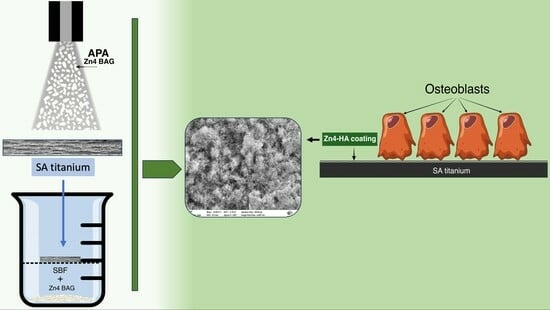
Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Titanium Substrates
2.2. Simulating Body Fluid Immersion
2.3. Surface Characterization
2.3.1. Surface Roughness
2.3.2. Contact Angle (CA) and Surface Free Energy (SFE) Calculations
2.3.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
2.4. Antimicrobial Activity Test
2.5. MC3T3-E1 Cell Culture
2.6. Focal Adhesion Staining
2.7. Cell Viability Assay with MTT
2.8. Statistical Analysis
3. Results
3.1. Surface Roughness, Contact Angle, and Surface Free Energy
3.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
3.3. Antibacterial Effect
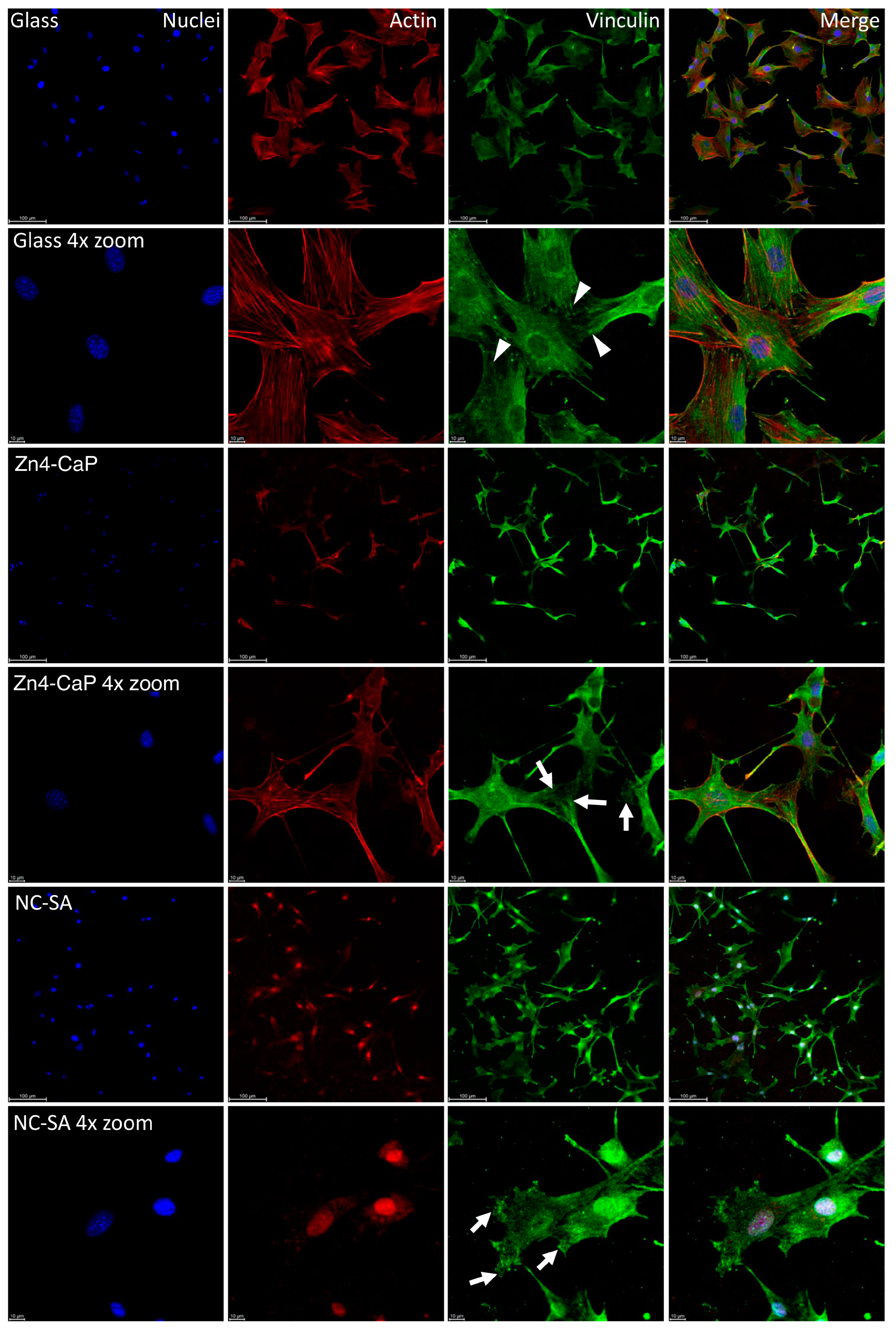
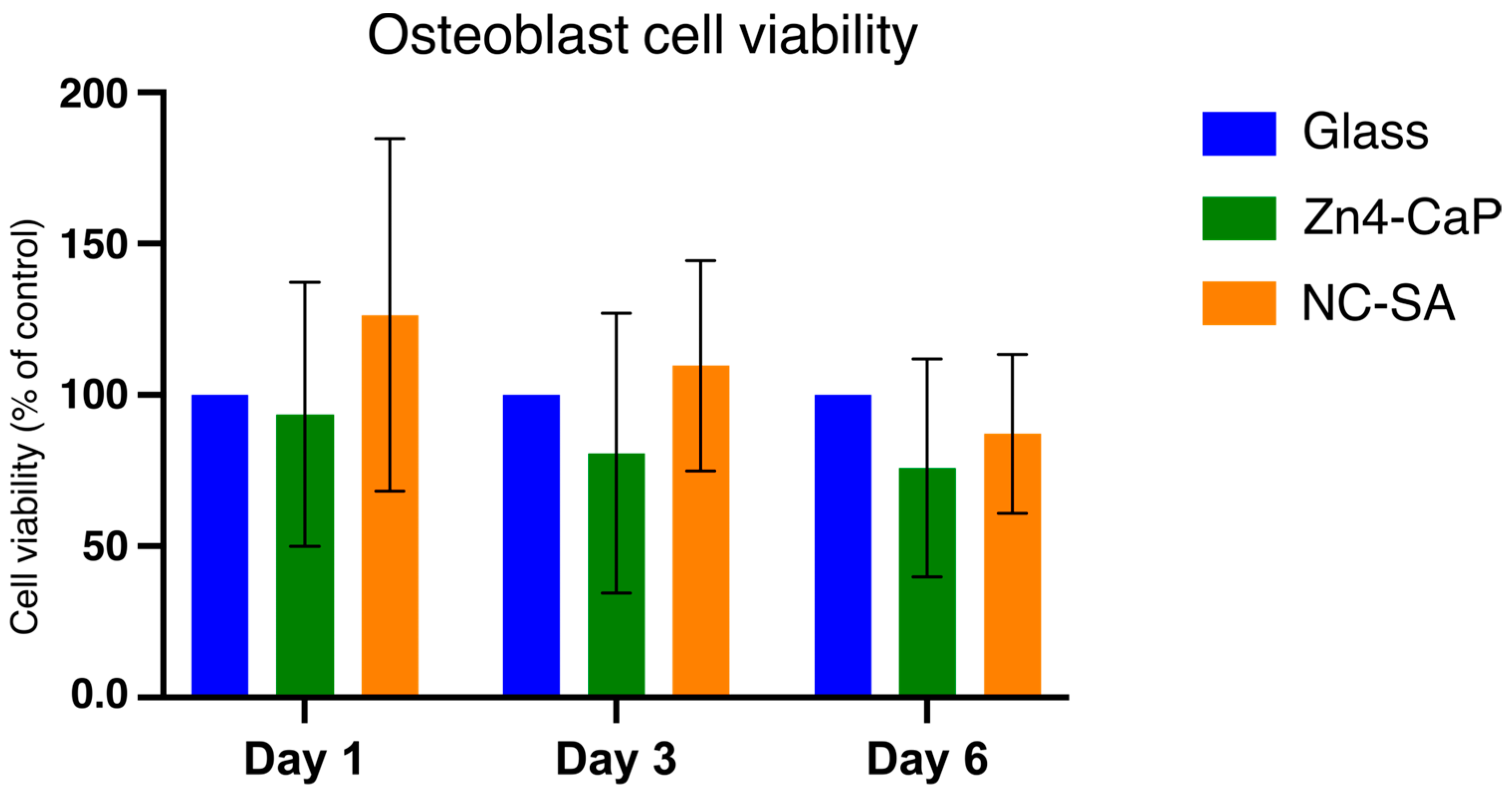
3.4. Focal Adhesion and MTT Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Suarez, F.; Monje, A.; Galindo-Moreno, P.; Wang, H.L. Implant surface detoxification: A comprehensive review. Implant. Dent. 2013, 22, 465–473. [Google Scholar] [CrossRef]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef]
- Estefanía-Fresco, R.; García-de-la-Fuente, A.M.; Egaña-Fernández-Valderrama, A.; Bravo, M.; Aguirre-Zorzano, L.A. One-year results of a nonsurgical treatment protocol for peri-implantitis. A retrospective case series. Clin. Oral Implants Res. 2019, 30, 702–712. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S237–S245. [Google Scholar] [CrossRef]
- Keim, D.; Nickles, K.; Dannewitz, B.; Ratka, C.; Eickholz, P.; Petsos, H. In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin. Oral Implants Res. 2019, 30, 550–558. [Google Scholar] [CrossRef]
- Moharrami, M.; Perrotti, V.; Iaculli, F.; Love, R.; Quaranta, A. Effects of air abrasive decontamination on titanium surfaces: A systematic review of in vitro studies. Clin. Implant Dent. Relat. Res. 2019, 21, 398–421. [Google Scholar] [CrossRef]
- Abushahba, F.; Söderling, E.; Aalto-Setälä, L.; Sangder, J.; Hupa, L.; Närhi, T.O. Antibacterial properties of bioactive glass particle abraded titanium against Streptococcus mutans. Biomed. Phys. Eng. Express 2018, 4, 045002. [Google Scholar] [CrossRef]
- Abushahba, F.; Söderling, E.; Aalto-Setälä, L.; Hupa, L.; Närhi, T.O. Air Abrasion with Bioactive Glass Eradicates Streptococcus mutans Biofilm From a Sandblasted and Acid-Etched Titanium Surface. J. Oral Implantol. 2019, 45, 444–450. [Google Scholar] [CrossRef]
- Abushahba, F.; Gürsoy, M.; Hupa, L.; Närhi, T.O. Effect of bioactive glass air-abrasion on Fusobacterium nucleatum and Porphyromonas gingivalis biofilm formed on moderately rough titanium surface. Eur. J. Oral Sci. 2021, 129, e12783. [Google Scholar] [CrossRef]
- Abushahba, F.; Tuukkanen, J.; Aalto-Setälä, L.; Miinalainen, I.; Hupa, L.; Närhi, T.O. Effect of bioactive glass air-abrasion on the wettability and osteoblast proliferation on sandblasted and acid-etched titanium surfaces. Eur. J. Oral Sci. 2020, 128, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Stoor, P.; Söderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef]
- Begum, S.; Johnson, W.E.; Worthington, T.; Martin, R.A. The influence of pH and fluid dynamics on the antibacterial efficacy of 45S5. Bioglass. Biomed. Mater. 2016, 11, 015006. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Particulate Bioglass reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin. Oral Implants Res. 2002, 13, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Gavinho, S.R.; Jakka, S.K.; Valente, M.A.; Graça, M.P.F.; Pádua, A.S.; Silva, J.C.; Sá-Nogueira, I.; Borges, J.P. Antibacterial Biomaterial Based on Bioglass Modified with Copper for Implants Coating. J. Funct. Biomater. 2023, 14, 369. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Sinitsyna, P.; Karlström, O.; Sevonius, C.; Hupa, L. In vitro dissolution and characterisation of flame-sprayed bioactive glass microspheres S53P4 and 13–93. J. Non-Cryst. Solids 2022, 591, 121736. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, W.; Li, B.; Wang, C.; Kuang, T.; Li, Y. Physical vapor deposition technology for coated cutting tools: A review. Ceram. Int. 2020, 46, 18373–18390. [Google Scholar] [CrossRef]
- Huang, C.H.; Yoshimura, M. Direct ceramic coating of calcium phosphate doped with strontium via reactive growing integration layer method on α-Ti alloy. Sci. Rep. 2020, 10, 10602. [Google Scholar] [CrossRef]
- Gross, K.A.; Berndt, C.C.; Herman, H. Amorphous phase formation in plasma-sprayed hydroxyapatite coatings. J. Biomed. Mater. Res. 1998, 3, 407–414. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Sprayed Hydroxylapatite-Based Coatings: Chemical, Mechanical, Microstructural, and Biomedical Properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Nahum, E.Z.; Lugovskoy, S.; Lugovskoy, A.; Kazanski, B.; Sobolev, A. The study of hydroxyapatite growth kinetics on CP—Tiand Ti65Zr treated by Plasma electrolytic oxidation process. J. Mater. Res. Technol. 2023, 24, 2169–2186. [Google Scholar] [CrossRef]
- Astaneh, S.H.; Faverani, L.P.; Sukotjo, C.; Takoudis, C.G. Atomic layer deposition on dental materials: Processing conditions and surface functionalization to improve physical, chemical, and Clinical Properties—A Review. Acta Biomater. 2021, 121, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Akhtach, S.; Tabia, Z.; Bricha, M.; El Mabrouk, K. Structural characterization, in vitro bioactivity, and antibacterial evaluation of low silver-doped bioactive glasses. Ceram. Int. 2021, 47, 29036–29046. [Google Scholar] [CrossRef]
- Luo, S.H.; Xiao, W.; Wei, X.J.; Jia, W.T.; Zhang, C.Q.; Huang, W.H.; Jin, D.X.; Rahaman, M.N.; Day, D.E. In vitro evaluation of cytotoxicity of silver-containing borate bioactive glass. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 441–448. [Google Scholar] [CrossRef]
- Mohd Bakhori, S.K.; Mahmud, S.; Ling, C.A.; Sirelkhatim, A.H.; Hasan, H.; Mohamad, D.; Masudi, S.M.; Seeni, A.; Abd Rahman, R. In-vitro efficacy of different morphology zinc oxide nanopowders on Streptococcus sobrinus and Streptococcus mutans. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 868–877. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. Int. J. Environ. Res. Public Health 2023, 26, 2197. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-edged effects and mechanisms of Zn2+microenvironments on osteogenic activity of BMSCs: Osteogenic differentiation or apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef]
- Koller, G.; Cook, R.J.; Thompson, I.D.; Watson, T.F.; Di Silvio, L. Surface modification of titanium implants using bioactive glasses with air abrasion technologies. J. Mater. Sci. Mater. Med. 2007, 18, 2291–2296. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- de Jong, H.P.; van Pelt, A.W.; Arends, J. Contact angle measurements on human enamel—An in vitro study of influence of pellicle and storage period. J. Dent. Res. 1982, 61, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Kylmäoja, E.; Holopainen, J.; Abushahba, F.; Ritala, M.; Tuukkanen, J. Osteoblast Attachment on Titanium Coated with Hydroxyapatite by Atomic Layer Deposition. Biomolecules 2022, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Abushahba, F.; Areid, N.; Gürsoy, M.; Willberg, J.; Laine, V.; Yatkin, E.; Hupa, L.; Närhi, T.O. Bioactive glass air-abrasion promotes healing around contaminated implant surfaces surrounded by circumferential bone defects: An experimental study in the rat. Clin. Implant Dent. Relat. Res. 2023, 25, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Aalto-Setälä, L.; Siekkinen, M.; Lindfors, N.; Hupa, L. Dissolution of Glass–Ceramic Scaffolds of Bioactive Glasses 45S5 and S53P4. Biomed. Mater. Devices 2023, 1, 871–881. [Google Scholar] [CrossRef]
- Siekkinen, M.; Karlström, O.; Hupa, L. Effect of local ion concentrations on the in vitro reactions of bioactive glass 45S5 particles. Int. J. Appl. Glass Sci. 2022, 13, 695–707. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Alharbi, A.A.; Al-Tuwaijri, M.M.; Makharita, R.R. The Antibacterial Activities and Characterizations of Biosynthesized Zinc Oxide Nanoparticles, and Their Coated with Alginate Derived from Fucus vesiculosus. Polymers 2023, 15, 2335. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Wu, S.; Zhou, R.; Zhang, K.; Zhang, Z.; Liu, J.; Qin, S.; Shi, J. Nanomaterial-Based Zinc Ion Interference Therapy to Combat Bacterial Infections. Front. Immunol. 2022, 13, 899992. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Yamamoto, O.; Takano, H.; Fukuda, M.; Iino, M. Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci. Rep. 2016, 6, 29462. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Cho, Y.E.; Kim, T.; Shin, H.I.; Kwun, I.S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef]
- Martinez, M.A.F.; Balderrama, Í.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface roughness of titanium disks influences the adhesion, proliferation and differentiation of osteogenic properties derived from human. Int. J. Implant Dent. 2020, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Fos-Parra, I.; Cabanillas-Balsera, D.; Gil, J.; Ortiz-García, I.; Giner, M.; Bocio-Núñez, J.; Montoya-García, M.J.; Jiménez-Guerra, Á. Osteoblastic Cell Behavior and Gene Expression Related to Bone Metabolism on Different Titanium Surfaces. Int. J. Mol. Sci. 2023, 24, 3523. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Kim, W.J.; Kim, S.; Ku, Y.; Ryoo, H.M. Surface Topography of Titanium Affects Their Osteogenic Potential through DNA Methylation. Int. J. Mol. Sci. 2021, 22, 2406. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The mucosal attachment to titanium implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Kohavi, D.; Badihi Hauslich, L.; Rosen, G.; Steinberg, D.; Sela, M.N. Wettability versus electrostatic forces in fibronectin and albumin adsorption to titanium surfaces. Clin. Oral Implants Res. 2013, 24, 1002–1008. [Google Scholar] [CrossRef]
- Ostrovskaya, L.; Perevertailo, V.; Ralchenko, V.; Dementjev, A.; Loginova, O. Wettability and surface energy of oxidized and hydrogen plasma-treated diamond films. Diam. Relat. Mater. 2002, 11, 845–850. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Komorowski, P.; Balcerzak, J.; Jastrzebski, K.; Przybyszewska, K.; Kaczmarek, A. Surface Characteristics and Biological Evaluation of Si-DLC Coatings Fabricated Using Magnetron Sputtering Method on Ti6Al7Nb Substrate. Nanomaterials 2019, 29, 812. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Latifi, S.; Shankar, R.; Donahue, H. Polydopamine Coating on Titanium Affects Osteoblastic Differentiation to a Greater Degree than Does Surface Roughness. Adv. Mater. Phys. Chem. 2020, 10, 339–349. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Foppiano, S.; Marshall, S.J.; Marshall, G.W.; Saiz, E.; Tomsia, A.P. Bioactive glass coatings affect the behavior of osteoblast-like cells. Acta Biomater. 2007, 3, 765–771. [Google Scholar] [CrossRef]
- Xynos, I.D.; Hukkanen, M.V.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nihonmatsu, S.; Okawara, T.; Onuki, H.; Sakagami, H.; Nakajima, H.; Takeishi, H.; Shimada, J. Adhesion and Proliferation of Osteoblastic Cells on Hydroxyapatite-dispersed Ti-based Composite Plate. In Vivo 2019, 33, 1067–1079. [Google Scholar] [CrossRef]
- Guo, H.; Wei, J.; Yuan, Y.; Liu, C. Development of calcium silicate/calcium phosphate cement for bone regeneration. Biomed. Mater. 2007, 2, 153–159. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef] [PubMed]



| Component | Concentration (g/L) |
|---|---|
| NaCl | 7.996 |
| NaHCO3 | 0.35 |
| KCl | 0.224 |
| K2HPO4·3H2O | 0.228 |
| MgCl2·6H2O | 0.305 |
| CaCl2 | 0.278 |
| Na2SO4 | 0.071 |
| (CH2OH)3CNH2 | 6.057 |
| Substrate | Ra | Rp | Rq | Rt | Rv |
|---|---|---|---|---|---|
| NC-SA | 3.695 (0.086) | 12.71 (0.64) | 4.53 (0.31) | 37.70 (3.90) | −24.98 (4.22) |
| Zn4-APA | 2.526 (0.054) *** | 9.23 (0.39) *** | 3.37 (0.14) *** | 27.64 (0.90) *** | −18.33 (0.95) * |
| Zn4-CaP | 2.565 (0.042) *** | 10.79 (1.58) *** | 3.52 (0.31) *** | 31.12 (3.73) ** | −20.71 (3.89) |
| Substrate | Contact Angles CA (°) | Surface Free Energy (SFE) | ||||
|---|---|---|---|---|---|---|
| Water | Diiodomethane | Formamide | Total (γtot) | Dispersive (γD) | Polar (γP) | |
| NC-SA | 77.19 (4.31) | 64.48 (3.32) | 40.73 (3.01) | 40.43 (1.14) | 30.14 (2.08) | 10.38 (1.46) |
| Zn4-CaP | 13.35 (1.58) *** | 11.06 (0.86) *** | 9.57 (1.20) *** | 71.13 (0.22) *** | 43.88 (0.14) *** | 27.22 (0.21) *** |
| Substrate | Na | Al | Ti | Cr | Cl | Si | P | Ca | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|
| NC-SA | Weight % | 0.44 | 5.69 | 92.51 | 0.88 | 0.17 | ||||
| Atom % | 0.87 | 9.63 | 88.25 | 0.77 | 0.22 | |||||
| Zn4-APA | Weight % | 5.76 | 2.49 | 45.35 | 0.38 | 0.32 | 3.27 | 0.13 | 2.39 | 0.54 |
| Atom % | 6.32 | 2.33 | 23.87 | 0.18 | 0.27 | 2.94 | 0.11 | 1.51 | 0.21 | |
| Zn4-CaP | Weight % | 0.63 | 18.73 | 0.19 | 0.49 | 15.46 | 3.32 | 9.48 | 3.22 | |
| Atom % | 0.53 | 8.88 | 0.11 | 0.31 | 12.50 | 2.43 | 5.37 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abushahba, F.; Kylmäoja, E.; Areid, N.; Hupa, L.; Vallittu, P.K.; Tuukkanen, J.; Närhi, T. Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating. Bioengineering 2024, 11, 74. https://doi.org/10.3390/bioengineering11010074
Abushahba F, Kylmäoja E, Areid N, Hupa L, Vallittu PK, Tuukkanen J, Närhi T. Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating. Bioengineering. 2024; 11(1):74. https://doi.org/10.3390/bioengineering11010074
Chicago/Turabian StyleAbushahba, Faleh, Elina Kylmäoja, Nagat Areid, Leena Hupa, Pekka K. Vallittu, Juha Tuukkanen, and Timo Närhi. 2024. "Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating" Bioengineering 11, no. 1: 74. https://doi.org/10.3390/bioengineering11010074
APA StyleAbushahba, F., Kylmäoja, E., Areid, N., Hupa, L., Vallittu, P. K., Tuukkanen, J., & Närhi, T. (2024). Osteoblast Attachment on Bioactive Glass Air Particle Abrasion-Induced Calcium Phosphate Coating. Bioengineering, 11(1), 74. https://doi.org/10.3390/bioengineering11010074