Abstract
Poly(lactic acid) (PLA) is widely used in the field of medicine due to its biocompatibility, versatility, and cost-effectiveness. Three-dimensional (3D) printing or the systematic deposition of PLA in layers has enabled the fabrication of customized scaffolds for various biomedical and clinical applications. In tissue engineering and regenerative medicine, 3D-printed PLA has been mostly used to generate bone tissue scaffolds, typically in combination with different polymers and ceramics. PLA’s versatility has also allowed the development of drug-eluting constructs for the controlled release of various agents, such as antibiotics, antivirals, anti-hypertensives, chemotherapeutics, hormones, and vitamins. Additionally, 3D-printed PLA has recently been used to develop diagnostic electrodes, prostheses, orthoses, surgical instruments, and radiotherapy devices. PLA has provided a cost-effective, accessible, and safer means of improving patient care through surgical and dosimetry guides, as well as enhancing medical education through training models and simulators. Overall, the widespread use of 3D-printed PLA in biomedical and clinical settings is expected to persistently stimulate biomedical innovation and revolutionize patient care and healthcare delivery.
1. Introduction
Poly(lactic acid) (PLA) is a versatile aliphatic polyester that can be synthesized from lactic acid monomers via direct polycondensation or ring-opening polymerization using a suitable catalyst []. Lactic acid, also known as 2-hydroxypropanoic acid, is one of the simplest hydroxy acids, and it contains one asymmetric carbon atom with two optically active configurations (L- and D-isomers). Hence, different stereochemical forms of PLA may be generated, which include poly(L-lactide), poly(D-lactide), and poly(DL-lactide). PLA was first synthesized in the 1930s by Wallace Carothers, a renowned American scientist credited with the development of neoprene and nylon at Dupont []. Since then, PLA has gained tremendous popularity in various fields due to its cost-effectiveness, versatility, and biocompatibility. It can be produced from starchy renewable sources including corn, wheat, and rice [], making it more sustainable and less reliant on fossil fuels. Compared to petroleum-based polymer alternatives like acrylonitrile butadiene styrene (ABS) and nylon, PLA is also cheaper and simpler to produce []. Moreover, its thermoplastic nature allows for easy processing using various techniques, including three-dimensional (3D) printing. Indeed, the tunable nature of PLA’s physicochemical properties has been harnessed for the 3D printing of materials for various industrial applications, such as food packaging, textiles, automotive, and electronics [,]. In particular, its biocompatibility and sterilizability have made it an attractive material for biomedical and clinical applications [,,]. PLA naturally undergoes degradation in situ via the process of hydrolysis, and its byproducts (i.e., lactic acid, water, and carbon dioxide) are easily metabolized by the body []. Hence, it can be used to fabricate medical devices that can safely interface with human tissues and organs. Overall, PLA’s affordability, combined with its versatility and biocompatibility, make it an ideal candidate for the fabrication of novel medical devices and delivery systems through 3D printing.
This review focuses on the wide range of the biomedical and clinical applications of 3D-printed PLA. While previous reviews have explored the general applications of 3D-printed PLA-based materials, this report consolidates the latest advancements in the 3D-printed PLA technology within the context of its transformative potential in patient care and healthcare delivery. This review aims to offer a comprehensive overview of the rapidly evolving field of 3D-printed PLA and its implications for the future of medicine by analyzing the diverse applications of this material in tissue engineering and regenerative medicine, drug delivery systems, medical devices, surgical instruments and guides, radiotherapy devices and phantoms, and training models.
2. PLA as a Versatile Material for 3D Printing
Three-dimensional printing, also referred to as additive manufacturing, is a revolutionary technology that enables the production of 3D objects from digital models [,]. This is accomplished by depositing successive layers of material until the entire object is formed, allowing the fabrication of more complex structures that would be difficult to produce using conventional manufacturing methods. Among the different materials used in 3D printing, thermoplastics, which become moldable above a specific temperature and return to a solid state upon cooling, have been widely used due to their ease of use and versatility. Specifically, thermoplastic PLA has become one of the most popular 3D printing feedstock materials because of its numerous advantageous qualities. As previously mentioned, PLA is biocompatible and is cheaper to produce than alternative petroleum-based polymers used in 3D printing. It has a glass transition temperature of around 55–60 °C, and it has a melting temperature of around 160–180 °C. In its capacity as a thermoplastic, it is capable of undergoing heating to its melting point, subsequent cooling, and re-heating without significant degradation []. It has good mechanical strength and may be combined with other substances to produce composites with novel characteristics. PLA filaments are also commercially available in a diverse array of colors and properties, providing users with a wide range of options and flexibility. This makes PLA a highly suitable material for use in a wide variety of 3D printing techniques. Several 3D printing methods, including binder jetting (BJT), material extrusion (MEX), material jetting (MJT), powder bed fusion (PBF), and vat photopolymerization (VP), have utilized PLA to produce biomedical and clinical constructs [,]. The standard terminologies and distinctions between these methodologies have been discussed in previous reviews [,].
Ultimately, the 3D printing of PLA has enabled the fabrication of intricate scaffold geometries that can be customized for various biomedical and clinical purposes. The use of PLA-based materials in biomedical and clinical applications can be broadly classified into tissue engineering and regenerative medicine, drug delivery systems, medical devices, surgical instruments and guides, radiotherapy devices and phantoms, and training models (Figure 1).
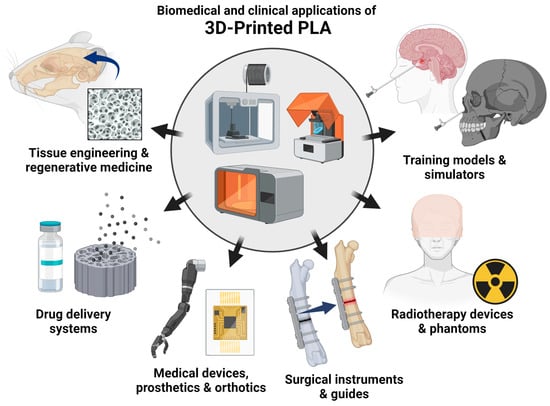
Figure 1.
Emerging biomedical and clinical applications of 3D-printed PLA. The biomedical and clinical uses of PLA-based materials are discussed in the subsequent chapters: tissue engineering and regenerative medicine, drug delivery systems, medical devices, surgical instruments and guides, radiotherapy devices and phantoms, and training models (created using BioRender.com).
3. Tissue Engineering and Regenerative Medicine
Three-dimensional-printed PLA has gained significant attention in the domains of tissue engineering and regenerative medicine, specifically in the realm of bone tissue engineering, owing to its durability and adaptability. Nonetheless, PLA alone may not fully mimic the complex architecture and composition of natural bone, which is a dynamic structure composed of vascular canals, mineralized and unmineralized connective tissue matrix, and specialized cells. Hence, recent studies have blended PLA with different materials, such as polymers, ceramics, stem cells, and growth factors to enhance its physicochemical properties and functionality. The efficacy of 3D-printed PLA-based composite materials in bone tissue engineering has been demonstrated in several preclinical studies (Table 1, Figure 2). Both natural and synthetic materials have been combined with PLA to match the native bone’s biomechanical properties [,,,,,,,]. Bioactive ceramics have been added to PLA to enhance not only its mechanical properties but also its osteoconductivity or ability to promote bone growth []. Examples of ceramic materials that have been blended with PLA in the context of bone tissue engineering include apatite-wollastonite [], beta-tricalcium phosphate [], bioactive glass [], and hydroxyapatite []. In addition, the incorporation of osteogenic cells and growth factors into the scaffolds has been shown to improve the regeneration of functional bone tissue. Several studies have shown that the addition of mesenchymal stem/stromal cells (MSCs) and extracellular vesicles (EVs), which have broad regenerative properties [,], can enhance the bone formation in rat calvarial defects [,,,]. Despite the recent numerous advances, several challenges need to be addressed to fully realize the potential of 3D-printed PLA-based materials in bone tissue engineering. These include replicating the intricate architecture of bone tissues, creating hierarchy and heterogeneity, and enhancing the vascularization. As previously mentioned, natural bone has a complex hierarchical structure that is difficult to reproduce using conventional techniques. Moreover, bone displays heterogeneity in structure and function across different locations. The development of high-resolution printing methods as well as strategies to improve oxygenation and nutrient transport, such as prevascularization and the incorporation of pro-angiogenic agents, can help overcome these challenges [,].
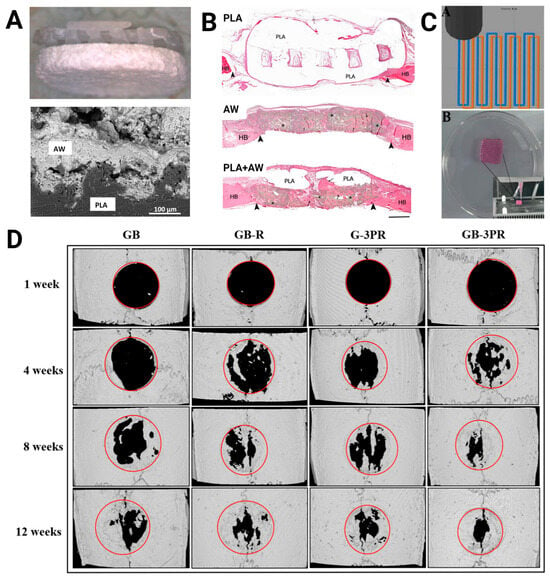
Figure 2.
Three-dimensional-printed PLA in bone tissue engineering. (A) Composite disks composed of PLA and the bioactive ceramic apatite-wollastonite (AW) (top: photograph, bottom: scanning electron microscopy image of interface) showed (B) higher bone formation than either PLA or AW alone in rat calvarial defects after 12 weeks as seen via hematoxylin and eosin staining (bar = 1 mm; HB, host bone; arrows, new bone; arrowheads, defect margins; *, residual AW). Images were adapted with permission from Tcacencu et al. []. (C) Dual extrusion 3D-printed in situ vascularized scaffolds containing bone marrow-derived mesenchymal stem cells (MSCs) and rat aortic endothelial cells (RAOECs) or GB-3PR (top: path planning; bottom: printed scaffold) (D) exhibited higher bone formation than the other groups (i.e., single-nozzle 3D-printed gel with BMSCs [GB], single-nozzle 3D-printed gel with BMSCs and RAOECs [GB-R], and dual-nozzle 3D-printed gel with RAOECs [G-3PR]) in rat calvarial defects after 12 weeks as seen via computed tomography. Images were adapted with permission from Shen et al. [].
In addition to its use in bone tissue engineering, 3D-printed PLA has also been recently studied for nerve, skin, and vascular tissue regeneration [,,,,]. In contrast to what is typically performed with bone scaffolds, PLA is combined with more pliable materials to mimic softer tissues. For example, to replicate the skin’s native architecture, PLA has been blended with softer natural polymers, such as hyaluronic acid and chitosan hydrogels [,]. Although the fabrication of soft tissues using PLA-based materials is still in its early stages, the functionality of these tissues is anticipated to be improved by future advancements in vascular tissue engineering and high-resolution printing. Overall, the aforementioned strategies highlight the adaptability of PLA in customizing scaffold characteristics to closely resemble the natural tissue environment and enhance the outcomes in tissue engineering and regenerative medicine.

Table 1.
Preclinical in vivo studies on 3D-printed PLA scaffolds for bone tissue engineering.
Table 1.
Preclinical in vivo studies on 3D-printed PLA scaffolds for bone tissue engineering.
| Scaffold Components | Printing Technique | Model | In Vivo Results | Reference |
|---|---|---|---|---|
| PLA, apatite-wollastonite | MEX, BJT | Rat | After 12 weeks, the scaffold with apatite-wollastonite showed a higher amount of newly formed bone in the calvarial defects than plain PLA (BA: ~20% vs. ~2%). | [] |
| PLA, HA, MSC | MEX | Rabbit | After 16 weeks, the scaffold loaded with cells demonstrated bone regeneration in the radial defects comparable to bone grafts (BV/TV: ~70%). | [] |
| PLA, MSCs | MEX | Rat | After 12 weeks, both the cell-free and cell-seeded scaffolds exhibited bone regeneration in the calvarial defects (BA: ~55% vs. ~60%). | [] |
| PLA, MSCs, EVs | MEX | Rat | After 6 weeks, the scaffold with MSCs and EVs showed the highest bone regeneration in the calvarial defects (BV/TV: 8.2%). | [] |
| PLA, MSCs, EVs | MEX | Rat | After 6 weeks, the scaffolds with MSCs and engineered EVs had higher bone formation in the calvarial defects compared to plain PLA (BA: 9.72% vs. 0%). | [] |
| PLA, nHA | MEX | Rabbit | After 12 weeks, the scaffold demonstrated new bone formation in the femoral defects (BV/TV: ~20%). | [] |
| PLA, nHA, gelatin, PRP | MEX | Rat | After 12 weeks, the scaffolds with nHA, gelatin, and PRP promoted better bone regeneration in the calvarial defects than plain PLA (BA: 83.68% vs. 71.08%). | [] |
| PLA, PDA | MEX | Rat | After 8 weeks, the etched scaffold with PDA promoted higher bone regeneration in the femoral defects compared to the untreated scaffold (BV/TV: ~30% vs. ~14%). | [] |
| PLA, PEG, gelMA, MSCs, ECs | MEX | Rat | After 12 weeks, the scaffolds with MSCs and ECs exhibited the highest bone formation in the calvarial defects (BV/TV: ~33%). | [] |
| PLA, PMMA, BG | MEX | Rabbit | After 4 weeks, the scaffolds with BG showed higher bone regeneration in the femoral defects compared to those without BG (BV/TV: 13–15% vs. 4–10%). | [] |
| PLA, PTMC, HA | MEX | Rat | After 8 weeks, the PLA/PTMC/HA scaffold demonstrated lower bone regeneration in the femoral defects than the PTMC/HA scaffolds (BV/TV: ~13% vs. ~16%). | [] |
| PLA, β-TCP | MEX | Rat | After 6 weeks, the scaffold with β-TCP showed significantly enhanced new bone formation in the femoral defects than plain PLA (BV/TV: ~38% vs. 25~). | [] |
Abbreviations: β-TCP, beta-tricalcium phosphate; BA, bone area; BG, bioactive glass; BJT, binder jetting; BV/TV, bone volume/total volume; EC, endothelial cell; EV, extracellular vesicles; gelMA, gelatin methacrylate; HA, hydroxyapatite; MEX, material extrusion; MSC, mesenchymal stem cell; nHA, nanohydroxyapatite; NP, nanoparticle; PDA, poly(dopamine); PLA, poly(lactic acid); PEG, poly(ethylene glycol); PMMA, poly(methyl methacrylate); PRP, platelet-rich plasma; PTMC, poly(trimethylene carbonate).
4. Drug Delivery Systems
PLA’s degradation kinetics can be controlled making it an excellent material for drug delivery. Three-dimensional-printed PLA has found use in drug-eluting bone scaffolds (Table 2), as well as medical devices and dosage forms (Table 3, Figure 3). By changing the composition of biodegradable scaffolds, the release rate of the encapsulated agents can be tailored to match the specific therapeutic needs, ensuring sustained and localized delivery with minimal toxicity. This capability is particularly valuable for chronic conditions or targeted therapies where precise dosage control is essential. In drug-eluting bone scaffolds, PLA matrices can be precisely engineered to deliver therapeutic agents such as growth factors, antibiotics, or anti-inflammatory drugs directly to the site of a bone defect, promoting tissue regeneration while preventing infection and inflammation. One of the most common agents loaded in bone scaffolds is bone morphogenetic protein 2 (BMP-2), an osteoinductive growth factor approved by the Food and Drug Administration (FDA) for human use []. Three-dimensional-printed PLA-based bone scaffolds loaded with BMP-2 have been shown to enhance the regeneration of bone in the calvarial defects in rodents [,,,], tibial defects in rabbits [], and mandibular defects in pigs []. Other agents that have been delivered through 3D-printed PLA-containing bone scaffolds include amoxicillin [], dexamethasone [], ketorolac [], and vancomycin [,].
Additionally, 3D-printed PLA-based medical devices, such as catheters [], dental retainers [], ocular inserts [], vaginal rings [], and vascular grafts [], have been tailored to release a wide variety of agents, such as antibiotics, antivirals, anti-hypertensives, chemotherapeutics, and hormones, which could offer enhanced therapeutic efficacy and patient compliance. PLA’s versatility has also allowed for the fabrication of various dosage forms for the delivery of 5-fluorouracil [], domperidone [], and riboflavin []. Overall, the efficacy of 3D-printed PLA in drug elution is attributed to its capacity to accurately regulate the kinetics of drug release and its compatibility with an extensive array of therapeutic substances. Nonetheless, further studies are needed to optimize the composition of scaffolds and devices for maximizing the stability, safety, and efficacy of the incorporated drugs.

Table 2.
Preclinical in vivo studies on 3D-printed drug-releasing PLA scaffolds for bone regeneration.
Table 2.
Preclinical in vivo studies on 3D-printed drug-releasing PLA scaffolds for bone regeneration.
| Indication | Scaffold Components | Printing Technique | Model | Drug Release | In Vivo Results | Reference |
|---|---|---|---|---|---|---|
| Alveolar defect | PLA, PLGA, ketorolac, amoxicillin | MEX | Rat | The scaffolds released levels of ketorolac and amoxicillin above the minimum effective concentration for 17 and 30 days, respectively. | After 4 weeks, the drug-loaded scaffolds revealed less inflammation compared to the plain scaffolds. | [] |
| Calvarial defect | PLA, alginate, collagen, BMP-2 | MEX | Mouse | The scaffold demonstrated an initial burst release phase for BMP-2 on the first day (670.61 ng) followed by a gradual release phase up to day 14 in vitro (cumulative release: 1132.30 ng). | After 4 weeks, the scaffold treated with 2 µg/mL of BMP-2 showed the highest bone regeneration in the calvarial defects. | [] |
| Calvarial defect | PLA, apatite, BMP-2 | MEX | Rat | None reported | After 6 months, the scaffold with BMP-2 showed higher bone regeneration in the calvarial defects than the non-drug-releasing counterpart (BA: 44.85% vs. 31.2%). | [] |
| Calvarial defect | PLA, Biogel, BMP-2 | MEX | Rat | The scaffold demonstrated an initial burst release phase for BMP-2 in the first 4 days (85%) followed by a gradual release phase up to day 15 in vitro. | After 8 weeks, the drug-releasing scaffold showed higher bone regeneration in the calvarial defects compared to the plain scaffold (BV/TV: 23.41% vs. 2.94%). | [] |
| Calvarial defect | PLA, PDA, alginate, heparin, BMP-2 | MEX | Rat | The microspheres demonstrated an initial burst phase followed by a plateau for 2 weeks and a more rapid release. The microspheres released 58.1–59.1% of the incorporated BMP-2 during the first month. | After 12 weeks, the scaffold with BMP-2-loaded microspheres showed higher bone regeneration in the calvarial defects compared to the plain scaffold (16.7% vs. 4.6%). | [] |
| Calvarial defect | PLA, PDA, PEI, pVEGF | MEX | Rat | The scaffold demonstrated a gradual release of pVEGF over 144 h (cumulative release: 15%). | After 4 weeks, the scaffold with pVEGF showed higher bone regeneration in the calvarial defects than the plain scaffold (~13% vs. ~8%). | [] |
| Calvarial defect | PLA, PEG, nHA, dexamethasone | MEX | Rat | The scaffold demonstrated a cumulative release of 8.33% of loaded dexamethasone over 2 weeks. | After 4 weeks, there were no differences in the bone regeneration in the calvarial defects among the treatment groups. | [] |
| Femoral defect | PLA, PLGA, nHA, vancomycin | MEX | Rabbit | The scaffold demonstrated an initial burst release phase for vancomycin on the first day (27%) followed by a gradual release phase up to day 24 in vitro (cumulative release: 62.3%). | After 4 weeks, there were no differences in the bone regeneration in the femoral defects between the drug-loaded and plain scaffolds. | [] |
| Mandibular defect | PLA, BMP-2 | MEX | Pig | None reported | After 3 months, the scaffold with 110 μg/cm3 BMP-2 demonstrated bone regeneration in the mandibular defects that are comparable to bone autografts (BA: ~20%). | [] |
| Tibial defect | PLA, Biogel, MSC, BMP-2 | MEX | Rabbit | None reported | After 4 weeks, the scaffold with BMP-2 and MSCs showed higher bone regeneration in the tibial defects than the plain scaffold (BV/TV: 7.9% vs. 4.31%). | [] |
Abbreviations: BA, bone area; BMP-2, bone morphogenetic protein 2; BV/TV, bone volume/total volume; HA, hydroxyapatite; MEX, material extrusion; MSC, mesenchymal stem cell; nHA, nanohydroxyapatite; NP, nanoparticle; PDA, polydopamine; PEI, polyethyleneimine; PLA, poly(lactic acid); PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol); pVEGF, vascular endothelial growth factor-encoding plasmid; SDF-1, stromal-derived factor 1.

Table 3.
Preclinical studies on 3D-printed drug-releasing PLA-containing medical devices and dosage forms.
Table 3.
Preclinical studies on 3D-printed drug-releasing PLA-containing medical devices and dosage forms.
| Device | Device Components | Printing Technique | Model | Drug Release | In Vivo Results | Reference |
|---|---|---|---|---|---|---|
| Catheter | PLA, gentamicin, methotrexate | MEX | In vitro | The catheter showed an initial burst release during the first few hours followed by a steady release for both gentamicin and methotrexate. The catheter also inhibited the growth of E. coli. | None reported | [] |
| Dental retainer | PLA, PCL, PEG, clonidine | MEX | In vitro | The washed retainer released 0.42 mg of clonidine after 24 h followed by a stable release at a rate of about 0.24 mg per day over 3 days. | None reported | [] |
| Implant | PLA, calcium carbonate, alginate, gemcitabine | MEX | Mouse | The implants demonstrated an initial burst release phase of gemcitabine for the first 10 h (24–29.3%) followed by a gradual release phase up to 3–7 days (cumulative release: 30–40%). | Over 4 weeks, the gemcitabine-loaded implants reduced the growth of subcutaneous MIA PaCa-2 tumors in mice. | [] |
| Implant | PLA, methotrexate | MEX | Mouse | The implants demonstrated an initial burst release phase of methotrexate for the first day (25%) followed by a gradual release phase over 30 days (cumulative release: ~80%). | Over 3 weeks, the methotrexate-loaded implants significantly reduced the growth of subcutaneous 4T1 tumors in mice. | [] |
| Implant | PLA, PCL, tetracycline | MEX | In vitro | The implants demonstrated a sustained release of tetracycline over 25 days (cumulative release: ~40–60%). The implants inhibited the growth of S. aureus and E. coli. | None reported | [] |
| Implant | PLA, β-TCP, titanium nitride, doxorubicin | MEX | Mouse | The implants demonstrated an initial burst release phase of doxorubicin for 24 h followed by a gradual release phase over 48 days (cumulative release: ~60%). | Over 18 days, the doxorubicin-loaded implants significantly reduced the growth of subcutaneous K7M2-WT tumors in mice. | [] |
| Ocular insert | PLA, glycosomes, ganciclovir | MEX | Rabbit | The glycerosomes demonstrated a sustained release of ganciclovir over 24 h in vitro. | There was a sustained release of ganciclovir over 5 days. The device did not cause gross or histological abnormalities. | [] |
| Tablet | PLA, alginate, 5-fluorouracil | MEX | In vitro | The tablets demonstrated an initial burst release phase of 5-fluorouracil for the first 2 h at pH 7.4 (~40%) followed by a gradual release phase up to 9 h (cumulative release: 80–100%). | None reported | [] |
| Tablet | PLA, PVA, domperidone | MEX | Rabbit | The tablet demonstrated an initial burst release phase of domperidone for the first 4 h (~65%) followed by a gradual release phase over 10 h (cumulative release: 98%). | The tablet floated in the rabbit’s stomach for at least 10 h. | [] |
| Tablet | PLA, riboflavin | MEX | Rabbit | The tablets demonstrated a cumulative release of riboflavin of 41–62% over 72 h. | The tablet floated in the rabbit’s stomach for over 72 h. | [] |
| Vaginal rings | PLA, PCL, PEG, progesterone | MEX | In vitro | The rings showed an initial burst release phase over 24 h followed by a gradual release phase over 7 days at a rate of 100–200 μg per day. | None reported | [] |
| Vascular graft | PLA, PCL, PEG, NO | MEX | Chick embryo | The graft demonstrated an initial burst release during the first day followed by a gradual release of NO in the physiological range over 14 days. | The NO-loaded graft significantly increased the number of vascular junctions in the chorioallantoic membrane of the chick embryo. | [] |
Abbreviations: β-TCP, beta-tricalcium phosphate; MEX, material extrusion; NO, nitric oxide; PCL, poly(caprolactone); PLA, poly(lactic acid); PEG, poly(ethylene glycol); PVA, poly(vinyl alcohol); VP, vat photopolymerization.
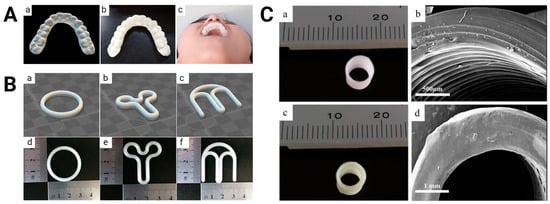
Figure 3.
Three-dimensional-printed PLA-based drug delivery systems. (A) Computer-aided design image (left), printed version (middle), and worn example (right) of clonidine-loaded orthodontic retainer. Images were adapted with permission from Jiang et al. []. (B) Computer-aided design images (top) and printed versions (bottom) of progesterone-loaded O- (left), Y- (middle), and M-shaped (right) vaginal rings. Images were adapted with permission from Fu et al. []. (C) Printed versions (left) and scanning electron microscopy images (right) of bare (top) and nitric oxide-loaded (bottom) vascular grafts. Images were adapted with permission from Kabirian et al. [].
5. Medical Devices, Prosthetics, and Orthotics
Three-dimensional-printed PLA has garnered considerable attention in the development of medical devices, prostheses, and orthoses due to its biocompatibility, adaptability, and affordability. The FDA defines a medical device as an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other related article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals [,]. PLA’s ability to be easily molded into complex shapes makes it ideal for fabricating different types of medical devices, such as detection electrodes, personal protective equipment, probes, stents, and swabs (Table 4). Three-dimensional-printed PLA is widely used in the medical device area for creating detection electrodes with a good limit of detection. In this application, PLA is typically mixed with conductive materials, such as carbon black, graphene, or graphite. PLA-based electrodes have been used to detect different substances, such as carbendazim [], creatinine [], dopamine [], hydroxychloroquine [], paraquat [], quetiapine [], and sulfanilamide []. Biological molecules, such as Hantavirus Araucaria nucleoprotein [], SARS-CoV-2 cDNA [], and SARS-CoV-2 S protein [], have also been detected using 3D-printed PLA electrodes. Drug-eluting medical devices have been discussed in the previous section, and the specific medical devices that have been developed for surgery and radiotherapy are discussed in the next sections.
Due to its strength and light weight, PLA is suitable for the fabrication of prosthetic and orthotic devices (Table 5, Figure 4), which are a subset of devices that enable people with physical impairments or functional limitations to live healthy, productive, independent, and dignified lives. Prosthetics is the science and art that focus on the development and application of prostheses, which are artificial replacements for absent or deficient bodily components []. Three-dimensional-printed PLA has been used to manufacture scleral [], dental [,], hand [,], and transtibial prostheses []. On the other hand, orthotics is the science and art that focus on the development and application of orthoses, which are externally applied devices used to modify the structural and functional characteristics of the neuromuscular and skeletal systems []. Similarly, 3D-printed PLA has been utilized to generate ankle–foot [], arm [], and hand orthoses []. Exoskeletons are a subset of orthotic devices that differ from conventional orthoses because they utilize an external power source to produce or supplement body movements through components that rely on controllers or sensors. Three-dimensional-printed PLA has been used to produce exoskeletons for the arms [] and hands [,].
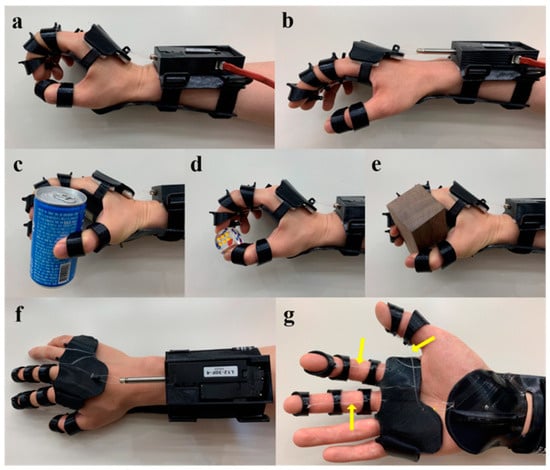
Figure 4.
Three-dimensional-printed PLA-based orthotic device. The electromyography-responsive hand orthosis has a linear motor that can be activated to assist in wrist extension and facilitate the tenodesis grasp (a). Once the motor goes back into place, the extended wrist relaxes back into a neutral position (b). The device has been used to pick up a can, dice, and a wooden block (c–e). Top view of the device (f). Bottom view of the device (g) showing the nylon threads (yellow arrow) connecting the splint and rings. Images were adapted with permission from Yoo et al. [].
One benefit of external devices, as opposed to implanted devices, is that they are easier to test in patients. Therefore, several prosthetic and orthotic devices have undergone testing in human subjects. For example, hand orthoses 3D-printed from PLA have been shown to produce patient satisfaction ratings comparable to those of commercially available hand splints []. In another study, a dynamic wrist–hand orthosis made from 3D-printed PLA was developed to assist a patient in performing active wrist extension against spastic flexor muscles, demonstrating the adaptability of this technology []. Hand exoskeletons made of 3D-printed PLA have also been tested in both healthy subjects and patients with spinal cord injury. A study on healthy subjects demonstrated that exoskeletons can grasp, hold, and release objects with good latency and high accuracy []. In another study, the incorporation of haptic stimulations into the exoskeleton enhanced the attention of participants during hand exercises, showcasing the potential for improving the therapeutic outcomes []. More importantly, it has been shown that an electromyography-responsive hand exoskeletons can significantly improve the hand function and eating independence in patients with spinal cord injury-induced weakness []. With the advancements in 3D printing technology, PLA will continue to play a crucial role in revolutionizing patient care and rehabilitation by offering personalized, high-quality, and affordable solutions in the field of medical devices, prosthetics, and orthotics. Nonetheless, future investigations should explore a wider range of orthotic and prosthetic devices for various indications and involve a larger and more diverse patient population to assess their long-term efficacy and impact on functional independence and the overall rehabilitation goals.

Table 4.
Recent studies on miscellaneous 3D-printed PLA-containing medical devices.
Table 4.
Recent studies on miscellaneous 3D-printed PLA-containing medical devices.
| Device | Device Components | Printing Technique | Results | Reference |
|---|---|---|---|---|
| Detection electrode | PLA, carbon black | MEX | The electrode showed a linear response for the detection of Hantavirus Araucaria nucleoprotein from 30 to 240 μg/mL in human serum (LOD: 22 μg/mL). | [] |
| Detection electrode | PLA, carbon black | MEX | The electrode showed a linear response for the detection of hydroxychloroquine from 0.4 to 7.5 μmol/L (LOD: 0.04 μmol/L). | [] |
| Detection electrode | PLA, carbon black | MEX | The electrode showed a linear response for the detection of quetiapine from 5 to 80 × 10−7 mol/L (LOD: 2 × 10−9 mol/L). | [] |
| Detection electrode | PLA, carbon black | MEX | The electrode showed a linear response for the detection of sulfanilamide from 1 to 39.2 μmol/L in breast milk, synthetic urine, and otologic solution samples (LOD: 12 nmol/L). | [] |
| Detection electrode | PLA, carbon black | MEX | The electrode showed a linear response for the detection of creatinine from 0.5 to 35 mmol/L (LOD: 37.3 μmol/L). | [] |
| Detection electrode | PLA, graphene | MEX | Using different electrochemical techniques, the LOD for dopamine was determined to be around 1.67–2.17 μmol/L. | [] |
| Detection electrode | PLA, graphene, gold particles | MEX | The LOD for creatinine and SARS-CoV-2 cDNA were 0.016 mmol/L and 0.3 µmol/L, respectively. | [] |
| Detection electrode | PLA, graphite | MEX | The electrode showed linear responses for the detection of paraquat (0.05–1 µmol/L and 1–50 µmol/L) and carbendazim (0.5–50 µmol/L). The LOD for paraquat and carbendazim were 0.01 and 0.03 µmol/L, respectively. | [] |
| Detection electrode | PLA, graphite | MEX | The electrode showed a linear response for the detection of SARS-CoV-2 S protein from 5 to 75 nmol/L (LOD: 1.36 nmol/L). | [] |
| Face mask | PLA, non-woven fabric | MEX | The mask showed a viral filtration efficiency of at least 80%. | [] |
| Face mask extenders | PLA | MEX | The mask extender, which can last for around 2 months, was well-received by healthcare providers. | [] |
| Face shield adapter | PLA | MEX | The headlight face shield adapter was used for 2 weeks and has been found to be useful for treating epistaxis, changing tracheostomy cannulas, and routine nasal and oral examinations while protecting the physicians from respiratory droplets, blood, sputum, and other fluids. | [] |
| Photothermal probe | PLA, graphene oxide | MEX | The probe demonstrated a photothermal conversion efficiency of up to 32.6% | [] |
| Stent | PLA | MEX | The stent exhibited shape recovery following thermomechanical programming. | [] |
| Stent | PLA, PU | MEX | The stent with spirals demonstrated self-expansion, anti-migration, and non-cytotoxicity. | [] |
| Swab | PLA | MEX | The printed swabs demonstrated threshold cycle values comparable to control swabs in terms of detecting RNase P expression. | [] |
| Swab | PLA | MEX | The overall concordance between the prototype and control swabs was 80.8%. | [] |
Abbreviations: LOD, limit of detection; MEX, material extrusion; PLA, poly(lactic acid); PU, poly(urethane).

Table 5.
Recent studies on 3D-printed PLA-containing prosthetics, orthotics, and sensors.
Table 5.
Recent studies on 3D-printed PLA-containing prosthetics, orthotics, and sensors.
| Device | Device Components | Printing Technique | Results | Reference |
|---|---|---|---|---|
| Ankle-foot orthosis | PLA, carbon fiber | MEX | The addition of carbon black increased the stiffness and mechanical strength of the device. The PLA and PLA-carbon black devices fractured at loads of 286.2 N and 345.4 N, respectively. | [] |
| Arm exoskeleton | PLA | MEX | The exoskeleton can move the arm into eight different positions. | [] |
| Arm orthosis | PLA | MEX | The use of perforations reduced the weight of the splints. In comparison to the solid splint, the topology-optimized and square-perforated hand splints were 26% and 12% lighter, respectively. | [] |
| Arm sensor | PLA, PU | MEX | The wearable forearm band allowed the acquisition of surface EMG signals for six different movements from 15 volunteers. The classification algorithm for the detected signals had an accuracy of 85%. | [] |
| Dental prosthesis | PLA | MEX | The deviation of PLA and wax dentures from the plaster model were comparable. | [] |
| Dental prosthesis | PLA | MEX | The temporary crowns were maintained in five patients without fracture, dislodging, or discomfort until the permanent prostheses were ready. | [] |
| General sensor | PLA | MEX | The device demonstrated a power density of ~25 mW/m2 and an ability to record the bone–joint motion, coughing action, and foot pressure distribution. | [] |
| Hand exoskeleton | PLA | MEX | The EMG-responsive device significantly improved the hand function and eating independence of patients with weakness due to spinal cord injury. | [] |
| Hand exoskeleton | PLA | MEX, MJT | The addition of haptic stimulations to the hand exoskeleton improved the attention of the participants during the conducting of hand exercises. | [] |
| Hand orthosis | PLA | MEX | The satisfaction ratings for the 3D-printed and commercially available splints were comparable among patients with an indication for immobilization of at least 4 weeks. | [] |
| Hand prosthesis | PLA, PU, Nylon | MEX | The body-powered prosthetic hand can perform different grasping patterns due to the adaptivity provided by the articulated fingers. | [] |
| Hand prosthesis | PLA | MEX | The prosthetic hand can perform fine movements and grasp different objects. | [] |
| Scleral prosthesis | PLA | MEX | A scleral cover shell prosthesis was developed from the corneoscleral topography profile of a volunteer patient. | [] |
| Transtibial prosthesis | PLA | MEX | The printed transtibial socket passed the ultimate static test force of 4025 N. | [] |
| Wrist hand orthosis | PLA | MEX | A dynamic orthosis was produced to help the patient perform an active extension of the wrist against the spastic flexor muscles. | [] |
Abbreviations: EMG, electromyography; MEX, material extrusion; MJT, material jetting; PLA, poly(lactic acid); PU, poly(urethane).
6. Surgical Instruments and Guides
The rapid prototyping and production of surgical instruments and guides (Table 6, Figure 5) has been made possible via the utilization of 3D printing technology with PLA. PLA’s compatibility with standard sterilization processes makes it suitable for use in sterile surgical environments. Surgical instruments used in septoplasty, such as a scalpel handle, needle holders, toothed forceps, a Cottle/Freer elevator, and a Killian’s speculum, have been produced from 3D-printed PLA []. In addition, recent studies have also demonstrated the ability of 3D-printed PLA to generate an external fixator [], orthopedic screws [], and a trocar spacer for vitreoretinal surgery [].
The ability of 3D-printed PLA to be precisely engineered into complex shapes has allowed the customization not only of surgical instruments to match specific surgical procedures and patient anatomy but also of surgical guides. A surgical guide is a custom-made model, typically generated from patient-specific medical imaging data, that aids in the precise placement of implants or the execution of surgical procedures with enhanced accuracy and efficiency. It can also minimize the risk of adverse reactions during surgical interventions, contributing to better patient outcomes. Examples of clinical procedures where 3D-printed PLA has been utilized as a guide include acetabular fracture surgery [], halo pin placement [], leg osteotomy [], microtia reconstruction [], pelvic reconstruction [], and subtalar joint arthrodesis []. For acetabular fracture surgery, the 3D-printed models of the fracture site have been used to preoperatively bend the reconstruction plate in a total of 12 cases []. For halo pin placement, PLA models have successfully guided safe placement in two pediatric patients with complex spinal conditions, leading to successful skull fusion []. For leg osteotomy, a PLA model was used to verify the cutting angle and positions for a patient with X-linked hypophosphatemia and 35-degree left genu varum []. For microtia reconstruction, PLA ear models proved valuable as intraoperative references []. Lastly, for subtalar joint arthrodesis, PLA surgical navigation guides reduced the procedure times without affecting the fusion outcomes [].
While several surgical procedures have seen success using 3D-printed PLA guides in human studies, more complex procedures like cervical screw fixation have only been tested in preclinical models [,]. However, these promising preclinical results pave the way for future investigations that could bridge the gap to clinical translation. Further research is crucial to expand the application of PLA surgical guides to a wider range of procedures, including highly complex surgeries like cardiovascular and neurosurgery where precise and personalized instrumentation is paramount. Addressing these challenges and expanding the scope of PLA’s applications will unlock its full potential in revolutionizing surgical practice.

Table 6.
Recent studies on 3D-printed PLA-based surgical guides.
Table 6.
Recent studies on 3D-printed PLA-based surgical guides.
| Procedure | Image Source | Guide Components | Printing Technique | Results | References |
|---|---|---|---|---|---|
| Acetabular fracture surgery | CT | PLA | MEX | The surface filtering pipeline decreased the printing time by 65%. The mean average deviation of the printed models from the computed model ranged from 0.67 to 1.06 mm. The mean reduction time of the fracture fragments during the operation was 12 min and 42 s. After 12 months, the average Harris Hip Score, Modified Harris Hip Score, and Merle d’Aubigne Score of nine patients were 71.6 points (fair), 75.7 points (fair), and 11.1 points (poor), respectively. | [] |
| Ankle, foot, and pelvic reconstruction | CT | PLA | MEX | The reconstruction of the deformities was planned using the printed models. | [] |
| Cervical screw fixation | CT | PLA | MEX | Patient-specific models and drill guides were fabricated for simulating C1/2 cervical pedicle screw fixation. The drill guides had an accuracy of 93.54%. | [] |
| Cervical screw fixation | CT | PLA | MEX | Patient-specific navigation templates were fabricated for lower cervical anterior transpedicular screw insertion. The axial and sagittal accuracies were 99.5% and 97%, respectively. | [] |
| Dental implant surgery | Surface scanning | PLA, PHA | MEX, MJT | The printed materials showed significant angle deviation and apex offset after sterilization. However, the errors were comparable to the levels reached using the materials that are routinely used in clinical settings. | [] |
| Halo pin placement | CT | PLA | MEX | The skull models were used to guide safe pin placement in two pediatric patients with diastrophic dysplasia requiring prolonged pre-fusion halo-gravity traction for staged fusion for progressive kyphoscoliosis. Both patients achieved fusion union by 9 months. | [] |
| Leg osteotomy | CT | PLA | MEX | The model was used to verify the cutting angle and positions of the osteotomy for a patient with X-linked hypophosphatemia and 35-degree left genu varum. After this, an arch osteotomy of the left femur and transverse osteotomy of the left upper tibiofibular bone were performed. The genu varum was repaired and the leg was fixed using an Ilizarov external fixator. | [] |
| Microtia reconstruction | Surface scanning | PLA | MEX | A patient-specific ear model was developed without additional ionizing radiation exposure and served as a helpful intraoperative reference for microtia reconstruction. | [] |
| Pelvic reconstruction | CT | PLA, titanium alloy | MEX | Compared to the common 3D-printed anatomic template, the modified 3D-printed anatomic template with a customized cutting block resulted in a shorter operating time (209 vs. 272 min) and less blood loss (1390 vs. 2248 mL) in patients requiring pelvic reconstruction after pelvic tumor resection. The modified strategy also reduced the local tumor recurrence (5.26 vs. 42.11%), but it is associated with a higher rate of implant loosening (21.05 vs 0%). | [] |
| Subtalar joint arthrodesis | CT | PLA | MEX | The surgical guide reduced the time that it took to drill the Kirschner wire to a satisfactory position in patients who had undergone subtalar joint arthrodesis compared to the control group (2.1 vs. 4.6 min). After 1 year, there was no significant difference observed in the subtalar fusion time and American Orthopaedic Foot & Ankle Society scores between the two groups. | [] |
Abbreviations: CT, computed tomography; EMG, electromyography; MEX, material extrusion; MJT, material jetting; PHU, poly(hydroxyalkanoate); PLA, poly(lactic acid).
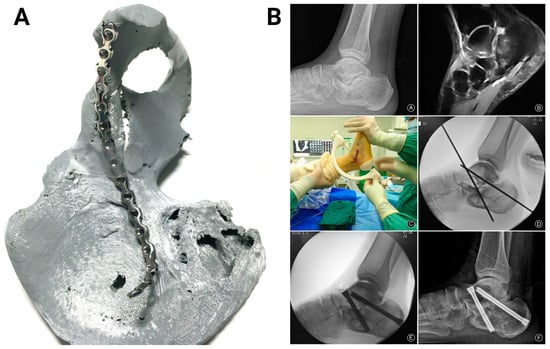
Figure 5.
Three-dimensional-printed PLA-based surgical guides. (A) The 3D-printed acetabular fracture model served as a guide for pre-bending the steel reconstruction plate prior to surgery. The image was adapted with permission from Weidert et al. []. (B) A clinical case showing the subtalar joint space upon radiography (top left) and osteoarthritis upon magnetic resonance imaging (top right). The 3D-printed guide was used to position the Kirschner wires (middle). Intraoperative fluoroscopy confirmed the satisfactory placement of the wires (bottom left). After 1 year, radiography showed subtalar bony fusion and obliteration of subtalar joint space (bottom right). Images were adapted with permission from Duan et al. [].
7. Radiotherapy Devices and Phantoms
Radiotherapy is a frequently used cancer treatment modality, and it involves the use of ionizing radiation to control or eradicate cancer cells. Three-dimensional printing has allowed the development of customized radiotherapy devices and phantoms that can help enhance the precision of treatment delivery while minimizing the exposure of healthy tissues to radiation (Table 7, Figure 6). Examples of radiotherapy devices that have been produced from 3D-printed PLA include a beam modifier for total skin electron beam treatment [], a bolus for postmastectomy radiation [], and an applicator for gynecologic brachytherapy []. Additionally, PLA has been combined with different polymers and radiopaque materials to construct 3D-printed phantoms for quality assurance and dosimetry calibration in the context of treatment planning and verification. Recent studies have reported the development of 3D-printed phantoms of the head [], cervical spine [], breast [,,], thorax [,], and pelvic organs [].
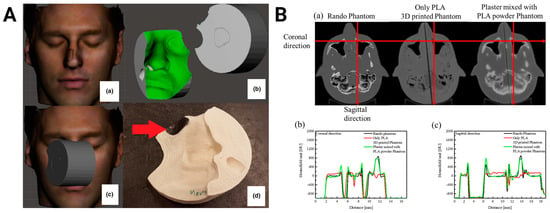
Figure 6.
Three-dimensional-printed PLA-based radiotherapy devices. (A) The process to produce a bolus includes an optical scan of the patient (a), a digital design of the material (b), optimization of treatment geometry (c), and printing and placing lead shielding as indicated by the red arrow (d). Images were adapted with permission from Sasaki et al. []. (B) Computed tomography scans of a commercial Rando head phantom, a head phantom printed using only PLA, and a head phantom printed with a mixture of plaster and PLA powder (a). Radiopacity profiles along the red line in the transverse (a) and coronal (b) planes. Images were adapted with permission from Kim et al. [].
The clinical impact of 3D-printed PLA in radiotherapy has been demonstrated in two recent studies. Digitally designed PLA boluses have been successfully used in nine patients with a complex surface anatomy for radiation, highlighting the clinical feasibility of personalized 3D-printed devices []. In another study, the use of PLA templates for radioactive seed implantation in lung cancer patients led to significantly improved dosimetric coverage of the tumor compared to the control group []. Building on these successes, ongoing innovations in composite materials can facilitate the development of even more personalized radiotherapy devices and complex phantoms. These advancements are poised to enhance the treatment precision and accuracy by closely mimicking the heterogeneity of the human anatomy, leading to more accurate dosimetry and treatment planning.

Table 7.
Recent studies on 3D-printed PLA-containing radiotherapy devices and phantoms.
Table 7.
Recent studies on 3D-printed PLA-containing radiotherapy devices and phantoms.
| Device | Image Source | Device Components | Printing Technique | Results | Reference |
|---|---|---|---|---|---|
| Beam modifier | N/A | PLA | MEX | The printed modifier shaped the electron beam as desired, resulting in an adequate field and skin coverage for total skin electron beam treatment with 10% and 3% inhomogeneity in the vertical and lateral dimensions, respectively. | [] |
| Bolus | CT | PLA | MEX | The printed bolus for postmastectomy radiation had a significantly lower frequency of air gaps of at least 5 mm in length compared to a standard sheet bolus (13% vs. 30%). The surface dose was within 3% for both bolus types. | [] |
| Bolus | CT, surface scanning | PLA | MEX | The digitally designed boluses had an average shape error of less than 0.5 mm and were used for the treatment of nine patients with a complex surface anatomy with no issues. | [] |
| Brachytherapy applicator | CT | PLA, tungsten | MEX | The patient-specific applicators can provide comparable doses while offering advanced healthy tissue sparing. The PLA-tungsten composite acted as the shielding material. | [] |
| Dosimetry guide | CT | PLA | MEX | The group of lung cancer patients with the printed template for the implantation of 125I radioactive seeds had a greater mean postoperative V90 value compared to the control group (93.8 vs. 88.42%). | [] |
| Phantom | CT | PLA | MEX | Compared to the computational phantom, the skin, adipose, glandular, and tumor tissues in the printed breast phantom had a percentage difference of 1.8%, 10.1%, 4.5%, and 12.3%, respectively. | [] |
| Phantom | CT | PLA, ABS | MEX | PLA (188 HU) and ABS (24 HU) were used to mimic glandular and adipose tissues in the breast, respectively. | [] |
| Phantom | CT | PLA, ABS, iron | MEX | The phantom slab demonstrated a similar range of HU that is comparable to the commercial phantom. The mean HU values for lung tissue, soft tissue, low-density bone, and high-density bone were –760, 50, 220, and 630, respectively. | [] |
| Phantom | CT | PLA, bismuth oxide | MEX | The addition of bismuth oxide significantly enhanced the radiopacity of the cervical spine phantom compared to plain PLA (612 HU vs. 119 HU). | [] |
| Phantom | CT | PLA, iron | MEX | The pediatric torso phantom demonstrated an excellent similarity to commercially available phantoms. The mean HU values for the heart, soft tissue remainder, right lung, and vertebral body were 94, 31, −417, and 1180, respectively. | [] |
| Phantom | CT | PLA, plaster | MEX | The head and neck phantom showed a mean difference of 61 HU for soft tissue and 109 HU for bone tissue compared to the Rando phantom. | [] |
| Phantom | CT | PLA, PP | MEX | The Pearson’s coefficients between the matched CT images of the breast phantom with those of the patients were 0.91–0.97. | [] |
| Phantom | CT | PLA, StoneFil | MEX | The HU values for the hip bone phantoms varied between 700 and 800. | [] |
| Phantom | N/A | PLA, PVC, silicone | MEX | PLA and heat-resistant silicone were used to fabricate the phantom case, prostate casting molds, probe insert, and prostate positioning tool, while PVC was used to fabricate the prostatic tissue. | [] |
| Phantom | N/A | PLA, zirconium oxide | MEX | The composites with a mere 6% weight fraction demonstrated a maximal radiopacity of 184 HU. | [] |
Abbreviations: ABS, acrylonitrile butadiene styrene; CT, computed tomography; HU, Hounsfield unit; MEX, material extrusion; PLA, poly(lactic acid); PP, poly(propylene); PVC, poly(vinyl chloride).
8. Training Models and Simulators
Three-dimensional-printed PLA has also played a pivotal role in the development of training models and simulators (Table 8, Figure 7). The versatility and affordability of PLA have enabled the creation of anatomically accurate models and simulators that reflect real-world clinical scenarios, allowing medical professionals and trainees to practice procedures and techniques in a controlled environment. PLA-based simulators have been developed to train medical personnel in a wide variety of procedures, such as bronchoscopy [], cervical myelography [], craniosynostosis correction [], dental implant surgery [], digital rectal examination [], endoscopic third ventriculostomy [], external ventricular drain placement [], intraarticular joint injection [], intraosseous access [,], mastoidectomy [,], maxillofacial surgery [], nasal osteotomy [], neuraxial anesthesia delivery [], pedicle screw insertion [], and transcranial ultrasonography [].
The use of PLA-based constructs offers a cost-effective, accessible, and safer means of enhancing medical training and skill development, which could ultimately improve patient care and safety. To fully unravel its potential in medical training and education, several key areas require further development. Optimizing the printing processes and material usage can enhance the cost-effectiveness and accessibility, making this technology more widely available, particularly in resource-limited settings. Additionally, establishing standardized evaluation protocols and validating the effectiveness of models and simulators across various medical specialties and training programs are crucial for broader adoption. Lastly, integrating 3D-printed models with cutting-edge technologies like virtual reality can create more immersive and interactive training environments that enhance learning experiences and improve skill acquisition. Overall, these strategies could revolutionize medical education by providing more realistic simulations, ultimately leading to well-trained healthcare providers and improved patient outcomes.

Table 8.
Recent studies on 3D-printed PLA-containing training models and simulators.
Table 8.
Recent studies on 3D-printed PLA-containing training models and simulators.
| Device | Image Source | Device Components | Printing Technique | Results | References |
|---|---|---|---|---|---|
| Aneurysm model | MRA | PLA | MEX | CT measurements of the intracranial aneurysm models were 0.32–0.35 mm higher than the MRA measurements. | [] |
| Bone model | CT | PLA | MEX | The ultimate strength of the printed bones (3920–8677 N) was closer to that of real bones (4992–13620 N) than the commercially available polyurethane-based products (1000–9119 N). | [] |
| Bone model | CT | PLA | MEX | The participants were 130 third-year medical students. The group with the printed craniofacial bone models had a higher gain score in anatomical knowledge than the cadaveric skull group (50 vs. 37.3). | [] |
| Bone model | CT | PLA | PBF | The correlation coefficient of the mechanical properties between the printed and actual trabeculae reached up to 0.94. | [] |
| Bone model | N/A | PLA, PVA | MEX | The vertebral models were successfully instrumented without hardware or material failures. | [] |
| Brain model | CT, MRI | PLA, PVA, calcium carbonate | MEX | The skull was printed using PLA with calcium carbonate. The brain was cast using a mixture of water, coolant, PVA, and barium sulfate. | [] |
| Brain model | MRI | PLA, PAM | MEX | The brain phantom was fabricated by extruding PAM into a PLA skull. | [] |
| Bronchoscopy simulator | CT | PLA, PVA, silicone | MEX | All the 17 participants had performed >100 bronchoscopies in the past. Of these, 77% thought that the printed stenotic airway model was better or much better for airway inspection when compared with the Broncho-Boy. A total of 94% reported that the model was accurate or very accurate for realism. | [] |
| Cervical myelography simulation | CT | PLA | MEX | The simulator adequately demonstrated the dimensions of the cervical canal in static and dynamic positions. | [] |
| Congenital heart disease model | CTA | PLA | MEX | The CT imaging of the models matched the original digital models. Independent reviewers correctly described 80 and 87% of the congenital heart disease models, respectively. | [] |
| Craniosynostosis correction simulator | CT | PLA | MEX | The participants were five attending physicians, four fellows, and nine residents. Of the participants, 100% and 94% reported that the model was a valuable training tool for open reconstruction and endoscopic suturectomy, respectively. | [] |
| Dental implant surgery simulator | CT | PLA | MEX, MJT, PBF, VP | The MJT model had the highest scores from five maxillofacial surgeons for drilling perception and corticotrabecular transition. | [] |
| Digital rectal examination simulator | N/A | PLA, silicone | MEX | The participants were five urologists. The participants gave a rating of 4.24 out of 5 to the task trainer with regard to appropriateness and usefulness in education. | [] |
| Endoscopic third ventriculostomy simulator | CT, MRI | PLA, silicone | VP | The participants were three attending physicians and 12 residents. A total of 87% strongly agreed that the simulator was useful for resident training, while 93% strongly agreed that the simulator helped them understand how to orient themselves with the endoscope. | [] |
| External ventricular drain placement simulator | N/A | PLA, agar | MEX | The participants were five neurosurgery residents and six EM residents. One hundred percent strongly agreed that the model was useful for their training and was a realistic simulation of the procedure. | [] |
| Intraarticular joint injection simulator | N/A | PLA, ballistics gel | MEX | The model reproduced the finding of an anterior shoulder dislocation upon ultrasound imaging and allowed for the visualization of the needle within the joint space. | [] |
| Intraosseous access simulator | N/A | PLA, silicone | MEX | The participants were 15 rural family medicine residents, six rural EM physicians, and six registered nurses. The majority of the physicians considered the adult intraosseus access simulator to be very effective as a training tool. | [] |
| Intraosseous access simulator | N/A | PLA, silicone | MEX | The participants were seven EM physicians, two family medicine residents, and three medical students. The majority of the physicians found the pediatric intraosseous access simulator to be “very effective”, whereas the medical students found it to be “effective to very effective”. | [] |
| Mastoidectomy simulator | CT | PLA | MEX | The participants were 10 junior residents. The ratings for the ease of use, safety, and value in training were 4.75/5, 4.5/5, and 4.35/5, respectively. | [] |
| Mastoidectomy simulator | CT | PLA, PETG, ABS, PC, nylon | MEX | The participants were surgeons with an average of 56.5 temporal bone procedures. PETG had the highest score for haptic feedback and appearance, which were 8.3/10 and 7.6/10, respectively. PLA was a reliable alternative with scores of 7.4/10 and 7.6/10. | [] |
| Maxillofacial surgery simulation | CT | PLA, ABS, silicone | MEX | The participants were 10 maxillofacial surgeons. The majority rated the haptics of bone and soft tissue simulation “good” and “moderate to good”, respectively. | [] |
| Nasal osteotomy simulator | CT | PLA | MEX | The evaluators were two attending facial plastic surgeons and one facial plastic surgery fellow. All of them strongly agreed that the model with a 10% infill density mimicked human bone better than the other models with different infill densities. | [] |
| Neuraxial anesthesia simulator | CT | PLA, gelatin, psyllium fiber | MEX | The participants were 22 anesthesiologists. Although the model was found to be less realistic in terms of surface palpation than the Simulab phantom, it had significantly better fidelity for the loss of resistance, dural puncture, and ultrasound imaging. | [] |
| Pedicle screw insertion simulator | CT | PLA, ABS, nylon | MEX | The surgeon authors reported that the ABS models were much more similar to human bone than the PLA models when cannulating pedicles and placing screws. | [] |
| Transcranial ultrasonography simulator | CT, MRI | PLA, PVC, photopolymer resin | MEX, VP | The transcranial ultrasonography of the phantom proved the possibility of B-mode imaging differentiation between the brain and particles of metal, bone tissue, and PLA. However, when examined through the temporal bone model, the particles in the sonogram were 3–10 times larger than their actual size. | [] |
Abbreviations: ABS, acrylonitrile butadiene styrene; CT, computed tomography; CTA, CT angiography; EM, emergency medicine; MEX, material extrusion; MJT, material jetting; MRI, magnetic resonance imaging; MRA, MR angiography; PAM, poly(acrylamide); PBF, powder bed fusion; PC, poly(carbonate); PETG, poly(ethylene terephthalate glycol); PLA, poly(lactic acid); PVA, poly(vinyl alcohol); PVC, poly(vinyl chloride); VP, vat photopolymerization.
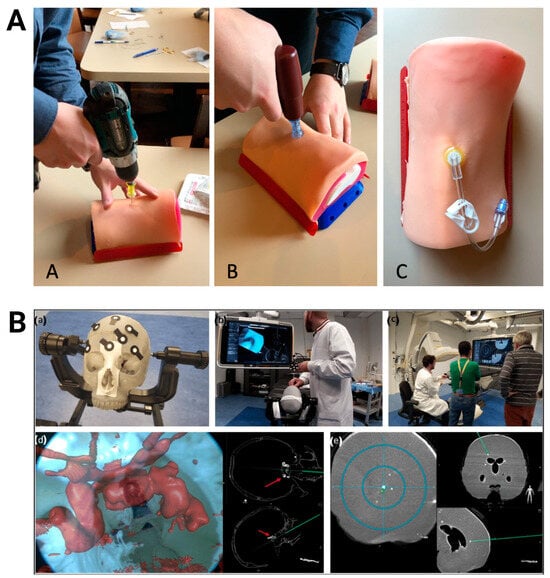
Figure 7.
Three-dimensional-printed PLA-based training models. (A) Testing of adult proximal tibia intraosseous trainer with a cordless drill (A), EZ-IO power driver (B), and needle insertion (C). Images were adapted with permission from Engelbrecht et al. []. (B) Anthropomorphic skull and brain model blocked on a clamp (a) for use in augmented reality-navigated endonasal skull-base surgery simulation (b/d) and brain biopsy simulations (c/e). Images were adapted with permission from Lai et al. [].
9. Conclusions
In summary, PLA’s adaptability, biocompatibility, and cost-effectiveness have made it an indispensable tool in addressing a wide range of medical challenges, from tissue engineering to medical education and training. While the progress is remarkable, further research is imperative to maximize the utility of 3D-printed PLA in medicine. In tissue engineering, reproducing the intricate architecture, hierarchy, and heterogeneity of native bone tissue remains a significant challenge. However, emerging high-resolution printing methods and strategies to enhance the vascularization offer promising solutions. In drug delivery, optimizing the scaffold and device composition to ensure the stability, safety, and efficacy of the incorporated drugs should be a key focus of further research. For medical devices, expanding the range of the 3D-printed PLA prosthetics and orthotics for diverse indications and patient populations is crucial to realizing their full potential. In the context of surgery, further research is needed to extend the application of PLA surgical guides to highly complex procedures where precision and personalization are of paramount importance. In the area of radiotherapy, the continued innovation in composite materials should facilitate the development of more personalized devices and phantoms that closely mimic the heterogeneity of the human anatomy, ultimately leading to more accurate dosimetry and treatment planning. Finally, in medical training and education, optimizing the printing processes, establishing standardized evaluation protocols, and integrating with virtual learning strategies, are key steps toward broader and more meaningful adoption. With these research opportunities, the 3D-printed PLA-based materials are expected to continue inspiring innovation, transforming patient care, and shaping the future of healthcare delivery and personalized medicine.
Author Contributions
Conceptualization, A.J.R.B., P.R., S.K., and K.T.; data curation, A.J.R.B. and K.T.; writing—original draft preparation, A.J.R.B. and K.T.; writing—review and editing, A.J.R.B., P.R., S.K., and K.T.; supervision, K.T.; funding acquisition, K.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sandanamsamy, L.; Harun, W.S.W.; Ishak, I.; Romlay, F.R.M.; Kadirgama, K.; Ramasamy, D.; Idris, S.R.A.; Tsumori, F. A comprehensive review on fused deposition modelling of polylactic acid. Prog. Addit. Manuf. 2023, 8, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Kallingal, A.; Suresh, A.M.; Mahapatra, D.K.; Hasanin, M.S.; Haponiuk, J.; Thomas, S. 3d printing of polylactic acid: Recent advances and opportunities. Int. J. Adv. Manuf. Technol. 2023, 125, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, J. Biodegradable and biobased polymers. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Davila, S.; González-Rodríguez, L.; Lama, R.; López-Álvarez, M.; Oliveira, A.L.; Serra, J.; Novoa, B.; Figueras, A.; González, P. 3d-printed PLA medical devices: Physicochemical changes and biological response after sterilisation treatments. Polymers 2022, 14, 4117. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties—From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- ISO/ASTM52900-21; Additive Manufacturing—General Principles—Fundamentals and Vocabulary. International Organization for Standardization: Geneva, Switzerland; American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- Barcena, A.J.R.; Dhal, K.; Patel, P.; Ravi, P.; Kundu, S.; Tappa, K. Current biomedical applications of 3d-printed hydrogels. Gels 2024, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Kadier, A.; Kalil, M.S.; Ibrahim, R.; Atikah, M.S.N.; Nurazzi, N.M.; Nazrin, A.; Lee, C.H.; Faiz Norrrahim, M.N.; et al. Chapter 8—Properties and characterization of PLA, pha, and other types of biopolymer composites. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Al-Oqla, F.M., Sapuan, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 111–138. [Google Scholar]
- Alexander, A.E.; Wake, N.; Chepelev, L.; Brantner, P.; Ryan, J.; Wang, K.C. A guideline for 3d printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print. Med. 2021, 7, 8. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Doostmohammadi, N.; Talebi, A.; Arab, S.; Alizadeh, A.; Ghanbari, A.; Salati, A. 3D printed polylactic acid/gelatin-nano-hydroxyapatite/platelet-rich PLAsma scaffold for critical-sized skull defect regeneration. Biomed. Eng. Online 2022, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, H.M.; Li, X.; Liu, W.; Chu, Y.X.; Wang, Y.; Wang, Y.; Xu, J.Z.; Li, Z.M.; Li, J.H. Simultaneously constructing nanotopographical and chemical cues in 3D-printed polylactic acid scaffolds to promote bone regeneration. Mater. Sci. Eng. C 2021, 118, 111457. [Google Scholar] [CrossRef]
- Shen, M.; Wang, L.; Gao, Y.; Feng, L.; Xu, C.; Li, S.; Wang, X.; Wu, Y.; Guo, Y.; Pei, G. 3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects. Mater. Today Bio 2022, 16, 100382. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, X.; Zhang, J.; Lv, Z.; Meng, X.; Yuan, Z.; Long, T.; Wang, Y. 3D printing polylactic acid polymer-bioactive glass loaded with bone cement for bone defect in weight-bearing area. Front. Bioeng. Biotechnol. 2022, 10, 947521. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jiang, X.; Liu, Z.; Liu, F.; Yan, G.; Li, F. Biodegradable 3D printed scaffolds of modified poly (trimethylene carbonate) composite materials with poly (l-lactic acid) and hydroxyapatite for bone regeneration. Nanomaterials 2021, 11, 3215. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, C.; Bu, W.; Yang, Y.; Guo, F.; Li, J.; Wang, E.; Mao, Y.; Mai, H.; You, H.; et al. 3D printing of polylactic acid/boron nitride bone scaffolds: Mechanical properties, biomineralization ability and cell responses. Ceram. Int. 2023, 49, 25886–25898. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Y.; Liu, C.; Bu, W.; Guo, F.; Li, J.; Wang, E.; Peng, Z.; Mai, H.; You, H.; et al. 3D printed TPMS structural PLA/GO scaffold: Process parameter optimization, porous structure, mechanical and biological properties. J. Mech. Behav. Biomed. Mater. 2023, 142, 105848. [Google Scholar] [CrossRef] [PubMed]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds—Current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar]
- Tcacencu, I.; Rodrigues, N.; Alharbi, N.; Benning, M.; Toumpaniari, S.; Mancuso, E.; Marshall, M.; Bretcanu, O.; Birch, M.; McCaskie, A.; et al. Osseointegration of porous apatite-wollastonite and poly(lactic acid) composite structures created using 3D printing techniques. Mater. Sci. Eng. C 2018, 90. [Google Scholar] [CrossRef] [PubMed]
- Fairag, R.; Li, L.; Ramirez-GarciaLuna, J.L.; Taylor, M.S.; Gaerke, B.; Weber, M.H.; Rosenzweig, D.H.; Haglund, L. A composite lactide-mineral 3D-printed scaffold for bone repair and regeneration. Front. Cell Dev. Biol. 2021, 9, 654518. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Y.; Zhang, L.; Wang, Y.; Guo, C.; Feng, K.; Yang, S.; Zhai, Z.; Chi, Y.; Zhao, J.; et al. The effect of induced membranes combined with enhanced bone marrow and 3D PLA-HA on repairing long bone defects in vivo. J. Tissue Eng. Regen. Med. 2020, 14, 1403–1414. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Owens, T.C.; Melancon, S.; Workeneh, I.; Tran Cao, H.S.; Vauthey, J.-N.; Huang, S.Y. Current perspectives and progress in preoperative portal vein embolization with stem cell augmentation (pvesa). Stem Cell Rev. Rep. 2024, 20, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Diomede, F.; Gugliandolo, A.; Chiricosta, L.; Bramanti, P.; Merciaro, I.; Orsini, T.; Mazzon, E.; Trubiani, O. 3D printing PLA/gingival stem cells/evs upregulate mir-2861 and-210 during osteoangiogenesis commitment. Int. J. Mol. Sci. 2019, 20, 3256. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M.; Talebi, A.; Doostmohammadi, N.; Arab, S.; Ghanbari, A.; Zarbakhsh, S. The healing of bone defects by cell-free and stem cell-seeded 3D-printed PLA tissue-engineered scaffolds. J. Orthop. Surg. Res. 2022, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Zhao, R.; Tang, W.; Yang, F.; Tian, H.; Peng, S.; Pan, H.; Shuai, C. Structural and functional adaptive artificial bone: Materials, fabrications, and properties. Adv. Funct. Mater. 2023, 33, 2214726. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, W.; Liu, H.; Chen, Y.; Li, B.; Gou, Z.; Li, X.; Gou, M. 3D printing of functional nerve guide conduits. Burn. Trauma. 2021, 9, tkab011. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, E.J.; Jang, W.B.; Kwon, S.M. Development of biocompatible 3D-printed artificial blood vessels through multidimensional approaches. J. Funct. Biomater. 2023, 14, 497. [Google Scholar] [CrossRef]
- Kabirian, F.; Brouki Milan, P.; Zamanian, A.; Heying, R.; Mozafari, M. Nitric oxide-releasing vascular grafts: A therapeutic strategy to promote angiogenic activity and endothelium regeneration. Acta Biomater. 2019, 92, 82–91. [Google Scholar] [CrossRef]
- Azadmanesh, F.; Pourmadadi, M.; Zavar Reza, J.; Yazdian, F.; Omidi, M.; Haghirosadat, B.F. Synthesis of a novel nanocomposite containing chitosan as a three-dimensional printed wound dressing technique: Emphasis on gene expression. Biotechnol. Prog. 2021, 37, e3132. [Google Scholar] [CrossRef]
- Metwally, W.M.; El-Habashy, S.E.; El-Hosseiny, L.S.; Essawy, M.M.; Eltaher, H.M.; El-Khordagui, L.K. Bioinspired 3D-printed scaffold embedding ddab-nano zno/nanofibrous microspheres for regenerative diabetic wound healing. Biofabrication 2023, 16, 015001. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivityin vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.R.; Kim, T.H.; Park, K.H.; Kang, J.; Lee, K.; Park, E.K.; Kwon, T.G.; Lim, J.O.; Oh, C.W. Rhbmp-2-conjugated three-dimensional-printed poly(l-lactide) scaffold is an effective bone substitute. Tissue Eng. Regen. Med. 2023, 20, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Maia-Pinto, M.O.; Brochado, A.C.B.; Teixeira, B.N.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.; Alves, G.G.; Calasans-Maia, M.D.; Thiré, R.M. Biomimetic mineralization on 3D printed PLA scaffolds: On the response of human primary osteoblasts spheroids and in vivo implantation. Polymers 2021, 13, 74. [Google Scholar] [CrossRef]
- Cha, M.; Jin, Y.Z.; Park, J.W.; Lee, K.M.; Han, S.H.; Choi, B.S.; Lee, J.H. Three-dimensional printed polylactic acid scaffold integrated with bmp-2 laden hydrogel for precise bone regeneration. Biomater. Res. 2021, 25, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Ye, G.; Wu, J.; Wang, L.; Fang, G.; Ye, Z.; Lai, G.; Qiu, X.; Sang, H. A 3D-printed bioactive polycaprolactone scaffold assembled with core/shell microspheres as a sustained bmp2-releasing system for bone repair. Biomater. Adv. 2022, 133, 112619. [Google Scholar] [CrossRef]
- Han, S.H.; Cha, M.; Jin, Y.Z.; Lee, K.M.; Lee, J.H. Bmp-2 and hmsc dual delivery onto 3D printed PLA-biogel scaffold for critical-size bone defect regeneration in rabbit tibia. Biomed. Mater. 2021, 16, 015019. [Google Scholar] [CrossRef]
- Bouyer, M.; Garot, C.; Machillot, P.; Vollaire, J.; Fitzpatrick, V.; Morand, S.; Boutonnat, J.; Josserand, V.; Bettega, G.; Picart, C. 3D-printed scaffold combined to 2d osteoinductive coatings to repair a critical-size mandibular bone defect. Mater. Today Bio 2021, 11, 100113. [Google Scholar] [CrossRef]
- Chou, P.Y.; Lee, D.; Chen, S.H.; Liao, C.T.; Lo, L.J.; Liu, S.J. 3D-printed/electrospun bioresorbable nanofibrous drug-eluting cuboid frames for repair of alveolar bone defects. Int. J. Pharm. 2022, 615, 121497. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/NHA/dexamethasone scaffold prepared by 3D printing for bone regeneration. Macromol. Biosci. 2018, 18, 1800068. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, K.; Du, Y.; Tang, X.; Liu, C.; Cao, S.; Zhao, B.; Huang, H.; Zhao, H.; Kong, W.; et al. Dual-nozzle 3D printed nano-hydroxyapatite scaffold loaded with vancomycin sustained-release microspheres for enhancing bone regeneration. Int. J. Nanomed. 2023, 18, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Davila, S.; Potel-Alvarellos, C.; Carballo, R.; González-Rodríguez, L.; López-Álvarez, M.; Serra, J.; Díaz-Rodríguez, P.; Landín, M.; González, P. Vancomycin-loaded 3D-printed polylactic acid–hydroxyapatite scaffolds for bone tissue engineering. Polymers 2023, 15, 4250. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.A.; Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Sumerel, J.; D’Agostino, H.B.; Mills, D.K.; Woodard, P.K. 3D printed antibiotic and chemotherapeutic eluting catheters for potential use in interventional radiology. Acad. Radiol. 2018, 26, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fu, J.; Li, M.; Wang, S.; Zhuang, B.; Sun, H.; Ge, C.; Feng, B.; Jin, Y. 3D-printed wearable personalized orthodontic retainers for sustained release of clonidine hydrochloride. AAPS PharmSciTech 2019, 20, 260. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.J.; Hassan, Y.R.; Abd-Elsalam, W.H. 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int. J. Pharm. 2021, 607, 121010. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Chatzitaki, A.T.; Karavasili, C.; Katsamenis, O.L.; Tzetzis, D.; Mystiridou, E.; Bouropoulos, N.; Fatouros, D.G. Controlled release of 5-fluorouracil from alginate beads encapsulated in 3D printed ph-responsive solid dosage forms. AAPS PharmSciTech 2018, 19, 3362–3375. [Google Scholar] [CrossRef]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Three-dimensional (3D)-printed devices composed of hydrophilic cap and hydrophobic body for improving buoyancy and gastric retention of domperidone tablets. Eur. J. Pharm. Sci. 2020, 155, 105555. [Google Scholar] [CrossRef]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018, 549, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chakka, J.L.; Acri, T.; Laird, N.Z.; Zhong, L.; Shin, K.; Elangovan, S.; Salem, A.K. Polydopamine functionalized vegf gene-activated 3D printed scaffolds for bone regeneration. RSC Adv. 2021, 11, 13282–13291. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Shim, I.K.; Foroughi, J.; Orive, G.; Vine, K.L.; Kim, S.C.; Wallace, G.G. 3D-printed coaxial hydrogel patches with mussel-inspired elements for prolonged release of gemcitabine. Polymers 2021, 13, 4367. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; He, C.; Gao, C.; Zhu, P.; Lu, G.; Li, H. 3D-printed degradable anti-tumor scaffolds for controllable drug delivery. Int. J. Bioprinting 2021, 7, 418. [Google Scholar] [CrossRef]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-printed reservoir-type implants containing poly(lactic acid)/poly(caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Yi, K.; Ju, E.; Jin, Y.; Xu, Y.; Wang, H.; Chen, W.C.; Wang, K.; Wang, Y.; Tao, Y.; et al. 3D printed bioceramic scaffolds as a universal therapeutic PLAtform for synergistic therapy of osteosarcoma. ACS Appl. Mater. Interfaces 2021, 13, 18488–18499. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.H.; Chen, W. Biomedical Devices: Materials, Design, and Manufacturing; Springer: Norwell, MA, USA, 2019. [Google Scholar]
- San Valentin, E.M.D.; Barcena, A.J.R.; Klusman, C.; Martin, B.; Melancon, M.P. Nano-embedded medical devices and delivery systems in interventional radiology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2023, 15, e1841. [Google Scholar] [CrossRef] [PubMed]
- Guterres Silva, L.R.; Santos Stefano, J.; Cornélio Ferreira Nocelli, R.; Campos Janegitz, B. 3D electrochemical device obtained by additive manufacturing for sequential determination of paraquat and carbendazim in food samples. Food Chem. 2023, 406, 135038. [Google Scholar] [CrossRef] [PubMed]
- Teekayupak, K.; Aumnate, C.; Lomae, A.; Preechakasedkit, P.; Henry, C.S.; Chailapakul, O.; Ruecha, N. Portable smartphone integrated 3D-printed electrochemical sensor for nonenzymatic determination of creatinine in human urine. Talanta 2023, 254, 124131. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Neumsteir, N.V.; Aparecido, G.D.O.; Ferraz, T.V.D.B.; Dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2020, 145, 1207–1218. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Rocha, R.G.; de Faria, L.V.; Richter, E.M.; Dantas, L.M.F.; da Silva, I.S.; Muñoz, R.A.A. Additively manufactured electrodes for the electrochemical detection of hydroxychloroquine. Talanta 2022, 250, 123727. [Google Scholar] [CrossRef] [PubMed]
- Ragazou, K.; Lougkovois, R.; Katseli, V.; Kokkinos, C. Fully integrated 3D-printed electronic device for the on-field determination of antipsychotic drug quetiapine. Sensors 2021, 21, 4753. [Google Scholar] [CrossRef]
- Lisboa, T.P.; Alves, G.F.; de Faria, L.V.; de Souza, C.C.; Matos, M.A.C.; Matos, R.C. 3D-printed electrode an affordable sensor for sulfanilamide monitoring in breast milk, synthetic urine, and pharmaceutical formulation samples. Talanta 2022, 247, 123610. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Gogola, J.L.; Budni, L.H.; Janegitz, B.C.; Marcolino-Junior, L.H.; Bergamini, M.F. 3D-printed electrode as a new PLAtform for electrochemical immunosensors for virus detection. Anal. Chim. Acta 2021, 1147, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.G.; Stefano, J.S.; Orzari, L.O.; Brazaca, L.C.; Carrilho, E.; Marcolino-Junior, L.H.; Bergamini, M.F.; Munoz, R.A.A.; Janegitz, B.C. Electrochemical biosensor for sars-cov-2 cdna detection using aups-modified 3D-printed graphene electrodes. Biosensors 2022, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.S.; Guterres e Silva, L.R.; Rocha, R.G.; Brazaca, L.C.; Richter, E.M.; Abarza Muñoz, R.A.; Janegitz, B.C. New conductive filament ready-to-use for 3D-printing electrochemical (bio)sensors: Towards the detection of sars-cov-2. Anal. Chim. Acta 2022, 1191, 339372. [Google Scholar] [CrossRef] [PubMed]
- ISO 8549-1:2020; Prosthetics and Orthotics—Vocabulary—Part 1: General Terms for External Limb Prostheses and External Orthoses. ISO: Geneva, Switzerland, 1989.
- Sanchez-Tena, M.A.; Alvarez-Peregrina, C.; Santos-Arias, F.; Villa-Collar, C. Application of 3D printing technology in scleral cover shell prosthesis. J. Med. Syst. 2019, 43, 149. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Chen, H.; Zhao, Y.; Zhou, Y.; Wang, Y.; Sun, Y. Evaluation of adaptation of the polylactic acid pattern of maxillary complete dentures fabricated by fused deposition modelling technology: A pilot study. PLoS ONE 2018, 13, e0201777. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Park, E.Y.; Kang, S. Three-dimensional printing of temporary crowns with polylactic acid polymer using the fused deposition modeling technique: A case series. J. Yeungnam Med. Sci. 2023, 40, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, J.S.; Plettenburg, D.; Zadpoor, A.A.; Breedveld, P.; Smit, G. Design of a 3D-printed hand prosthesis featuring articulated bio-inspired fingers. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 235, 336–345. [Google Scholar] [CrossRef]
- Dunai, L.; Novak, M.; Espert, C.G. Human hand anatomy-based prosthetic hand. Sensors 2021, 21, 137. [Google Scholar] [CrossRef]
- van der Stelt, M.; Verhamme, L.; Slump, C.H.; Brouwers, L.; Maal, T.J.J. Strength testing of low-cost 3D-printed transtibial prosthetic socket. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 367–375. [Google Scholar] [CrossRef]
- Raj, R.; Dixit, A.R.; Łukaszewski, K.; Wichniarek, R.; Rybarczyk, J.; Kuczko, W.; Górski, F. Numerical and experimental mechanical analysis of additively manufactured ankle–foot orthoses. Materials 2022, 15, 6130. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.H.; Umer, U.; Moiduddin, K.; Alkhalefah, H. Finite element analysis of upper limb splint designs and materials for 3D printing. Polymers 2023, 15, 2993. [Google Scholar] [CrossRef] [PubMed]
- Waldburger, L.; Schaller, R.; Furthmüller, C.; Schrepfer, L.; Schaefer, D.J.; Kaempfen, A. 3D-printed hand splints versus thermoplastic splints: A randomized controlled pilot feasibility trial. Int. J. Bioprinting 2022, 8, 474. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, J.; Todoh, M. Development of a portable assistive exoskeleton for human arm movements. Electron. Lett. 2023, 59, e12785. [Google Scholar] [CrossRef]
- Yoo, H.J.; Lee, S.; Kim, J.; Park, C.; Lee, B. Development of 3D-printed myoelectric hand orthosis for patients with spinal cord injury. J. NeuroEngineering Rehabil. 2019, 16, 162. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; He, G.; Cui, L.; Chen, C.; Secco, E.L.; Yao, W.; Xie, J.; Xu, G.; Wurdemann, H. Attention enhancement for exoskeleton-assisted hand rehabilitation using fingertip haptic stimulation. Front. Robot. AI 2021, 8, 602091. [Google Scholar] [CrossRef] [PubMed]
- Thomann, G.; de Carvalho, V.A. Personalized upper limb orthosis necessitates variety of tools during the development process: Hemiplegic child case study. Disabil. Rehabil. Assist. Technol. 2021, 16, 188–195. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Bouteraa, Y. A newly-designed wearable robotic hand exoskeleton controlled by emg signals and ros embedded systems. Robotics 2023, 12, 95. [Google Scholar] [CrossRef]
- Ohara, Y.; Kanie, J.; Hori, K. Fabrication of a highly protective 3D-printed mask and evaluation of its viral filtration efficiency using a human head mannequin. HardwareX 2022, 11, e00314. [Google Scholar] [CrossRef]
- O’Connor, Z.; Huellewig, D.; Sithiyopasakul, P.; Morris, J.A.; Gan, C.; Ballard, D.H. 3D printed mask extenders as a supplement to isolation masks to relieve posterior auricular discomfort: An innovative 3D printing response to the covid-19 pandemic. 3D Print. Med. 2020, 6, 27. [Google Scholar] [CrossRef]
- Viera-Artiles, J.; Valdiande, J.J. 3D-printable headlight face shield adapter. Personal protective equipment in the covid-19 era. Am. J. Otolaryngol.—Head Neck Med. Surg. 2020, 41, 102576. [Google Scholar] [CrossRef] [PubMed]
- Vence, J.; Gil, C.; González-Rodríguez, L.; López-Álvarez, M. Thermal behavior of graphene oxide deposited on 3D-printed polylactic acid for photothermal therapy: An experimental–numerical analysis. J. Funct. Biomater. 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Slavkovic, V.; Palic, N.; Milenkovic, S.; Zivic, F.; Grujovic, N. Thermo-mechanical characterization of 4d-printed biodegradable shape-memory scaffolds using four-axis 3D-printing system. Materials 2023, 16, 5186. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Firoozi, N.; Tsai, C.T.; Wallace, M.B.; Kang, Y. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater. 2019, 83, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Suaréz, V.; Villalobos-Osnaya, A.; García-García, J.A.; De León-Hernández, A.; Sánchez-Pérez, C.; Espinosa-García, A.M. Validation of 3D-printed swabs for sampling in sars-cov-2 detection: A pilot study. Ann. Biomed. Eng. 2023, 51, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Alghounaim, M.; Almazeedi, S.; Youha, S.A.; Papenburg, J.; Alowaish, O.; Abdulhussain, G.; Al-Shemali, R.; Albuloushi, A.; Alzabin, S.; Al-Wogayan, K.; et al. Low-cost polyester-tipped three-dimensionally printed nasopharyngeal swab for the detection of severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2). J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Correa, M.; Cruz-Ortiz, D. Low-cost wearable band sensors of surface electromyography for detecting hand movements. Sensors 2022, 22, 5931. [Google Scholar] [CrossRef]
- Saini, D.; Kumar, A.; Mishra, H.K.; Gupta, V.; Mondal, B.; Mallick, Z.; Mandal, D. Surface potential modulation for improved mechanical energy harvesting and sensing in 3D printed biopolymer thermoelectret. Sens. Actuators A Phys. 2024, 365, 114858. [Google Scholar] [CrossRef]
- Zaidi, S.; Naik, P.; Ahmed, S. Three-dimensional printed instruments used in a septoplasty: A new paradigm in surgery. Laryngoscope Investig. Otolaryngol. 2021, 6, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Skelley, N.W. Design and development of a novel 3-d printed external fixation device for fracture stabilization. 3D Print. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Hari Raj, K.; Gnanavel, S.; Ramalingam, S. Investigation of 3D printed biodegradable PLA orthopedic screw and surface modified with nanocomposites (Ti–Zr) for biocompatibility. Ceram. Int. 2023, 49, 7299–7307. [Google Scholar] [CrossRef]
- Di-Luciano, A.; Gómez-Nuñez, R.; Acosta, F.; Rivas-Vega, L.; Morales-Cantón, V.; Trujillo-Alvarez, M.; Cernichiaro-Espinosa, L. Trocar shortening for pediatric vitreoretinal surgery with a 3D printed trocar spacer: Report of two cases. Arch. De La Soc. Española De Oftalmol. (Engl. Ed.) 2022, 97, 473–476. [Google Scholar] [CrossRef]
- Weidert, S.; Andress, S.; Linhart, C.; Suero, E.M.; Greiner, A.; Böcker, W.; Kammerlander, C.; Becker, C.A. 3D printing method for next-day acetabular fracture surgery using a surface filtering pipeline: Feasibility and 1-year clinical results. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 565–575. [Google Scholar] [CrossRef]
- Sidhu, V.S.; Cheng, T.L.; Lillia, J.; Bridge, C.; Little, D.G.; Gray, R.J. 3D printed models can guide safe halo pin PLAcement in patients with diastrophic dysplasia. Spine Deform. 2021, 9, 841–849. [Google Scholar] [CrossRef]
- Jin, J.Y.; Zhang, L.Y.; Guo, S.; Tang, K.; Zeng, L.; Xiang, R.; Liang, J.Y. Genetic analysis combined with 3D-printing assistant surgery in diagnosis and treatment for an x-linked hypophosphatemia patient. J. Clin. Lab. Anal. 2022, 36, e24243. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Liu, Y.C.C.; Silva, R.C. Fabrication of 3D models for microtia reconstruction using smartphone-based technology. Ann. Otol. Rhinol. Laryngol. 2022, 131, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Lu, W.; Liao, S.; Du, Q.; Deng, Z.; Lu, W. Combined application of modified three-dimensional printed anatomic templates and customized cutting blocks in pelvic reconstruction after pelvic tumor resection. J. Arthroplast. 2019, 34, 338–345.e1. [Google Scholar] [CrossRef]
- Duan X j Fan H q Wang F y He, P.; Yang, L. Application of 3D-printed customized guides in subtalar joint arthrodesis. Orthop. Surg. 2019, 11, 405–413. [Google Scholar] [CrossRef]
- Malikov, A.; Secen, A.E.; Divanlioglu, D.; Gunerhan, G.; Ocal, O.; Gunduz, U.K. The feasibility of creating image-based patient-specific drill guides for the atlantoaxial instabilities using open-source cad software and desktop 3D printers. World Neurosurg. 2022, 163, e377–e383. [Google Scholar] [CrossRef]
- Li, F.; Huang, X.; Wang, K.; Luo, B.; Zhang, F.; Chen, Z.; Li, Q.; Zhang, Y.; Qi, K.; Jin, C.; et al. Preparation and assessment of an individualized navigation template for lower cervical anterior transpedicular screw insertion using a three-dimensional printing technique. Spine 2018, 43, E348–E356. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, M.; Jain, V.; Gupta, V. Three dimensional printing of deformed ankle foot and pelvis using poly lactic acid for pre surgical PLAnning. Int. J. Interact. Des. Manuf. (IJIDeM) 2023. [Google Scholar] [CrossRef]
- Gielisch, M.; Heimes, D.; Thiem, D.G.E.; Boesing, C.; Krumpholtz, M.; Al-Nawas, B.; Kämmerer, P.W. Steam-sterilized and degradable fused filament fabrication-printed polylactide/polyhydroxyalkanoate surgical guides for dental implants: Are they accurate enough for static navigation? Int. J. Bioprint. 2023, 9, 655. [Google Scholar] [CrossRef]
- Diamantopoulos, S.; Kagkiouzis, I.; Patatoukas, G.; Kypraiou, E.; Kouloulias, V.; Efstathopoulos, E.; Platoni, K. Three dimensional printed electron beam modifier for total skin electron treatments. Med. Dosim. 2019, 44, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Robar, J.L.; Moran, K.; Allan, J.; Clancey, J.; Joseph, T.; Chytyk-Praznik, K.; MacDonald, R.L.; Lincoln, J.; Sadeghi, P.; Rutledge, R. Intrapatient study comparing 3D printed bolus versus standard vinyl gel sheet bolus for postmastectomy chest wall radiation therapy. Pract. Radiat. Oncol. 2018, 8, 221–229. [Google Scholar] [CrossRef]
- Semeniuk, O.; Cherpak, A.; Robar, J. Design and evaluation of 3D printable patient-specific applicators for gynecologic hdr brachytherapy. Med. Phys. 2021, 48, 4053–4063. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.W.; Park, J.; Yea, J.W.; Oh, S.A. Fabrication of 3D printed head phantom using PLAster mixed with polylactic acid powder for patient-specific QA in intensity-modulated radiotherapy. Sci. Rep. 2022, 12, 17500. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Sarkar, K.; Smith, D. 3D printed bismuth oxide-polylactic acid composites for radio-mimetic computed tomography spine phantoms. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2021, 109, 789–796. [Google Scholar] [CrossRef]
- Varallo, A.; Sarno, A.; Castriconi, R.; Mazzilli, A.; Loria, A.; del Vecchio, A.; Orientale, A.; Pilotti, I.A.M.; D’Andria, P.; Bliznakova, K.; et al. Fabrication of 3D printed patient-derived anthropomorphic breast phantoms for mammography and digital breast tomosynthesis: Imaging assessment with clinical x-ray spectra. Phys. Medica 2022, 98, 88–97. [Google Scholar] [CrossRef]
- Okkalidis, N.; Bliznakova, K. A voxel-by-voxel method for mixing two filaments during a 3D printing process for soft-tissue replication in an anthropomorphic breast phantom. Phys. Med. Biol. 2022, 67, 245019. [Google Scholar] [CrossRef]
- Tino, R.B.; Yeo, A.U.; Brandt, M.; Leary, M.; Kron, T. A customizable anthropomorphic phantom for dosimetric verification of 3D-printed lung, tissue, and bone density materials. Med. Phys. 2022, 49, 52–69. [Google Scholar] [CrossRef]
- Mille, M.M.; Griffin, K.T.; Maass-Moreno, R.; Lee, C. Fabrication of a pediatric torso phantom with multiple tissues represented using a dual nozzle thermoplastic 3D printer. J. Appl. Clin. Med. Phys. 2020, 21, 226–236. [Google Scholar] [CrossRef]
- Chiu, T.; Xiong, Z.; Parsons, D.; Folkert, M.R.; Medin, P.M.; Hrycushko, B. Low-cost 3D print–based phantom fabrication to facilitate interstitial prostate brachytherapy training program. Brachytherapy 2020, 19, 800–811. [Google Scholar] [CrossRef]
- Sasaki, D.K.; McGeachy, P.; Alpuche Aviles, J.E.; McCurdy, B.; Koul, R.; Dubey, A. A modern mold room: Meshing 3D surface scanning, digital design, and 3D printing with bolus fabrication. J. Appl. Clin. Med. Phys. 2019, 20, 78–85. [Google Scholar] [CrossRef]
- Han, X.; Fang, S.; Sheng, R.; Wang, Y.; Zhou, J.; Wang, J. Dosimetry verification of three-dimensional printed polylactic acid template-guided precision 125i seed implantation for lung cancer using a desktop three-dimensional printer. J. Appl. Clin. Med. Phys. 2021, 22, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Dukov, N.; Bliznakova, K.; Okkalidis, N.; Teneva, T.; Encheva, E.; Bliznakov, Z. Thermoplastic 3D printing technology using a single filament for producing realistic patient-derived breast models. Phys. Med. Biol. 2022, 67, 045008. [Google Scholar] [CrossRef] [PubMed]
- Okkalidis, N.; Bliznakova, K.; Kolev, N. A filament 3D printing approach for ct-compatible bone tissues replication. Phys. Medica 2022, 102, 96–102. [Google Scholar] [CrossRef]
- Thomazi, E.; Roman Jr, C.; Gamba, T.O.; Perottoni, C.A.; Zorzi, J.E. Pla-based ceramic composites for 3D printing of anthropomorphic simulators. Int. J. Adv. Manuf. Technol. 2023, 128, 5289–5300. [Google Scholar] [CrossRef]
- Chee, A.; Sierra-Ruiz, M.; Parikh, M.S.; Majid, A. Comparison of flexible 3D printed stenotic airway model versus standard model for therapeutic bronchoscopy training a proof of concept. J. Bronchol. Interv. Pulmonol. 2021, 28, 124–129. [Google Scholar] [CrossRef]
- Clifton, W.; Nottmeier, E.; Damon, A.; Dove, C.; Pichelmann, M. The future of biomechanical spine research: Conception and design of a dynamic 3D printed cervical myelography phantom. Cureus 2019, 11, e4591. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Yuan, M.; Perera, I.; O’Connor, A.; Evins, A.I.; Imahiyerobo, T.; Souweidane, M.; Hoffman, C. Developing a 3D composite training model for cranial remodeling. J. Neurosurg. Pediatr. 2019, 24, 632–641. [Google Scholar] [CrossRef]
- Wang, X.; Shujaat, S.; Shaheen, E.; Jacobs, R. Quality and haptic feedback of three-dimensionally printed models for simulating dental implant surgery. J. Prosthet. Dent. 2022, 131, 660–667. [Google Scholar] [CrossRef]
- DeZeeuw, J.; O’Regan, N.B.; Goudie, C.; Organ, M.; Dubrowski, A. Anatomical 3D-printed silicone prostate gland models and rectal examination task trainer for the training of medical residents and undergraduate medical students. Cureus 2020, 12, e9020. [Google Scholar] [CrossRef]
- Garling, R.J.; Jin, X.; Yang, J.; Khasawneh, A.H.; Harris, C.A. Low-cost endoscopic third ventriculostomy simulator with mimetic endoscope. J. Neurosurg. Pediatr. 2018, 22, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Podkovik, S.; Patchana, T.; Farr, S.; Brazdzionis, J.; Marino, M.; Savla, P.; Kashyap, S.; Chin, B.; Crouch, A.; Miulli, D.E. External ventricular drain (evd) PLAcement using a hands-on training session on a simple three-dimensional (3D) model. Cureus 2022, 14, e28014. [Google Scholar] [CrossRef]
- Risler, Z.; Magee, M.A.; Mazza, J.M.; Goodsell, K.; Au, A.K.; Lewiss, R.E.; Pugliese, R.S.; Ku, B. A three-dimensional printed low-cost anterior shoulder dislocation model for ultrasound-guided injection training. Cureus 2018, 10, e3536. [Google Scholar] [CrossRef]
- Engelbrecht, R.; Patey, C.; Dubrowski, A.; Norman, P. Development and evaluation of a 3D-printed adult proximal tibia model for simulation training in intraosseous access. Cureus 2020, 12, e12180. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.E.; McCullum, B.; Patey, C.; Dubrowski, A. Development and evaluation of a three-dimensional-printed pediatric intraosseous infusion simulator to enhance medical training. Cureus 2022, 14, e21080. [Google Scholar] [CrossRef]
- Gadaleta, D.J.; Huang, D.; Rankin, N.; Hsue, V.; Sakkal, M.; Bovenzi, C.; Huntley, C.T.; Willcox, T.; Pelosi, S.; Pugliese, R.; et al. 3D printed temporal bone as a tool for otologic surgery simulation. Am. J. Otolaryngol.—Head Neck Med. Surg. 2020, 41, 102273. [Google Scholar] [CrossRef]
- Haffner, M.; Quinn, A.; Hsieh, T.Y.; Strong, E.B.; Steele, T. Optimization of 3D print material for the recreation of patient-specific temporal bone models. Ann. Otol. Rhinol. Laryngol. 2018, 127, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Werz, S.M.; Zeichner, S.J.; Berg, B.I.; Zeilhofer, H.F.; Thieringer, F. 3D printed surgical simulation models as educational tool by maxillofacial surgeons. Eur. J. Dent. Educ. 2018, 22, e500–e505. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Goldfarb, J.; Moayer, R.; Nwagu, U.; Ganti, R.; Krein, H.; Heffelfinger, R.; Hutchinson, M.L. Design and printing of a low-cost 3D-printed nasal osteotomy training model: Development and feasibility study. JMIR Med. Educ. 2020, 6, e19792. [Google Scholar] [CrossRef] [PubMed]
- Mashari, A.; Montealegre-Gallegos, M.; Jeganathan, J.; Yeh, L.; Hiansen, J.Q.; Meineri, M.; Mahmood, F.; Matyal, R. Low-cost three-dimensional printed phantom for neuraxial anesthesia training: Development and comparison to a commercial model. PLoS ONE 2018, 13, e0191664. [Google Scholar] [CrossRef] [PubMed]
- Bohl, M.A.; Morgan, C.D.; Mooney, M.A.; Repp, G.J.; Lehrman, J.N.; Kelly, B.P.; Chang, S.W.; Turner, J.D.; Kakarla, U.K. Biomechanical testing of a 3D-printed l5 vertebral body model. Cureus 2019, 11, e3893. [Google Scholar] [CrossRef] [PubMed]
- Leonov, D.; Kodenko, M.; Leichenco, D.; Nasibullina, A.; Kulberg, N. Design and validation of a phantom for transcranial ultrasonography. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Acar, T.; Karakas, A.B.; Ozer, M.A.; Koc, A.M.; Govsa, F. Building three-dimensional intracranial aneurysm models from 3D-tof mra: A validation study. J. Digit. Imaging 2019, 32, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Nägl, K.; Reisinger, A.; Pahr, D.H. The biomechanical behavior of 3D printed human femoral bones based on generic and patient-specific geometries. 3D Print. Med. 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Tseng, H.-C.; Liu, C.-H.; Tsai, S.-Y.; Chen, J.-H.; Chu, Y.-H.; Li, S.-T.; Lee, J.-J.; Liao, W.-C. Effects of the individual three-dimensional printed craniofacial bones with a quick response code on the skull spatial knowledge of undergraduate medical students. Anat. Sci. Educ. 2023, 16, 858–869. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, X.; Li, C.; Li, P.; Lin, Z.; Huang, S. 3D printed trabeculae conditionally reproduce the mechanical properties of the actual trabeculae—A preliminary study. Heliyon 2022, 8, e12101. [Google Scholar] [CrossRef]
- Clifton, W.; Nottmeier, E.; Damon, A.; Dove, C.; Chen, S.G.; Pichelmann, M. A feasibility study for the production of three-dimensional-printed spine models using simultaneously extruded thermoplastic polymers. Cureus 2019, 11, e4440. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Skyrman, S.; Kor, F.; Homan, R.; El-Hajj, V.G.; Babic, D.; Edström, E.; Elmi-Terander, A.; Hendriks, B.H.W.; de With, P.H.N. Development of a CT-compatible, anthropomorphic skull and brain phantom for neurosurgical PLAnning, training, and simulation. Bioengineering 2022, 9, 537. [Google Scholar] [CrossRef]
- Kerwin, J.; Yücesoy, A.; Vidhate, S.; Dávila-Montero, B.M.; Van Orman, J.L.; Pence, T.J.; Tartis, M.; Mejía-Alvarez, R.; Willis, A.M. Sulcal cavitation in linear head acceleration: Possible correlation with chronic traumatic encephalopathy. Front. Neurol. 2022, 13, 832370. [Google Scholar] [CrossRef] [PubMed]
- Seckeler, M.D.; Boe, B.A.; Barber, B.J.; Berman, D.P.; Armstrong, A.K. Use of rotational angiography in congenital cardiac catheterisations to generate three-dimensional-printed models. Cardiol. Young 2021, 31, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).