Erectile Dysfunction Treatment Using Stem Cell Delivery Patch in a Cavernous Nerve Injury Rat Model
Abstract
1. Introduction
2. Materials and Methods
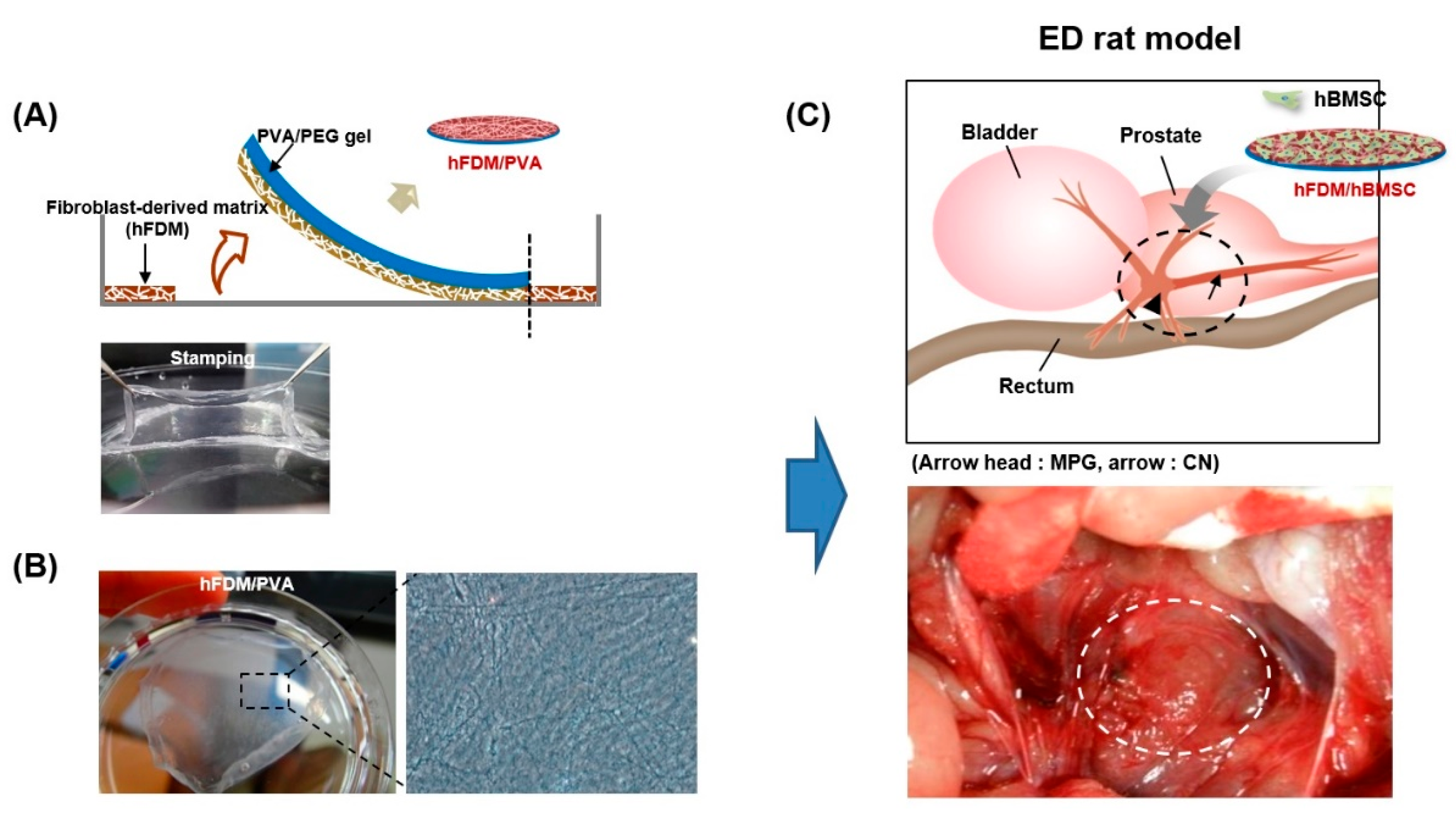
2.1. Preparation of Human Fibroblast-Derived ECM (hFDM) Patch
2.2. Cell Culture and Differentiation
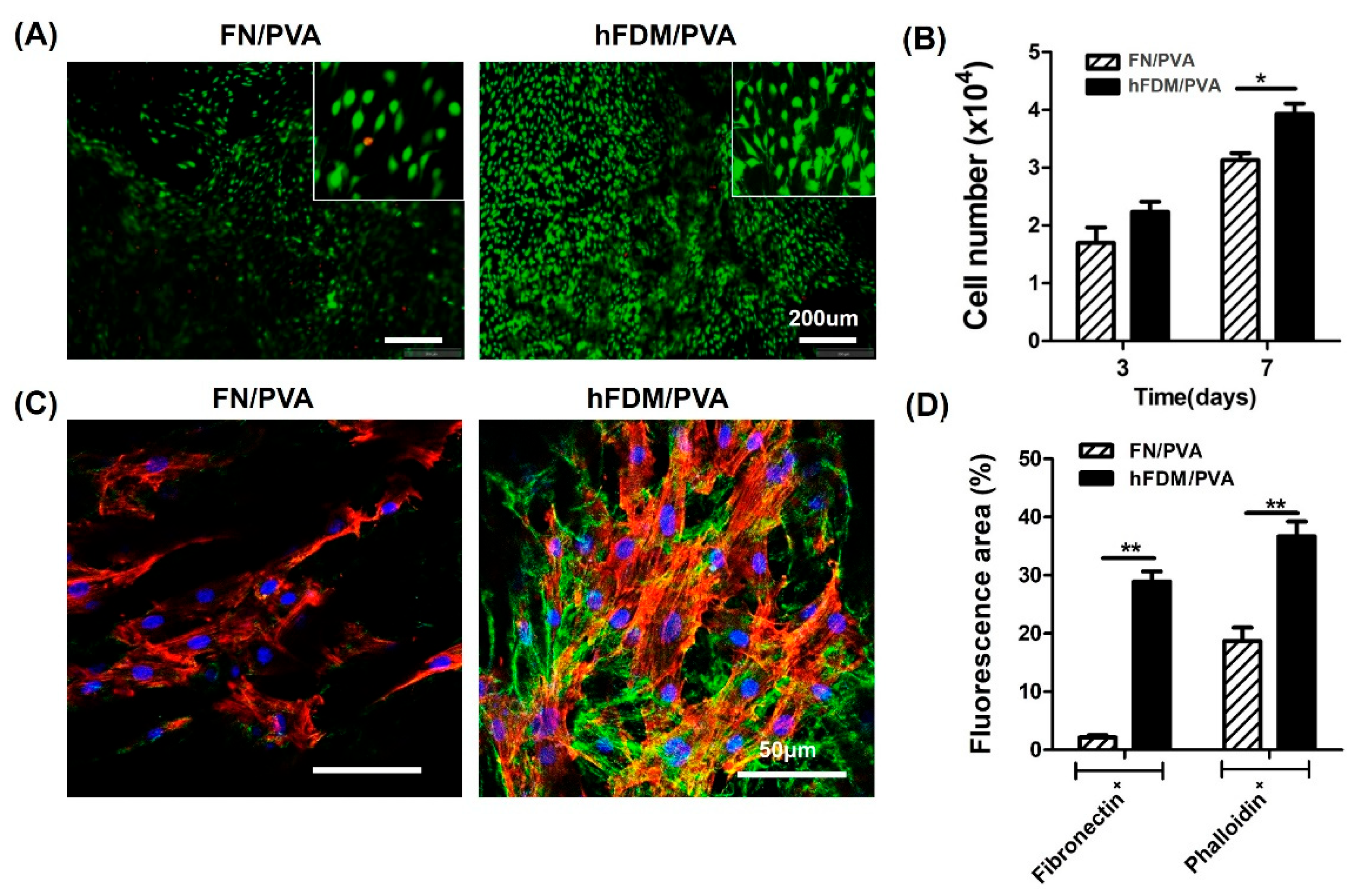
2.3. Cell Adhesion and Proliferation on FN and hFDM Patch
2.4. Immunocytochemistry
2.5. Quantitative Reverse Transcription-PCR (qRT-PCR)
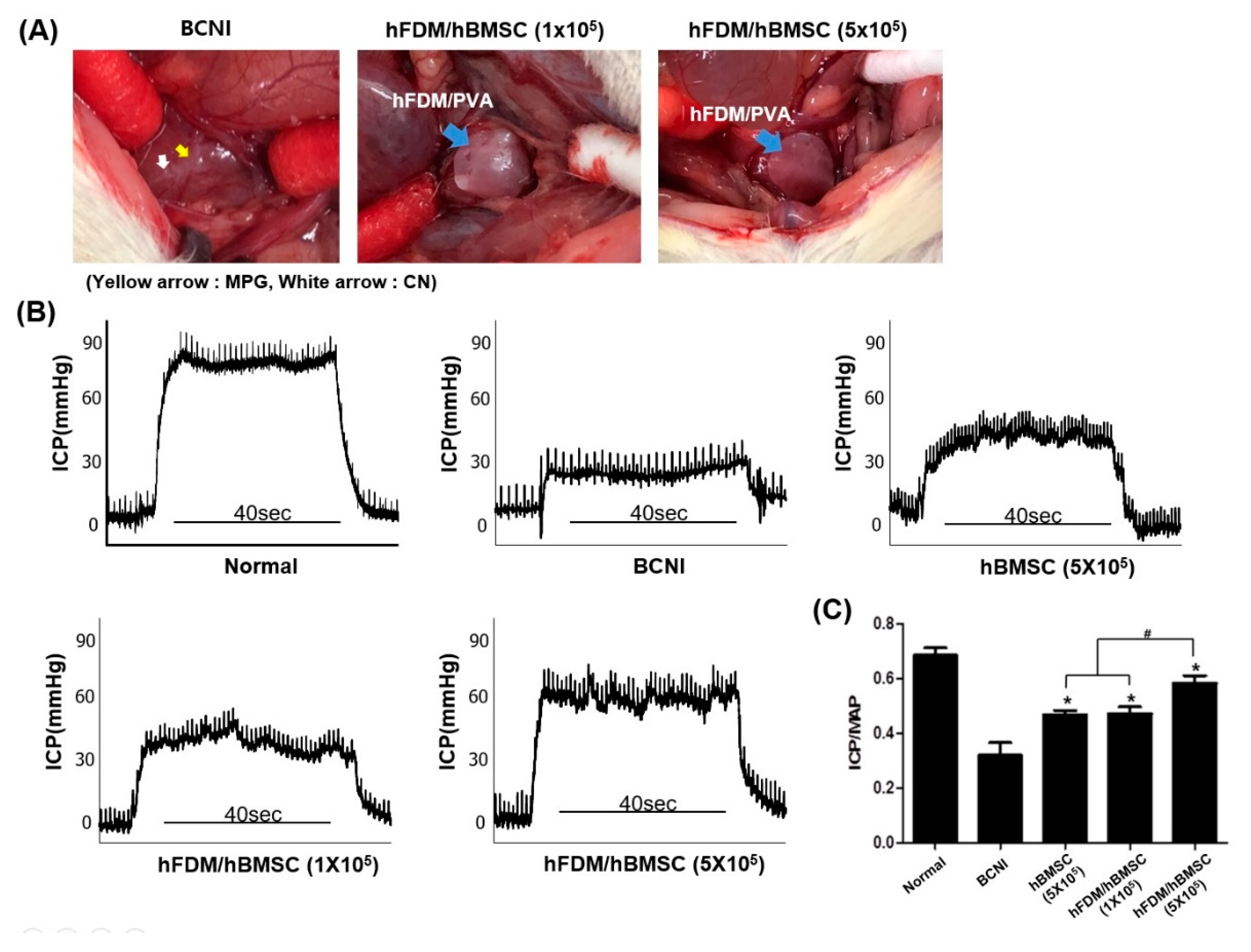
2.6. Animal Experiments
2.7. Erectile Function Measurement
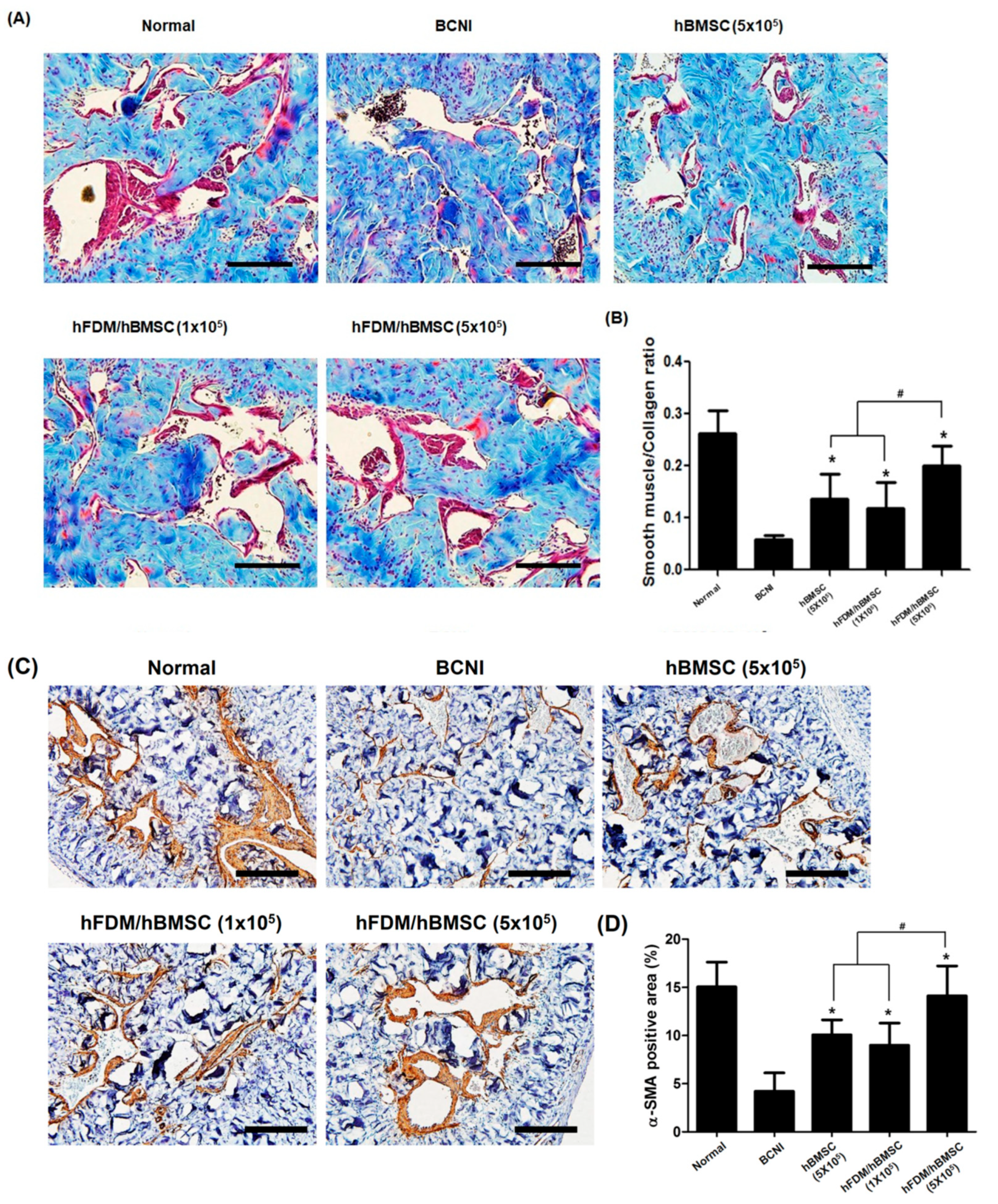
2.8. Immunohistochemistry
2.9. Measurement of Cyclic Guanosine Monophosphate (cGMP) Levels
2.10. Statistical Analyses
3. Results
3.1. Characterization of PVA/hFDM
3.2. Cell Viability and Cell Adhesion of hBMSC Seeded on Patch
3.3. Cell Differentiation of hBMSC Seeded on Patch
3.4. The hBMSCs Seeded on Patches Improves Erectile Function
3.5. hBMSCs Seeded in the Patch Increases Smooth Muscle Cells in the Corpus Cavernosum
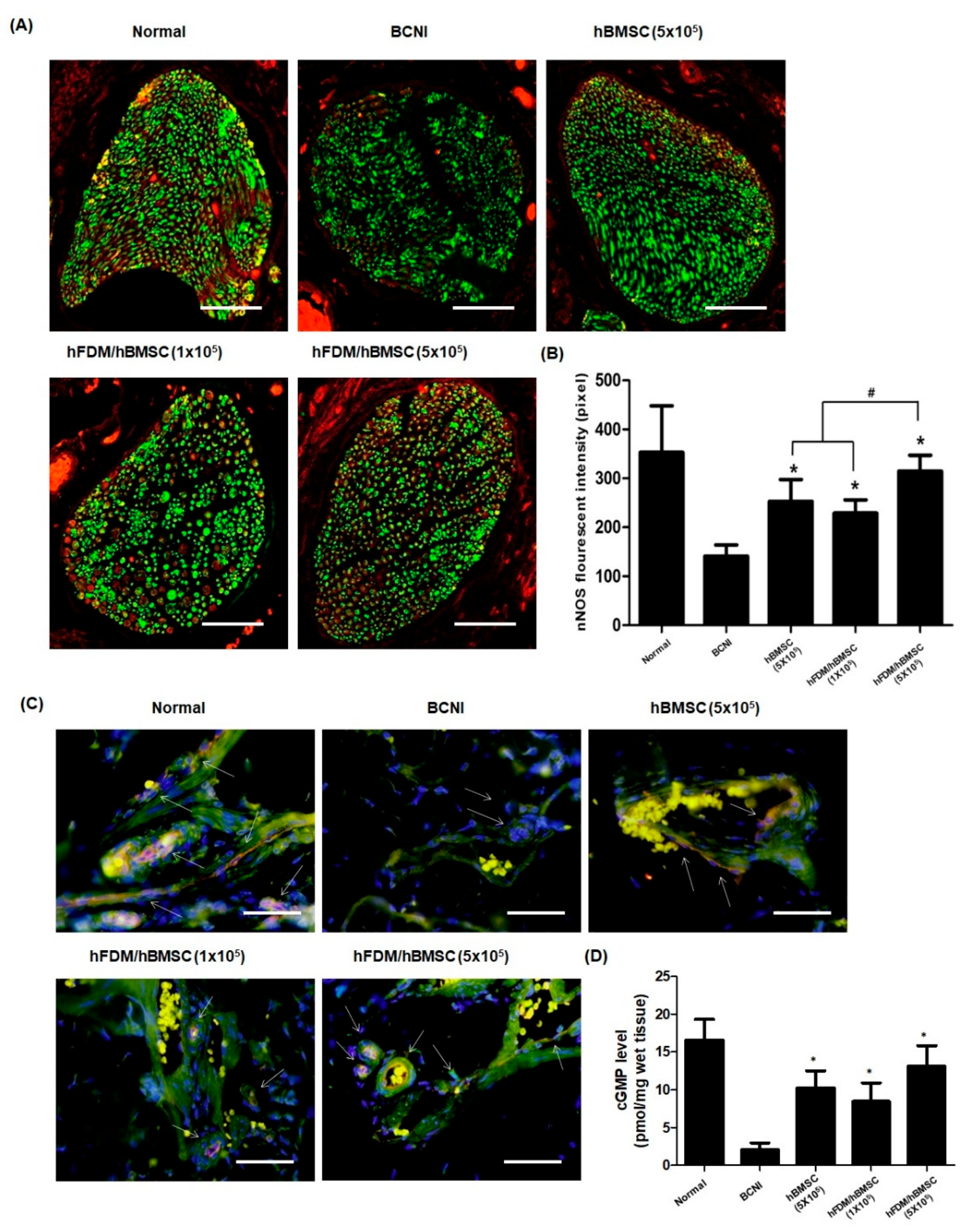
3.6. hBMSCs Seeded in the Patch Restores Nitric Oxide (NO)/cGMP Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, P.C.; Donker, P.J. Impotence following radical prostatectomy: Insight into etiology and prevention. J. Urol. 1982, 128, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Head, L.; Witiuk, K.; Punjani, N.; Mallick, R.; Cnossen, S.; Fergusson, D.A.; Cagiannos, I.; Lavallée, L.T.; Morash, C.; et al. The Risks and Benefits of Cavernous Neurovascular Bundle Sparing during Radical Prostatectomy: A Systematic Review and Meta-Analysis. J. Urol. 2017, 198, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Kilminster, S.; Müller, S.; Menon, M.; Joseph, J.V.; Ralph, D.J.; Patel, H.R. Predicting erectile function outcome in men after radical prostatectomy for prostate cancer. BJU Int. 2012, 110, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Matz, E.L.; Terlecki, R.; Zhang, Y.; Jackson, J.; Atala, A. Stem Cell Therapy for Erectile Dysfunction. Sex. Med. Rev. 2019, 7, 321–328. [Google Scholar] [CrossRef]
- Patel, V.R.; Samavedi, S.; Bates, A.S.; Kumar, A.; Coelho, R.; Rocco, B.; Palmer, K. Dehydrated Human Amnion/Chorion Membrane Allograft Nerve Wrap Around the Prostatic Neurovascular Bundle Accelerates Early Return to Continence and Potency Following Robot-assisted Radical Prostatectomy: Propensity Score-matched Analysis. Eur. Urol. 2015, 67, 977–980. [Google Scholar] [CrossRef]
- Bunpetch, V.; Wu, H.; Zhang, S.; Ouyang, H. From “Bench to Bedside”: Current Advancement on Large-Scale Production of Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 1662–1673. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Reinders, M.E.; de Fijter, J.W.; Roelofs, H.; Bajema, I.M.; de Vries, D.K.; Schaapherder, A.F.; Claas, F.H.; van Miert, P.P.; Roelen, D.L.; van Kooten, C.; et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Transl. Med. 2013, 2, 107–111. [Google Scholar] [CrossRef]
- Fode, M.; Nadler, N.; Lund, L.; Azawi, N. Feasibility of minimally invasive, same-day injection of autologous adipose-derived stem cells in the treatment of erectile dysfunction. Scand. J. Urol. 2022, 57, 1–5. [Google Scholar] [CrossRef]
- Yiou, R.; Hamidou, L.; Birebent, B.; Bitari, D.; Lecorvoisier, P.; Contremoulins, I.; Khodari, M.; Rodriguez, A.M.; Augustin, D.; Roudot-Thoraval, F.; et al. Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction: An Open Dose-Escalation Pilot Study. Eur. Urol. 2016, 69, 988–991. [Google Scholar] [CrossRef]
- Castiglione, F.; Cakir, O.O.; Satchi, M.; Fallara, G.; Pang, K.H. The Current Role and Implications of Stem Cell Therapy in Erectile Dysfunction: A Transformation from Caterpillar to Butterfly Is Required. Eur. Urol. Focus 2022, 9, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Siregar, S.; Novesar, A.R.; Mustafa, A. Application of Stem Cell in Human Erectile Dysfunction—A Systematic Review. Res. Rep. Urol. 2022, 14, 379–388. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Jang, M.J.; Lee, J.; Suh, N.; Jeong, I.G.; Sohn, D.W.; Kim, S.W.; Ahn, T.Y.; Kim, C.S. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate 2013, 73, 278–286. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Xie, Y.; Deng, C.; Liu, G.; Zhang, X. Comparative study of different transplantation methods of adipose tissue-derived stem cells in the treatment of erectile dysfunction caused by cavernous nerve injury. Andrologia 2021, 53, e13950. [Google Scholar] [CrossRef] [PubMed]
- Gur, S.; Hellstrom, W.J.G. Harnessing Stem Cell Potential for the Treatment of Erectile Function in Men with Diabetes Mellitus: From Preclinical/Clinical Perspectives to Penile Tissue Engineering. Curr. Stem Cell Res. Ther. 2020, 15, 308–320. [Google Scholar] [CrossRef]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Yang, L.; Wei, M.; Xing, B.; Zhang, C. Extracellular matrix and synapse formation. Biosci. Rep. 2023, 43, BSR20212411. [Google Scholar] [CrossRef]
- Jung, A.R.; Kim, R.Y.; Kim, H.W.; Shrestha, K.R.; Jeon, S.H.; Cha, K.J.; Park, Y.H.; Kim, D.S.; Lee, J.Y. Nanoengineered Polystyrene Surfaces with Nanopore Array Pattern Alters Cytoskeleton Organization and Enhances Induction of Neural Differentiation of Human Adipose-Derived Stem Cells. Tissue Eng. Part A 2015, 21, 2115–2124. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Kim, I.G.; Hwang, M.P.; Park, J.S.; Kim, S.H.; Kim, J.H.; Kang, H.J.; Subbiah, R.; Ko, U.H.; Shin, J.H.; Kim, C.H.; et al. Stretchable ECM Patch Enhances Stem Cell Delivery for Post-MI Cardiovascular Repair. Adv. Healthc. Mater. 2019, 8, e1900593. [Google Scholar] [CrossRef]
- Suhaeri, M.; Noh, M.H.; Moon, J.H.; Kim, I.G.; Oh, S.J.; Ha, S.S.; Lee, J.H.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.S.; Song, E.S.; Du, P.; Suhaeri, M.; Lee, J.H.; Park, K. Novel ECM Patch Combines Poly(vinyl alcohol), Human Fibroblast-Derived Matrix, and Mesenchymal Stem Cells for Advanced Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Suhaeri, M.; Subbiah, R.; Van, S.Y.; Du, P.; Kim, I.G.; Lee, K.; Park, K. Cardiomyoblast (h9c2) differentiation on tunable extracellular matrix microenvironment. Tissue Eng. Part A 2015, 21, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Huang, X.; Zhou, Y.; Jin, R.; Li, Q. Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation. Stem Cells Transl. Med. 2016, 5, 960–969. [Google Scholar] [CrossRef]
- Fandel, T.M.; Albersen, M.; Lin, G.; Qiu, X.; Ning, H.; Banie, L.; Lue, T.F.; Lin, C.S. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur. Urol. 2012, 61, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; You, D.; Kim, K.; Aum, J.; Kim, Y.S.; Jang, M.J.; Moon, K.H.; Kang, H.W. Therapeutic Effect of Human Mesenchymal Stem Cell-Conditioned Medium on Erectile Dysfunction. World J. Mens Health 2022, 40, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dhanani, N.; Tseng, H.; Souza, G.R.; Wang, G.; Cao, Y.; Ko, T.C.; Jiang, H.; Wang, R. Nanoparticle Improved Stem Cell Therapy for Erectile Dysfunction in a Rat Model of Cavernous Nerve Injury. J. Urol. 2016, 195, 788–795. [Google Scholar] [CrossRef]
- Zhang, H.B.; Chen, F.Z.; He, S.H.; Liang, Y.B.; Wang, Z.Q.; Wang, L.; Chen, Z.R.; Ding, W.; Zhao, S.C.; Wei, A.Y. In vivo tracking on longer retention of transplanted myocardin gene-modified adipose-derived stem cells to improve erectile dysfunction in diabetic rats. Stem Cell Res. Ther. 2019, 10, 208. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef]
- Yiou, R.; Mahrouf-Yorgov, M.; Trébeau, C.; Zanaty, M.; Lecointe, C.; Souktani, R.; Zadigue, P.; Figeac, F.; Rodriguez, A.M. Delivery of human mesenchymal adipose-derived stem cells restores multiple urological dysfunctions in a rat model mimicking radical prostatectomy damages through tissue-specific paracrine mechanisms. Stem Cells 2016, 34, 392–404. [Google Scholar] [CrossRef]
- You, D.; Jang, M.J.; Lee, J.; Jeong, I.G.; Kim, H.S.; Moon, K.H.; Suh, N.; Kim, C.S. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology 2013, 81, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Weyne, E.; Ilg, M.M.; Cakir, O.O.; Muneer, A.; Roussel, D.B.; Albersen, M.; Angulo, J.; Corona, G.; Bettocchi, C.; Reisman, Y.; et al. European Society for Sexual Medicine Consensus Statement on the Use of the Cavernous Nerve Injury Rodent Model to Study Postradical Prostatectomy Erectile Dysfunction. Sex. Med. 2020, 8, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Albersen, M.; Fiorenzo, S.; Hedlund, P.; Cakir, O.O.; Pavone, C.; Alnajjar, H.M.; Joniau, S.; Muneer, A. Long-term consequences of bilateral cavernous crush injury in normal and diabetic rats: A functional study. Int. J. Impot. Res. 2022, 34, 781–785. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.W.; Kim, I.G.; Kim, M.Y.; Jung, A.R.; Park, K.; Lee, J.Y. Erectile Dysfunction Treatment Using Stem Cell Delivery Patch in a Cavernous Nerve Injury Rat Model. Bioengineering 2023, 10, 635. https://doi.org/10.3390/bioengineering10060635
Moon HW, Kim IG, Kim MY, Jung AR, Park K, Lee JY. Erectile Dysfunction Treatment Using Stem Cell Delivery Patch in a Cavernous Nerve Injury Rat Model. Bioengineering. 2023; 10(6):635. https://doi.org/10.3390/bioengineering10060635
Chicago/Turabian StyleMoon, Hyong Woo, In Gul Kim, Mee Young Kim, Ae Ryang Jung, Kwideok Park, and Ji Youl Lee. 2023. "Erectile Dysfunction Treatment Using Stem Cell Delivery Patch in a Cavernous Nerve Injury Rat Model" Bioengineering 10, no. 6: 635. https://doi.org/10.3390/bioengineering10060635
APA StyleMoon, H. W., Kim, I. G., Kim, M. Y., Jung, A. R., Park, K., & Lee, J. Y. (2023). Erectile Dysfunction Treatment Using Stem Cell Delivery Patch in a Cavernous Nerve Injury Rat Model. Bioengineering, 10(6), 635. https://doi.org/10.3390/bioengineering10060635




