Bioremediation of Neonicotinoid Pesticide, Imidacloprid, Mediated by Bacillus cereus
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Preparation of Growth Culture
2.3. Screening of Microbes
2.4. Optimization of Experimental Variables
2.5. Conditions for HPLC Analysis
2.6. LC-MS/MS Conditions
2.7. Experimental Design/Statistical Analysis
3. Results and Discussion
3.1. Screening of Bacillus Strains
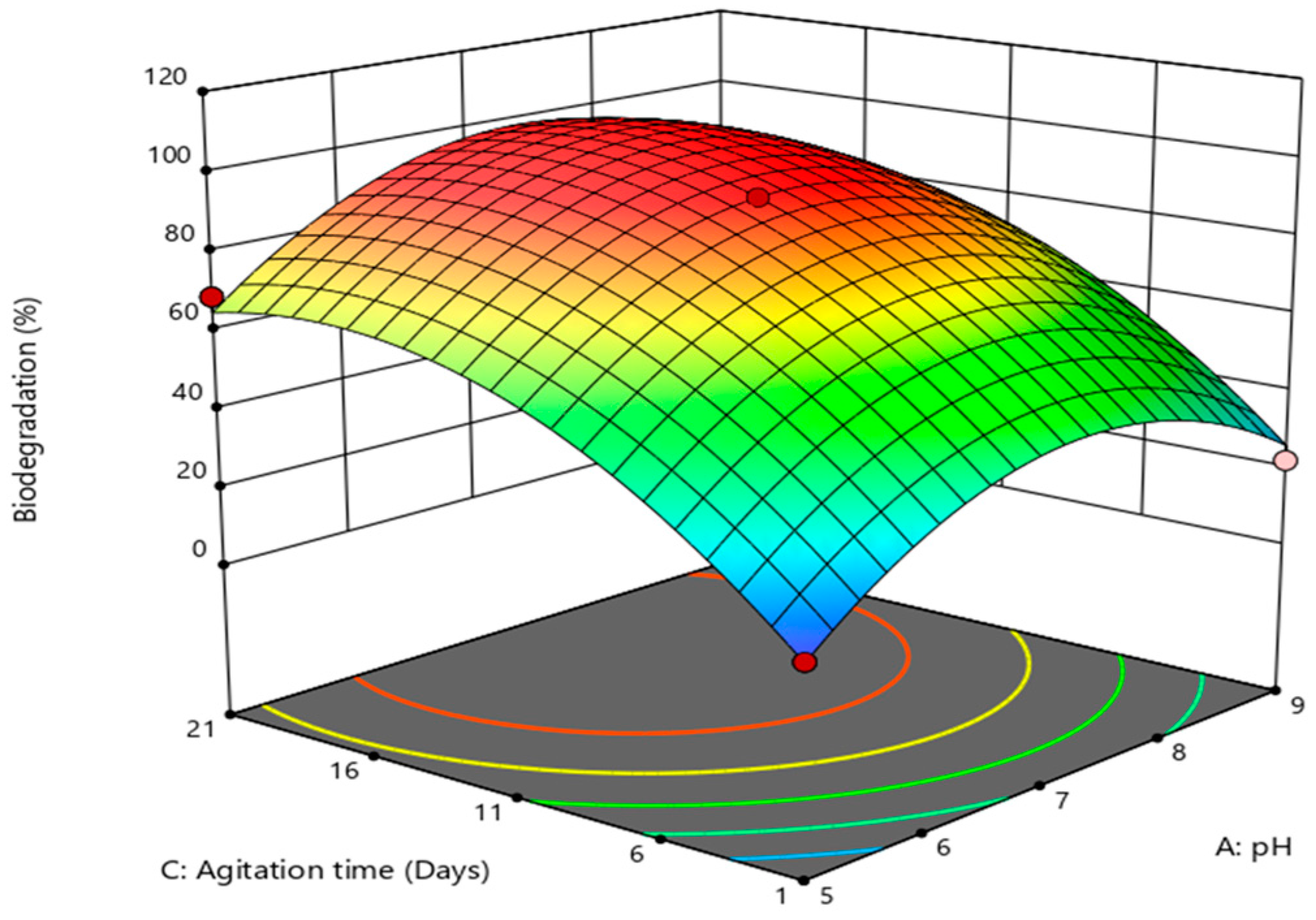
3.2. Optimization of Experimental Variable with Box–Behnken Design
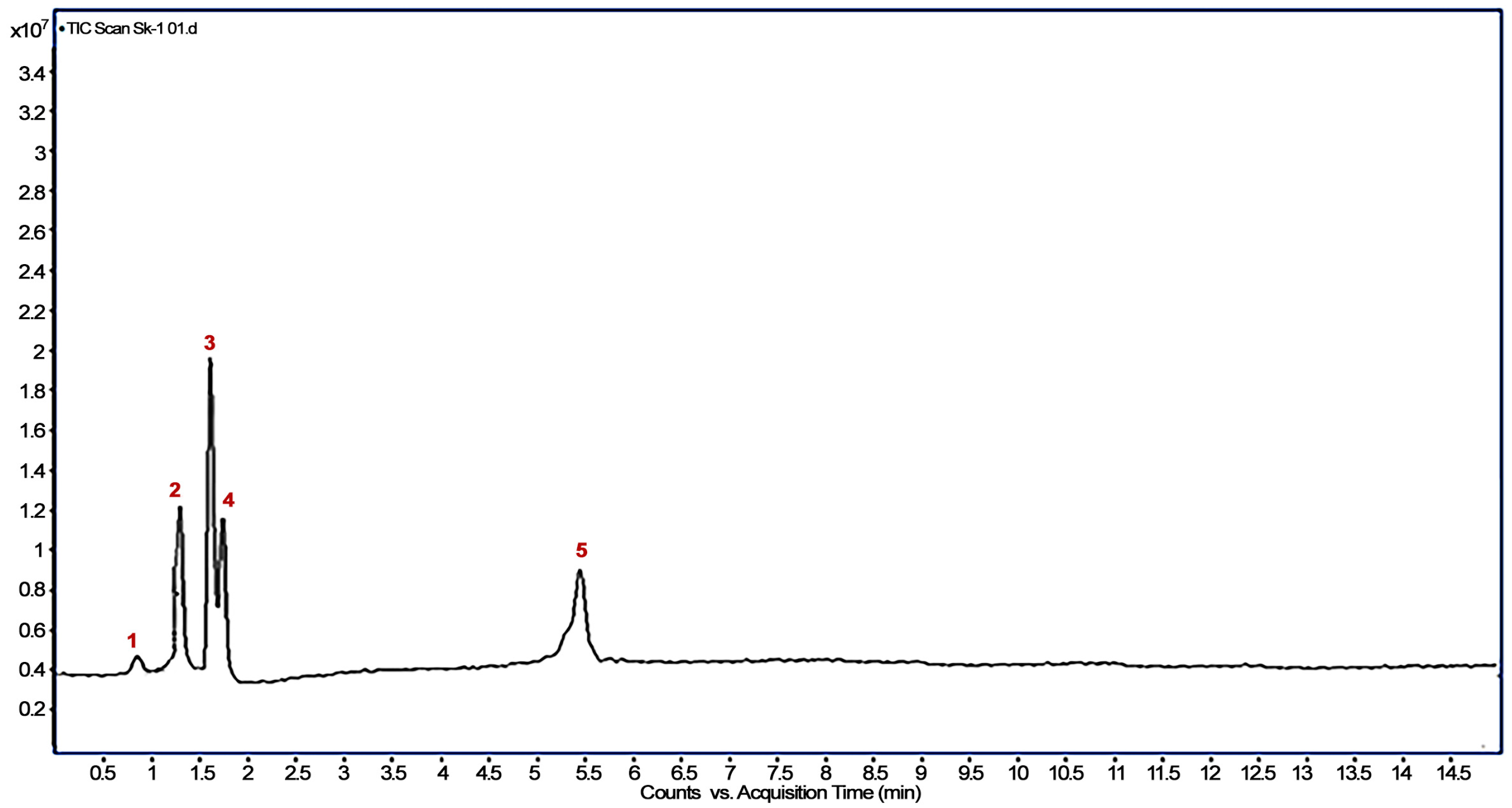
3.3. Identification of Imidacloprid Metabolites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, K.; Dhania, G. Bioremediation and biodegradation of pesticide from contaminated soil and water—A noval approach. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 23–33. [Google Scholar]
- Sultana, J.; Syed, J.H.; Mahmood, A.; Ali, U.; Rehman, M.Y.A.; Malik, R.N.; Li, J.; Zhang, G. Investigation of organochlorine pesticides from the Indus Basin, Pakistan: Sources, air–soil exchange fluxes and risk assessment. Sci. Total Environ. 2014, 497, 113–122. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Fenoll, J.; Ruiz, E.; Hellín, P.; Flores, P.; Navarro, S. Heterogeneous photocatalytic oxidation of cyprodinil and fludioxonil in leaching water under solar irradiation. Chemosphere 2011, 85, 1262–1268. [Google Scholar] [CrossRef]
- Xuqing, L.; Yayun, L.; Chao, L.; Li, Z.; Hao, Y.; Chao, F.; Ashraf, M. Identification of residual non-biodegradable organic compounds in wastewater effluent after two-stage biochemical treatment. Open Life Sci. 2016, 11, 396–401. [Google Scholar] [CrossRef]
- Zalom, F.; Toscano, N.; Byrne, F. Managing resistance is critical to future use of pyrethroids and neonicotinoids. Calif. Agric. 2005, 59, 11–15. [Google Scholar] [CrossRef][Green Version]
- Guzsvány, V.J.; Papp, Z.J.; Lazić, S.D.; Gaál, F.F.; Bjelica, L.J.; Abramović, B.F. A rapid spectrophotometric determination of imidacloprid in selected commercial formulations in the presence of 6-chloronicotinic acid. J. Serb. Chem. Soc. 2009, 74, 1455–1465. [Google Scholar] [CrossRef]
- Casida, J. Neonicotinoids and other insect nicotinic receptor competitive modulators: Progress and prospects. Annu. Rev. Entomol. 2018, 63, 125–144. [Google Scholar] [CrossRef]
- Carrington, D. EU Agrees Total Ban on Bee-Harming Pesticides. The Guardian. Available online: https://www.theguardian.com/environment/2018/apr/27/eu-agrees-total-ban-on-bee-harming-pesticides (accessed on 27 April 2023).
- Khan, M.I.; Shoukat, M.A.; Cheema, S.A.; Arif, H.N.; Niazi, N.K.; Azam, M.; Bashir, S.; Ashraf, I.; Qadri, R. Use, contamination and exposure of pesticides in Pakistan: A review. Pak. J. Agric. Sci. 2020, 57, 131–149. [Google Scholar]
- Baig, S.A.; Akhter, N.A.; Ashfaq, M.; Asi, M.R.; Ashfaq, U. Imidacloprid residues in vegetables, soil and water in the southern Punjab, Pakistan. J. Agric. Technol. 2012, 8, 903–916. [Google Scholar]
- Khooharo, A.A.; Memon, R.A.; Mallah, M.U. An empirical analysis of pesticide marketing in Pakistan. Pak. Econ. Soc. Rev. 2008, 46, 57–74. [Google Scholar]
- Sarkar, M.; Biswas, P.; Roy, S.; Kole, R.; Chowdhury, A. Effect of pH and type of formulation on the persistence of imidacloprid in water. Bull. Environ. Contam. Toxicol. 1999, 63, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, W.; Ma, Y.; Liu, K.K. Sorption and degradation of imidacloprid in soil and water. J. Environ. Sci. Health Part B 2006, 41, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Pankaj, S.; Sharma, A. In situ biodegradation of endosulfan, imidacloprid, and carbendazim using indigenous bacterial cultures of agriculture fields of Uttarakhand, India. Int. J. Biol. Food Vet. Agric. Eng. 2014, 8, 935–943. [Google Scholar]
- Kluser, S.; Peduzzi, P. Global Pollinator Decline: A Literature Review; UNEP/GRID: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, H.; Siddiqi, R.; Aijaz, P. Pesticide poisoning in Pakistan: The need for public health reforms. Public Health 2016, 141, 185. [Google Scholar] [CrossRef]
- Hussain, A.M.; Sultan, S.T. Organophosphorus insecticide poisoning: Management in surgical intensive care unit. J. Coll. Physicians Surg.—Pak. JCPSP 2005, 15, 100–102. [Google Scholar]
- Millot, F.; Decors, A.; Mastain, O.; Quintaine, T.; Berny, P.; Vey, D.; Lasseur, R.; Bro, E. Field evidence of bird poisonings by imidacloprid-treated seeds: A review of incidents reported by the French SAGIR network from 1995 to 2014. Environ. Sci. Pollut. Res. 2017, 24, 5469–5485. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Pesticides in the Modern World—Pesticides Use and Management; InTech Open: Rijeka, Croatia, 2011. [Google Scholar]
- Francis, M.; Redondo, A.; Burns, J.; Graham, J. Soil application of imidacloprid and related SAR-inducing compounds produces effective and persistent control of citrus canker. Eur. J. Plant Pathol. 2009, 124, 283–292. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Kulkarni, S.V.; Mulani, M.S.; Baig, U.I. Biodegradation of imidacloprid, the new generation neurotoxic insecticide. Biodegradation 2014, 3, 16301–16307. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Gao, H.; Yue, W.; Xiong, M.; Li, F.; Zhang, H.; Ge, W. Co-metabolic biodegradation of acetamiprid by Pseudoxanthomonas sp. AAP-7 isolated from a long-term acetamiprid-polluted soil. Bioresour. Technol. 2013, 150, 259–265. [Google Scholar] [CrossRef]
- Sabale, S.; Tamhankar, B.; Dongare, M.; Mohite, B. Extraction, determination and bioremediation of heavy metal ions and pesticide residues from lake water. J. Bioremed. Biodegrad. 2012, 3, 143. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Wang, J. A brief study on the degradation kinetics of seven organophosphorus pesticides in skimmed milk cultured with Lactobacillus spp. at 42 °C. Food Chem. 2012, 131, 300–304. [Google Scholar] [CrossRef]
- Uqab, B.; Mudasir, S.; Nazir, R. Review on bioremediation of pesticides. J. Bioremediat. Biodegrad. 2016, 7, 2. [Google Scholar] [CrossRef]
- Farhan, M.; Ahmad, M.; Kanwal, A.; Butt, Z.A.; Khan, Q.F.; Raza, S.A.; Qayyum, H.; Wahid, A. Biodegradation of chlorpyrifos using isolates from contaminated agricultural soil, its kinetic studies. Sci. Rep. 2021, 11, 10320. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rai, P.; Pandey, A.; Pandey, A. Exploring the potential of Bacillus licheniformis AP1 for fermentive biohydrogen production using starch substrate: BBD based process parameter optimization. Fuel 2022, 319, 123668. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Demir, A.S.; Cebeci, A.; Jawaid, S. A highly selective whole cell biocatalysis method for the production of two major bioactive conjugated linoleic acid isomers. Biocatal. Agric. Biotechnol. 2013, 2, 328–332. [Google Scholar] [CrossRef]
- Surhio, M.A.; Talpur, F.N.; Nizamani, S.M.; Amin, F.; Bong, C.W.; Lee, C.W.; Ashraf, M.; Shah, M.R. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. 2014, 4, 55960–55966. [Google Scholar] [CrossRef]
- Bhurgri, S.; Talpur, F.N.; Nizamani, S.M.; Afridi, H.I.; Surhio, M.A.; Shah, M.R.; Bong, C.W. Isolation of Bacillus cereus from botanical soil and subsequent biodegradation of waste engine oil. Int. J. Environ. Sci. Technol. 2018, 15, 1453–1466. [Google Scholar] [CrossRef]
- Pollard, D.; Telari, K.; Lane, J.; Humphrey, G.; McWilliams, C.; Nidositko, S.; Salmon, P.; Moore, J. Asymmetric reduction of α, β-unsaturated ketone to (R) allylic alcohol by Candida chilensis. Biotechnol. Bioeng. 2006, 93, 674–686. [Google Scholar] [CrossRef]
- Qambrani, S.; Talpur, F.N.; Panhwar, A.A.; Afridi, H.I.; Talpur, M.K.; Khan, A.; Hab, S.A. Development of guar gum-based coating with castor oil for improved postharvest quality of fresh mangoes using response surface methodology. Appl. Food Res. 2022, 2, 100220. [Google Scholar] [CrossRef]
- Maheswari, C.; Ramya, A.; Priya, B.M.; Sudhahar, S.; Raj, B.P.; Lokesh, B.; Ramani, G. Analysis and optimization on the biodegradable plate making process parameters using RSM-based Box–Behnken Design method. J. Mater. Cycles Waste Manag. 2021, 23, 2255–2265. [Google Scholar] [CrossRef]
- Asim, N.; Hassan, M.; Shafique, F.; Ali, M.; Nayab, H.; Shafi, N.; Khawaja, S.; Manzoor, S. Characterizations of novel pesticide-degrading bacterial strains from industrial wastes found in the industrial cities of Pakistan and their biodegradation potential. PeerJ 2021, 9, e12211. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Maqbool, A.; Zhou, R.; Arsalan, M.; Sun, K.; Si, Y. Optimized degradation of bisphenol A by immobilized laccase from Trametes versicolor using Box-Behnken design (BBD) and artificial neural network (ANN). J. Environ. Chem. Eng. 2022, 10, 107331. [Google Scholar] [CrossRef]
- Goldberg, K.; Schroer, K.; Lütz, S.; Liese, A. Biocatalytic ketone reduction—A powerful tool for the production of chiral alcohols—Part II: Whole-cell reductions. Appl. Microbiol. Biotechnol. 2007, 76, 249–255. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Gupta, V. Biodegradation of imidacloprid by consortium of two soil isolated Bacillus sp. Bull. Environ. Contam. Toxicol. 2014, 93, 637–642. [Google Scholar] [CrossRef]
- Phugare, S.S.; Jadhav, J.P. Biodegradation of acetamiprid by isolated bacterial strain Rhodococcus sp. BCH2 and toxicological analysis of its metabolites in silkworm (Bombax mori). CLEAN–Soil Air Water 2015, 43, 296–304. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, S. Biodegradation of omethoate by Bacillus sp. YB-10: Optimization of culture conditions and degradation characteristics. Environ. Eng. Res. 2021, 26, 200235. [Google Scholar] [CrossRef]
- Shetti, A.; Kaliwal, B. Imidacloprid induced intoxication in soil isolate Brevundimonas Sp. MJ 15. Life 2012, 50, 105. [Google Scholar]
- Guo, L.; Dai, Z.; Guo, J.; Yang, W.; Ge, F.; Dai, Y. Oligotrophic bacterium Hymenobacter latericoloratus CGMCC 16346 degrades the neonicotinoid imidacloprid in surface water. AMB Express 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Prosenc, F.; Piechocka, J.; Škufca, D.; Heath, E.; Bulc, T.; Istenič, D.; Buttiglieri, G. Microalgae-based removal of contaminants of emerging concern: Mechanisms in Chlorella vulgaris and mixed algal-bacterial cultures. J. Hazard. Mater. 2021, 418, 126284. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, J.-H. Asymmetric reduction of aryl ketones with a new isolate Rhodotorula Sp. AS2. 2241. J. Mol. Catal. B Enzym. 2002, 18, 233–241. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, J.-H.; Xu, Y. Isolation of a Bacillus strain producing ketone reductase with high substrate tolerance. Bioresour. Technol. 2010, 101, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Khalid, A.; Chowdury, A.; Sarfraz, M.; Balkhair, K.; Ashraf, M. Impacts of azo dye on ammonium oxidation process and ammonia oxidizing soil bacteria. RSC Adv. 2015, 5, 34812–34820. [Google Scholar] [CrossRef]
- Mateen, F.; Javed, I.; Rafique, U.; Tabassum, N.; Sarfraz, M.; Safi, S.Z.; Yusoff, I.; Ashraf, M.A. New method for the adsorption of organic pollutants using natural zeolite incinerator ash (ZIA) and its application as an environmentally friendly and cost-effective adsorbent. Desalination Water Treat. 2016, 57, 6230–6238. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Gupta, V. Assessment of imidacloprid degradation by soil-isolated Bacillus alkalinitrilicus. Environ. Monit. Assess. 2014, 186, 7183–7193. [Google Scholar] [CrossRef] [PubMed]
- Anhalt, J.C.; Moorman, T.B.; Koskinen, W.C. Biodegradation of imidacloprid by an isolated soil microorganism. J. Environ. Sci. Health Part B 2007, 42, 509–514. [Google Scholar] [CrossRef]
- Kulkarni, M.; Chaudhari, A. Biodegradation of p-nitrophenol by P. putida. Bioresour. Technol. 2006, 97, 982–988. [Google Scholar] [CrossRef]
- Błaszak, M.; Pełech, R.; Graczyk, P. Screening of microorganisms for biodegradation of simazine pollution (Obsolete Pesticide Azotop 50 WP). Water Air Soil Pollut. 2011, 220, 373–385. [Google Scholar] [CrossRef][Green Version]
- Farouk, M.; Hussein, L.A.A.; El Azab, N.F. Different techniques for determination of imidacloprid insecticide residues. Int. J. Environ. Anal. Chem. 2014, 94, 194–209. [Google Scholar] [CrossRef]
- Ma, Y.; Zhai, S.; Mao, S.Y.; Sun, S.; Wang, Y.; Liu, Z.H.; Dai, Y.J.; Yuan, S. Co-metabolic transformation of the neonicotinoid insecticide imidacloprid by the new soil isolate Pseudoxanthomonas indica CGMCC 6648. J. Environ. Sci. Health Part B 2014, 49, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Akoijam, R.; Singh, B. Biodegradation of imidacloprid in sandy loam soil by Bacillus aerophilus. Int. J. Environ. Anal. Chem. 2015, 95, 730–743. [Google Scholar] [CrossRef]



| Independent Variables | Levels | ||
|---|---|---|---|
| Low (−1) | Centre (0) | High (+1) | |
| A: pH | 5 | 7 | 9 |
| B: Imidacloprid Concentration (mM) | 0.01 | 0.03 | 0.05 |
| C: Agitation time (days) | 1 | 12 | 21 |
| Run | Experimental Variables | Biodegradation (%) | ||
|---|---|---|---|---|
| Factor A; pH | Factor B; Imidacloprid Conc. (mM) | Factor C; Agitation Time (Days) | ||
| 1 | 9 | 0.05 | 11 | 55 |
| 2 | 7 | 0.05 | 1 | 25 |
| 3 | 7 | 0.01 | 1 | 48 |
| 4 | 7 | 0.05 | 21 | 87 |
| 5 | 9 | 0.03 | 21 | 80 |
| 6 | 5 | 0.03 | 21 | 69 |
| 7 | 7 | 0.03 | 11 | 92 |
| 8 | 7 | 0.01 | 21 | 86 |
| 9 | 5 | 0.03 | 1 | 12 |
| 10 | 5 | 0.05 | 11 | 41 |
| 11 | 9 | 0.03 | 1 | 22 |
| 12 | 5 | 0.01 | 11 | 55 |
| 13 | 9 | 0.01 | 11 | 79 |
| Regression Coefficient | Coefficient in Term of Variable Factors |
|---|---|
| Intercept | 92.00 |
| A-pH | −7.50 * |
| B-Imidacloprid conc. | 26.88 * |
| C-Agitation time (days) | −2.50 * |
| AB | 0.2500 |
| AC | 6.00 |
| BC | −25.12 |
| A2 | −9.37 * |
| B2 | −21.13 |
| C2 | −7.50 * |
| Mean | 57.77 |
| R2 | 0.9890 |
| Adjusted R2 | 0.9559 |
| Model F-value | 29.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talpur, F.N.; Unar, A.; Bhatti, S.K.; Alsawalha, L.; Fouad, D.; Bashir, H.; Afridi, H.I.; Ataya, F.S.; Jefri, O.A.; Bashir, M.S. Bioremediation of Neonicotinoid Pesticide, Imidacloprid, Mediated by Bacillus cereus. Bioengineering 2023, 10, 951. https://doi.org/10.3390/bioengineering10080951
Talpur FN, Unar A, Bhatti SK, Alsawalha L, Fouad D, Bashir H, Afridi HI, Ataya FS, Jefri OA, Bashir MS. Bioremediation of Neonicotinoid Pesticide, Imidacloprid, Mediated by Bacillus cereus. Bioengineering. 2023; 10(8):951. https://doi.org/10.3390/bioengineering10080951
Chicago/Turabian StyleTalpur, Farah Naz, Ahsanullah Unar, Sana Kanwal Bhatti, Laila Alsawalha, Dalia Fouad, Humaira Bashir, Hassan Imran Afridi, Farid Shokry Ataya, Ohoud A. Jefri, and Muhammad Sohail Bashir. 2023. "Bioremediation of Neonicotinoid Pesticide, Imidacloprid, Mediated by Bacillus cereus" Bioengineering 10, no. 8: 951. https://doi.org/10.3390/bioengineering10080951
APA StyleTalpur, F. N., Unar, A., Bhatti, S. K., Alsawalha, L., Fouad, D., Bashir, H., Afridi, H. I., Ataya, F. S., Jefri, O. A., & Bashir, M. S. (2023). Bioremediation of Neonicotinoid Pesticide, Imidacloprid, Mediated by Bacillus cereus. Bioengineering, 10(8), 951. https://doi.org/10.3390/bioengineering10080951






