1. Introduction
Dental implants are a robust solution for tooth replacement. However, post-implantation failures, notably due to peri-implantitis, remain a concern [
1,
2]. Peri-implantitis, an inflammatory disorder induced by microbial infections, leads to the degradation of the supporting alveolar bone [
2]. Microorganisms engulf the surface of the implant [
3], creating a bacterial biofilm that provokes an inflammatory response in adjacent soft tissues. If left untreated, progressive destruction of the marginal bone ensues [
3,
4]. Numerous studies highlighted the role of microbial deposition in peri-implantitis [
1,
2,
3,
4] as well as the need to eradicate bacterial biofilm for effective peri-implantitis management. However, optimal strategies to decontaminate the compromised implant surface and to rejuvenate peri-implant tissues remain elusive.
Traditional methods for removing microbial deposits from dental implants predominantly involve mechanical debridement using tools such as plastic curettes and titanium brushes [
5]. Several chemical agents, including chlorhexidine and minocycline, were recommended for targeted applications [
6]. Air-abrasive devices are also used to detoxify the implant surface; however, efficacy wanes for deeply situated rough implant surfaces with infrabony defects [
7]. Due to their inherently bactericidal properties, lasers can be used to disinfect implant surfaces. Unlike conventional mechanical devices, lasers offer several benefits: tissue ablation, hemostasis, and decontamination [
8]. Their subdued noise and vibration profiles enhance patient comfort while minimizing collateral tissue damage, reducing post-procedural discomfort, and improving prognosis [
8]. Moritz et al. showed that laser interventions can drastically diminish the risk of infection and reduce the prevalence of pathogens such as
Aggregatibacter actinomycetemcomitans,
Prevotella intermedia, and
Porphyromonas gingivalis [
9].
Although Er:YAG and Er,Cr:YSGG lasers are primarily used for dental caries and soft tissue treatments, their hemostatic effects are limited [
10]. Diode and CO
2 lasers are suitable for soft tissue surgeries despite risks of extensive thermal repercussions [
10]. The surface of peri-implantitis-afflicted implants must be cleaned without being modified. Kreisler et al. assessed how various laser types affect titanium implant surfaces and found that Nd:YAG and Ho:YAG lasers modified the surface and are therefore unsuitable for peri-implantitis treatments [
11]. Likewise, Romanos et al., observed deleterious effects of Nd:YAG lasers on implant surfaces [
12]. Conversely, in vitro studies showed the antimicrobial efficacy of Er:YAG, CO
2, and diode lasers against bacteria on titanium surfaces [
13]. Notably, CO
2 and diode lasers were found to be benign against titanium, but Er:YAG lasers eliminated surface microorganisms without modifying the surface [
14,
15].
The architecture of the implant surface significantly influences osseointegration [
16]. Therefore, a variety of surface treatments were proposed to optimize implant success rates and osseointegration [
17,
18], including standalone sandblasting and hybrid techniques incorporating acid etching. Notably, the sandblasted, large-grit, and acid-etched (SLA) surface treatment enhances cellular proliferation and bone implant juxtapositions while possibly reducing treatment timelines [
19,
20]. Buser et al., showed how the SLA surface fosters osseointegration in porcine models [
21]; their subsequent studies echoed these findings and the efficacy of the SLA surface in torque retention [
22]. Vorobyev et al. illustrated the potential of the femtosecond laser to fabricate intricate nanostructures on titanium surfaces that support cell adhesion and proliferation [
23,
24].
A previous study sought to identify optimal power and exposure durations for diode lasers to cleanse titanium disc surfaces sans damage [
25]. This study evaluated surface alterations on a variety of implant surfaces post-laser periodontal treatments, particularly Er,Cr:YSGG and diode lasers. Electrocautery, another viable option for peri-implant tissue management, was also assessed. Within the vast landscape of implant surfaces, we compared the less characterized femtosecond laser-treated surfaces with machined surfaces and SLA surface implants. The purpose of this study was to investigate the effects of different peri-implantitis treatment methods (Er,Cr:YSGG laser, diode laser, and electrocautery) on various titanium implant surfaces: machined; sandblasted, large-grit, and acid-etched; and femtosecond laser-treated surfaces. The null hypothesis of this study was that different peri-implantitis treatment methods (Er, Cr:YSGG laser, diode laser, and electrocautery) do not effect various titanium implant surfaces (machined; sandblasted, large-grit, and acid-etched; and femtosecond laser-treated surfaces).
2. Materials and Methods
2.1. Implant Fixture Preparation and Surface Treatment of Titanium Disk
Machined surface group: Grade 4 titanium discs with a diameter of 10 mm and a thickness of 1 mm were fabricated by a dental implant manufacturer (DENTIS, Daegu, Republic of Korea) and processed using a CNC milling machine. The discs were composed of 0.08% carbon (C), 0.5% iron (Fe), 0.015% hydrogen (H), 0.05% nitrogen (N), 0.40% oxygen (O), and 98.9% titanium (Ti).
SLA surface group: Titanium discs were sandblasted with a large volume of alumina particles (250–500 μm) at a pressure of 4 bar for 20 min. They were then oxidized using a mixture of HCl (65%) and H2SO4 (9%) (in a 1:1 volume ratio) at 60 °C for 30 min, followed by rinsing with distilled water and air-drying at ambient conditions.
Femtosecond laser surface group: Titanium disc surfaces were treated using a femtosecond laser operating at a wavelength of 343 nm, a scanning speed of 10 mm/s, and a repetition frequency of 200 kHz. As a result, lines were patterned at intervals of 50 μm on the titanium (
Table 1).
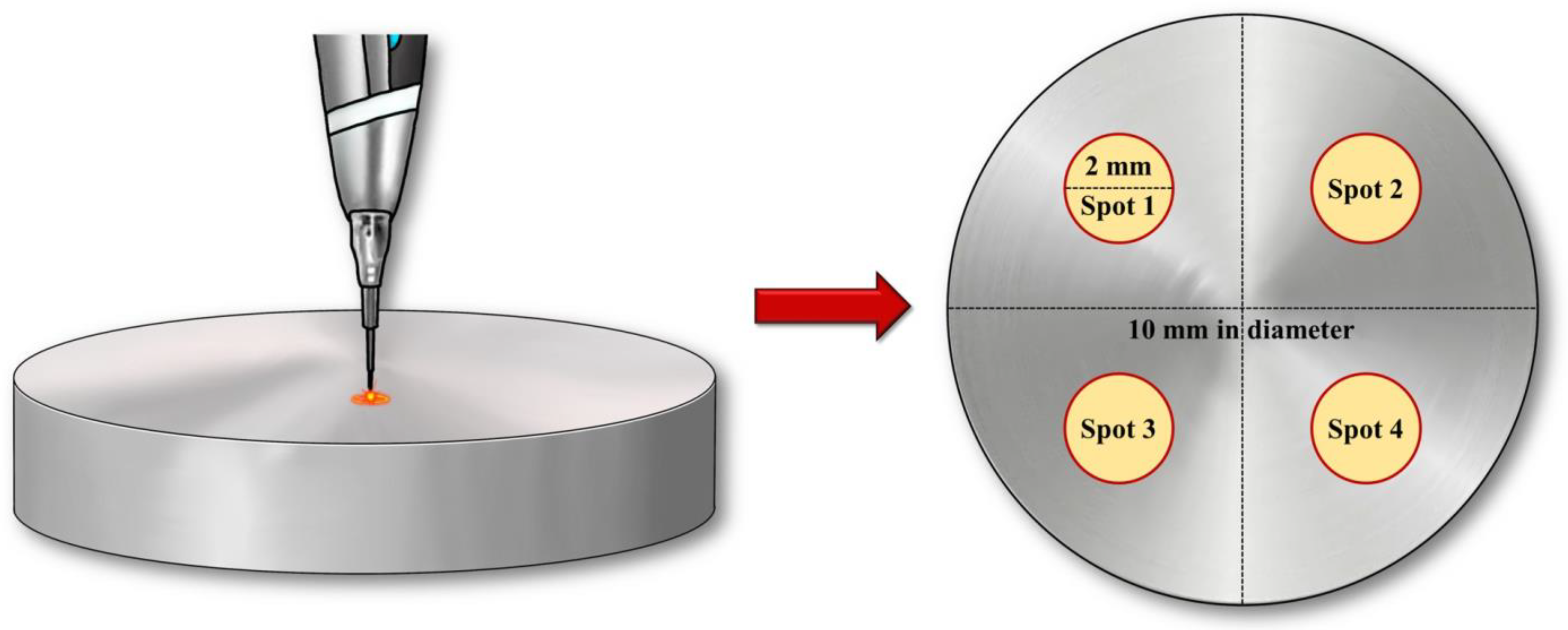
After conducting pilot experiments prior to the current study, we determined that 3 samples per group would be appropriate based on the following results obtained using power analysis software (G*Power v3.1.9.2; Heinrich Heine University, Dusseldorf, Germany): actual power = 81.7%; and power = 80%. To minimize potential investigator-induced variations, experienced researchers performed repeated experiments under identical conditions, and all utilized devices were calibrated in accordance with the manufacturer’s instructions prior to use. Additionally, all experiments were conducted under consistent conditions (
Figure 1).
2.2. Dental Laser and Electrocautery
All peri-implantitis treatment methods in the present study were performed by one investigator (J.-S.L.) skilled in the technique. Three dental diode lasers ranging from 940 nm to 980 nm were selected based on a previous study [
24]. Here, two types of lasers and an electrocautery device were used:
Epic 10TM (Biolase, Inc., Foothill Ranch, CA, USA) for diode laser at a wavelength of 940 nm;
Waterlase iPlus® (Biolase, Inc., Foothill Ranch, CA, USA) for the Er,Cr:YSGG laser at a wavelength of 2780 nm;
Bovie Surgitron FfpF (Ellman, Hicksville, NY, USA) for electrocautery. Ten-second treatments were administered as described in
Table 2.
The laser and electrocautery tips were chosen to be identical in size; they remained static and made direct perpendicular contact with the titanium disc surface for 10 s (
Figure 2). All lasers operated in continuous wave mode using a flexible fiber, generating a focused spot whose diameter was 0.4 mm. The laser parameters are listed in
Table 2.
2.3. Surface Roughness Assessment via Scanning Electron and Confocal Scanning Microscopy
Titanium discs were thoroughly cleaned in an ultrasonic bath using a mixture of acetone, ethanol, and distilled water (1:1:1 w/v/v) for 15 min at each step. The discs were then oven-dried at 60 °C for 2 h and analyzed using a scanning electron microscope (SEM; Hitachi SU8230; Hitachi, Tokyo, Japan) at an acceleration voltage of 5 kV and 100× magnification.
Confocal microscopy images were captured at 10× magnification using a laser scanning confocal microscope (LEXT OLS4100; Olympus, Tokyo, Japan). The surface roughness of the treated titanium discs was quantified using the arithmetic mean roughness (Ra) and arithmetical mean height (Sa) derived from the microscopy images. Each sample was measured thrice to evaluate how each surface treatment affected implant texture.
2.4. Wettability Assessment
Titanium disc wettability was measured using a contact angle goniometer (Phoenix-MT; SEO, Suwon, Republic of Korea) and the following procedure was conducted as prior study [
26]. A two-microliter droplet of deionized water was placed on each sample using a micro syringe. The contact angle between the liquid droplet and the solid disc surface was measured within 10 s. This procedure was repeated five times for every sample to obtain an average contact angle.
2.5. Energy-Dispersive Spectrometry
Energy-dispersive X-ray spectroscopy (EDS) was conducted using a field emission SEM coupled with an Oxford ULTIM MAX 100 (SU8230, Hitachi, Ltd., Tokyo, Japan) to characterize the chemical properties of the scanned disks. This method provided atomic and weight percentage ratios for titanium, oxygen, and carbon.
2.6. Statistical Analysis
Non-parametric statistical evaluations were conducted using SPSS software (version 26; IBM Corp., Armonk, NY, USA). The Kruskal–Wallis H test was performed (α = 0.05) to assess differences in surface roughness and contact angles across the three implant surface treatments. In the case of significant differences, subsequent pairwise comparisons were executed using post-hoc tests and the Bonferroni correction. Significant differences between the implant surface treatments were denoted using uppercase letters (α = 0.05).
The Kruskal–Wallis H test was also used (α = 0.05) to assess differences in wettability across the implant surface treatments. As stated above, pairwise examinations were conducted with the Bonferroni correction. Significant differences between the implant surface treatments were denoted using uppercase letters (α = 0.05).
4. Discussion
Implant-related infections such as peri-implantitis and mucositis often lead to implant failure [
3,
4]. Therefore, this study initially aimed to investigate the effects of laser and electrocautery, which are currently used for peri-implantitis treatment, on the titanium surface. Additionally, we sought to determine if there were any differences in treatment outcomes based on the type of implant surface. Despite the relatively minimal impact of peri-implantitis treatment using lasers on titanium implant surfaces, we anticipated variations based on the type of laser used in the experiment. We also expected different responses when applying lasers to three different implant surfaces, and to investigate this, we analyzed surface roughness, chemical composition, and wettability. Ultimately, while there were differences observed in roughness analysis using Ra and Sa depending on the type of implant surface, these differences did not show statistically significant results. However, there were cases where statistically significant differences were observed depending on the type of laser used, specifically with the Er,Cr:YSGG laser (Ra) and electrocautery (Sa). In the wettability test, the use of the Er,Cr:YSGG laser on femtosecond laser-treated surfaces showed statistically significant differences compared to other treatment methods.
The implant surfaces were characterized qualitatively and quantitatively using SEM and confocal microscopy. Treatment with the Er,Cr:YSGG laser at a modest power of 2 W for 10 s significantly altered the implant surface, which was also shown previously by Park et al., especially beyond the 3 W power threshold [
27]. These results urge caution when using Er,Cr:YSGG lasers in clinical settings. Conversely, the diode laser only slightly altered the surface, corroborating findings from Lollobrigida et al. [
28,
29] and others [
25]. A 10 s treatment in continuous wave mode negligibly altered the surface of a small disk, consistent with Castro [
28,
29]. Although the diode laser only slightly altered the surface, its decontamination efficiency may differ and should be explored in a future study. Additionally, the skill of the operator administering the laser and the variations in laser parameters can introduce differences in the results. It is also crucial to consider the heat generation resulting from the use of laser equipment. While this heat generation may aid in contamination removal, a significant temperature rise can potentially affect the surrounding teeth and bone formation. Further studies are needed to account for and explore these effects.
EDS was used to characterize the chemical compositions of the disks after surface treatments. Specifically, the machined and SLA surfaces displayed elevated titanium (Ti) and reduced oxygen (O) levels, whereas the femtosecond laser-treated surface showed enhanced oxygen levels. Given titanium’s tendency to bond with atmospheric elements such as carbon (C), oxygen (O), and nitrogen (N), an oxidized titanium surface results in a titanium oxide (TiO
2) layer. Augmented polarization enhances oxygen levels, promoting the formation of the TiO
2 layer [
30,
31,
32]. A pronounced TiO
2 layer on the femtosecond laser-treated surface suggested enhanced biocompatibility that could enhance cellular responses to the implant surface. When treated with the Er,Cr:YSGG laser, there was a common trend of increased oxygen levels, and it is believed that this result also occurred due to surface oxidation reactions. In the research of Scarano et al., the irradiation of the SLA disk surface with Er:YAG laser depicted similar results, leading to an increase in TiO
2 layer [
33]. Additionally, the experiment of Ercan et al. showed treatment with the Er,Cr:YSGG laser at settings above 2 W resulted in an increase in oxygen levels and a decrease in titanium levels due to oxidation [
34,
35]. The effects of the chemical composition of the disks on cell and tissue behaviors warrant comprehensive in vitro and in vivo studies.
Contact angles were measured to determine how various treatments affect surface wettability [
36,
37,
38,
39,
40,
41,
42,
43]. After implantation, interactions between implants and fluids affect the tissue microenvironment as well as the surface of the implant. MacDonald’s research showed that implants with higher wettability promote osseointegration [
44]. Our data suggest that femtosecond laser-treated surfaces may be more favorable than SLA surfaces for osseointegration by increasing hydrophilicity.
After exploring how laser treatment and electrocautery affect peri-implantitis treatment, Ra and Sa values revealed minimal differences between groups. Therefore, the resistance of specific implant surfaces to laser-induced changes remains unclear. However, the potential benefits of femtosecond laser-treated surfaces, such as enhanced osseointegration due to complex surface compositions [
36] and the flexible modification of surface patterns across scales, warrant further consideration.
Ultimately, the degree of contamination on the titanium surface determines mechanical stability and osteoinductive properties [
40]. Even if chemically treated implant surfaces remained uncontaminated, mechanical processes often introduce foreign substances [
41]. Gaggl et al., found that femtosecond laser applications optimize surface structure without introducing contaminants, which agrees with our findings [
42]. Higher surface purity may enhance osseointegration and therefore implant stability. Cunha et al., observed reduced
S. aureus adhesion and biofilm formation on femtosecond laser-treated implants [
43]. Thus, femtosecond laser texturing could render titanium implants antimicrobial, mitigating the risks associated with implant infections. While femtosecond laser texturing may reduce biofilm formation associated with peri-implantitis, the present study focused solely on evaluating the morphology of implant surfaces that underwent femtosecond laser texturing and were then subjected to different peri-implantitis treatment methods. Future research should apply these peri-implantitis treatment methods and assess the attachment levels of various biofilm types to determine the impact of post-treatment modified implant surfaces on biofilm formation.
This study has several limitations. First, unlike mechanical treatment, the area of application on the surface is very small, resulting in a relatively minimal impact. Additionally, there is a limitation in that the output and application times of the laser used in the study are not diverse, but restricted to one setting. These issues could potentially be addressed by creating larger specimens for experimentation, applying the laser to multiple areas, and refining the laser settings. Furthermore, due to the nature of in vitro studies, the evaluation of the materials used in the experiment itself constitutes a significant portion of the research. Although our study characterized the effects of various implant decontamination strategies on surface properties, clinical validations are needed. Future studies should explore the resistance of surface modifications to detoxification, particularly the implications of laser-induced changes for toxin elimination and biocompatibility. In previous research, the adhesion of osteoblasts to modified implant surfaces was investigated to assess osseointegration after specific treatments [
18]. Moreover, other studies evaluated the attachment of biofilms to altered surfaces to determine the influence of modified implant surfaces on peri-implantitis [
2,
14]. The present study qualitatively and quantitatively analyzed the surface roughness and morphology following different peri-implantitis treatment methods, including Er,Cr:YSGG laser, diode laser, and electrocautery. Future research is needed to evaluate the adhesion of osteoblasts and biofilms, further elucidating the effects of modified implant surfaces on osseointegration and peri-implantitis.