Effects of Oral Cavity Stem Cell Sources and Serum-Free Cell Culture on Hydrogel Encapsulation of Mesenchymal Stem Cells for Bone Regeneration: An In Vitro Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Compliance with Ethical Standards
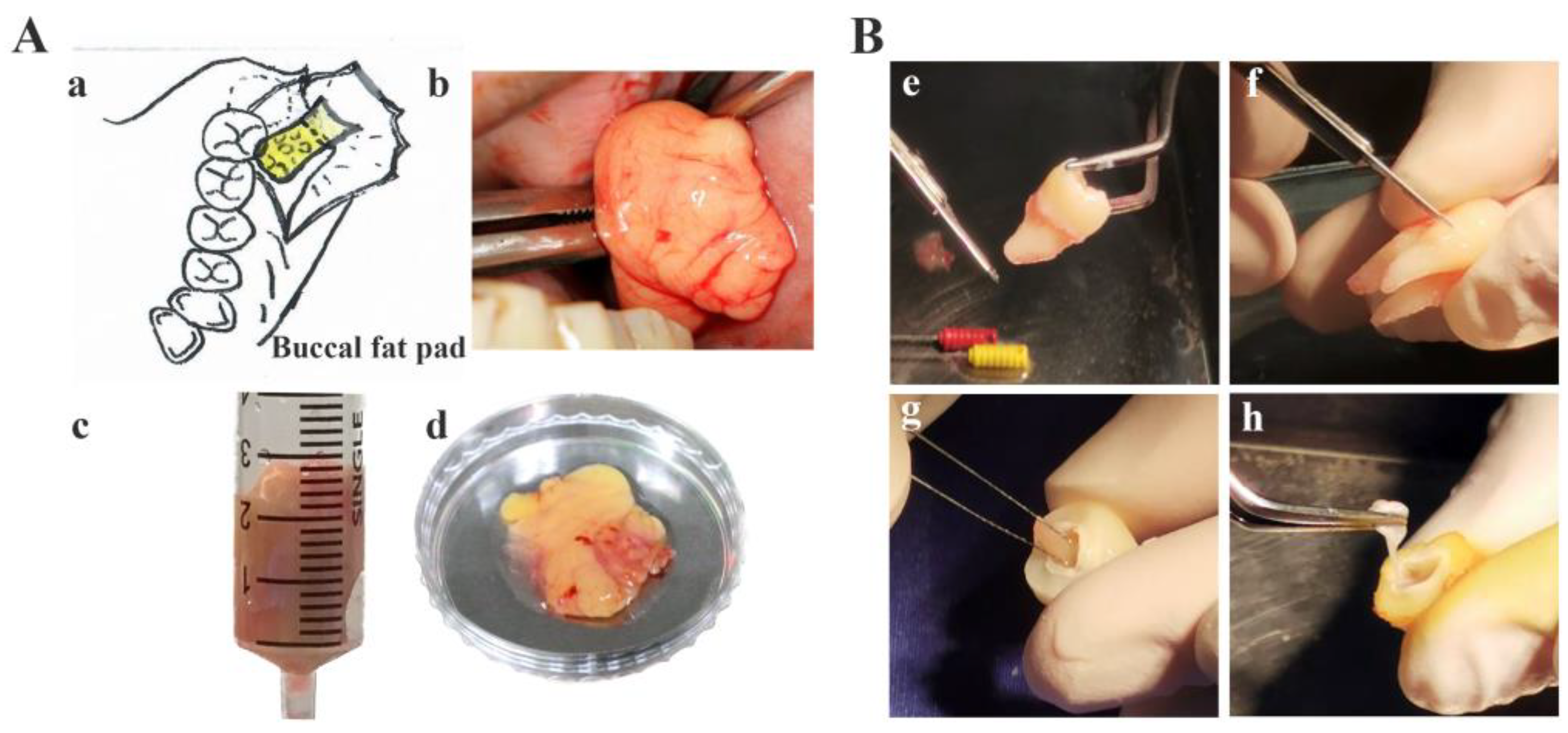
2.2. Sample Collection
2.3. Human Buccal Fat Pad, Periodontal Ligament, and Dental Pulp Isolation and Expansion
2.4. Types of Sera and Cell Batches
2.5. Study Groups
2.6. Hydrogel Cell Encapsulation
2.7. Flow Cytometry Analysis of Cell Surface Antigens
2.8. Cell Proliferation
2.9. Cell Viability and Live/Dead Cell Staining Assays
2.10. Alkaline Phosphatase and Alizarin Red Staining
2.11. Measuring Total Protein Levels and Alkaline Phosphatase Activity
2.12. Measuring Calcium Levels in the Extracellular Matrix
2.13. Measuring Osteocalcin Levels in Culture Medium
2.14. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.15. Statistical Analysis
3. Results
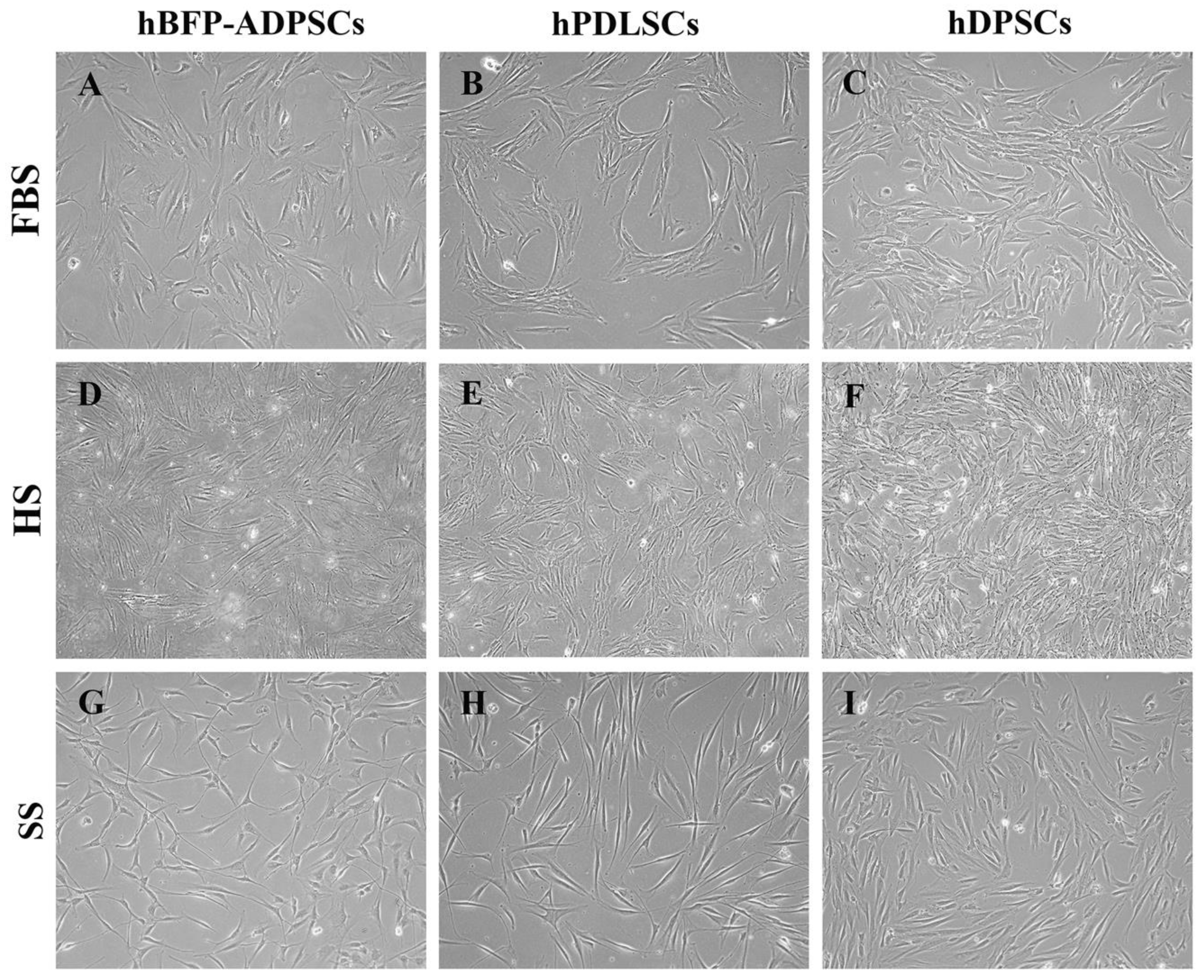
3.1. Cell Morphology and Expression of MSC Surface Antigens
3.2. Effects of Stem Cell Sources on Osteogenic Differentiation in Synthetic Serum
3.3. Effects of Sera on Growth and Osteogenic Differentiation
3.4. Effects of the Hydrogel Cell Encapsulation Model on Growth and Osteogenic Differentiation
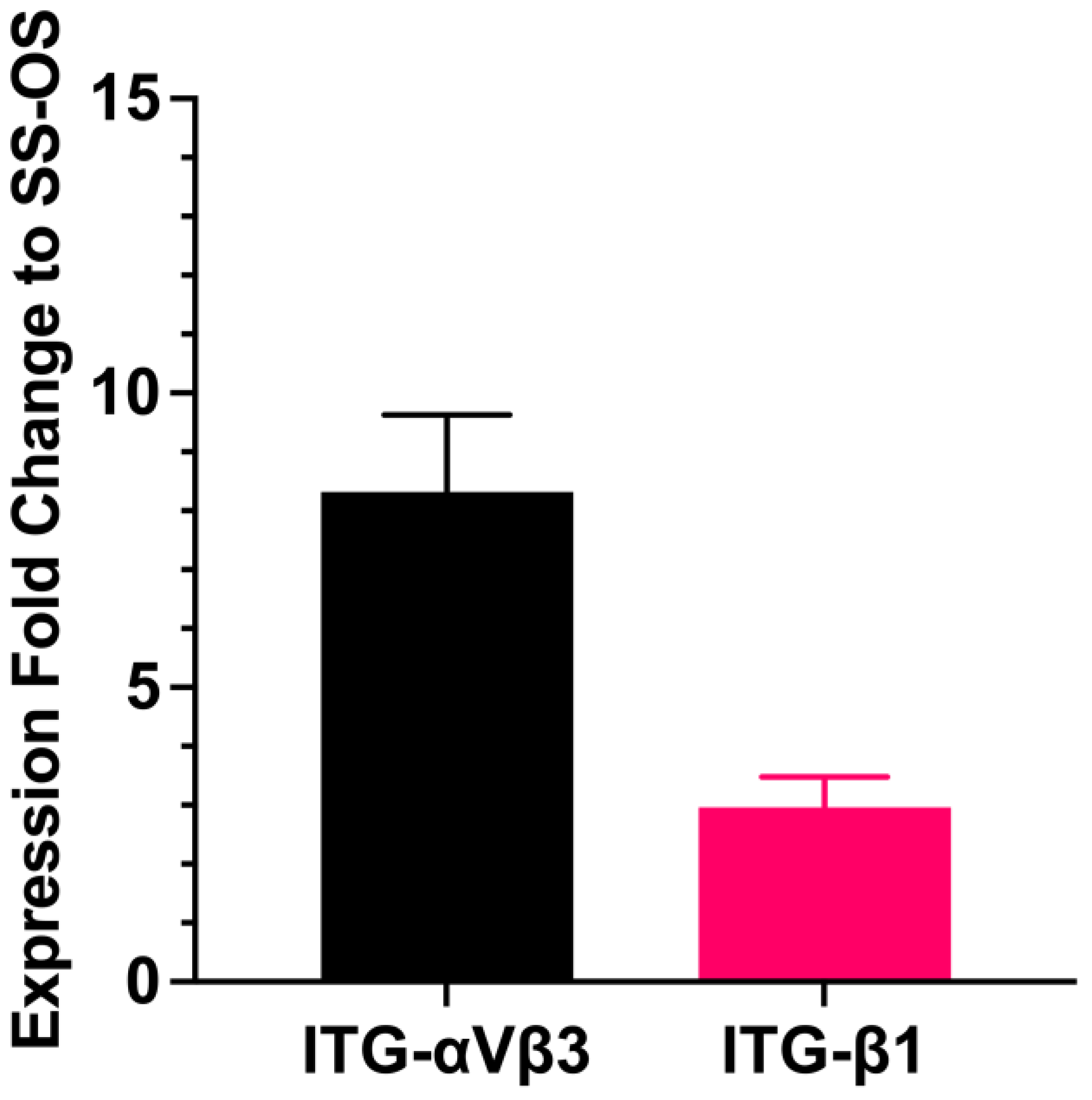
3.5. Effects of Sera on the Synthesis of an Extracellular Matrix and Cell Adhesion Molecules of the Encapsulated Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurniawan, A.; Ivansyah, M.D.; Dilogo, I.H.; Hutami, W.D. Umbilical cord mesenchymal stem cells combined with secretome for treating congenital pseudarthrosis of the Tibia: A case series. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Jiang, Y.; Qian, Y.; Du, J.; Yu, X.; Luo, S.; Chen, Z. The Emerging Biological Functions of Exosomes from Dental Tissue-Derived Mesenchymal Stem Cells. Cell Reprogram. 2023, 25, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Yassin, M.A.; Rashad, A.; Espedal, H.; Idris, S.B.; Finne-Wistrand, A.; Mustafa, K.; Vindenes, H.; Fristad, I. Comparison of bone regenerative capacity of donor-matched human adipose-derived and bone marrow mesenchymal stem cells. Cell Tissue Res. 2021, 383, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Chua, J.; Prasadh, S.; Lim, J.; Saigo, L.; Goh, B.T. Alveolar Ridge Augmentation with a Novel Combination of 3D-Printed Scaffolds and Adipose-Derived Mesenchymal Stem Cells-A Pilot Study in Pigs. Biomedicines 2023, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Uri, O.; Behrbalk, E.; Folman, Y. Local implantation of autologous adipose-derived stem cells increases femoral strength and bone density in osteoporotic rats: A randomized controlled animal study. J. Orthop. Surg. 2018, 26, 2309499018799534. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.; Fierravanti, L.; Nunez, J.; Vignoletti, F.; Gonzalez-Zamora, M.; Santamaria, S.; Suarez-Sancho, S.; Fernandez-Santos, M.E.; Figuero, E.; Herrera, D.; et al. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J. Clin. Periodontol. 2020, 47, 1391–1402. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng. Part A. 2014, 20, 611–621. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, W.; Liu, C.; Wang, Z.; Liu, Y.; Yu, Y.; Jian, C.; Yu, A. Exosomes Derived from Adipose Stem Cells Enhance Bone Fracture Healing via the Activation of the Wnt3a/beta-Catenin Signaling Pathway in Rats with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 4852. [Google Scholar] [CrossRef]
- Khojasteh, A.; Hosseinpour, S.; Rad, M.R.; Alikhasi, M. Buccal Fat Pad-Derived Stem Cells in Three-Dimensional Rehabilitation of Large Alveolar Defects: A Report of Two Cases. J. Oral. Implantol. 2019, 45, 45–54. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Tudela-Mulero, M.R.; Navarro, J.A.; Buendia, A.J.; Mercado-Diaz, A.M. Use of buccal fat pad-derived stem cells cultured on bioceramics for repair of critical-sized mandibular defects in healthy and osteoporotic rats. Clin. Oral. Investig. 2022, 26, 5389–5408. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Hsu, Y.K.; Chuang, M.H.; Chu, C.M.; Lin, P.C.; Liao, J.H.; Lin, S.Z.; Kuo, T.F. Enhanced Bone Formation in Osteoporotic Mice by a Novel Transplant Combined with Adipose-derived Stem Cells and Platelet-rich Fibrin Releasates. Cell Transplant 2020, 29, 963689720927398. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Ito, K.; Hofmann, S. Development of serum substitute medium for bone tissue engineering. J. Biomed. Mater. Res. A 2023, 111, 1423–1440. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz, N.; Hoffman, E.; Clements, J.; Cruickshank, R.; Doherty, A.; Ebner, D.; Elloway, J.; Fu, J.; Kelsall, J.; Millar, V.; et al. Longitudinal characterization of TK6 cells sequentially adapted to animal product-free, chemically defined culture medium: Considerations for genotoxicity studies. Front. Toxicol. 2023, 5, 1177586. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Joyce, E.J.; Kao, W.J. Fetal bovine serum xenoproteins modulate human monocyte adhesion and protein release on biomaterials in vitro. Acta Biomater. 2011, 7, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Aussel, C.; Busson, E.; Vantomme, H.; Peltzer, J.; Martinaud, C. Quality assessment of a serum and xenofree medium for the expansion of human GMP-grade mesenchymal stromal cells. Peer J. 2022, 10, e13391. [Google Scholar] [CrossRef]
- Shahdadfar, A.; Fronsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef]
- Chen, S.; Meng, L.; Wang, S.; Xu, Y.; Chen, W.; Wei, J. Effect assessment of a type of xeno-free and serum-free human adipose-derived mesenchymal stem cells culture medium by proliferation and differentiation capacities. Cytotechnology 2023, 75, 403–420. [Google Scholar] [CrossRef]
- Lensch, M.; Muise, A.; White, L.; Badowski, M.; Harris, D. Comparison of Synthetic Media Designed for Expansion of Adipose-Derived Mesenchymal Stromal Cells. Biomedicines 2018, 6, 54. [Google Scholar] [CrossRef]
- Hoang, V.T.; Trinh, Q.M.; Phuong, D.T.M.; Bui, H.T.H.; Hang, L.M.; Ngan, N.T.H.; Anh, N.T.T.; Nhi, P.Y.; Nhung, T.T.H.; Lien, H.T.; et al. Standardized xeno- and serum-free culture platform enables large-scale expansion of high-quality mesenchymal stem/stromal cells from perinatal and adult tissue sources. Cytotherapy 2021, 23, 88–99. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res. Ther. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Jia, L.; Xu, N.; Xiao, Y.; Li, Q. The Application of Stem Cells in Tissue Engineering for the Regeneration of Periodontal Defects in Randomized Controlled Trial: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2022, 22, 101713. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, H.; Ni, X.; Deng, Y.; Li, Z.; Xing, X.; Du, M. Programmed release of vascular endothelial growth factor and exosome from injectable chitosan nanofibrous microsphere-based PLGA-PEG-PLGA hydrogel for enhanced bone regeneration. Int. J. Biol. Macromol. 2023, 253, 126721. [Google Scholar] [CrossRef] [PubMed]
- Renaud, M.; Bousquet, P.; Macias, G.; Rochefort, G.Y.; Durand, J.O.; Marsal, L.F.; Cuisinier, F.; Cunin, F.; Collart-Dutilleul, P.Y. Allogenic Stem Cells Carried by Porous Silicon Scaffolds for Active Bone Regeneration In Vivo. Bioengineering 2023, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Akhlaghi, F.; Hesami, N.; Rad, M.R.; Nazeman, P.; Fahimipour, F.; Khojasteh, A. Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction. J. Craniomaxillofac Surg. 2019, 47, 1266–1273. [Google Scholar] [CrossRef]
- Perczel-Kovach, K.; Hegedus, O.; Foldes, A.; Sangngoen, T.; Kallo, K.; Steward, M.C.; Varga, G.; Nagy, K.S. STRO-1 positive cell expansion during osteogenic differentiation: A comparative study of three mesenchymal stem cell types of dental origin. Arch. Oral. Biol. 2021, 122, 104995. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef]
- Rathod, N.; Khobaragade, B.; Ganesan, K. Use of the temporal extension of the buccal fat pad for closure of oro-antral communications. Int. J. Oral. Maxillofac. Surg. 2021, 50, 1638–1642. [Google Scholar] [CrossRef]
- Broccaioli, E.; Niada, S.; Rasperini, G.; Ferreira, L.M.; Arrigoni, E.; Yenagi, V.; Brini, A.T. Mesenchymal Stem Cells from Bichat’s Fat Pad: In Vitro Comparison with Adipose-Derived Stem Cells from Subcutaneous Tissue. Biores. Open Access 2013, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Meshram, M.; Anchlia, S.; Shah, H.; Vyas, S.; Dhuvad, J.; Sagarka, L. Buccal Fat Pad-Derived Stem Cells for Repair of Maxillofacial Bony Defects. J. Maxillofac. Oral. Surg. 2019, 18, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Rezai Rad, M.; Bohloli, M.; Akhavan Rahnama, M.; Anbarlou, A.; Nazeman, P.; Khojasteh, A. Impact of Tissue Harvesting Sites on the Cellular Behaviors of Adipose-Derived Stem Cells: Implication for Bone Tissue Engineering. Stem Cells Int. 2017, 2017, 2156478. [Google Scholar] [CrossRef] [PubMed]
- Haumer, A.; Bourgine, P.E.; Occhetta, P.; Born, G.; Tasso, R.; Martin, I. Delivery of cellular factors to regulate bone healing. Adv. Drug Deliv. Rev. 2018, 129, 285–294. [Google Scholar] [CrossRef]
- Sareethammanuwat, M.; Boonyuen, S.; Arpornmaeklong, P. Effects of beta-tricalcium phosphate nanoparticles on the properties of a thermosensitive chitosan/collagen hydrogel and controlled release of quercetin. J. Biomed. Mater. Res. A 2021, 109, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Pripatnanont, P.; Suwatwirote, N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int. J. Oral. Maxillofac. Surg. 2008, 37, 357–366. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Jaiman, N.; Apinyauppatham, K.; Fuongfuchat, A.; Boonyuen, S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers 2023, 15, 416. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef]
- Khojasteh, A.; Sadeghi, N. Application of buccal fat pad-derived stem cells in combination with autogenous iliac bone graft in the treatment of maxillomandibular atrophy: A preliminary human study. Int. J. Oral. Maxillofac. Surg. 2016, 45, 864–871. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sutthitrairong, C.; Jantaramanant, P.; Pripatnanont, P. Allogenic human serum, a clinical grade serum supplement for promoting human periodontal ligament stem cell expansion. J. Tissue Eng. Regen. Med. 2018, 12, 142–152. [Google Scholar] [CrossRef]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral. Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Piattelli, A.; Gatta, V.; Marchisio, M.; Diomede, F.; D’Aurora, M.; Merciaro, I.; Pierdomenico, L.; Maraldi, N.M.; Zini, N. Assessment of an efficient xeno-free culture system of human periodontal ligament stem cells. Tissue Eng. Part C Methods 2015, 21, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Nakahara, T. Establishment of xenogeneic serum-free culture methods for handling human dental pulp stem cells using clinically oriented in-vitro and in-vivo conditions. Stem Cell Res. Ther. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Brown, S.E.; Wang, Z.; Krebsbach, P.H. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009, 18, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Kocaoemer, A.; Kern, S.; Kluter, H.; Bieback, K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007, 25, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; You, H.; Qin, N.; Zuo, W. Interleukin-10 Modulates the Metabolism and Osteogenesis of Human Dental Pulp Stem Cells. Cell Reprogram. 2021, 23, 270–276. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Pripatnanont, P.; Chookiatsiri, C.; Tangtrakulwanich, B. Effects of Titanium Surface Microtopography and Simvastatin on Growth and Osteogenic Differentiation of Human Mesenchymal Stem Cells in Estrogen-Deprived Cell Culture. Int. J. Oral. Maxillofac. Implants. 2017, 32, e35–e46. [Google Scholar] [CrossRef][Green Version]
- Arpornmaeklong, P.; Wang, Z.; Pressler, M.J.; Brown, S.E.; Krebsbach, P.H. Expansion and characterization of human embryonic stem cell-derived osteoblast-like cells. Cell Reprogram. 2010, 12, 377–389. [Google Scholar] [CrossRef]
- Arrigoni, E.; Lopa, S.; de Girolamo, L.; Stanco, D.; Brini, A.T. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: From small to large animal models. Cell Tissue Res. 2009, 338, 401–411. [Google Scholar] [CrossRef]
- Aubin, J.E. Advances in the osteoblast lineage. Biochem. Cell Biol. 1998, 76, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S. Development of the osteoblast phenotype: Molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop. J. 1995, 15, 118–140. [Google Scholar] [PubMed]
- Cimino, M.; Parreira, P.; Bidarra, S.J.; Goncalves, R.M.; Barrias, C.C.; Martins, M.C.L. Effect of surface chemistry on hMSC growth under xeno-free conditions. Colloids Surf. B Biointerfaces 2020, 189, 110836. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, P.; Moshrefi, M.; Eslaminejad, T. An Overview on Mesenchymal Stem Cells Derived from Extraembryonic Tissues: Supplement Sources and Isolation Methods. Stem Cells Cloning. 2020, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, Y.; Ning, X.; Li, H.; Li, Q.; Wu, J. The functional effects of Piezo channels in mesenchymal stem cells. Stem Cell Res. Ther. 2023, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Panella, S.; Muoio, F.; Jossen, V.; Harder, Y.; Eibl-Schindler, R.; Tallone, T. Chemically Defined Xeno- and Serum-Free Cell Culture Medium to Grow Human Adipose Stem Cells. Cells 2021, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Sagara, H.; Nakahara, T. Type I collagen facilitates safe and reliable expansion of human dental pulp stem cells in xenogeneic serum-free culture. Stem Cell Res. Ther. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gong, Y.; Cai, Z.; Deng, Y.; Shi, X.; Pan, H.; Xu, L.; Zhang, H. A composite membrane with microtopographical morphology to regulate cellular behavior for improved tissue regeneration. Acta Biomater. 2023, 168, 125–143. [Google Scholar] [CrossRef]
- Fernandez-Pernas, P.; Rodriguez-Lesende, I.; de la Fuente, A.; Mateos, J.; Fuentes, I.; De Toro, J.; Blanco, F.J.; Arufe, M.C. CD105+-mesenchymal stem cells migrate into osteoarthritis joint: An animal model. PLoS ONE 2017, 12, e0188072. [Google Scholar] [CrossRef]
- Qu, C.; Brohlin, M.; Kingham, P.J.; Kelk, P. Evaluation of growth, stemness, and angiogenic properties of dental pulp stem cells cultured in cGMP xeno-/serum-free medium. Cell Tissue Res. 2020, 380, 93–105. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Yamada, Y.; Okano, T.; Orita, K.; Makino, T.; Shima, F.; Nakamura, H. 3D-cultured small size adipose-derived stem cell spheroids promote bone regeneration in the critical-sized bone defect rat model. Biochem. Biophys. Res. Commun. 2022, 603, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Laaksonen, T.; Koivuniemi, R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10890. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, Y.; Wu, X.; Zhao, B.; Ji, Y.; Xu, X. A comprehensive study on donor-matched comparisons of three types of mesenchymal stem cells-containing cells from human dental tissue. J. Periodontal Res. 2019, 54, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Bohlouli, M.; Bastami, F.; Nokhbatolfoghahei, H.; Khojasteh, A. Tissue buccal fat pad-stromal vascular fraction as a safe source in maxillofacial bone regeneration: A clinical pilot study. J. Plast. Reconstr. Aesthet. Surg. 2023, 79, 111–121. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable hydrogels for cartilage and bone tissue regeneration: A review. Int. J. Biol. Macromol. 2023, 246, 125674. [Google Scholar] [CrossRef]
- Xia, B.; Chen, G. Research progress of natural tissue-derived hydrogels for tissue repair and reconstruction. Int. J. Biol. Macromol. 2022, 214, 480–491. [Google Scholar] [CrossRef]
- Abd El-Azeem, S.H.; Khalil, A.A.; Ibrahim, M.A.; Gamal, A.Y. The use of integrin binding domain loaded hydrogel (RGD) with minimally invasive surgical technique in treatment of periodontal intrabony defect: A randomized clinical and biochemical study. J. Appl. Oral. Sci. 2023, 31, e20230263. [Google Scholar] [CrossRef]
- Basoli, V.; Della Bella, E.; Kubosch, E.J.; Alini, M.; Stoddart, M.J. Effect of expansion media and fibronectin coating on growth and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Sci. Rep. 2021, 11, 13089. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, Y.H.; Park, K.D.; Jee, K.S.; Shin, J.W.; Hahn, S.B. Importance of integrin beta1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials 2004, 25, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Moghadasi, M.H.; Hajifathali, A.; Azad, M.; Rahmani, M.; Soleimani, M. Expansion of cord blood stem cells in fibronectin-coated microfluidic bioreactor. Hematol. Transfus. Cell Ther. 2021, 10, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Rach, J.; Handke, W.; Seltsam, A.; Pepelanova, I.; Strauss, S.; Vogt, P.; Scheper, T.; Lavrentieva, A. Comparative Analysis of Mesenchymal Stem Cell Cultivation in Fetal Calf Serum, Human Serum, and Platelet Lysate in 2D and 3D Systems. Front. Bioeng. Biotechnol. 2020, 8, 598389. [Google Scholar] [CrossRef] [PubMed]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf. B Biointerfaces 2015, 128, 191–200. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, D.J.; Zheng, H.; Deng, J.; Gou, Z.; Gao, C. Adsorption of serum proteins on titania nanotubes and its role on regulating adhesion and migration of mesenchymal stem cells. J. Biomed. Mater. Res. A 2020, 108, 2305–2318. [Google Scholar] [CrossRef]





| Sets I/III | hBFP-ADSCs | hPDLSCs | hDPSCs | ||||
|---|---|---|---|---|---|---|---|
| Set II | Mono | Encap | Mono | Encap | Mono | Encap | |
| FBS | Group 1m | Group 1e | Group 2m | - | Group 3m | - | |
| HS | Group 4m | Group 4e | Group 5m | - | Group 6m | - | |
| SS | Group 7m | Group 7e | Group 8m | G8e | Group 9m | Group 9e | |
| Cell Culture Conditions (Study Groups) | Investigated Parameters | Findings |
|---|---|---|
SC sources
| MSC characteristics
|
|
| Effects of SC sources in the SS | OS differentiation potential
|
|
|
| |
| Effects of Types of Sera (FBS and SS) on hADSCs | OS differentiation potential
|
|
|
| |
Levels of adhesion molecules
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arpornmaeklong, P.; Boonyuen, S.; Apinyauppatham, K.; Pripatnanont, P. Effects of Oral Cavity Stem Cell Sources and Serum-Free Cell Culture on Hydrogel Encapsulation of Mesenchymal Stem Cells for Bone Regeneration: An In Vitro Investigation. Bioengineering 2024, 11, 59. https://doi.org/10.3390/bioengineering11010059
Arpornmaeklong P, Boonyuen S, Apinyauppatham K, Pripatnanont P. Effects of Oral Cavity Stem Cell Sources and Serum-Free Cell Culture on Hydrogel Encapsulation of Mesenchymal Stem Cells for Bone Regeneration: An In Vitro Investigation. Bioengineering. 2024; 11(1):59. https://doi.org/10.3390/bioengineering11010059
Chicago/Turabian StyleArpornmaeklong, Premjit, Supakorn Boonyuen, Komsan Apinyauppatham, and Prisana Pripatnanont. 2024. "Effects of Oral Cavity Stem Cell Sources and Serum-Free Cell Culture on Hydrogel Encapsulation of Mesenchymal Stem Cells for Bone Regeneration: An In Vitro Investigation" Bioengineering 11, no. 1: 59. https://doi.org/10.3390/bioengineering11010059
APA StyleArpornmaeklong, P., Boonyuen, S., Apinyauppatham, K., & Pripatnanont, P. (2024). Effects of Oral Cavity Stem Cell Sources and Serum-Free Cell Culture on Hydrogel Encapsulation of Mesenchymal Stem Cells for Bone Regeneration: An In Vitro Investigation. Bioengineering, 11(1), 59. https://doi.org/10.3390/bioengineering11010059





