Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus aureus, Escherichia coli, and Candida albicans for Improved Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Photosensitizer and Light Source
2.2. Biofilm Formation
2.3. Treatment Groups
2.4. Plate Count Method
2.5. Measuring Reactive Oxygen Species (ROS)
2.6. Statistical Analysis
3. Results
3.1. Effects of Treatment Groups on the Cell Viability
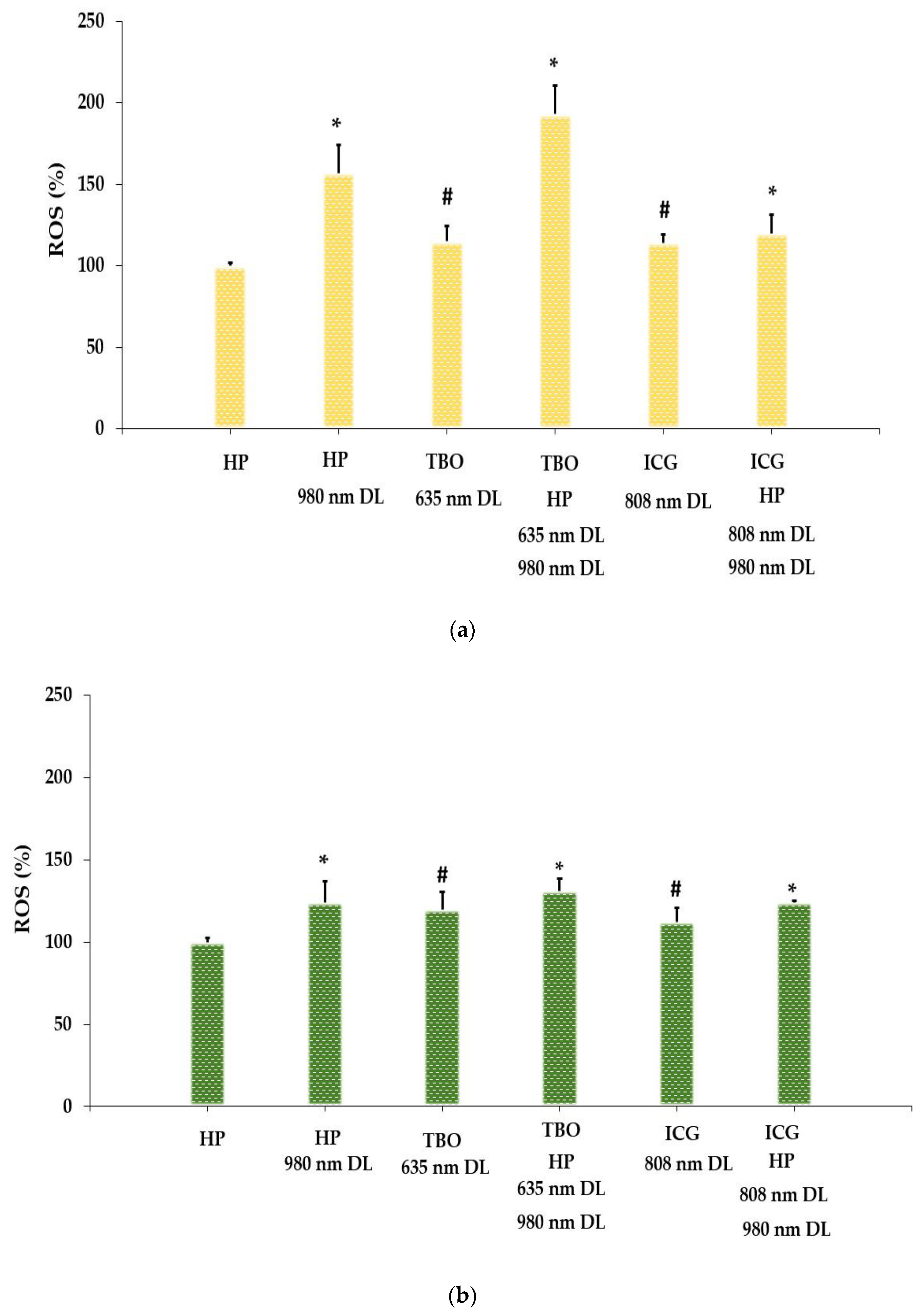
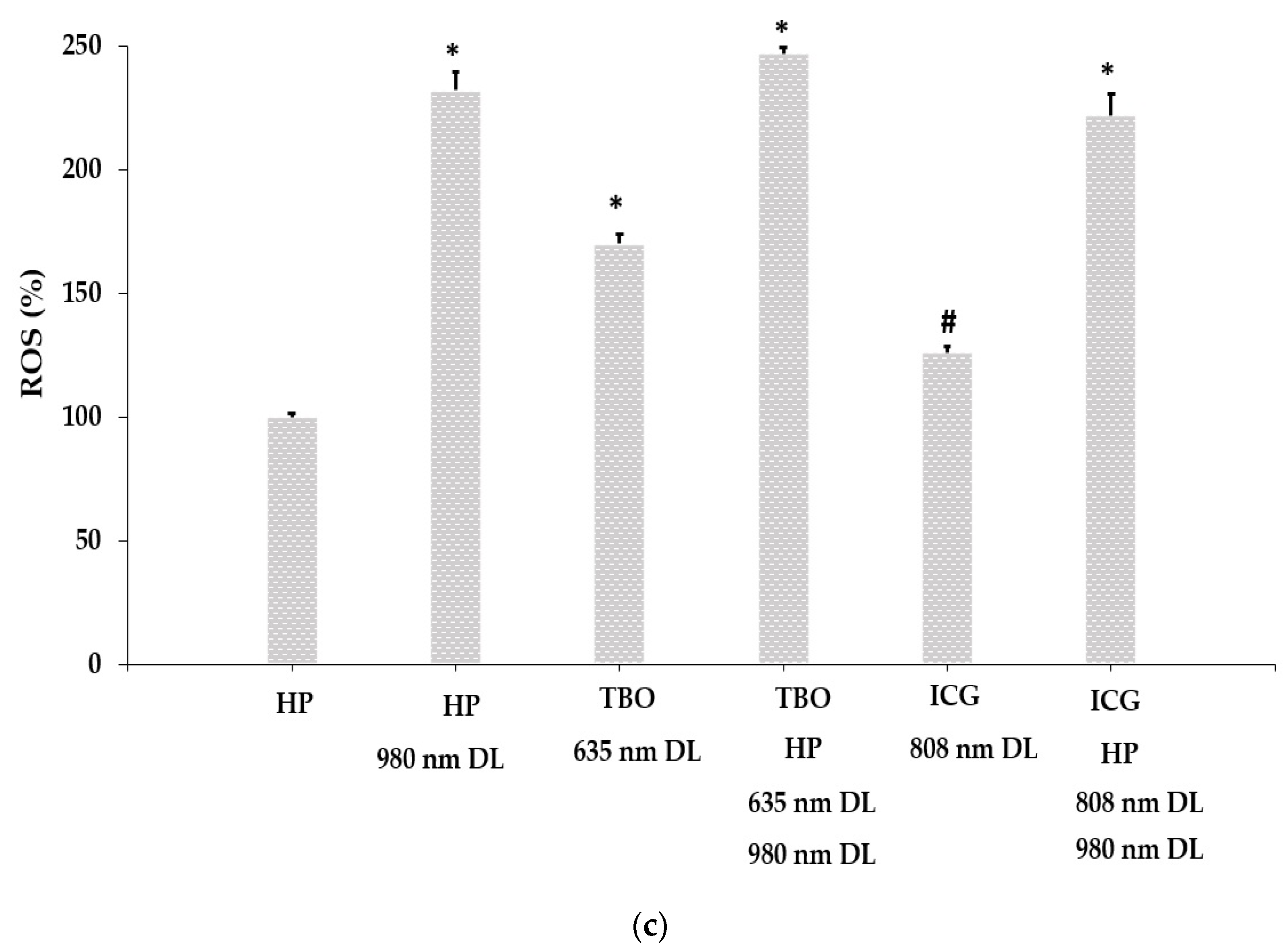
3.2. Intracellular ROS Generation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Körtvélyessy, G.; Tarjányi, T.; Baráth, Z.L.; Minarovits, J.; Tóth, Z. Bioactive coatings for dental implants: A review of alternative strategies to prevent peri-implantitis induced by anaerobic bacteria. Anaerobe 2021, 70, 102404. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Huang, Y.-W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.S.; Feres, M.; Figueiredo, L.C.; Shibli, J.A.; Ramiro, F.S.; Faveri, M. Microbiological diversity of peri-implantitis biofilm by S anger sequencing. Clin. Oral Implants Res. 2014, 25, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Alonso, F.; Salinas, J.; Sánchez-Siles, M.; Pato-Mourelo, J.; Cotrina-Veizaga, B.D.; Ortega, N. Synergistic antimicrobial effect of photodynamic therapy and chitosan on the titanium-adherent biofilms of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa: An in vitro study. J. Periodontol. 2022, 93, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Alrabiah, M.; Alshagroud, R.S.; Alsahhaf, A.; Almojaly, S.A.; Abduljabbar, T.; Javed, F. Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2019, 21, 781–785. [Google Scholar] [CrossRef]
- Leonhardt, Å.; Dahlén, G.; Renvert, S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J. Periodontol. 2003, 74, 1415–1422. [Google Scholar] [CrossRef]
- Coman, A.N.; Mare, A.; Tanase, C.; Bud, E.; Rusu, A. Silver-deposited nanoparticles on the titanium nanotubes surface as a promising antibacterial material into implants. Metals 2021, 11, 92. [Google Scholar] [CrossRef]
- de Fátima Balderrama, Í.; de Toledo Stuani, V.; Cardoso, M.V.; Oliveira, R.C.; Lopes, M.M.R.; Greghi, S.L.A. The influence of implant surface roughness on decontamination by antimicrobial photodynamic therapy and chemical agents: A preliminary study in vitro. Photodiagnosis Photodyn. Ther. 2021, 33, 102105. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: In vitro study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Valente, N.A.; Mang, T.; Hatton, M.; Mikulski, L.; Andreana, S. Effects of two diode lasers with and without photosensitization on contaminated implant surfaces: An ex vivo study. Photomed. Laser Surg. 2017, 35, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Park, H.-W.; Lee, J.-H.; Seo, H.-W.; Lee, S.-Y. The photodynamic therapy on Streptococcus mutans biofilms using erythrosine and dental halogen curing unit. Int. J. Oral Sci. 2012, 4, 196–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Araújo, N.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med. Sci. 2014, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic efficiency: From molecular photochemistry to cell death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light sources and dosimetry techniques for photodynamic therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Rismanchian, M.; Nosouhian, S.; Shahabouee, M.; Davoudi, A.; Nourbakhshian, F. Effect of conventional and contemporary disinfectant techniques on three peri-implantitis associated microbiotas. Am. J. Dent. 2017, 30, 23–26. [Google Scholar]
- Li, Y.; Du, J.; Huang, S.; Wang, S.; Wang, Y.; Cai, Z.; Lei, L.; Huang, X. Hydrogen peroxide potentiates antimicrobial photodynamic therapy in eliminating Candida albicans and Streptococcus mutans dual-species biofilm from denture base. Photodiagnosis Photodyn. Ther. 2022, 37, 102691. [Google Scholar] [CrossRef]
- Caccianiga, G.; Cambini, A.; Rey, G.; Paiusco, A.; Fumagalli, T.; Giacomello, M. The use of laser diodes superpulses in implantology. Eur. J. Inflamm. 2012, 10 (Suppl. 2), 97–100. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Chiniforush, N. An in vitro study on the efficacy of hydrogen peroxide mediated high-power photodynamic therapy affecting Enterococcus faecalis biofilm formation and dispersal. Photodiagnosis Photodyn. Ther. 2023, 41, 103310. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Parker, S.; Chiniforush, N. Synergistic Antimicrobial Effect of Photodynamic Inactivation and SWEEPS in Combined Treatment against Enterococcus faecalis in a Root Canal Biofilm Model: An In Vitro Study. Appl. Sci. 2023, 13, 5668. [Google Scholar] [CrossRef]
- Caccianiga, G.; Baldoni, M.; Ghisalberti, C.A.; Paiusco, A. A preliminary in vitro study on the efficacy of high-power photodynamic therapy (hllt): Comparison between pulsed diode lasers and superpulsed diode lasers and impact of hydrogen peroxide with controlled stabilization. BioMed Res. Int. 2016, 138, 6158. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, Z.; Ehtesabi, H.; Hallaji, Z.; Aminoroaya, N.; Tavana, H.; Behroodi, E.; Rahimifard, M.; Abdollahi, M.; Latifi, H. On-chip analysis of carbon dots effect on yeast replicative lifespan. Anal. Chim. Acta 2018, 1033, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Parab, S.; Achalla, P.K.; Yanamandala, N.; Singhvi, G.; Kesharwani, P.; Dubey, S.K. Sensitizers in photodynamic therapy. In Nanomaterials for Photodynamic Therapy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 81–103. [Google Scholar]
- Dörtbudak, O.; Haas, R.; Bernhart, T.; Mailath-Pokorny, G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin. Oral Implant. Res. 2001, 12, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Saffarpour, A.; Fekrazad, R.; Heibati, M.N.; Bahador, A.; Saffarpour, A.; Rokn, A.R.; Iranparvar, A.; KharaziFard, M.J. Bactericidal Effect of Erbium-Doped Yttrium Aluminum Garnet Laser and Photodynamic Therapy on Aggregatibacter Actinomycetemcomitans Biofilm on Implant Surface. Int. J. Oral Maxillofac. Implant. 2016, 31, 71–78. [Google Scholar] [CrossRef]
- Nastri, L.; Donnarumma, G.; Porzio, C.; De Gregorio, V.; Tufano, M.; Caruso, F.; Mazza, C.; Serpico, R. Effects of toluidine blue-mediated photodynamic therapy on periopathogens and periodontal biofilm: In vitro evaluation. Int. J. Immunopathol. Pharmacol. 2010, 23, 1125–1132. [Google Scholar] [CrossRef]
- Fox, I.J.; Wood, E.H. Indocyanine green: Physical and physiolofic properties. Proc. Staff. Meet. Mayo Clin. 1960, 35, 732–744. [Google Scholar]
- Shafirstein, G.; Bäumler, W.; Hennings, L.J.; Siegel, E.R.; Friedman, R.; Moreno, M.A.; Webber, J.; Jackson, C.; Griffin, R.J. Indocyanine green enhanced near-infrared laser treatment of murine mammary carcinoma. Int. J. Cancer 2012, 130, 1208–1215. [Google Scholar] [CrossRef]
- Nikinmaa, S.; Alapulli, H.; Auvinen, P.; Vaara, M.; Rantala, J.; Kankuri, E.; Sorsa, T.; Meurman, J.; Pätilä, T. Dual-light photodynamic therapy administered daily provides a sustained antibacterial effect on biofilm and prevents Streptococcus mutans adaptation. PLoS ONE 2020, 15, e0232775. [Google Scholar] [CrossRef]
- Giraudeau, C.; Moussaron, A.; Stallivieri, A.; Mordon, S.; Frochot, C. Indocyanine green: Photosensitizer or chromophore? Still a debate. Curr. Med. Chem. 2014, 21, 1871–1897. [Google Scholar] [CrossRef]
- Jao, Y.; Ding, S.-J.; Chen, C.-C. Antimicrobial photodynamic therapy for the treatment of oral infections: A systematic review. J. Dent. Sci. 2023, 18, 1453–1466. [Google Scholar] [CrossRef]
- Xu, X.; Liu, B.; Wu, H.; Zhang, Y.; Tian, X.; Tian, J.; Liu, T. Poly Lactic-co-Glycolic Acid-Coated Toluidine Blue Nanoparticles for the Antibacterial Therapy of Wounds. Nanomaterials 2021, 11, 3394. [Google Scholar] [CrossRef]
- Rahman, B.; Acharya, A.B.; Siddiqui, R.; Verron, E.; Badran, Z. Photodynamic therapy for Peri-implant diseases. Antibiotics 2022, 11, 918. [Google Scholar] [CrossRef]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of toluidine blue—Mediated antimicrobial photodynamic therapy on candida spp. A systematic review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- George, S.; Kishen, A. Augmenting the antibiofilm efficacy of advanced noninvasive light activated disinfection with emulsified oxidizer and oxygen carrier. J. Endod. 2008, 34, 1119–1123. [Google Scholar] [CrossRef]
- von Ohle, C.; Gieseke, A.; Nistico, L.; Decker, E.M.; DeBeer, D.; Stoodley, P. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl. Environ. Microbiol. 2010, 76, 2326–2334. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núnez, S.C.; Baptista, M.S.; Daghastanli, N.A.; Itri, R.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial mechanisms behind photodynamic effect in the presence of hydrogen peroxide. Photochem. Photobiol. Sci. 2011, 10, 483–490. [Google Scholar] [CrossRef]
- Katalinić, I.; Smojver, I.; Morelato, L.; Vuletić, M.; Budimir, A.; Gabrić, D. Evaluation of the Photoactivation Effect of 3% Hydrogen Peroxide in the Disinfection of Dental Implants: In Vitro Study. Biomedicines 2023, 11, 1002. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Y.; Wang, Y.; Chen, S.; Jiang, S.; Ge, H.; Lei, L.; Huang, X. Antimicrobial effects of photodynamic therapy with antiseptics on Staphylococcus aureus biofilm on titanium surface. Photodiagnosis Photodyn. Ther. 2019, 25, 382–388. [Google Scholar] [CrossRef]
- Wiedmer, D.; Petersen, F.C.; Lönn-Stensrud, J.; Tiainen, H. Antibacterial effect of hydrogen peroxide-titanium dioxide suspensions in the decontamination of rough titanium surfaces. Biofouling 2017, 33, 451–459. [Google Scholar] [CrossRef]
- Bürgers, R.; Witecy, C.; Hahnel, S.; Gosau, M. The effect of various topical peri-implantitis antiseptics on Staphylococcus epidermidis, Candida albicans, and Streptococcus sanguinis. Arch. Oral Biol. 2012, 57, 940–947. [Google Scholar] [CrossRef]
- De Angelis, N.; Hanna, R.; Signore, A.; Amaroli, A.; Benedicenti, S. Effectiveness of dual-wavelength (Diodes 980 nm and 635 nm) laser approach as a non-surgical modality in the management of periodontally diseased root surface: A pilot study. Biotechnol. Biotechnol. Equip. 2018, 32, 1575–1582. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Tang, J.; Li, P.; Su, R.; Zhong, H.; Su, W. Dual-mode antibacterial core-shell gold nanorod@ mesoporous-silica/curcumin nanocomplexes for efficient photothermal and photodynamic therapy. J. Photochem. Photobiol. A Chem. 2022, 425, 113722. [Google Scholar] [CrossRef]
- Wen, F.; Li, P.; Meng, H.; Yan, H.; Huang, X.; Cui, H.; Su, W. Nitrogen-doped carbon dots/curcumin nanocomposite for combined Photodynamic/photothermal dual-mode antibacterial therapy. Photodiagnosis Photodyn. Ther. 2022, 39, 103033. [Google Scholar] [CrossRef]
- Leanse, L.G.; Goh, X.S.; Cheng, J.-X.; Hooper, D.C.; Dai, T. Dual-wavelength photo-killing of methicillin-resistant Staphylococcus aureus. JCI Insight 2020, 5, e134343. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Garcia-Diaz, M.; Huang, Y.-Y.; Hamblin, M.R. Use of fluorescent probes for ROS to tease apart Type I and Type II photochemical pathways in photodynamic therapy. Methods 2016, 109, 158–166. [Google Scholar] [CrossRef]
- Atsumi, T.; Iwakura, I.; Fujisawa, S.; Ueha, T. The production of reactive oxygen species by irradiated camphorquinone–related photosensitizers and their effect on cytotoxicity. Arch. Oral Biol. 2001, 46, 391–401. [Google Scholar] [CrossRef]
- Tedesco, A.C.; Primo, F.L.; de Jesus, P.d.C.C. Chapter 2—Antimicrobial Photodynamic Therapy (APDT) Action Based on Nanostructured Photosensitizers. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 9–29. ISBN 978-0-323-52725-5. [Google Scholar]
- Elagin, V.; Budruev, I.; Antonyan, A.; Bureev, P.; Ignatova, N.; Streltsova, O.; Kamensky, V. Enhancement of the Efficacy of Photodynamic Therapy against Uropathogenic Gram-Negative Bacteria Species. Photonics 2023, 10, 310. [Google Scholar] [CrossRef]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg. Med. 2003, 33, 311–319. [Google Scholar] [CrossRef] [PubMed]



| Staphylococcus aureus | Mean (lg CFU/mL) | Std. Deviation | Sig. * | Lower Bound | Upper Bound |
|---|---|---|---|---|---|
| Treatments | |||||
| Control | 5.86 | 0.11 | |||
| HP | 5.23 | 0.03 | <0.001 | 0.45 | 0.92 |
| HP-PDT | 4.46 | 0.13 | <0.001 | 0.94 | 1.83 |
| TBO-PDT | 4.56 | 0.32 | <0.001 | 0.84 | 1.73 |
| HP-TBO-PDT | 3.96 | 0.22 | <0.001 | 1.41 | 2.37 |
| ICG-PDT | 4.97 | 0.21 | <0.001 | 0.40 | 1.36 |
| HP-ICG-PDT | 4.33 | 0.11 | <0.001 | 1.08 | 1.97 |
| Escherichia coli | |||||
| Treatments | |||||
| Control | 6.65 | 0.05 | |||
| HP | 6.06 | 0.03 | <0.001 | 0.38 | 0.86 |
| HP-PDT | 5.26 | 0.10 | <0.001 | 1.18 | 1.60 |
| TBO-PDT | 5.55 | 0.05 | <0.001 | 0.89 | 1.29 |
| HP-TBO-PDT | 4.98 | 0.08 | <0.001 | 1.45 | 1.90 |
| ICG-PDT | 5.67 | 0.06 | <0.001 | 0.41 | 0.87 |
| HP-ICG-PDT | 5.21 | 0.12 | <0.001 | 1.23 | 1.65 |
| Candida albicans | |||||
| Treatments | |||||
| Control | 6.40 | 0.46 | |||
| HP | 6.01 | 0.13 | 0.019 | 0.07 | 0.70 |
| HP-PDT | 4.55 | 0.21 | <0.001 | 1.53 | 2.39 |
| TBO-PDT | 5.04 | 0.12 | 0.001 | 0.59 | 2.12 |
| HP-TBO-PDT | 3.95 | 0.22 | <0.001 | 1.69 | 3.22 |
| ICG-PDT | 5.50 | 0.26 | 0.011 | 0.19 | 1.61 |
| HP-ICG-PDT | 4.34 | 0.31 | <0.001 | 1.29 | 2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrasiabi, S.; Benedicenti, S.; Signore, A.; Arshad, M.; Chiniforush, N. Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus aureus, Escherichia coli, and Candida albicans for Improved Antimicrobial Photodynamic Therapy. Bioengineering 2024, 11, 48. https://doi.org/10.3390/bioengineering11010048
Afrasiabi S, Benedicenti S, Signore A, Arshad M, Chiniforush N. Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus aureus, Escherichia coli, and Candida albicans for Improved Antimicrobial Photodynamic Therapy. Bioengineering. 2024; 11(1):48. https://doi.org/10.3390/bioengineering11010048
Chicago/Turabian StyleAfrasiabi, Shima, Stefano Benedicenti, Antonio Signore, Mahnaz Arshad, and Nasim Chiniforush. 2024. "Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus aureus, Escherichia coli, and Candida albicans for Improved Antimicrobial Photodynamic Therapy" Bioengineering 11, no. 1: 48. https://doi.org/10.3390/bioengineering11010048
APA StyleAfrasiabi, S., Benedicenti, S., Signore, A., Arshad, M., & Chiniforush, N. (2024). Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus aureus, Escherichia coli, and Candida albicans for Improved Antimicrobial Photodynamic Therapy. Bioengineering, 11(1), 48. https://doi.org/10.3390/bioengineering11010048









