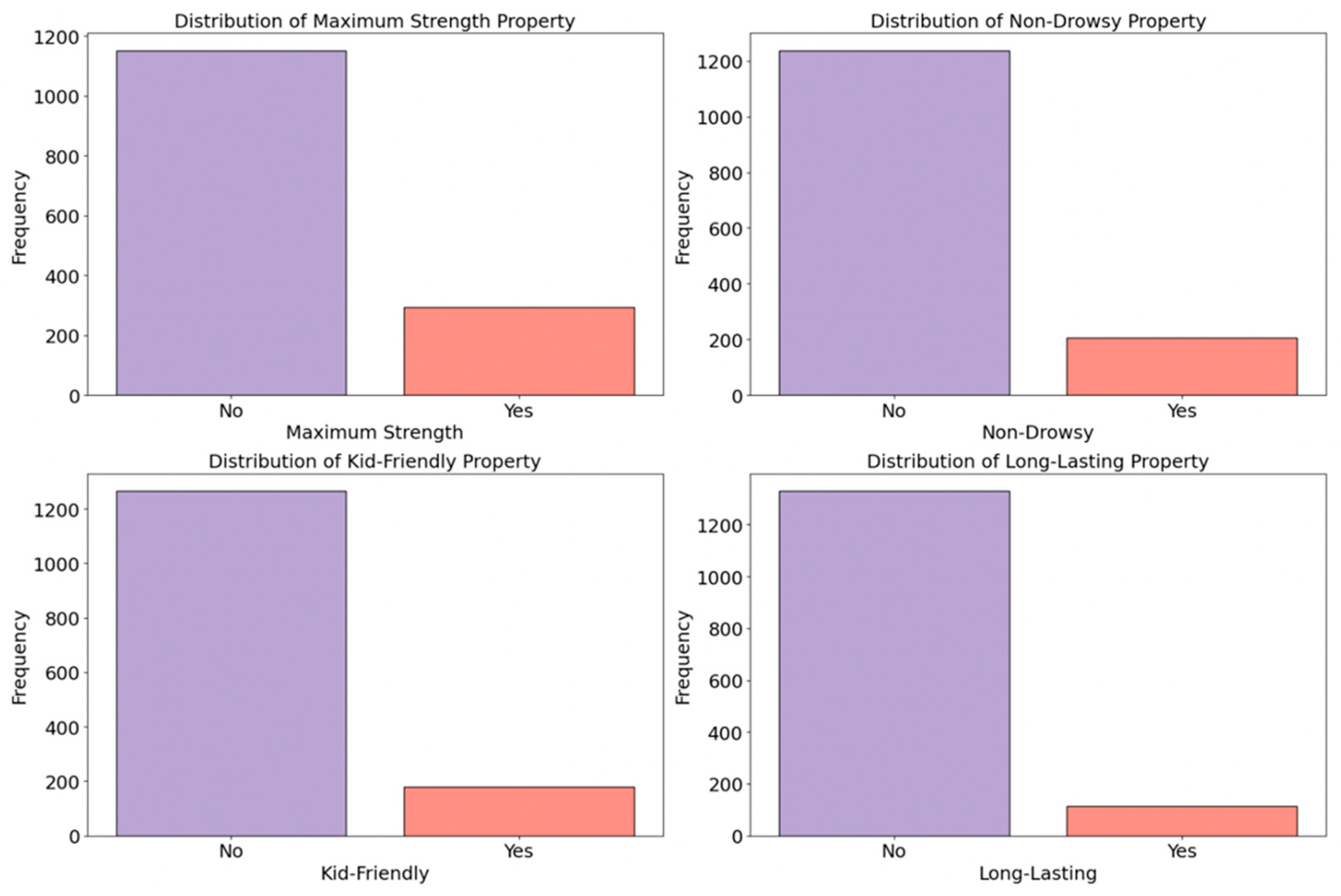
2.3.6. Special Effects
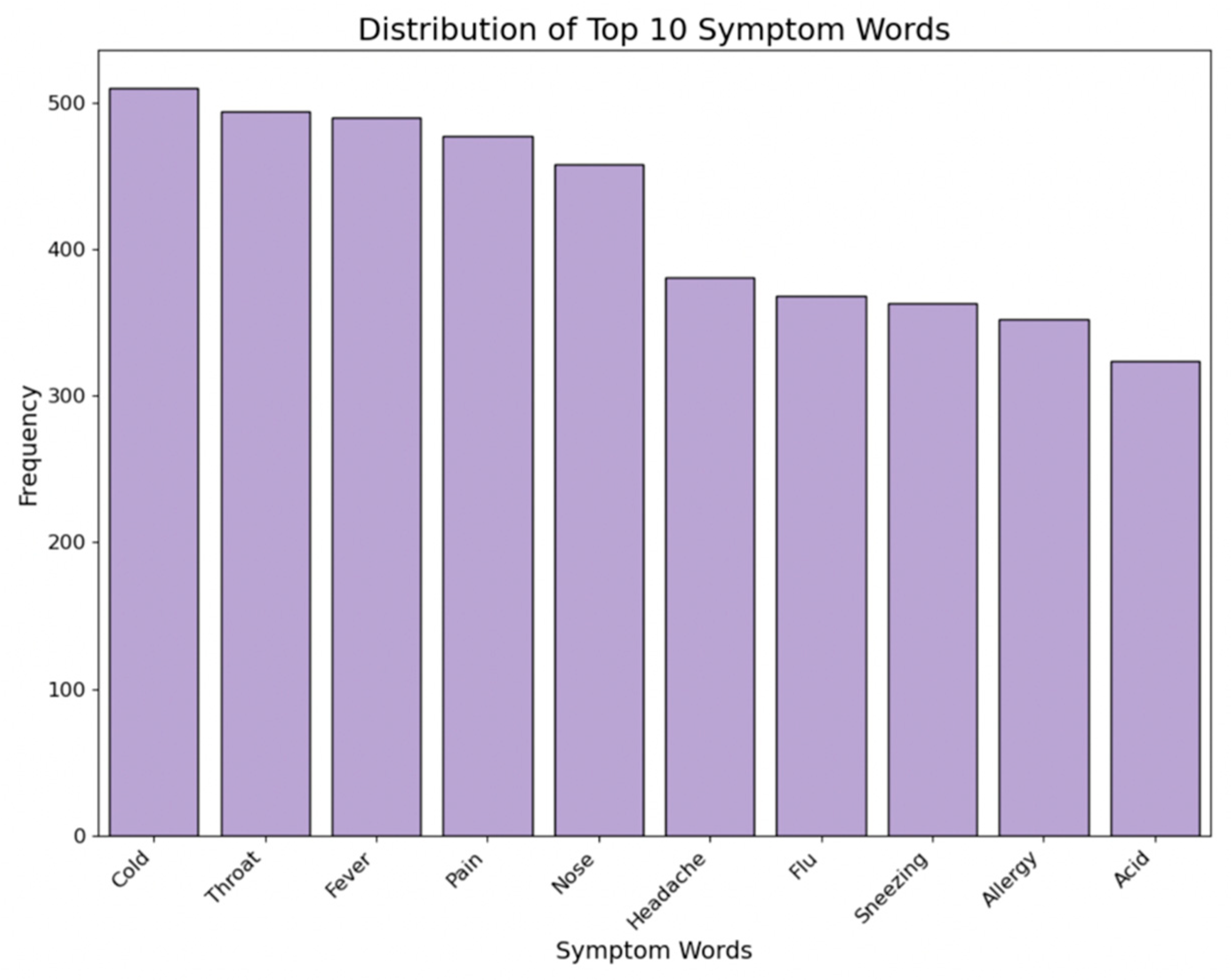
2.3.7. Symptom Treats
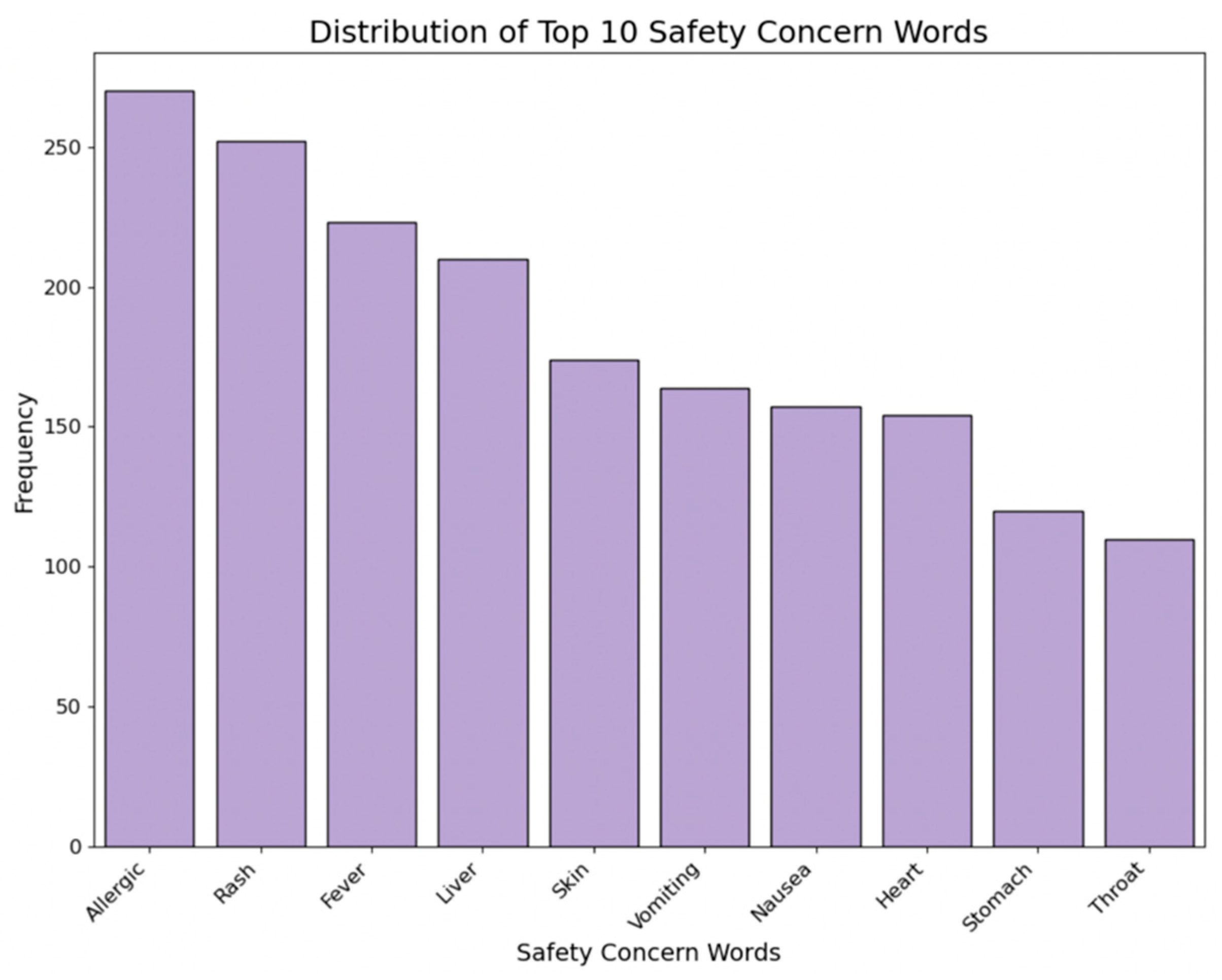
2.3.8. Safety Warnings
2.4. Machine Learning Modeling and Impact Assessment of Key Factors
2.4.1. Machine Learning Models for Predicting CER and Performance Metrics
2.4.2. SHAP Values and Logistic Regression Coefficients for Identifying Factor Impact
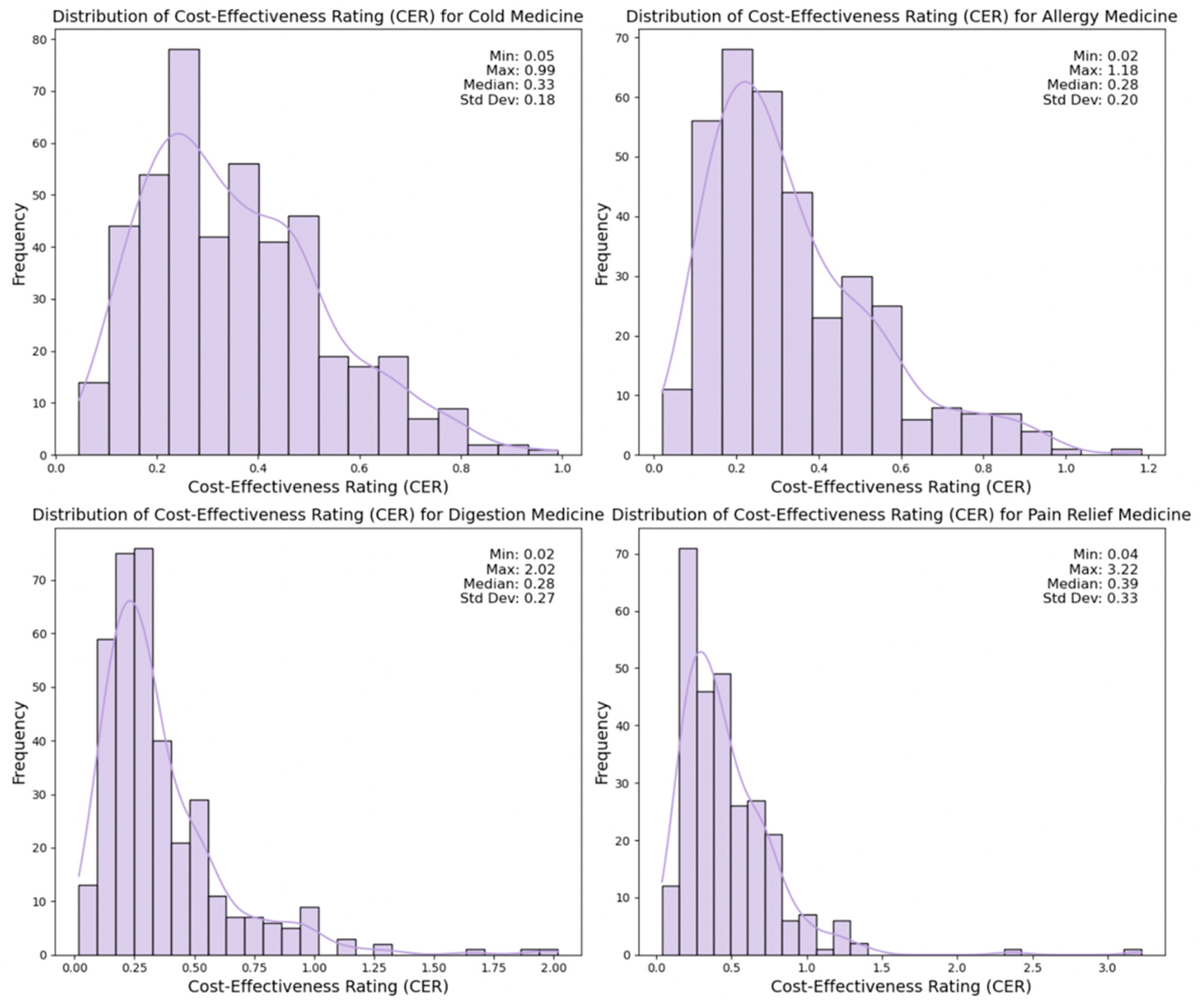
3. Results
3.1. Machine Learning Classifiers for CER Across Medicine Types
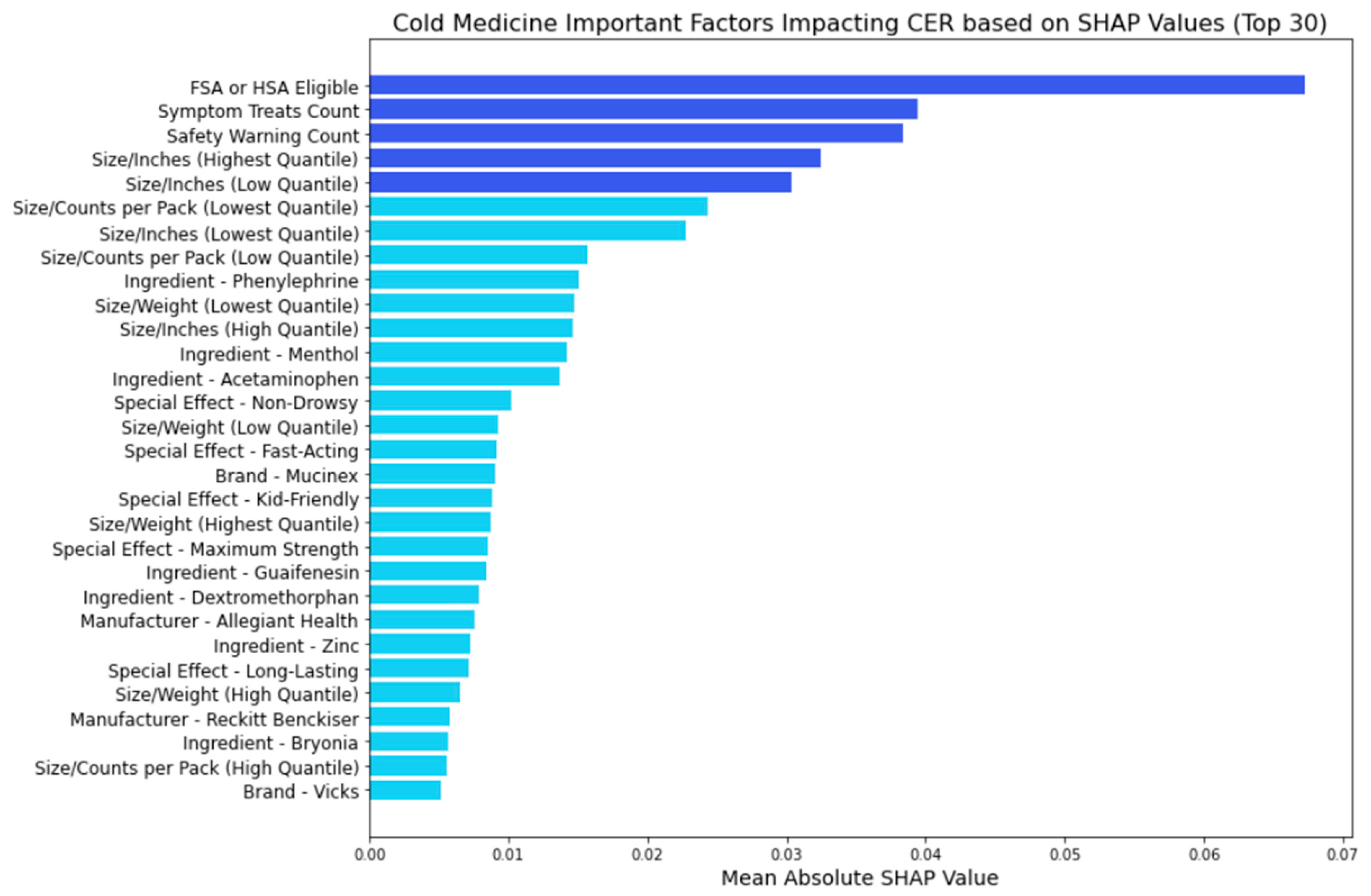
3.2. Key Feature Categories Influencing CER across Medicine Types
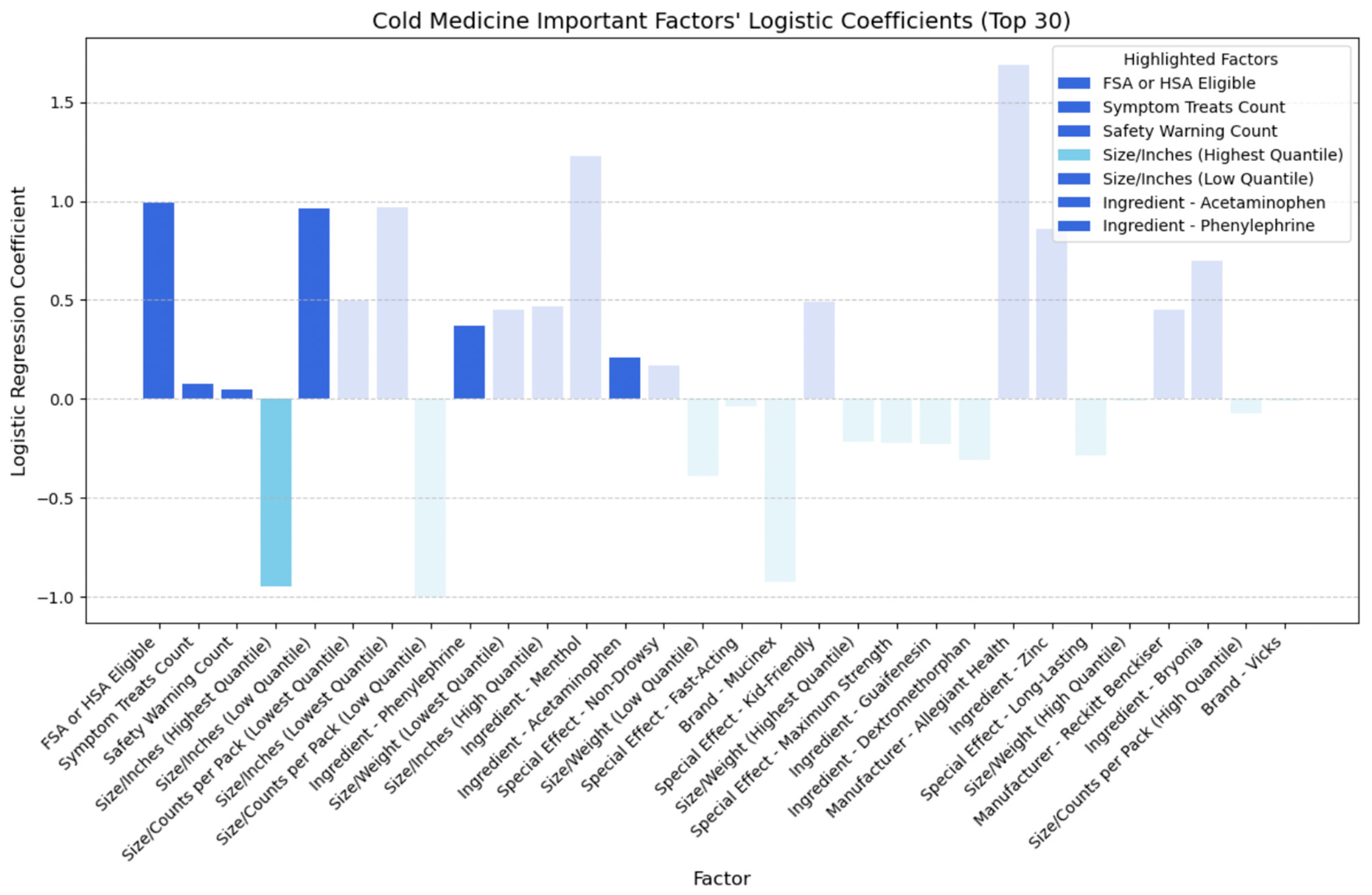
- FSA or HSA eligibility: This signifies the potential for consumers to utilize pre-tax funds for medication purchases, which is more cost-effective.
- Symptom treats: The number of symptoms treated emerges as a significant contributor to the CER, underscoring the importance of efficacy considerations.
- Safety warnings: Safety warnings also significantly contribute to cost-effectiveness ratings, emphasizing the importance of safety considerations.
- Size metrics: Both lower and higher quantiles of inches play a significant role in influencing the CER, suggesting that the physical dimensions of the medication packaging impact its cost-effectiveness.
- Size metrics: Smaller packaging or lighter weight contribute to cost-effectiveness.
- Manufacturer influence: Specific manufacturers like Johnson & Johnson, Bayer, Sanofi, Major, and Perrigo exert notable influence, indicating that brand reputation and trustworthiness may affect consumer ratings when adjusting the cost
- Special effects: Attributes like being kid-friendly influence the CER, enhance safety perceptions, and influence actual cost-effectiveness.
- Symptom treats: Like cold medicine, the medication’s ability to address a broader range of symptoms impacts the CER.
- FSA or HSA eligibility: Similar to cold medicine, this suggests the potential for pre-tax fund utilization to be more cost-effective.
- Size metrics: Similar to allergy medicine, smaller-sized packaging or lighter weight mainly affects actual cost-effectiveness.
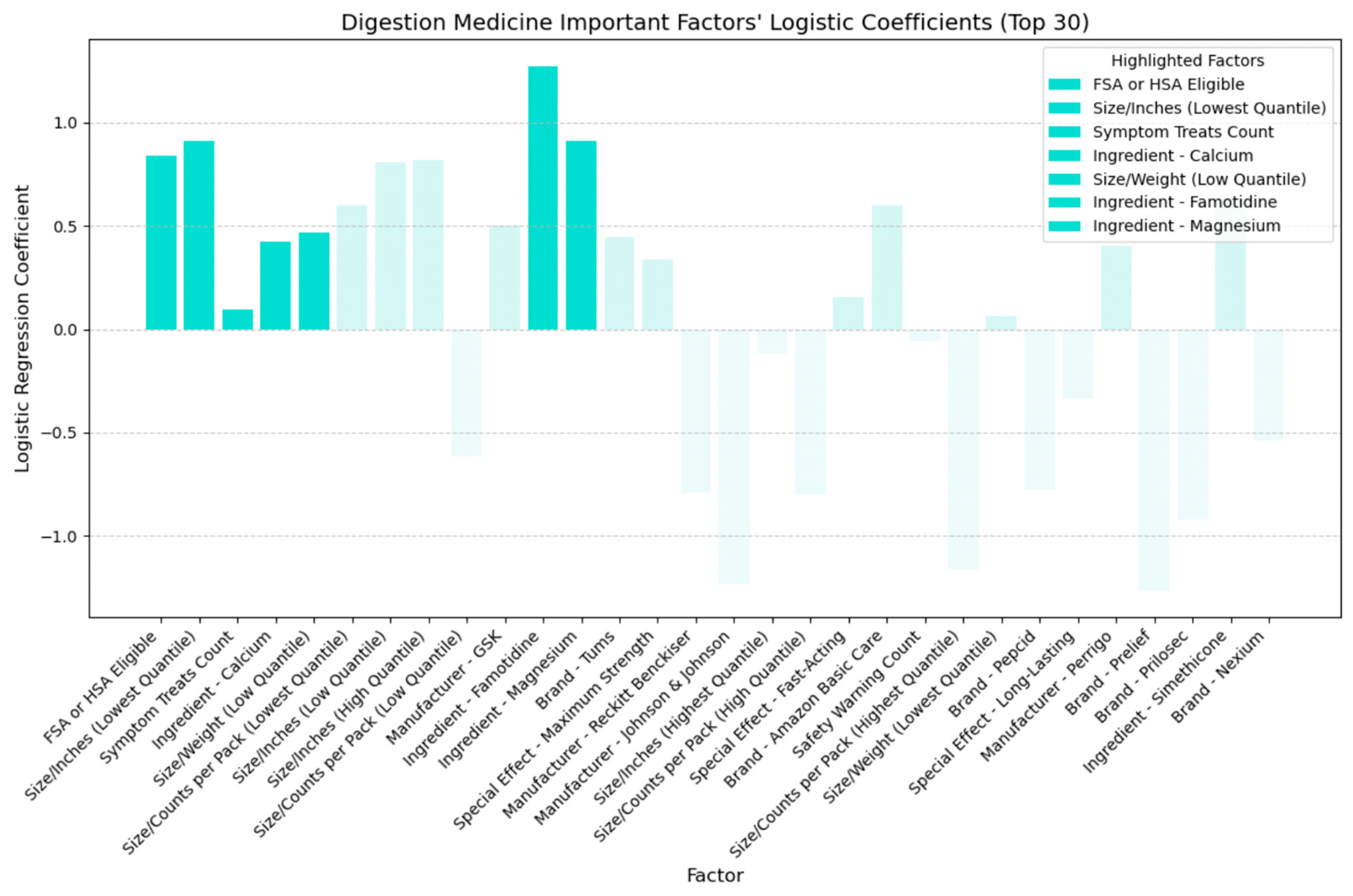
- Symptom treats: Like cold and allergy medicine, the medication’s effectiveness in treating various symptoms influences cost-adjusted ratings.
- Active ingredients: Specific ingredients like calcium, famotidine, and magnesium influence perceived cost-effectiveness.
- Size metrics: Both lower and higher quantiles of inches impact packaging dimensions.
- FSA or HSA eligibility: Signifying potential pre-tax fund usage to be more cost-effective.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statista. OTC Pharmaceuticals—United States. Available online: https://www.statista.com/outlook/hmo/otc-pharmaceuticals/united-states (accessed on 22 June 2024).
- Karimi, S.; Papamichail, K.N.; Holland, C.P. The effect of prior knowledge and decision-making style on the online purchase decision-making process: A typology of consumer shopping behaviour. Decis. Support Syst. 2015, 77, 137–147. [Google Scholar] [CrossRef]
- Mason, A.; Narcum, J.; Mason, K. Changes in consumer decision-making resulting from the COVID-19 pandemic. J. Cust. Behav. 2020, 19, 299–321. [Google Scholar] [CrossRef]
- Alipour, J.; Sharifian, R.; Haghighi, J.D.; Hashemzehi, M.; Karimi, A. Patients’ perceptions, experiences, and satisfaction with e-prescribing system: A cross-sectional study. Int. J. Med. Inform. 2024, 181, 105282. [Google Scholar] [CrossRef] [PubMed]
- Temechewu, M.W.; Gebremedhin, M. Factors affecting consumers’ purchase decision of over-the-counter (OTC) medicines: Empirical evidences from community pharmacies in Ethiopia. J. Med. Physiol. Biophys. 2020, 65, 8–25. [Google Scholar]
- Jha, M.; Bhowmick, A.; Rajkumar, M. Impact of consumers attitudes, subjective norms, and perceived behavioural control on consumer purchase behaviour towards OTC allopathic medicine. Acad. Mark. Stud. J. 2023, 27, 1–13. [Google Scholar]
- Limbu, Y.B.; Huhmann, B.A. What influences consumers’ online medication purchase intentions and behavior? A scoping review. Front. Pharmacol. 2024, 15, 1356059. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.D.; Nguyen, T.D. The impact of security, individuality, reputation, and consumer attitudes on purchase intention of online shopping: The evidence in Vietnam. Cogent Psychol. 2022, 9, 2035530. [Google Scholar] [CrossRef]
- Cohen, A.; Vakharia, S.P.; Netherland, J.; Frederique, K. How the war on drugs impacts social determinants of health beyond the criminal legal system. Ann. Med. 2022, 54, 2024–2038. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.S.; Tan, M.L. Exploring OTC drug consumers’ perception towards online shopping and digital marketing through qualitative interviews: A sample from Malaysia. Int. J. Healthc. Manag. 2024, 17, 168–176. [Google Scholar] [CrossRef]
- López Vila, E.D.; Buts, C.; Jegers, M. A quantitative classification of OTC medicines regulations in 30 European countries: Dispensing restrictions, distribution, pharmacy ownership, and pricing systems. J. Pharm. Policy Pract. 2023, 16, 19. [Google Scholar] [CrossRef]
- Hanaysha, J.R. An examination of the factors affecting consumer’s purchase decision in the Malaysian retail market. PSU Res. Rev. 2018, 2, 7–23. [Google Scholar] [CrossRef]
- Bloor, S. Factors That Influence Consumer Purchasing Decisions. Available online: https://pimberly.com/blog/factors-influence-consumer-purchasing-decisions/#:~:text=Key%20Points%3A%20Consumer%20Purchasing%20Decisions,-Multiple%20factors%20influence&text=Common%20factors%20include%20personal%20preferences,the%20marketing%20campaigns%20retailers%20leverage (accessed on 22 June 2024).
- Blue Monarch Group. The Psychology of Pricing: Influencing Consumer Behavior through Price Perception. Available online: https://bluemonarchgroup.com/blog/the-psychology-of-pricing-influencing-consumer-behavior-through-price-perception/ (accessed on 22 June 2024).
- Gao, S.; Yang, Y.; Krogstie, J. The Adoption of Smartphones Among Older Adults in China. In Proceedings of the 16th IFIP WG 8.1 International Conference on Informatics and Semiotics in Organisations, Toulouse, France, 19–20 March 2015. [Google Scholar]
- Monroe, K.B.; Chapman, J.D. Framing effects on buyers’ subjective product evaluations. Adv. Consum. Res. 1987, 14, 193. [Google Scholar]
- Monroe, K.B. Price and customers’ perceptions of value. Adv. Bus. Mark. Purch. 2012, 19, 129–152. [Google Scholar] [CrossRef]
- Kumar, A.; Grisaffe, D.B. Effects of extrinsic attributes on perceived quality, customer value, and behavioral intentions in B2B settings: A comparison across goods and service industries. J. Bus. Bus. Mark. 2004, 11, 43–74. [Google Scholar] [CrossRef]
- Nelson, P. Information and consumer behavior. J. Polit. Econ. 1970, 78, 311–329. [Google Scholar] [CrossRef]
- Creyer, E.H.; Ross, W.T. Tradeoffs between price and quality: How a value index affects. J. Consum. Aff. 1997, 31, 280–302. [Google Scholar] [CrossRef]
- Zeithaml, V.A. Consumer perceptions of price, quality, and value: A means-end model and synthesis of evidence. J. Mark. 1988, 52, 2–22. [Google Scholar] [CrossRef]
- Yoon, S.; Oh, S.; Song, S.; Kim, K.K.; Kim, Y. Higher quality or lower price? How value-increasing promotions affect retailer reputation via perceived value. J. Bus. Res. 2014, 67, 2088–2096. [Google Scholar] [CrossRef]
- Wineinger, N.E.; Zhang, Y.; Topol, E.J. Trends in prices of popular brand-name prescription drugs in the United States. JAMA Netw. Open 2019, 2, e194791. [Google Scholar] [CrossRef]
- Myshko, D. IQVIA: Medicine Spending Expected to Grow 4% to 7%. Available online: https://www.managedhealthcareexecutive.com/view/iqvia-medicine-spending-expected-to-grow-4-to-7- (accessed on 22 June 2024).
- Shailaja, K.; Seetharamulu, B.; Jabbar, M.A. Machine Learning in Healthcare: A Review. In Proceedings of the 2018 Second International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 29–31 March 2018. [Google Scholar]
- Kumar, M.R.; Venkatesh, J.; Rahman, A.M.J.M.Z. Data mining and machine learning in retail business: Developing efficiencies for better customer retention. J. Ambient Intell. Humaniz. Comput. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Balaji, T.K.; Annavarapu, C.S.R.; Bablani, A. Machine learning algorithms for social media analysis: A survey. Comput. Sci. Rev. 2021, 40, 100395. [Google Scholar] [CrossRef]
- Long, B.; Lai, S.W.; Wu, J.; Bellur, S. Predicting phase 1 lymphoma clinical trial durations using machine learning: An in-depth analysis and broad application insights. Clin. Pract. 2024, 14, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Navada, A.; Ansari, A.N.; Patil, S.; Sonkamble, B.A. Overview of Use of Decision Tree Algorithms in Machine Learning. In Proceedings of the 2011 IEEE Control and System Graduate Research Colloquium, Shah Alam, Malaysia, 27–28 June 2011. [Google Scholar]
- Liu, Y.; Wang, Y.; Zhang, J. New Machine Learning Algorithm: Random Forest. In Proceedings of the Third International Conference, ICICA 2012, Chengde, China, 14–16 September 2012. [Google Scholar]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Sharma, A.; Paliwal, K.K. Linear discriminant analysis for the small sample size problem: An overview. Int. J. Mach. Learn. Cybern. 2015, 6, 443–454. [Google Scholar] [CrossRef]
- Kramer, O. K-nearest neighbors. In Dimensionality Reduction with Unsupervised Nearest Neighbors; Kramer, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 13–23. [Google Scholar]
- Orrù, P.F.; Zoccheddu, A.; Sassu, L.; Mattia, C.; Cozza, R.; Arena, S. Machine learning approach using MLP and SVM algorithms for the fault prediction of a centrifugal pump in the oil and gas industry. Sustainability 2020, 12, 4776. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Mitchell, M.N.; Chen, X. Visualizing main effects and interactions for binary logit models. Stata J. 2005, 5, 64–82. [Google Scholar] [CrossRef]
- Lee, K.S.; Kassab, Y.W.; Taha, N.A.; Zainal, Z.A. Factors impacting pharmaceutical prices and affordability: Narrative review. Pharmacy 2020, 9, 1. [Google Scholar] [CrossRef]
- Dong, J.; Rudin, C. Exploring the cloud of variable importance for the set of all good models. Nat. Mach. Intell. 2020, 2, 810–824. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Menard, S. Applied Logistic Regression Analysis; Sage: London, UK, 2002; Volume 106. [Google Scholar]
- Multum, C. Acetaminophen, Dextromethorphan, and Phenylephrine. Available online: https://www.drugs.com/mtm/acetaminophen-dextromethorphan-and-phenylephrine.html (accessed on 22 June 2024).
- Mayo Clinic. Cold Remedies: What Works, What Doesn’t, What Can’t Hurt. Available online: https://www.mayoclinic.org/diseases-conditions/common-cold/in-depth/cold-remedies/art-20046403 (accessed on 22 June 2024).
- Everyday Health. Mucinex Sinus-Max (Acetaminophen, Dextromethorphan, and Phenylephrine). Available online: https://www.everydayhealth.com/drugs/mucinex-sinus-max (accessed on 22 June 2024).
- Frost, J. Understanding Interaction Effects in Statistics. Available online: https://statisticsbyjim.com/regression/interaction-effects/ (accessed on 22 June 2024).
- Huang, S.C.; Lee, L. The 5S’s of Consumer Health: A Framework and Curation of JCR Articles on Health and Medical Decision-Making. J. Consum. Res. 2023, 49, 926–939. [Google Scholar] [CrossRef]








| Column | Value |
|---|---|
| Product Name | DayQuil and NyQuil Combo Pack, Cold & Flu Medicine, Powerful Multi-Symptom Daytime And Nighttime Relief For Headache, Fever, Sore Throat, Cough, 72 Count, 48 DayQuil, 24 NyQuil Liquicaps |
| Price | USD 22.99 |
| Rating | 4.80 |
| Number of Reviews | 7081 |
| % 5 Star Reviews | 86% |
| % 4 Star Reviews | 10% |
| % 3 Star Reviews | 3% |
| % 2 Star Reviews | 1% |
| % 1 Star Reviews | 1% |
| Size | 72 count (pack of 1) |
| Item Weight | 0.01 ounces |
| Item Dimension | 4.38 × 3 × 3.38 inches |
| Product Dimension | 4.38 × 3 × 3.38 inches; 0.01 ounces |
| FSA or HSA Eligible | Yes |
| Brand | Vicks |
| Manufacturer | Procter & Gamble—HABA Hub |
| Ingredients | DayQuil Cold & Flu Active Ingredients (In Each Liquicap): Acetaminophen 325 mg (Pain Reliever/Fever Reducer), Dextromethorphan HBr 10 mg (Cough Suppressant), Phenylephrine HCl 5 mg (Nasal Decongestant) Inactive Ingredients: FD&C Red No. 40, FD&C Yellow No. 6, Gelatin, … (See full list in original text) |
| Special Feature | Non-drowsy |
| Product Benefit | Cough, Cold & Flu Relief, Sore Throat, Fever, & Congestion Relief |
| Special Use | Cold, Cough, Sore Throat, Fever |
| About | About this item—FAST, POWERFUL MULTI-SYMPTOM RELIEF: Use non-drowsy DayQuil for daytime relief and at night try NyQuil for fast relief so you can rest EFFECTIVE COLD & FLU SYMPTOM RELIEF: DayQuil and NyQuil Cold & Flu medicine temporarily relieve common cold & flu symptoms FEEL BETTER FAST: Just one dose starts working fast… (See full description in original text) |
| Item Description | Knock your cold out with Vicks DayQuil and NyQuil SEVERE Cold & Flu Liquid medicine. Just one dose starts working fast to relieve 9 of your worst cold and flu symptoms, to help take you from 9 to none. From the world’s #1 selling OTC cough and cold brand, Vicks DayQuil and NyQuil SEVERE provide fast, powerful, maximum strength relief… (See full description in original text) |
| Safety Information | Safety Information DayQuil Cold & Flu: Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take: • More than 4 doses in 24 h, which is the maximum daily amount for this product • Other drugs containing acetaminophen • 3 or more alcoholic drinks every day while using this product. Sore throat warning: If sore throat is severe… (see full safety information in original text) |
| Directions | Take only as directed—see Overdose warning. Do not exceed 4 doses per 24 h. Adults and children 12 years and over: 2 LiquiCaps with water every 4 h… (See full directions in original text) |
| ASIN | B00796NI1Q |
| Link | https://www.amazon.com/Vicks-Medicine-Multi-Symptom-Nighttime-Liquicaps/dp/B00796NI1Q/ref=sr_1_22?c=ts&keywords=Cold+%26+Flu+Medicine&qid=1699298540&refinements=p_85%3A2470955011&refresh=1&rps=1&s=hpc&sr=1-22&ts_id=3761171 (accessed on 8 January 2024). |
| Feature Category | Feature | Explanation | Feature Type |
|---|---|---|---|
| FSA or HSA Eligible | FSA or HSA Eligible | Indicates if the medicine item is a Flexible Spending Account (FSA) or Health Savings Account (HSA) eligible item (yes/no) | Binary |
| Size | Counts per Pack | Indicates if the counts per pack belong to the lowest/low/high/highest quantile | Binary |
| Weight | Indicates if the weight of the item (in ounces) belongs to the lowest/low/high/highest quantile | Binary | |
| Inches | Indicates if the dimensions of the item (in inches) belong to the lowest/low/high/highest quantile | Binary | |
| Brand | Brand | Indicates the brand of the item (yes for corresponding one-hot-encoded brand column, no for others) | Binary |
| Manufacturer | Manufacturer | Indicates the manufacturer of the item (yes for corresponding one-hot-encoded manufacturer column, no for others) | Binary |
| Ingredients | Active Ingredients | Indicates the presence of active ingredients (yes for corresponding one-hot-encoded ingredient columns, no if an ingredient is absent) | Binary |
| Special Effect | Fast-Acting | Indicates if the item qualifies as a fast-acting property | Binary |
| Long-Lasting | Indicates if the item qualifies as a long-lasting property | Binary | |
| Maximum Strength | Indicates if the item has maximum strength property | Binary | |
| Non-Drowsy | Indicates if the item qualifies as non-drowsy property | Binary | |
| Kid-Friendly | Indicates if the item qualifies as a kid-friendly property | Binary | |
| Symptom Treats | Symptom Treats Count | Number of symptom words this medicine item treats | Numerical |
| Safety Warnings | Safety Warning Count | Number of safety concern words this medicine item has | Numerical |
| ROC-AUC | Accuracy | Precision | Recall | F1-Score | |
|---|---|---|---|---|---|
| Random Forest | 0.7428 ± 0.0863 | 0.6897 ± 0.0743 | 0.7076 ± 0.0914 | 0.6667 ± 0.1849 | 0.6703 ± 0.1142 |
| XGBoost | 0.7256 ± 0.0886 | 0.6853 ± 0.0723 | 0.7026 ± 0.0797 | 0.6533 ± 0.1798 | 0.6619 ± 0.1186 |
| Logistic Regression | 0.7064 ± 0.0867 | 0.6364 ± 0.0844 | 0.6386 ± 0.1037 | 0.6311 ± 0.2092 | 0.6188 ± 0.1320 |
| Linear Discriminant Analysis | 0.7030 ± 0.0831 | 0.6187 ± 0.0674 | 0.6151 ± 0.0613 | 0.6178 ± 0.1888 | 0.6046 ± 0.1108 |
| Multi-Layer Perceptron | 0.6843 ± 0.0650 | 0.6322 ± 0.0825 | 0.6304 ± 0.0790 | 0.7200 ± 0.1719 | 0.6560 ± 0.0791 |
| Gaussian Naïve Bayes | 0.6473 ± 0.0483 | 0.5675 ± 0.0568 | 0.6456 ± 0.1173 | 0.2844 ± 0.1074 | 0.3880 ± 0.1127 |
| K-Nearest Neighbors | 0.6351 ± 0.0612 | 0.5944 ± 0.0702 | 0.6276 ± 0.1004 | 0.5422 ± 0.2064 | 0.5541 ± 0.1196 |
| Decision Tree | 0.6252 ± 0.0527 | 0.6322 ± 0.0557 | 0.6386 ± 0.1037 | 0.6311 ± 0.2092 | 0.6188 ± 0.1320 |
| ROC-AUC | Accuracy | Precision | Recall | F1-Score | |
|---|---|---|---|---|---|
| Logistic Regression | 0.7548 ± 0.045 | 0.6793 ± 0.054 | 0.6859 ± 0.082 | 0.6997 ± 0.099 | 0.6849 ± 0.049 |
| Linear Discriminant Analysis | 0.7449 ± 0.044 | 0.6480 ± 0.050 | 0.6630 ± 0.070 | 0.6373 ± 0.131 | 0.6394 ± 0.065 |
| Multi-Layer Perceptron | 0.7269 ± 0.023 | 0.6734 ± 0.038 | 0.6569 ± 0.030 | 0.7278 ± 0.086 | 0.6884 ± 0.045 |
| Random Forest | 0.7223 ± 0.037 | 0.6736 ± 0.053 | 0.6730 ± 0.068 | 0.6994 ± 0.064 | 0.6823 ± 0.043 |
| XGBoost | 0.7160 ± 0.054 | 0.6679 ± 0.051 | 0.6780 ± 0.068 | 0.6598 ± 0.084 | 0.6641 ± 0.053 |
| Gaussian Naïve Bayes | 0.7158 ± 0.013 | 0.5738 ± 0.044 | 0.7340 ± 0.179 | 0.2503 ± 0.103 | 0.3596 ± 0.109 |
| Decision Tree | 0.6131 ± 0.058 | 0.6137 ± 0.053 | 0.6219 ± 0.056 | 0.5798 ± 0.077 | 0.5988 ± 0.061 |
| K-Nearest Neighbors | 0.6044 ± 0.069 | 0.5828 ± 0.066 | 0.6153 ± 0.105 | 0.5002 ± 0.068 | 0.5454 ± 0.056 |
| ROC-AUC | Accuracy | Precision | Recall | F1-Score | |
|---|---|---|---|---|---|
| Random Forest | 0.7081 ± 0.071 | 0.6641 ± 0.035 | 0.7008 ± 0.075 | 0.6323 ± 0.155 | 0.6455 ± 0.058 |
| XGBoost | 0.7023 ± 0.046 | 0.6587 ± 0.045 | 0.6848 ± 0.082 | 0.6547 ± 0.125 | 0.6535 ± 0.044 |
| Logistic Regression | 0.7004 ± 0.062 | 0.6150 ± 0.059 | 0.6254 ± 0.069 | 0.6335 ± 0.063 | 0.6233 ± 0.022 |
| Linear Discriminant Analysis | 0.6777 ± 0.070 | 0.6178 ± 0.076 | 0.6220 ± 0.076 | 0.6505 ± 0.034 | 0.6328 ± 0.044 |
| Gaussian Naïve Bayes | 0.6410 ± 0.027 | 0.5494 ± 0.051 | 0.5410 ± 0.048 | 0.8243 ± 0.109 | 0.6455 ± 0.020 |
| K-Nearest Neighbors | 0.6351 ± 0.088 | 0.5604 ± 0.074 | 0.6031 ± 0.105 | 0.3974 ± 0.115 | 0.4680 ± 0.102 |
| Multi-Layer Perceptron | 0.6351 ± 0.088 | 0.6148 ± 0.054 | 0.5773 ± 0.036 | 0.8743 ± 0.051 | 0.6947 ± 0.036 |
| Decision Tree | 0.6018 ± 0.059 | 0.5986 ± 0.052 | 0.6030 ± 0.060 | 0.6114 ± 0.049 | 0.6043 ± 0.037 |
| ROC-AUC | Accuracy | Precision | Recall | F1-Score | |
|---|---|---|---|---|---|
| Random Forest | 0.8022 ± 0.050 | 0.7576 ± 0.055 | 0.7748 ± 0.072 | 0.7185 ± 0.069 | 0.7433 ± 0.056 |
| Linear Discriminant Analysis | 0.7884 ± 0.063 | 0.7432 ± 0.066 | 0.7543 ± 0.093 | 0.7259 ± 0.050 | 0.7363 ± 0.055 |
| Logistic Regression | 0.7874 ± 0.064 | 0.7179 ± 0.076 | 0.7326 ± 0.098 | 0.6889 ± 0.055 | 0.7070 ± 0.065 |
| Gaussian Naïve Bayes | 0.7867 ± 0.061 | 0.6594 ± 0.082 | 0.8042 ± 0.148 | 0.3852 ± 0.127 | 0.5168 ± 0.145 |
| XGBoost | 0.7577 ± 0.055 | 0.7286 ± 0.058 | 0.7240 ± 0.066 | 0.7259 ± 0.065 | 0.7235 ± 0.057 |
| Multi-Layer Perceptron | 0.7139 ± 0.091 | 0.6598 ± 0.084 | 0.6337 ± 0.072 | 0.7407 ± 0.105 | 0.6798 ± 0.075 |
| K-Nearest Neighbors | 0.6542 ± 0.030 | 0.5869 ± 0.024 | 0.5817 ± 0.027 | 0.5556 ± 0.081 | 0.5652 ± 0.049 |
| Decision Tree | 0.6373 ± 0.039 | 0.6378 ± 0.040 | 0.6450 ± 0.065 | 0.6000 ± 0.049 | 0.6186 ± 0.034 |
| Active Ingredient | Chi-Square Statistic | p-Value | Item Count |
|---|---|---|---|
| Dextromethorphan | 41.3911 | 1.25 × 10−10 | 131 |
| Acetaminophen | 40.7375 | 1.74 × 10−10 | 112 |
| Phenylephrine | 35.3099 | 2.81 × 10−9 | 106 |
| Guaifenesin | 5.9919 | 1.44 × 10−2 | 85 |
| Doxylamine | 39.091 | 4.05 × 10−10 | 40 |
| Hydrobromide | 17.634 | 2.68 × 10−5 | 32 |
| Bryonia | 5.4334 | 1.98 × 10−2 | 23 |
| Phosphorus | 3.9605 | 4.66 × 10−2 | 17 |
| Gelsemium | 5.9838 | 1.44 × 10−2 | 15 |
| Ipecacuanha | 4.4107 | 3.57 × 10−2 | 14 |
| Eupatorium | 8.8677 | 2.90 × 10−3 | 13 |
| Perfoliatum | 6.9362 | 8.45 × 10−3 | 12 |
| Manufacturer | Average Price | Average Rating | Average CER |
|---|---|---|---|
| Johnson & Johnson | USD 12.4 | 4.68 | 0.47 |
| Bayer | USD 19.68 | 4.57 | 0.35 |
| Sanofi | USD 11.74 | 4.71 | 0.51 |
| Major | USD 25.66 | 4.72 | 0.22 |
| Perrigo | USD 22.55 | 4.73 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, B.; Zhou, J.; Tan, F.; Bellur, S. Deciphering Factors Contributing to Cost-Effective Medicine Using Machine Learning. Bioengineering 2024, 11, 818. https://doi.org/10.3390/bioengineering11080818
Long B, Zhou J, Tan F, Bellur S. Deciphering Factors Contributing to Cost-Effective Medicine Using Machine Learning. Bioengineering. 2024; 11(8):818. https://doi.org/10.3390/bioengineering11080818
Chicago/Turabian StyleLong, Bowen, Jinfeng Zhou, Fangya Tan, and Srikar Bellur. 2024. "Deciphering Factors Contributing to Cost-Effective Medicine Using Machine Learning" Bioengineering 11, no. 8: 818. https://doi.org/10.3390/bioengineering11080818
APA StyleLong, B., Zhou, J., Tan, F., & Bellur, S. (2024). Deciphering Factors Contributing to Cost-Effective Medicine Using Machine Learning. Bioengineering, 11(8), 818. https://doi.org/10.3390/bioengineering11080818







