Development of an Oral Epithelial Ex Vivo Organ Culture Model for Biocompatibility and Permeability Assessment of Biomaterials
Abstract
1. Introduction
2. Materials and Methods
- A.
- Epi-ExPer device
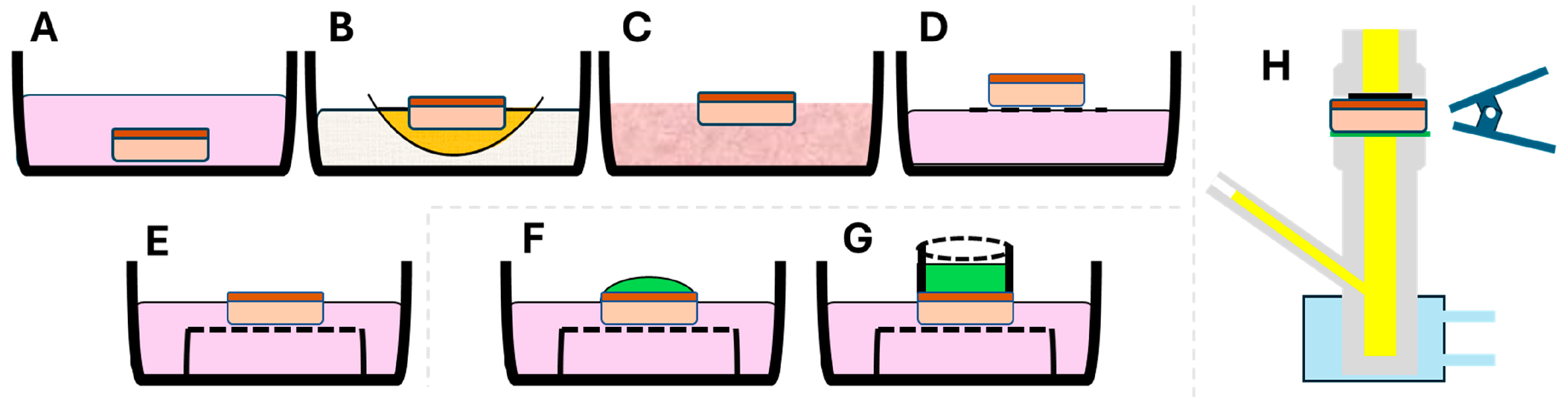
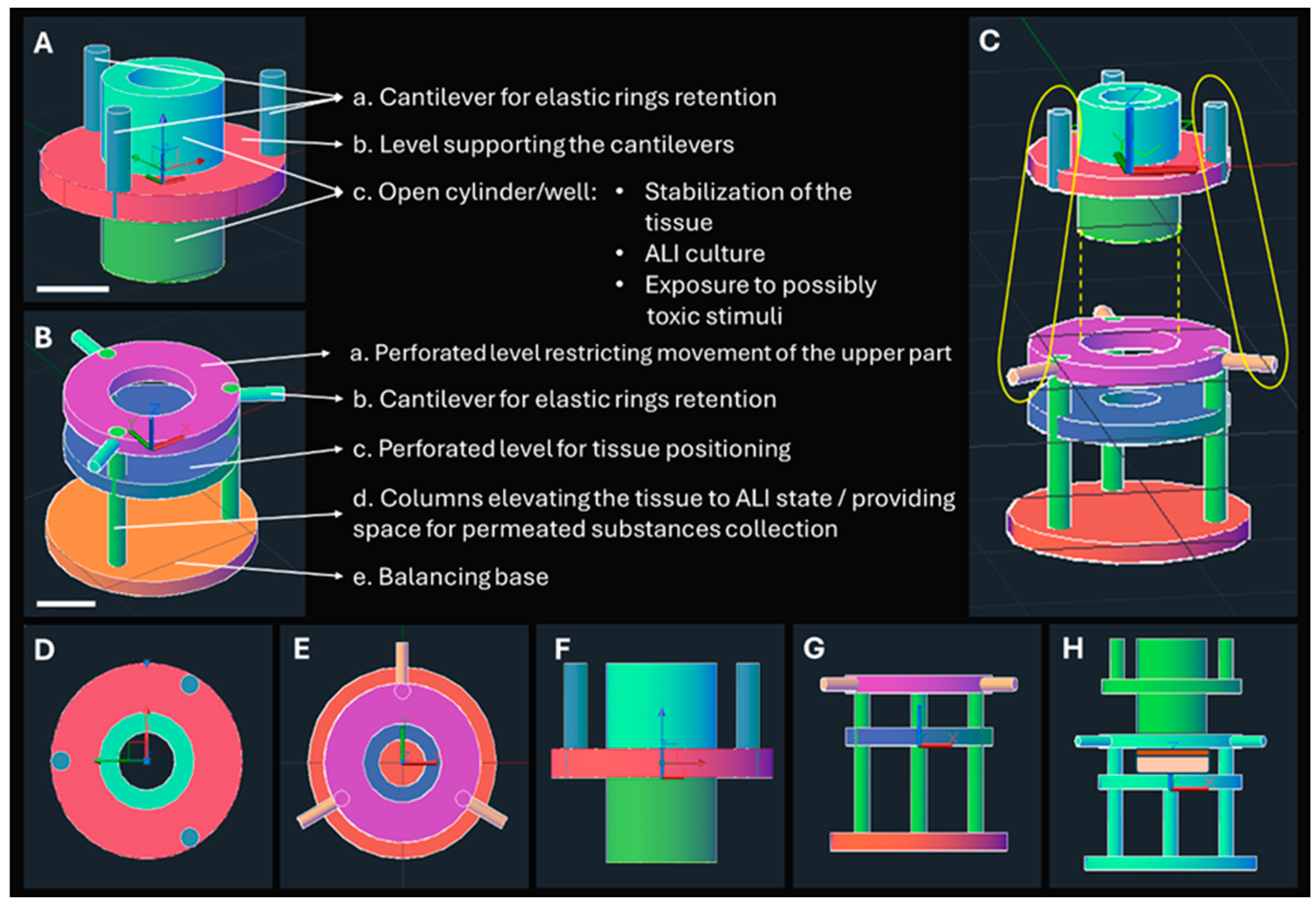
2.1. Design of the Epi-ExPer Device
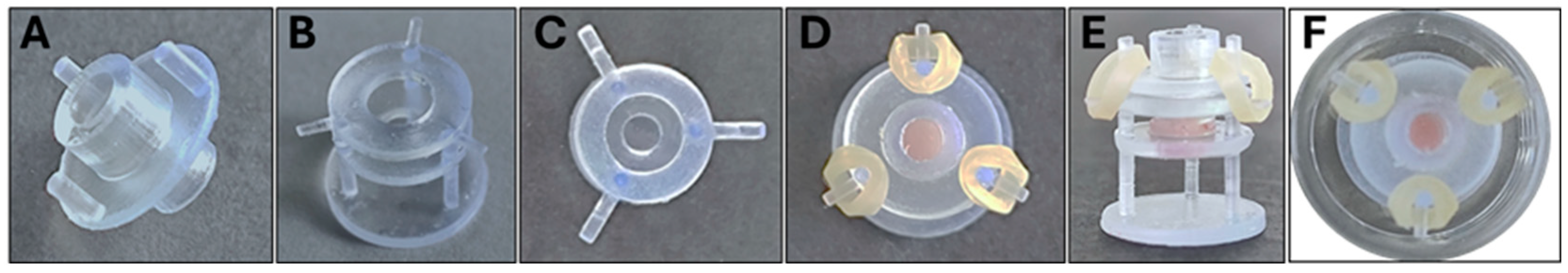
2.2. Additive Manufacturing and Assembly of the Epi-ExPer Device
- B.
- Evaluation of toxic stimuli on the Epi-ExPer device
2.3. Epithelial Organ/Tissue Biopsy and Cultivation
2.4. Exposure of the Epithelial Tissue to Common Toxic Stimuli
2.5. Transepithelial Permeation of Calcein
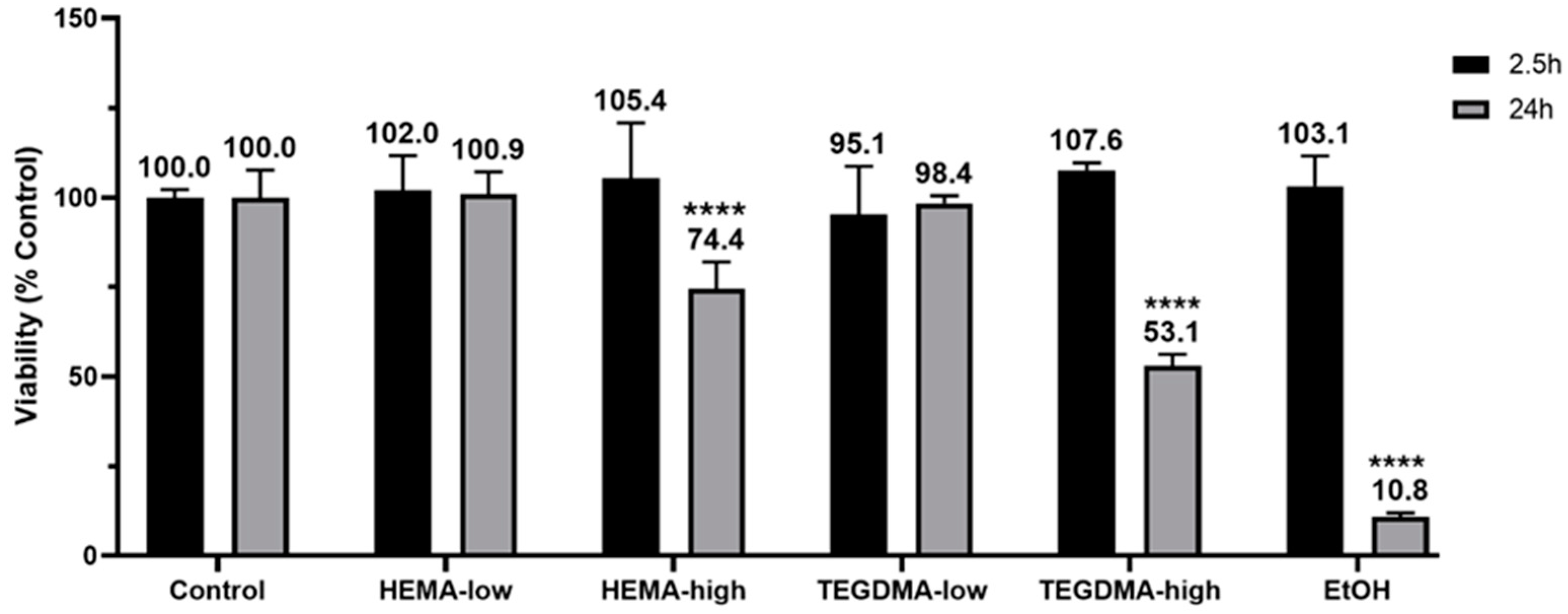
2.6. Effects of Resinous Monomers on Tissue Viability
2.7. Histological Assessment
2.8. Whole Mount Architecture Evaluation via 3D X-ray Histology
2.9. Histological Sectioning and Imaging
2.10. Immunofluorescence (IF)
2.11. Statistical Analysis
3. Results
3.1. Design of Epi-ExPer Device
3.2. Manufactured and Assembled Epi-ExPer Device
3.3. Transepithelial Permeation of Calcein Assay
3.4. Effect of Resinous Monomers on Tissue Viability
3.5. Whole Mount Architecture Evaluation via 3D X-ray Histology
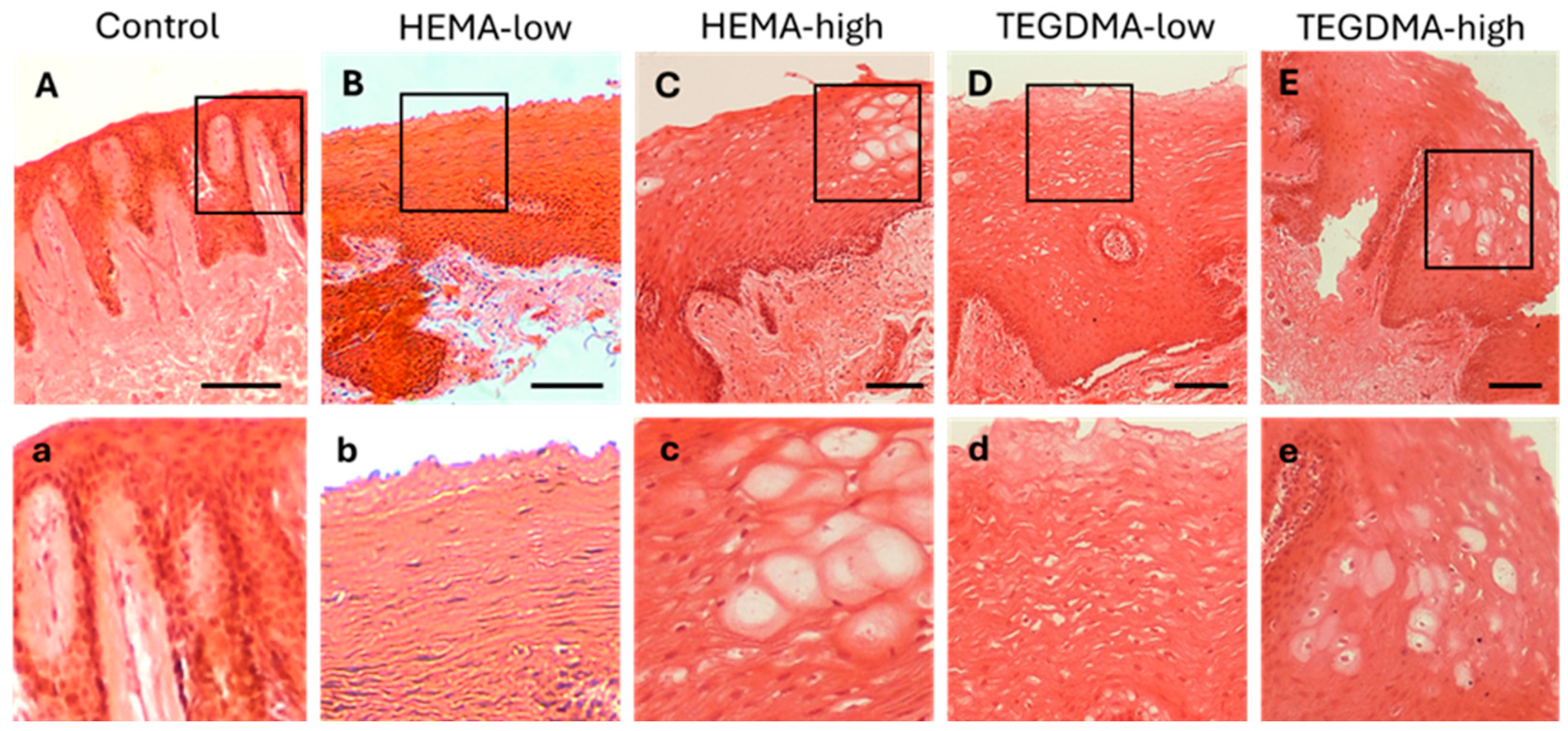
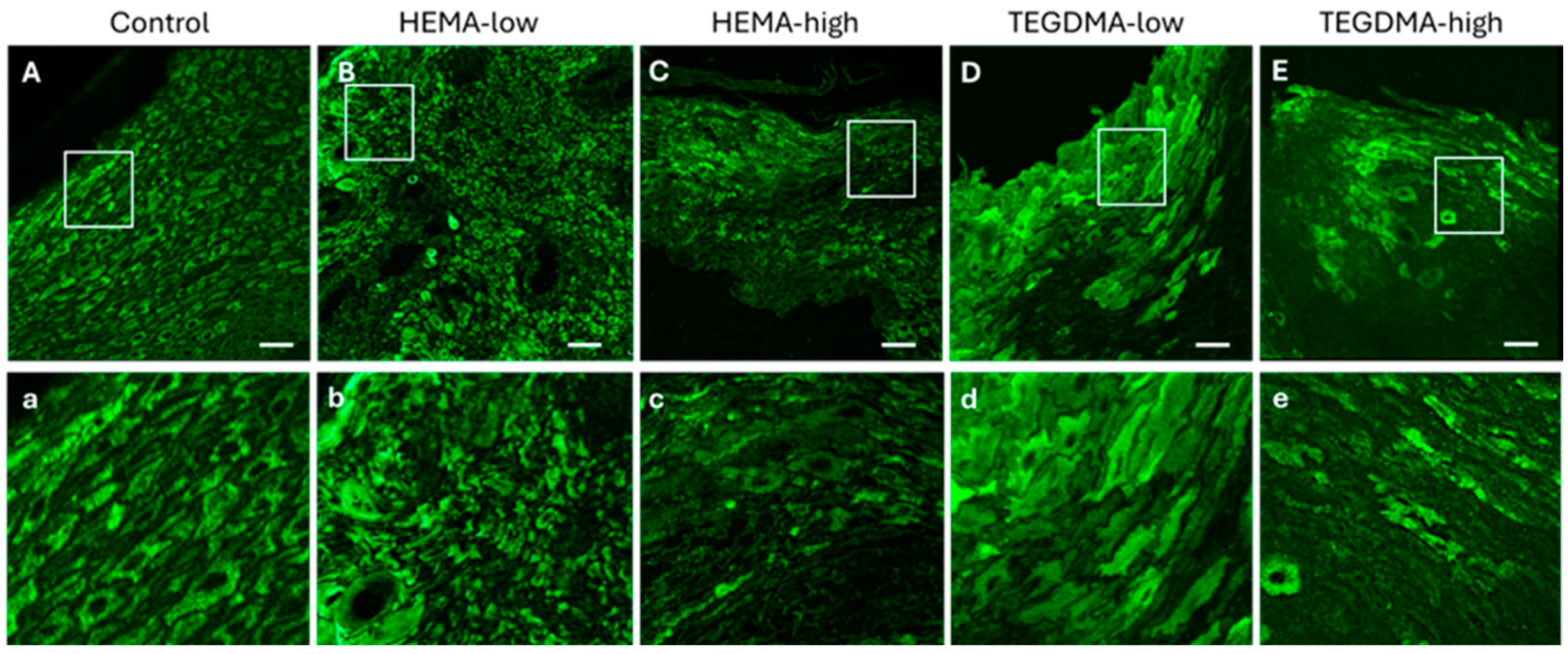
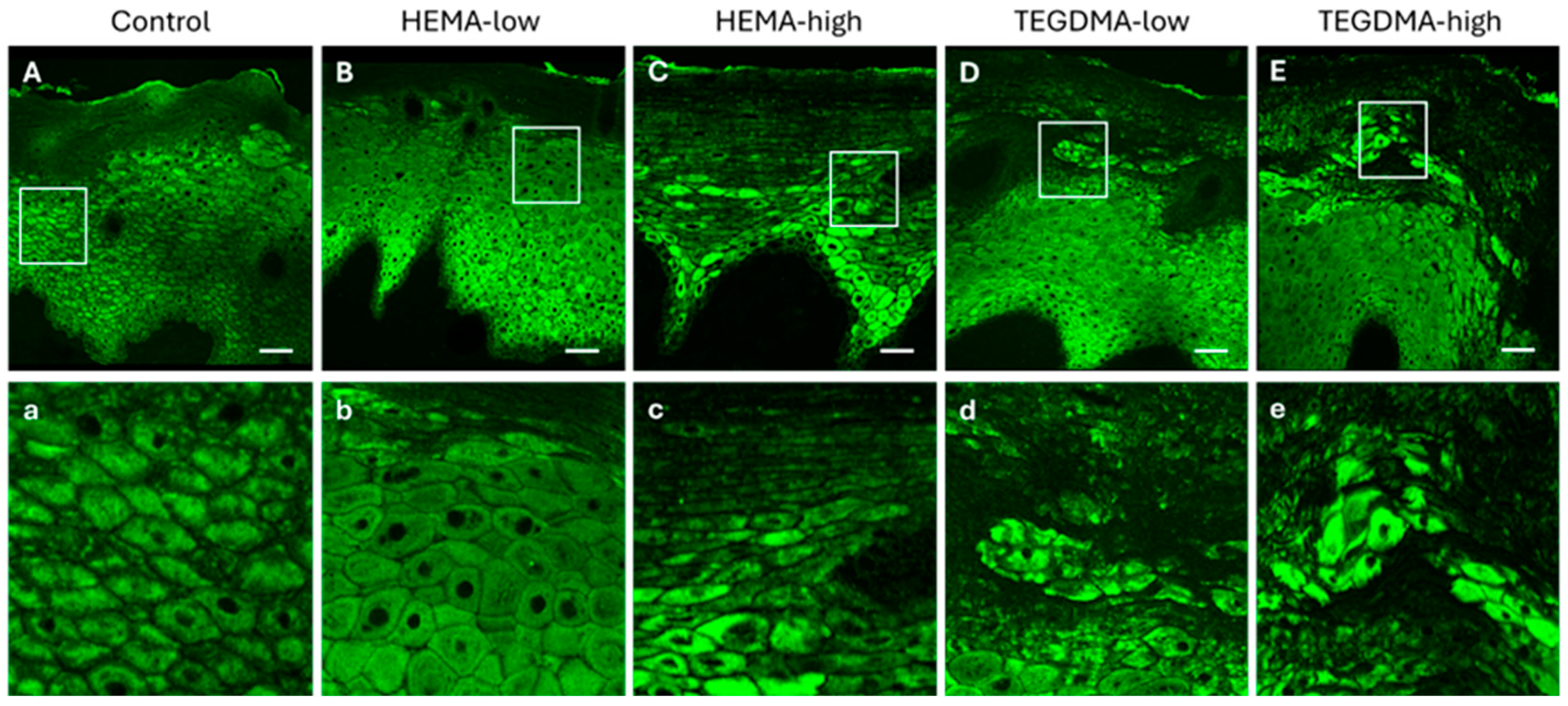
3.6. Histological and IF Analyses of Exposed Human Epithelial Tissues
3.7. Histological and IF Analyses of Exposed Porcine Epithelial Tissues
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şenel, S. An Overview of Physical, Microbiological and Immune Barriers of Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 7821. [Google Scholar] [CrossRef]
- Afonso, M.; Buzalaf, R.; Reis Hannas, A.; Kato, M.T. Saliva and Dental Erosion. J. Appl. Oral Sci. 2012, 20, 493–502. [Google Scholar]
- Reinhart, J.P.; Stoopler, E.T.; Crawford, G.H. Oral Hypersensitivity Reactions. Dermatol. Clin. 2020, 38, 467–476. [Google Scholar] [CrossRef]
- Hensten-Pettersen, A. Casting Alloys: Side-Effects. Adv. Dent. Res. 1992, 6, 38–43. [Google Scholar] [CrossRef]
- Sato, H.; Yamada, K.; Miyake, M.; Onoue, S. Recent Advancements in the Development of Nanocarriers for Mucosal Drug Delivery Systems to Control Oral Absorption. Pharmaceutics 2023, 15, 2708. [Google Scholar] [CrossRef]
- Díaz, L.; Zambrano-González, E.; Flores, M.E.; Contreras, M.; Crispín, J.C.; Alemán, G.; Bravo, C.; Armenta-Espinosa, A.; Valdés, V.J.; Tovar, A.; et al. Ethical Considerations in Animal Research: The Principle of 3R’s. Rev. Investig. 2021, 73, 199–209. [Google Scholar] [CrossRef]
- Adashi, E.Y.; O’Mahony, D.P.; Cohen, I.G. The FDA Modernization Act 2.0: Drug Testing in Animals Is Rendered Optional. Am. J. Med. 2023, 136, 853–854. [Google Scholar] [CrossRef]
- Bostanci, N.; Bao, K.; Wahlander, A.; Grossmann, J.; Thurnheer, T.; Belibasakis, G.N. Secretome of Gingival Epithelium in Response to Subgingival Biofilms. Mol. Oral Microbiol. 2015, 30, 323–335. [Google Scholar] [CrossRef]
- Thurnheer, T.; Belibasakis, G.N.; Bostanci, N. Colonisation of Gingival Epithelia by Subgingival Biofilms In Vitro: Role of “Red Complex” Bacteria. Arch. Oral Biol. 2014, 59, 977–986. [Google Scholar] [CrossRef]
- Hu, S.; Muniraj, G.; Mishra, A.; Hong, K.; Lum, J.L.; Hong, C.H.L.; Rosa, V.; Sriram, G. Characterization of Silver Diamine Fluoride Cytotoxicity Using Microfluidic Tooth-on-a-Chip and Gingival Equivalents. Dent. Mater. 2022, 38, 1385–1394. [Google Scholar] [CrossRef]
- Schmalz, G.; Schweikl, H.; Hiller, K. Release of Prostaglandin E2, IL-6 and IL-8 from Human Oral Epithelial Culturemodels after Exposure to Compounds of Dental Materials. Eur. J. Oral Sci. 2000, 108, 442–448. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Franklin, K.L.; Brook, I.M.; van Noort, R. Biologic Assessment of Antiseptic Mouthwashes Using a Three-Dimensional Human Oral Mucosal Model. J. Periodontol. 2009, 80, 769–775. [Google Scholar] [CrossRef]
- Ingendoh-Tsakmakidis, A.; Mikolai, C.; Winkel, A.; Szafrański, S.P.; Falk, C.S.; Rossi, A.; Walles, H.; Stiesch, M. Commensal and Pathogenic Biofilms Differently Modulate Peri-Implant oral Mucosa in an Organotypic Model. Cell. Microbiol. 2019, 21, e13078. [Google Scholar] [CrossRef]
- Buskermolen, J.K.; Janus, M.M.; Roffel, S.; Krom, B.P.; Gibbs, S. Saliva-Derived Commensal and Pathogenic Biofilms in a Human Gingiva Model. J. Dent. Res. 2018, 97, 201–208. [Google Scholar] [CrossRef]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Janus, M.M.; Krom, B.P.; Roffel, S.; Waaijman, T.; van Loveren, C.; Crielaard, W.; Gibbs, S. Multi-Species Oral Biofilm Promotes Reconstructed Human Gingiva Epithelial Barrier Function. Sci. Rep. 2018, 8, 16061. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A.M.; Smith, K.G.; Thornhill, M.H. Development, Optimization and Characterization of a Full-Thickness Tissue Engineered Human Oral Mucosal Model for Biological Assessment of Dental Biomaterials. J. Mater. Sci. Mater. Med. 2008, 19, 1793–1801. [Google Scholar] [CrossRef]
- Muniraj, G.; Tan, R.H.S.; Dai, Y.; Wu, R.; Alberti, M.; Sriram, G. Microphysiological Modeling of Gingival Tissues and Host-Material Interactions Using Gingiva-on-Chip. Adv. Healthc. Mater. 2023, 12, e2301472. [Google Scholar] [CrossRef]
- Jaroch, K.; Jaroch, A.; Bojko, B. Cell Cultures in Drug Discovery and Development: The Need of Reliable In Vitro-In Vivo Extrapolation for Pharmacodynamics and Pharmacokinetics Assessment. J. Pharm. Biomed. Anal. 2018, 147, 297–312. [Google Scholar] [CrossRef]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New Developments and Opportunities in Oral Mucosal Drug Delivery for Local and Systemic Disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Organ, Histotypic and Organotypic Culture, and Tissue Engineering. In Practical Approach to Mammalian Cell and Organ Culture; Springer Nature: Singapore, 2023; pp. 687–727. [Google Scholar]
- Dame, M.K.; Bhagavathula, N.; Mankey, C.; DaSilva, M.; Paruchuri, T.; Aslam, M.N.; Varani, J. Human Colon Tissue in Organ Culture: Preservation of Normal and Neoplastic Characteristics. Vitr. Cell. Dev. Biol.–Anim. 2010, 46, 114–122. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Paus, R.; Lu, Z. The Renaissance of Human Skin Organ Culture: A Critical Reappraisal. Differentiation 2018, 104, 22–35. [Google Scholar] [CrossRef]
- Nunes, S.F.; Murcia, P.R.; Tiley, L.S.; Brown, I.H.; Tucker, A.W.; Maskell, D.J.; Wood, J.L.N. An Ex Vivo Swine Tracheal Organ Culture for the Study of Influenza Infection. Influ. Other Respir. Viruses 2010, 4, 7–15. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Sloan, A.E. An Agarose-Based Cloning-Ring Anchoring Method for Isolation of Viable Cell Clones. BioTechniques 2009, 46, 305–307. [Google Scholar] [CrossRef]
- Sil, B.C.; Alvarez, M.P.; Zhang, Y.; Kung, C.; Hossain, M.; Iliopoulos, F.; Luo, L.; Crowther, J.M.; Moore, D.J.; Hadgraft, J.; et al. 3D-Printed Franz Type Diffusion Cells. Int. J. Cosmet. Sci. 2018, 40, 604–609. [Google Scholar] [CrossRef]
- Katsamenis, O.L.; Basford, P.J.; Robinson, S.K.; Boardman, R.P.; Konstantinopoulou, E.; Lackie, P.M.; Page, A.; Ratnayaka, J.A.; Goggin, P.M.; Thomas, G.J.; et al. A High-Throughput 3D X-ray Histology Facility for Biomedical Research and Preclinical Applications. Wellcome Open Res. 2023, 8, 366. [Google Scholar] [CrossRef]
- Franca, C.M.; Balbinot, G.d.S.; Cunha, D.; Saboia, V.d.P.A.; Ferracane, J.; Bertassoni, L.E. In-Vitro Models of Biocompatibility Testing for Restorative Dental Materials: From 2D Cultures to Organs on-a-Chip. Acta Biomater. 2022, 150, 58–66. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Hirashita, Y.; Shibata, T.; Fumoto, S.; Kurogi, S.; Nakada, C.; Kinoshita, K.; Fuchino, T.; Murakami, K.; Inomata, M.; et al. Patient-Derived Ex Vivo Cultures and Endpoint Assays with Surrogate Biomarkers in Functional Testing for Prediction of Therapeutic Response. Cancers 2023, 15, 4104. [Google Scholar] [CrossRef]
- Morales, M.; Pérez, D.; Correa, L.; Restrepo, L. Evaluation of Fibrin-Based Dermal-Epidermal Organotypic Cultures for In Vitro Skin Corrosion and Irritation Testing of Chemicals According to OECD TG 431 and 439. Toxicol. Vitr. 2016, 36, 89–96. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Brook, I.M.; Scutt, A.M.; Thornhill, M.H.; Van Noort, R. Mucotoxicity of Dental Composite Resins on a Tissue-Engineered Human Oral Mucosal Model. J. Dent. 2008, 36, 331–336. [Google Scholar] [CrossRef]
- Tigani, E.K.; Skrtic, D.; Valerio, M.S.; Kaufman, G. Assessing the Effect of Triethyleneglycol Dimethacrylate on Tissue Repair in 3D Organotypic Cultures. J. Appl. Toxicol. 2019, 39, 247–259. [Google Scholar] [CrossRef]
- Jain, N. Integrating Sustainability into Scientific Research. Nat. Rev. Methods Prim. 2022, 2, 35. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, W.; Dicolandrea, T.; Finlay, D.; Supp, D.; Boyce, S.; Wei, K.; Kadekaro, A.L.; Zhang, Y. An Improved Human Skin Explant Culture Method for Testing and Assessing Personal Care Products. J. Cosmet. Dermatol. 2023, 22, 1585–1594. [Google Scholar] [CrossRef]
- Demetriou, C.; Abid, N.; Butterworth, M.; Lezina, L.; Sandhu, P.; Howells, L.; Powley, I.R.; Pringle, J.H.; Sidat, Z.; Qassid, O.; et al. An Optimised Patient-Derived Explant Platform for Breast Cancer Reflects Clinical Responses to Chemotherapy and Antibody-Directed Therapy. Sci. Rep. 2024, 14, 12833. [Google Scholar] [CrossRef]
- Lu, Z.; Hasse, S.; Bodo, E.; Rose, C.; Funk, W.; Paus, R. Towards the Development of a Simplified Long-Term Organ Culture Method for Human Scalp Skin and Its Appendages under Serum-Free Conditions. Exp. Dermatol. 2007, 16, 37–44. [Google Scholar] [CrossRef]
- Vairo, S.; Van den Broeck, W.; Favoreel, H.; Scagliarini, A.; Nauwynck, H. Development and Use of a Polarized Equine Upper Respiratory Tract Mucosal Explant System to Study the Early Phase of Pathogenesis of a European Strain of Equine Arteritis Virus. Vet. Res. 2013, 44, 22. [Google Scholar] [CrossRef]
- Companjen, A.R.; van der Wel, L.I.; Wei, L.; Laman, J.D.; Prens, E.P. A Modified Ex Vivo Skin Organ Culture System for Functional Studies. Arch. Dermatol. Res. 2001, 293, 184–190. [Google Scholar] [CrossRef]
- Pagar, R.R.; Musale, S.R.; Pawar, G.; Kulkarni, D.; Giram, P.S. Comprehensive Review on the Degradation Chemistry and Toxicity Studies of Functional Materials. ACS Biomater. Sci. Eng. 2022, 8, 2161–2195. [Google Scholar] [CrossRef]
- Mazzinelli, E.; Favuzzi, I.; Arcovito, A.; Castagnola, R.; Fratocchi, G.; Mordente, A.; Nocca, G. Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics 2023, 15, 1559. [Google Scholar] [CrossRef]
- Munt, D.J.; Qi, Y.; Dash, A.K. Comparative Evaluation of the Munt-Dash Air-Interface Diffusion Chamber and Franz Chamber for the In Vitro Examination of Topical Spray Formulations. Int. J. Pharm. 2021, 608, 121127. [Google Scholar] [CrossRef]
- Neil, J.E.; Brown, M.B.; Williams, A.C. Human Skin Explant Model for the Investigation of Topical Therapeutics. Sci. Rep. 2020, 10, 21192. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular Toxicology of Substances Released from Resin–Based Dental Restorative Materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and Cellular Toxicology of Dental Resin Monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Samuelsen, J.T.; Dahl, J.E.; Karlsson, S.; Morisbak, E.; Becher, R. Apoptosis Induced by the Monomers HEMA and TEGDMA Involves Formation of ROS and Differential Activation of the MAP-Kinases p38, JNK and ERK. Dent. Mater. 2007, 23, 34–39. [Google Scholar] [CrossRef]
- Schmalz, G.; Krifka, S.; Schweikl, H. Toll-like Receptors, LPS, and Dental Monomers. Adv. Dent. Res. 2011, 23, 302–306. [Google Scholar] [CrossRef]
- Schweikl, H.; Widbiller, M.; Krifka, S.; Klement, J.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Buchalla, W. Interaction between LPS and a Dental Resin Monomer on Cell Viability in Mouse Macrophages. Dent. Mater. 2016, 32, 1492–1503. [Google Scholar] [CrossRef]
- Durner, J.; Kreppel, H.; Zaspel, J.; Schweikl, H.; Hickel, R.; Reichl, F.X. The Toxicokinetics and Distribution of 2-Hydroxyethyl Methacrylate in Mice. Biomaterials 2009, 30, 2066–2071. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, A.; Guo, J. Types and Evaluation of In Vitro Penetration Models for Buccal Mucosal Delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 102122. [Google Scholar] [CrossRef]
- Sa, G.; Xiong, X.; Wu, T.; Yang, J.; He, S.; Zhao, Y. Histological Features of Oral Epithelium in Seven Animal Species: As a Reference for Selecting Animal Models. Eur. J. Pharm. Sci. 2016, 81, 10–17. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Jeong, H.; Lee, N.; Hur, S.; Lee, N.; Han, J.J.; Jang, H.W.; Choi, W.K.; Nam, K.T.; Lim, K.-M. Ex Vivo Live Full-Thickness Porcine Skin Model as a Versatile In Vitro Testing Method for Skin Barrier Research. Int. J. Mol. Sci. 2021, 22, 657. [Google Scholar] [CrossRef]
- Pittermann, W.; Hopfgarten, F.; Kietzmann, M. The Skin Compatibility of Distilled Tall Oils: Evaluation with the Bovine Udder Skin In Vitro Model System. Altern. Lab. Anim. 2009, 37, 69–76. [Google Scholar] [CrossRef]
- Kandárová, H.; Liebsch, M.; Spielmann, H.; Genschow, E.; Schmidt, E.; Traue, D.; Guest, R.; Whittingham, A.; Warren, N.; Gamer, A.O.; et al. Assessment of the Human Epidermis Model SkinEthic RHE for In Vitro Skin Corrosion Testing of Chemicals According to New OECD TG 431. Toxicol. Vitr. 2006, 20, 547–559. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Meng, S.; Wang, Y.; Liu, D.; Gao, Z.; Zuo, A.; Guo, J. A Method for Evaluating Drug Penetration and Absorption through Isolated Buccal Mucosa with Highly Accuracy and Reproducibility. Drug Deliv. Transl. Res. 2022, 12, 2875–2892. [Google Scholar] [CrossRef]
- Gallorini, M.; Cataldi, A.; di Giacomo, V. HEMA-Induced Cytotoxicity: Oxidative Stress, Genotoxicity and Apoptosis. Int. Endod. J. 2014, 47, 813–818. [Google Scholar] [CrossRef]
- Squier, C.A. The Permeability of Oral Mucosa. Crit. Rev. Oral Biol. Med. 1991, 2, 13–32. [Google Scholar] [CrossRef]
- Italo Giannola, L.; De Caro, V.; Giandalia, G.; Gabriella Siragusa, M.; Paderni, C.; Campisi, G.; Maria Florena, A. 5-Fluorouracil Buccal Tablets for Locoregional Chemotherapy of Oral Squamous Cell Carcinoma: Formulation, Drug Release and Histological Effects on Reconstituted Human Oral Epithelium and Porcine Buccal Mucosa. Curr. Drug Deliv. 2010, 7, 109–117. [Google Scholar] [CrossRef]
- Khan, S.; Trivedi, V.; Boateng, J. Functional Physico-Chemical, Ex Vivo Permeation and Cell Viability Characterization of Omeprazole Loaded Buccal Films for Paediatric Drug Delivery. Int. J. Pharm. 2016, 500, 217–226. [Google Scholar] [CrossRef]
- Tazrart, A.; Bolzinger, M.A.; Moureau, A.; Molina, T.; Coudert, S.; Angulo, J.F.; Briancon, S.; Griffiths, N.M. Penetration and Decontamination of Americium-241 Ex Vivo Using Fresh and Frozen Pig Skin. Chem. Interact. 2017, 267, 40–47. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Farias, S.; Boateng, J.S. In Vitro, Ex Vivo and In Vivo Evaluation of Taste Masked Low Dose Acetylsalicylic Acid Loaded Composite Wafers as Platforms for Buccal Administration in Geriatric Patients with Dysphagia. Int. J. Pharm. 2020, 589, 119807. [Google Scholar] [CrossRef]
- Holm, R.; Meng-Lund, E.; Andersen, M.B.; Jespersen, M.L.; Karlsson, J.-J.; Garmer, M.; Jørgensen, E.B.; Jacobsen, J. In Vitro, Ex Vivo and In Vivo Examination of Buccal Absorption of Metoprolol with Varying pH in TR146 Cell Culture, Porcine Buccal Mucosa and Göttingen Minipigs. Eur. J. Pharm. Sci. 2013, 49, 117–124. [Google Scholar] [CrossRef]
- Bibi, H.A.; Holm, R.; Bauer-Brandl, A. Use of Permeapad® for Prediction of Buccal Absorption: A Comparison to In Vitro, Ex Vivo and In Vivo Method. Eur. J. Pharm. Sci. 2016, 93, 399–404. [Google Scholar] [CrossRef]




| Group | Jss · 10−4 ± SD (μg × cm−2 × min−1) | Papp · 10−6 ± SD (cm × h−1) |
|---|---|---|
| CCM—2.5 h | 0.72 ± 0.18 | 1.97 ± 0.14 |
| Control—2.5 h | 0.49 ± 0.04 | 2.90 ± 0.71 |
| HEMA-low—2.5 h | 0.55 ± 0.05 | 2.19 ± 0.21 |
| HEMA-high—2.5 h | 0.44 ± 0.05 | 1.76 ± 0.20 |
| TEGDMA-low—2.5 h | 0.53 ± 0.10 | 2.11 ± 0.42 |
| TEGDMA-high—2.5 h | 0.72 ± 0.46 | 2.87 ± 1.85 |
| EtOH—2.5 h | 0.66 ± 0.21 | 2.66 ± 0.84 |
| CCM—24 h | 0.98 ± 0.58 | 3.94 ± 2.31 |
| Control—24 h | 0.61 ± 0.04 | 2.46 ± 0.14 |
| HEMA-low—24 h | 0.85 ± 0.45 | 3.40 ± 1.80 |
| HEMA-high—24 h | 2.56 ± 2.73 | 10.25 ± 10.92 |
| TEGDMA-low—24 h | 0.44 ± 0.07 | 1.77 ± 0.27 |
| TEGDMA-high—24 h | 7.38 ± 0.50 | 29.51 ± 1.98 |
| EtOH—24 h | 16.73 ± 5.50 | 66.91 ± 21.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machla, F.; Bekiari, C.; Monou, P.K.; Kofidou, E.; Theodosaki, A.M.; Katsamenis, O.L.; Zisis, V.; Kokoti, M.; Bakopoulou, A.; Fatouros, D.; et al. Development of an Oral Epithelial Ex Vivo Organ Culture Model for Biocompatibility and Permeability Assessment of Biomaterials. Bioengineering 2024, 11, 1035. https://doi.org/10.3390/bioengineering11101035
Machla F, Bekiari C, Monou PK, Kofidou E, Theodosaki AM, Katsamenis OL, Zisis V, Kokoti M, Bakopoulou A, Fatouros D, et al. Development of an Oral Epithelial Ex Vivo Organ Culture Model for Biocompatibility and Permeability Assessment of Biomaterials. Bioengineering. 2024; 11(10):1035. https://doi.org/10.3390/bioengineering11101035
Chicago/Turabian StyleMachla, Foteini, Chrysanthi Bekiari, Paraskevi Kyriaki Monou, Evangelia Kofidou, Astero Maria Theodosaki, Orestis L. Katsamenis, Vasileios Zisis, Maria Kokoti, Athina Bakopoulou, Dimitrios Fatouros, and et al. 2024. "Development of an Oral Epithelial Ex Vivo Organ Culture Model for Biocompatibility and Permeability Assessment of Biomaterials" Bioengineering 11, no. 10: 1035. https://doi.org/10.3390/bioengineering11101035
APA StyleMachla, F., Bekiari, C., Monou, P. K., Kofidou, E., Theodosaki, A. M., Katsamenis, O. L., Zisis, V., Kokoti, M., Bakopoulou, A., Fatouros, D., & Andreadis, D. (2024). Development of an Oral Epithelial Ex Vivo Organ Culture Model for Biocompatibility and Permeability Assessment of Biomaterials. Bioengineering, 11(10), 1035. https://doi.org/10.3390/bioengineering11101035









