Appendix A. Substrate Analysis
Substrate analysis is performed using gas chromatography time-of-flight mass spectrometry (GC-ToF-MS) as published in de Wit et al. (2022) [
96] for qualitative analysis of impurities. For quantitative analysis, inductively coupled plasma optical emission spectroscopy (ICP-OES) is used for cations while anions are quantified via ion exchange chromatography (IC). Additionally, NH
4+ and PO
43− concentration are determined by confirmatory factor analysis (CFA). HPLC according to
Section 2.2.4 is used with a column of 30 cm length, 8 mm in diameter at a flowrate of 0.6 mL/min to quantify sugar and lactic acid content. The resulting data is depicted in
Table A1. Density is determined to 1.3310 ± 0.0023 g/cm³ with a DMA48 density meter (Anton Paar, Graz, Austria) at 20 °C. Even though pigments are not detected using GC-ToF-MS, ICP-OES, IC, CFA or HPLC, thick juice shows a light to medium brown color. As it can be found in the literature, colorization of thick juice occurs during sugar production from the alkaline degradation of hexose sugars and Maillard reactions forming melanoidins [
83]. Both compound groups have a large molecule size of over 2 kDa [
83] and contain a variety of active groups such as carboxyl and aldehyde groups [
83,
84]. Furthermore, colourants not related to sucrose, e.g., phenols, flavonoids and melanin are present in crude substrates [
78]. In both cases, due to their size and active groups, most of these molecules are expected to be of a more hydrophobic nature at low pH values.
Table A1.
Composition of thick juice.
Table A1.
Composition of thick juice.
| Analyte | Measurement | Unit | Method |
|---|
| Sucrose | 842 ± 8 | g/L | HPLC |
| Glucose | >1 | g/L | HPLC |
| Fructose | >1 | g/L | HPLC |
| Lactic acid | 1.17 ± 0.13 | g/L | HPLC |
| Cl− | 0.048 ± 0.000 | wt% | IC |
| NO3− | 0.0524 ± 0.01 | wt% | IC |
| SO42− | 0.0833 ± 0.01 | wt% | IC |
| Ca | 72.93 ± 1.00 | mg/kg | ICP-OES |
| Fe | <2 | mg/kg | ICP-OES |
| K | 6660 ± 20 | mg/kg | ICP-OES |
| Mg | 12.11 ± 0.16 | mg/kg | ICP-OES |
| Na | 547 ± 3 | mg/kg | ICP-OES |
| P | 35 ± 3 | mg/kg | ICP-OES |
| NH4+ | 0.0122 | wt% | CFA |
| PO33− | >0.0008 | wt% | CFA |
Appendix B. Calculations of Yield, STY, CTR, OTR and RQ for Fermentation Evaluation
To evaluate fermentative performance, yield, STY, CTR, OTR and RQ are calculated based on mass balances. Equation (
A1) shows how to calculate the mass balance for a compound from fermentation.
With [g] as the calculated mass at time point t, () [g/L] as the concentration of x at the beginning of the fermentation, (t) [L] as the initial filling volume, [g/L] as the concentration of x in the feed and [L] as the added feeding volume at time t. Sampling is considered by summing up the substrate concentration (t) [g/L] times the corresponding sample volume [L].
The yield
[g
ITA/g
substrate] can be calculated by dividing the mass of ITA produced by the summed up mass of the substrates glucose, fructose and sucrose (Equation (
A2)).
STY [g
ITA/(L·h)] is calculated by dividing the mass of ITA
[g] by the sum of the filling volume
[L] of the fermenter and the total sample volume
[L] times the fermentation time
t [h] (Equation (
A3)).
Equations (
A4) and (
A5) describe the calculation of CTR and OTR using the gas flow rate into the fermenter
[L/h], the fermentation volume
[L], the molar volume of an ideal gas
[L/mmol] at 25 °C and the O
2 and CO
2 fractions in the inlet and outlet
,
,
and
, [-], respectively.
Equation (
A6) describes the calculation of the RQ corresponding to CTR and OTR.
Equations (
A7) and (
A8) describe the RQ of growth on pure glucose. Calculations are based on the biomass composition from Klement et al. (2012) [
76].
Equations (
A9) and (
A10) describe the theoretical RQ of ITA production on pure glucose.
ITA, sucrose, glucose and fructose concentration and CDW are analyzed in triplicates, mapping the error of pipetting and concentration measurements. Volumes are determined once. From there, fermentation KPIs are calculated and the mean is given. The standard deviation from the mean is also calculated and is below 5% for all values. OTR, CTR, DOT and pH are determined once for the corresponding fermentation time point.
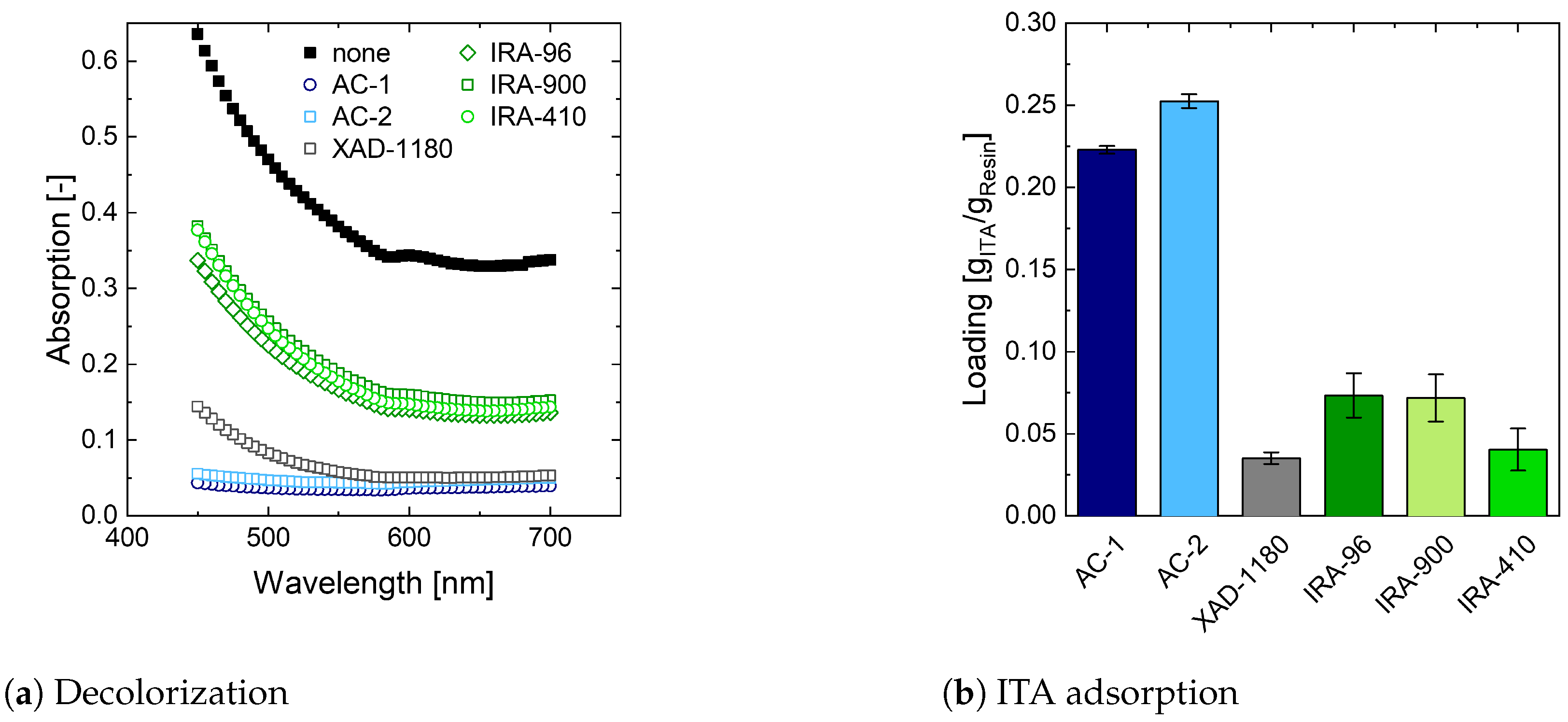
Appendix C. Establishing a Protocol for Screening of Decolorization Resins
For characterization of the decolorization performance, adsorption is determined between 450 nm and 700 nm. ITA does not absorb light in this wavelength range and therefore does not contribute to the overall signal [
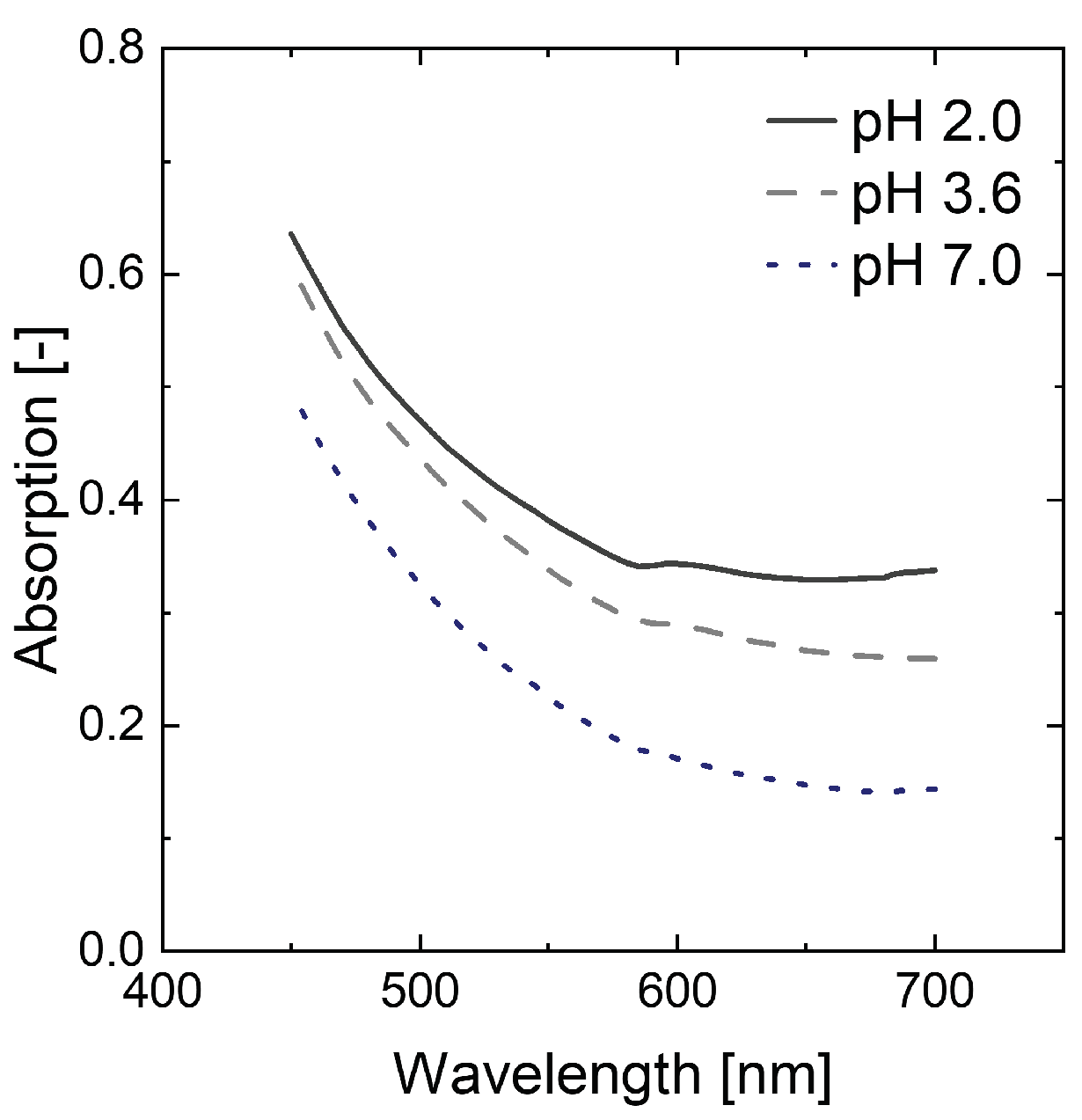
97]. When the absorption of the artificially prepared broth prior to decolorization is investigated, no clear peaks can be identified (
Figure A1). This suggests multiple compounds being involved in the colorization of the fermentation broth [
79,
83,
84]. Furthermore, a pH dependence in adsorption is observed, indicating the presence of some pH sensitive compounds. As dilution of samples leads to a change in pH and thus adsorption, samples are measured in undiluted form. The remaining 5% of wash water are considered by diluting the reference solution accordingly.
Figure A1.
Differences in adsorption from artificially prepared broth depending on pH.
Figure A1.
Differences in adsorption from artificially prepared broth depending on pH.
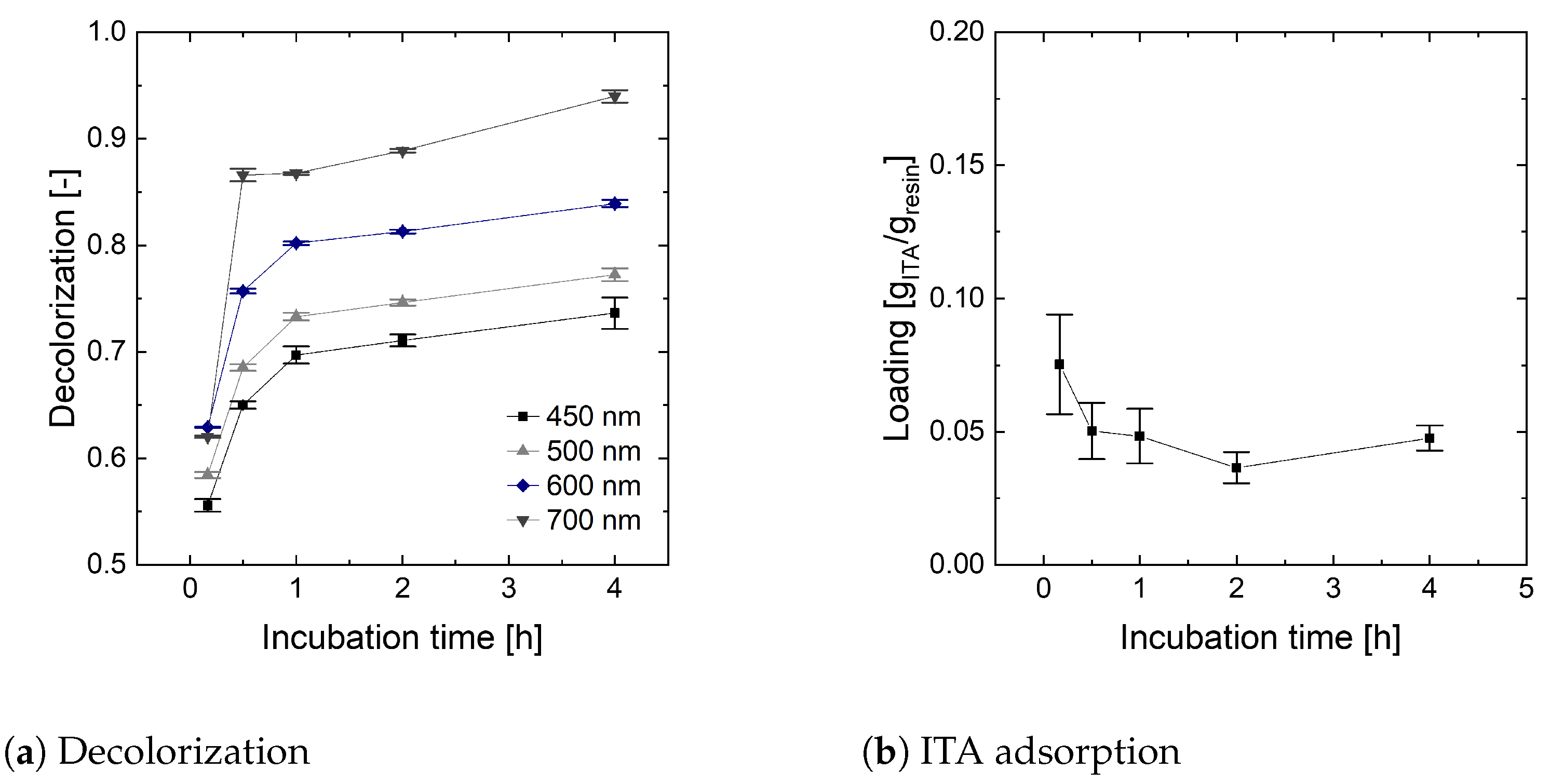
To determine the minimum incubation time, the XAD-1180 resin is used with artificially prepared broth in an exemplary study. As decolorization and ITA adsorption are constant after 4 h incubation time, the minimum incubation time is set accordingly (
Figure A2).
Figure A2.
Determination of minimum incubation time for decolorization.
Figure A2.
Determination of minimum incubation time for decolorization.
To calculate the resin loading with ITA
[g
ITA/g
resin], the remaining volume of washing water
[L] is considered in combination with the volume of the solution added for decolorization
[L], the ITA concentration before
and after incubation
and the amount of resin used
[g]. (Equation (
A11)).
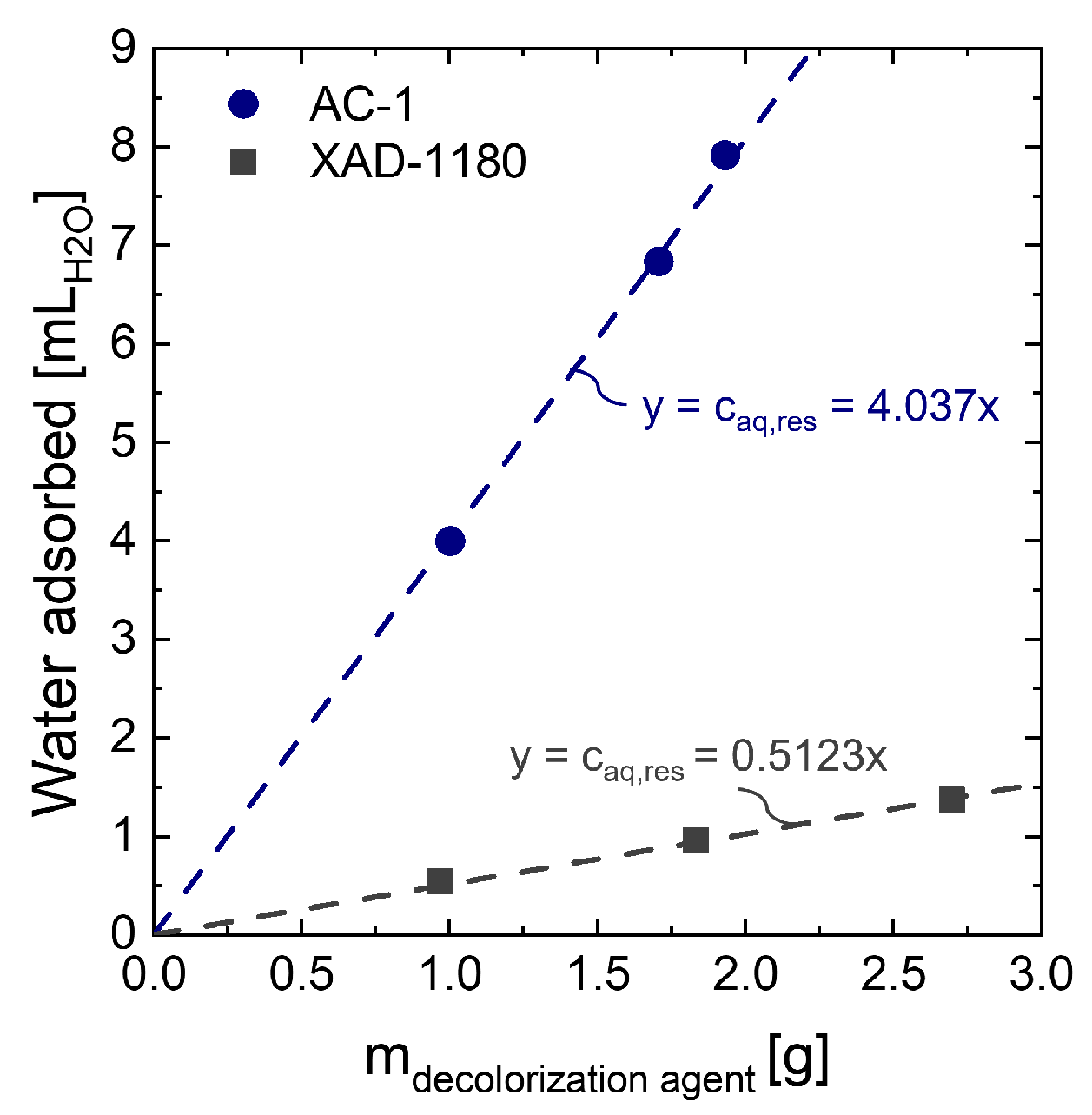
For yield calculations in decolorization depending on resin concentration, the amount of liquid remaining in decolorization agents after filtration and therefore contributing to yield loss is considered. Otherwise, process yield is overestimated. To determine the amount of liquid bound in the decolorization agent after filtration, different amounts of decolorization agent are incubated with water similar to the washing step in the screening experiments mentioned above. After vacuum filtration, the recovered water is quantified. To subtract the amount of water lost in the filter, the same filtration protocol is conducted with water only. In each filter, 4.0 ± 0.1 g distilled water are incorporated.
Yield calculation with yield loss due to water
is performed using the amount of water lost per g resin
, which can be deduced from the slope of the linear function in
Figure A3 (Equation (
A12)).
Figure A3.
Water retained in XAD-1180 resin and AC-1 after incubation with distilled water.
Figure A3.
Water retained in XAD-1180 resin and AC-1 after incubation with distilled water.
Appendix D. Calculation of Yield and Purity for Downstream Process Evaluation
A yield calculation for each unit operation is performed by dividing the mass of ITA after the unit operation
[g] by the mass of ITA before the unit operation
[g] to close the mass balance for each unit operation. The mass of ITA is calculated from the concentration
[g/L], the density of the aqueous phase
[g/L] and the mass of the aqueous phase
[g] (Equation (
A13)). The overall process yield was calculated based on the amount of ITA after cell separation. The standard deviations reflect errors in HPLC measurements.
Purity of ITA,
[-], is calculated dividing the ITA concentration by the gravimetrically determined crystal concentration
[g/L].
Similar to fermentation, ITA, sucrose, glucose and fructose concentration are analyzed in triplicates, mapping the error of pipetting and concentration measurements. Absorption at different wavelengths and volumes are determined once. From there, DSP KPIs are calculated and the mean is given. The standard deviation from the mean is also calculated and is below 5% for all values.
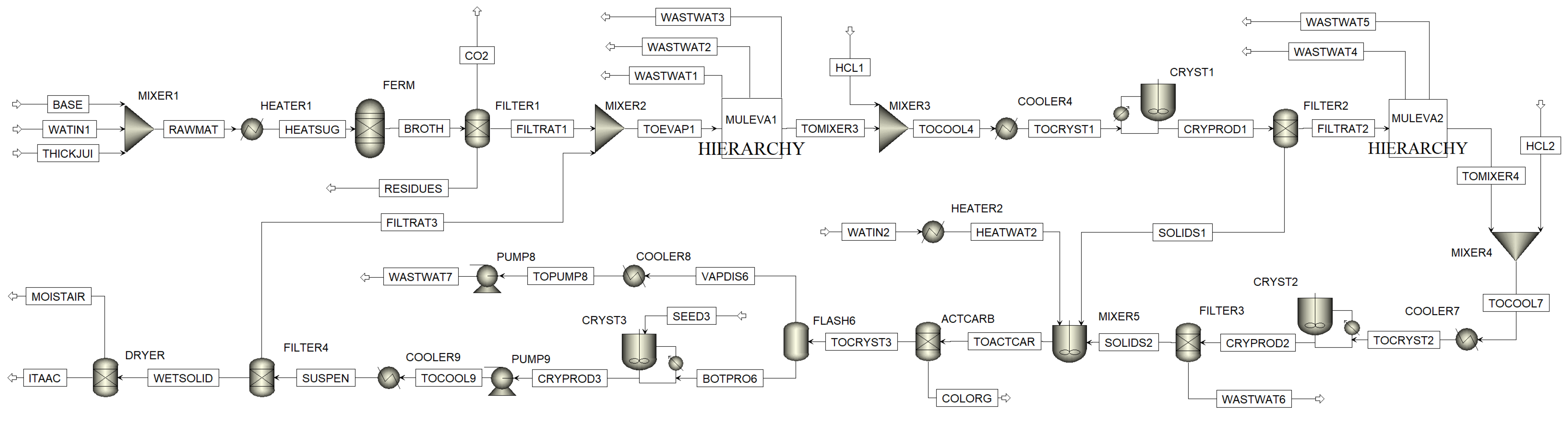
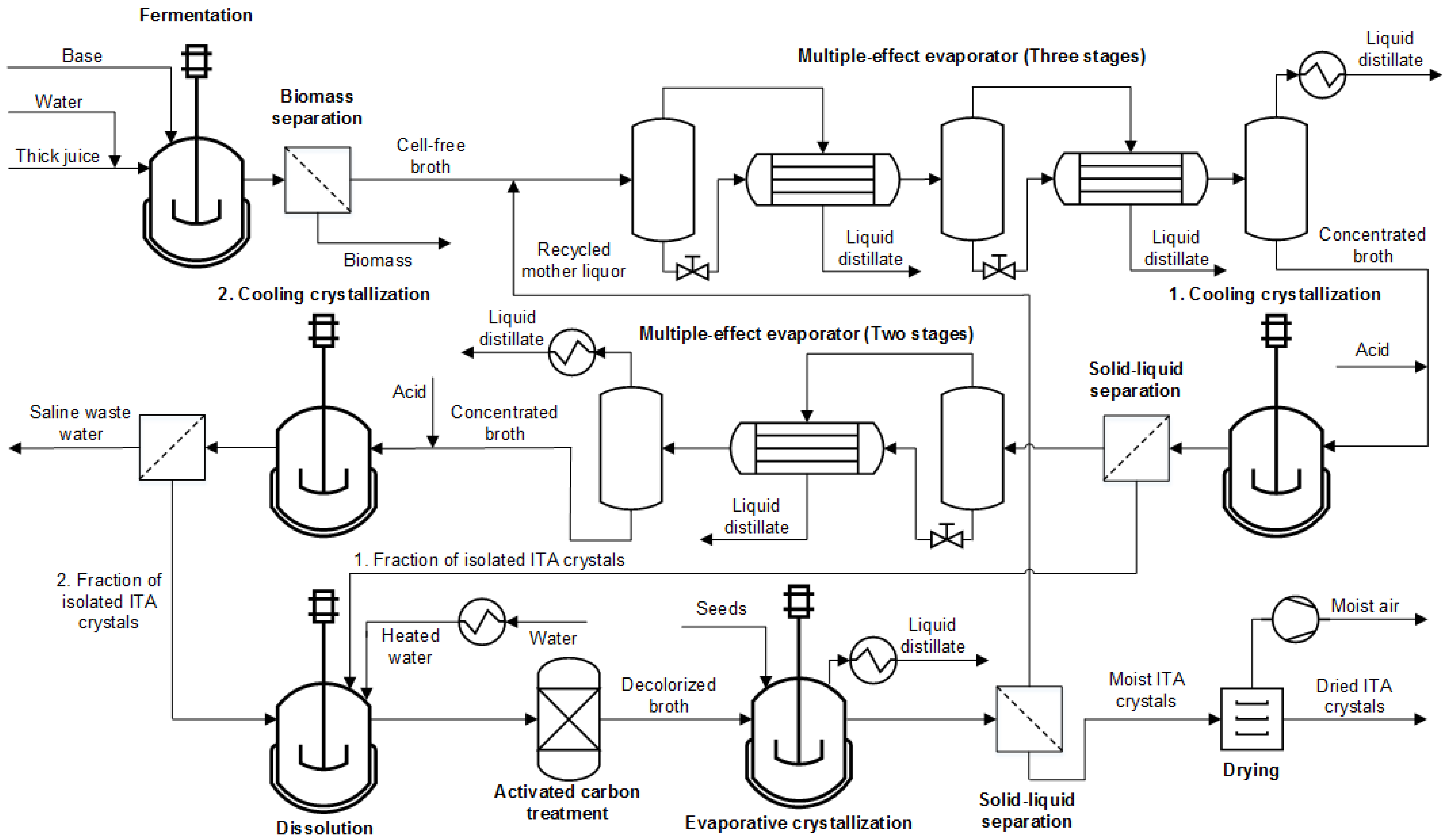
Appendix F. Process Modeling in Aspen Plus and Techno-Economic Analysis
The flowsheet model is set up in Aspen Plus and displayed in
Figure A5. ELECNRTL is used as global property method. UNIFAC is applied to determine missing binary interaction parameters. The Elec Wizard allowed the implementation of dissociation and precipitation behavior of inorganic components. Deprotonated ITA species are not available in Aspen component databanks. Thus, they are added by molecular structure. Respective dissociation reactions are set up in the global chemistry environment. For crystallization blocks, the method is locally adapted to SOLIDS.
Figure A5.
Aspen process flowsheet of multiple crystallization process.
Figure A5.
Aspen process flowsheet of multiple crystallization process.
The feed is modeled as glucose–water mixture representing the typical sugar content of thick juice (0.65 wt%). The water feed and base mass flows are defined by product titer and pH during product formation, respectively, (
Table 4). The fermenter is implemented as RYIELD modeling the reaction as de-lumping of glucose to ITA and CO
2. The overall yields in
Table 4 are used as reaction coefficients. A full consumption of the sugar content is assumed. CO
2 is isolated from the broth in a separator model. RESIDUES contains the residual sugar content that is not converted to ITA or CO
2 and would in a real fermentation be used for biomass formation or maintenance metabolism. Additional nutrients such as NH
4Cl are not considered in the simulation.
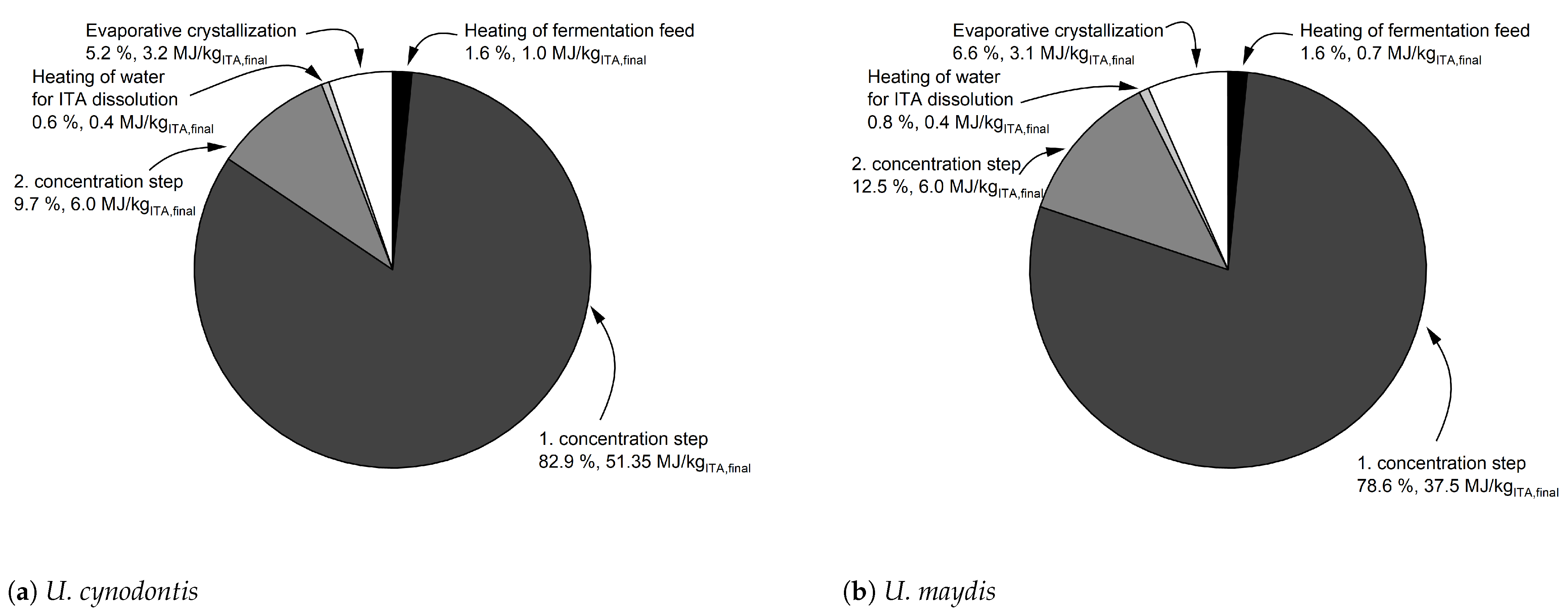
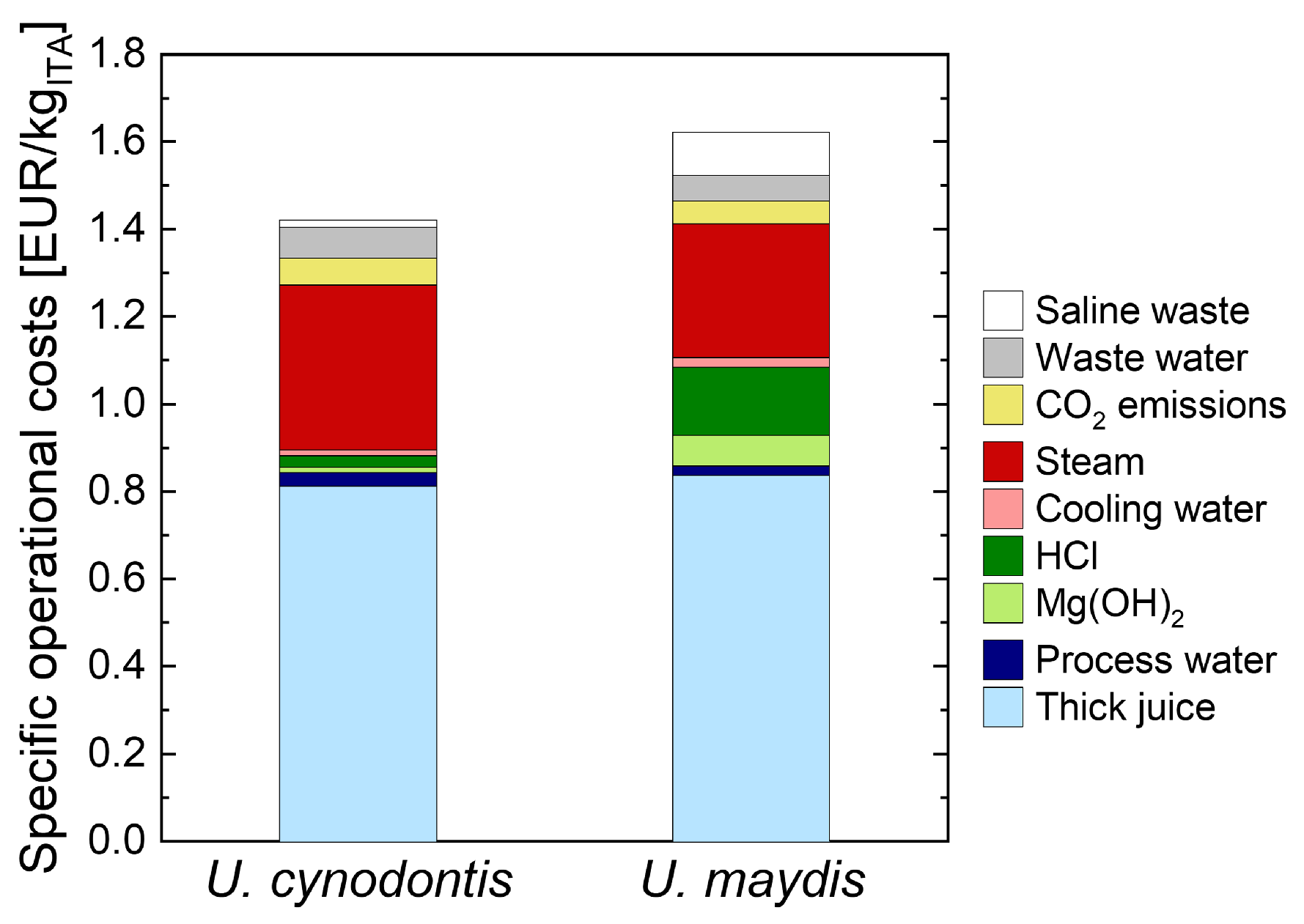
Steam is identified as the major energy cost driver in the presented process concept (
Figure 2).
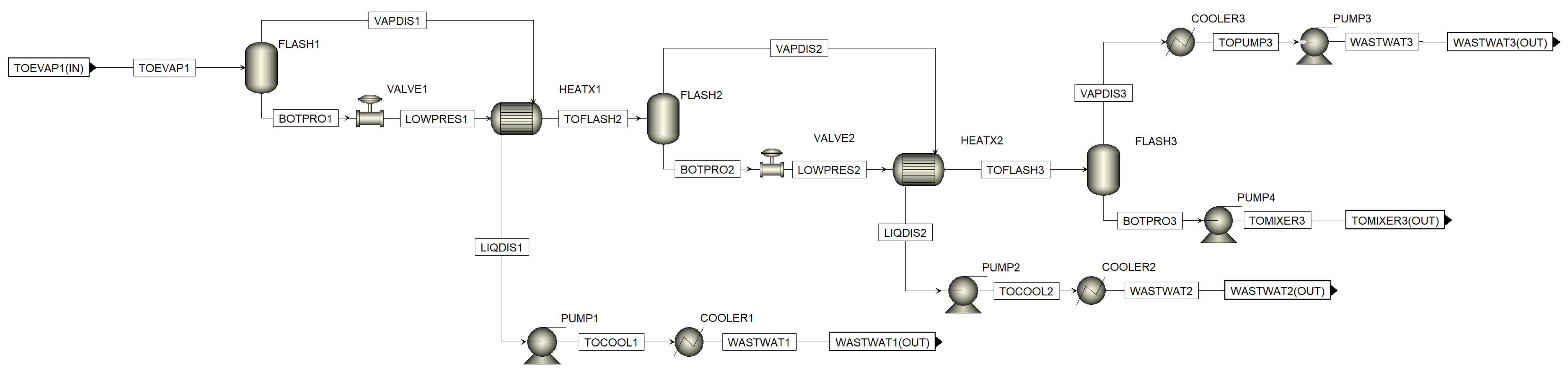
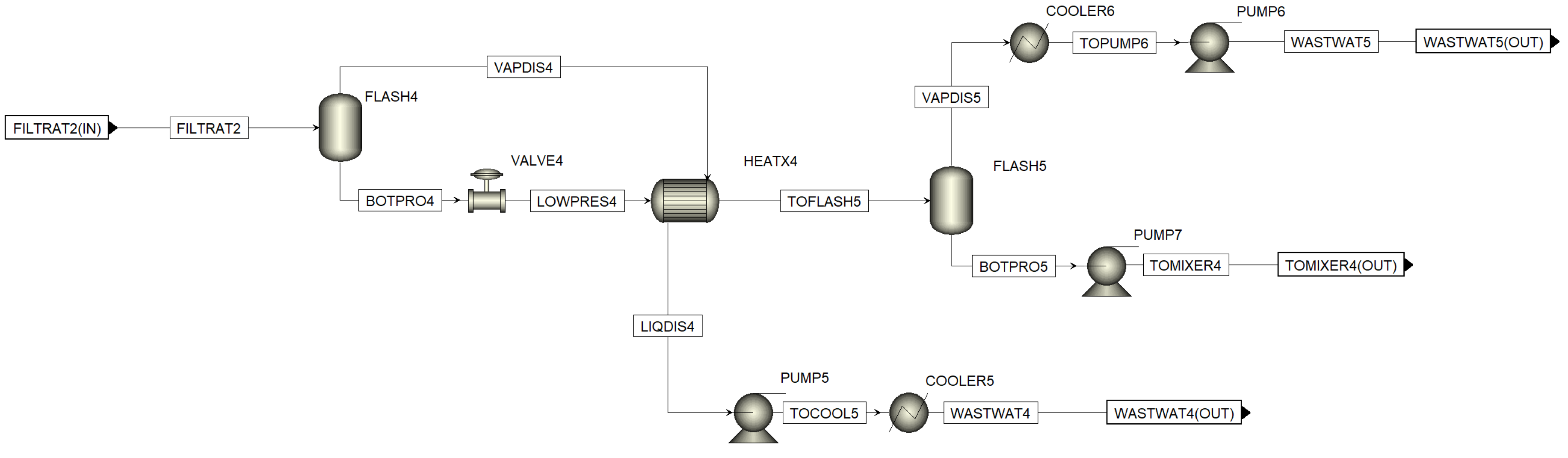
Figure A6 displays the steam consuming process steps. The evaporation steps in the first and second cooling crystallization sequence require the largest fractions of the total steam demand. Thus, we implement multiple-effect evaporators (MEE) for those concentration units to mitigate the energy requirement of the proposed process. For the purpose of flowsheet modeling, a three-stage and a two-stage MEE are implemented in the first and second cooling crystallization sequence reducing steam requirement by approximately 60% and 40% in comparison to simple flash evaporators, respectively. The pressure drop in each throttle is adjusted to just meet the minimum temperature requirement of 10 K in the heat exchangers. As described in
Section 3.1.1, in the first MEE the final stage concentrates the broth up to a concentration of 350 g
ITA/L. However, in the second MEE the broth is concentrated so that the solubility limit of the co-salt MgCl
2 is just not exceeded during the subsequent crystallization. That way, overall ITA yield is maximized. The Aspen flowsheets for the MEEs used in this process flowsheet are displayed in
Figure A7 and
Figure A8.
Figure A6.
Gross steam requirement (without energy integration measures).
Figure A6.
Gross steam requirement (without energy integration measures).
Figure A7.
Aspen process flowsheet of MEE in the first cooling crystallization sequence.
Figure A7.
Aspen process flowsheet of MEE in the first cooling crystallization sequence.
Figure A8.
Aspen process flowsheet of MEE in the second cooling crystallization sequence.
Figure A8.
Aspen process flowsheet of MEE in the second cooling crystallization sequence.
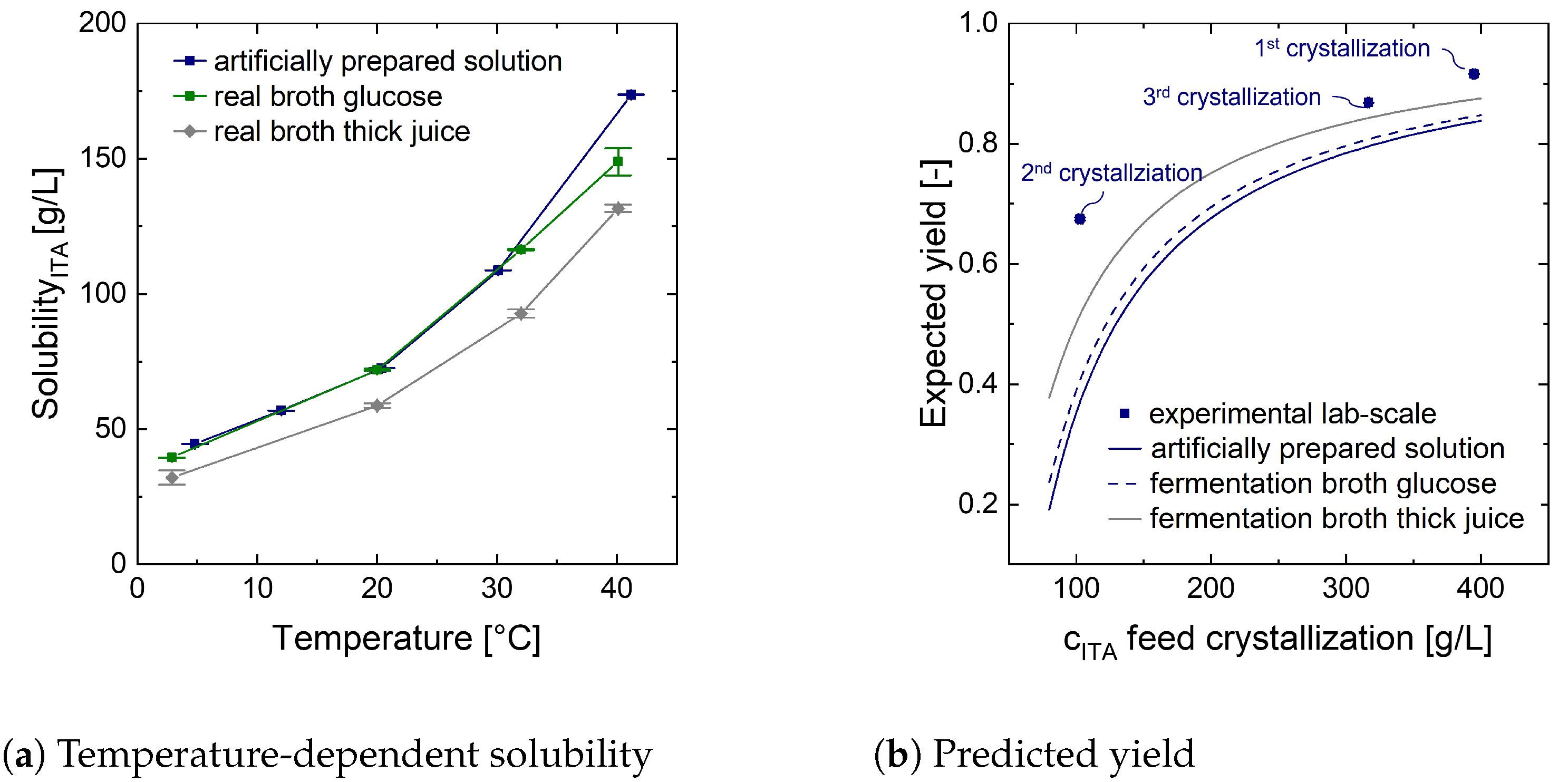
The cooling crystallization steps lower the temperature to 15 °C at 1 bar. The effluents are at solid–liquid equilibrium. The according data is experimentally determined with artificially prepared ITA systems (
Figure A4a) and supplied to the model. After the second cooling crystallization sequence only the liquid fraction contains the co-salt which is then to be disposed. The saline mass fraction of this stream is priced with 110 EUR/tonne (
Table A2). The solid fractions of the cooling crystallization sequences are mixed. Just enough water is added to the system until all solids are dissolved at 80 °C. The elevated temperature limits the amount of water to be evaporated in succeeding process steps. Afterwards the system is subject to an activated carbon treatment, which is only symbolically added to the Aspen flowsheet. Thus, the COLORG stream is a zero-flow. The stream is then fed to the evaporative crystallization which is operated at 80 °C. To reach an ITA yield of 90% after the crystallization, the vacuum pressure is selected accordingly. The seeds added amount to 5 wt% of the solids produced by the crystallizer. After solid–liquid separation, the mother liquor is recycled to the beginning of the DSP. Similar to the activated carbon adsorber, a dryer for ITA crystals is added to the flowsheet for symbolic reasons. Thus, MOISTAIR stream is a zero-flow.
To retrieve heat/cooling duties from crystallizers in Aspen Plus poses difficulties. Thus, coolers and a flash evaporator are integrated into the flowsheet before the cooling and the evaporative crystallizer, respectively. The operating parameters of the coolers and the flash evaporator are aligned with the crystallizer to obtain the duties required by the crystallization steps. Since ITA crystallization only occurs in the crystallizers, the energetic duty resulting from crystal formation is calculated separately using the mass flow of crystal product and the enthalpy of fusion for ITA (
C) = 32.2 kJ/mol [
98]).
All solid–liquid phase separations in the process are calculated ideally. Pumps are only integrated in the flowsheet when an elevated pressure has to be generated in a liquid phase. Coolers operate nearly isobaric. Waste water streams are cooled down by air and leave the system at ambient pressure.
As the simulation is solely applied for an operative cost analysis, the mass flows are scaled with respect to the amount of ITA produced by the process. Stream and utility prices used for the operative cost analysis are displayed in
Table A2. Detailed parameterization for
U. cynodontis ITA Max pH and
U. maydis K14 simulation can be retrieved from
Table A3.
Table A2.
Stream, waste and utility prices.
Table A2.
Stream, waste and utility prices.
| Stream/Utility | Composition | Price | Unit | Reference |
|---|
| Thick juice | 65 wt% | 211.54 | EUR/tonne | 1 |
| Mg(OH)2 | 40 wt% | 100.00 | EUR/tonne | 2 |
| HCl | 30 wt% | 80.00 | EUR/tonne | 2 |
| Process water | 100 wt% | 1.40 | EUR/tonne | 2 |
| Waste water | - | 2.90 | EUR/tonne | 2 |
| Saline waste water | - | 110 | EUR/tonne | 2,3 |
| Electricity | - | 0.0849 | EUR/kWh | [99] |
| Low pressure steam | - | 27.00 | EUR/tonne | 2 |
| Cooling water | - | 0.05 | EUR/kWh | 2,4 |
| Process and utility-related CO2 emissions | - | 27.37 | EUR/tonne | [100] |
Table A3.
Parameterization of U. cynodontis ITA Max pH and U. maydis K14 simulations.
Table A3.
Parameterization of U. cynodontis ITA Max pH and U. maydis K14 simulations.
| Parameter | U. cynodontis ITA Max pH | U. maydis K14 |
|---|
| Thick juice mass flow | 3.84 kg/kgITA,final | 3.95 kg/kgITA,final |
| Water feed mass flow | 20.94 kg/kgITA,final | 14.76 kg/kgITA,final |
| Base mass flow | 0.12 kg/kgITA,final | 0.70 kg/kgITA,final |
| HEATER1 Temperature | 35.00 °C | 35.00 °C |
| HEATER1 Outlet pressure | 1.00 bar | 1.00 bar |
| De-lumping coefficient ITA | 0.39 gITA/gglucose | 0.42 gITA/gglucose |
| De-lumping coefficient CO2 | 0.1323 gCO2/gglucose | 0.1425 gCO2/gglucose |
| FLASH1 Temperature | 100.00 °C | 100.00 °C |
| FLASH1 Vapor mass fraction | 0.27 | 0.25 |
| VALVE1 Outlet pressure | 0.59 bar | 0.57 bar |
| HEATX1 Hot stream outlet temperature | 97.00 K | 97.00 K |
| HEATX1 Tmin | 10.00 K | 10.00 K |
| FLASH2 Temperature | 86.00 °C | 87.00 °C |
| FLASH2 Vapor mass fraction | 0.39 | 0.35 |
| VALVE2 Outlet pressure | 0.33 bar | 0.30 bar |
| HEATX2 Hot stream outlet temperature | 82.00 K | 82.00 K |
| HEATX2 Tmin | 10.00 K | 10.00 K |
| FLASH3 Temperature | 72.28 °C | 76.20 °C |
| FLASH3 Vapor mass fraction | 0.67 | 0.56 |
| HCl (1. cooling crystallization) | 0.33 kg/kgITA | 1.93 kg/kgITA |
| COOLER4 Temperature | 15.00 °C | 15.00 °C |
| COOLER4 Pressure | 1.00 bar | 1.00 bar |
| CRYST1 Temperature | 15.00 °C | 15.00 °C |
| CRYST1 Pressure | 1.00 bar | 1.00 bar |
| FLASH4 Temperature | 100.00 °C | 100.00 °C |
| FLASH4 Vapor mass fraction | 0.39 | 0.20 |
| VALVE4 Outlet pressure | 0.39 bar | 0.29 bar |
| HEATX4 Hot stream outlet temperature | 97.00 K | 87.00 K |
| HEATX4 Tmin | 10.00 K | 10.00 K |
| FLASH5 Temperature | 86.07 °C | 85.77 °C |
| FLASH5 Vapor mass fraction | 0.64 | 0.27 |
| HCl (2. cooling crystallization) | 0.007 kg/kgITA,final | 0.002 kg/kgITA,final |
| COOLER7 Temperature | 15.00 °C | 15.00 °C |
| COOLER7 Pressure | 1.00 bar | 1.00 bar |
| CRYST2 Temperature | 15.00 °C | 15.00 °C |
| CRYST2 Pressure | 1.00 bar | 1.00 bar |
| Water added for dissolution | 1.68 kg/kgITA,final | 1.64 kg/kgITA,final |
| MIXER 5 Temperature | 80.00 °C | 80.00 °C |
| MIXER 5 Pressure | 1.00 bar | 1.00 bar |
| FLASH6 Temperature | 80.00 °C | 80.00 °C |
| FLASH6 Vapor mass fraction | 0.55 | 0.54 |
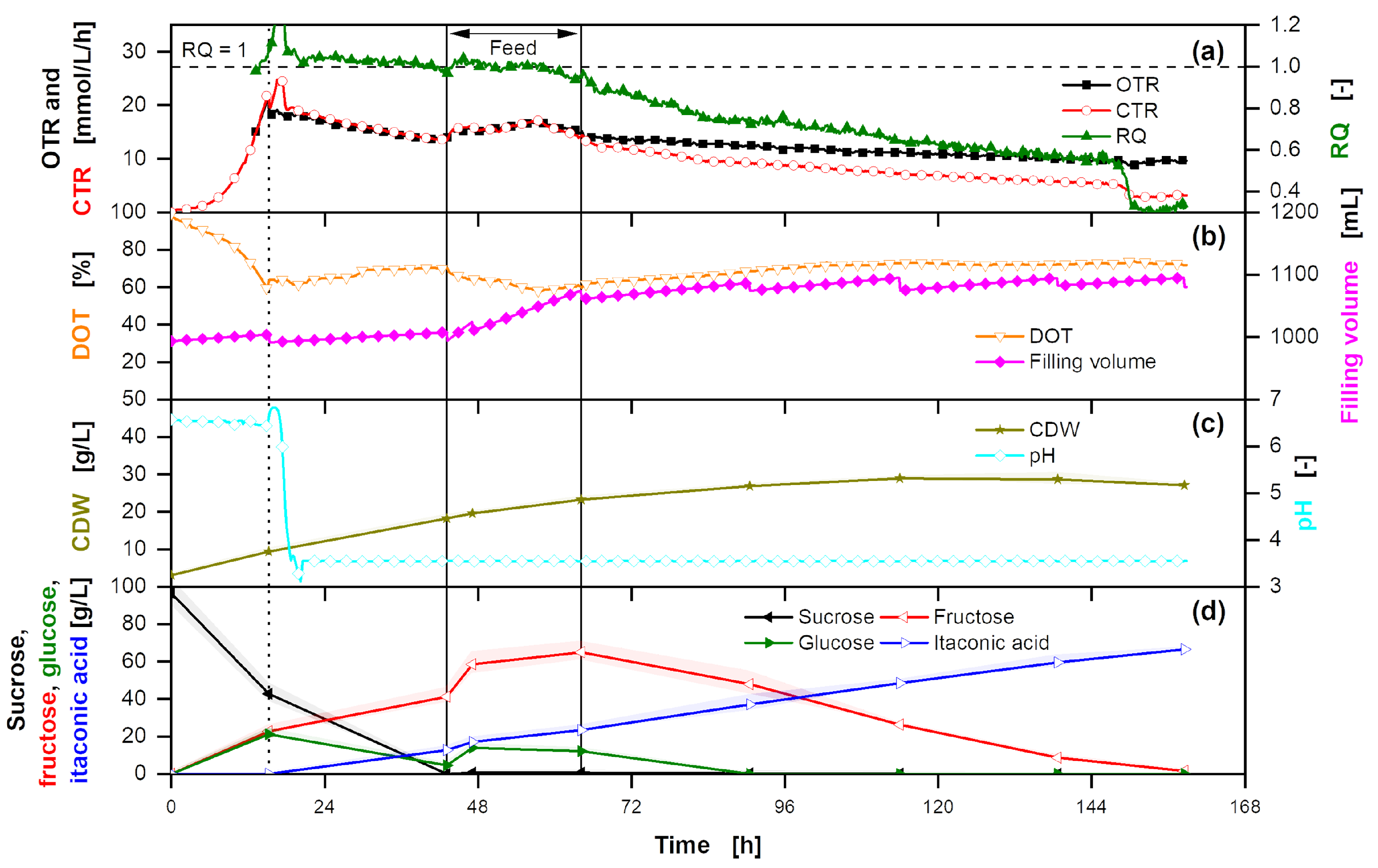
Appendix G. Overfed Fermentation of U. cynodontis ITA Max pH
A fermentation with an elongated feed profile with thick juice is depicted in
Figure A9. The product yield is low, as not all substrate is used from the fermentation broth. However, this is not due to substrate inhibition. Even with high sugar concentrations of over 200 g/L sucrose, a maximum titer of 80 g/L, similiar to fermentations on glucose [
74] and to the fermentation shown in
Figure 3, is reached. Therefore, product inhibition by weak organic acid stress [
74,
89] is responsible for reduced cell viability and prevents further substrate conversion. Furthermore, no limitation in oxygen transfer due to high substrate concentration and high medium osmolarity is observed [
102].
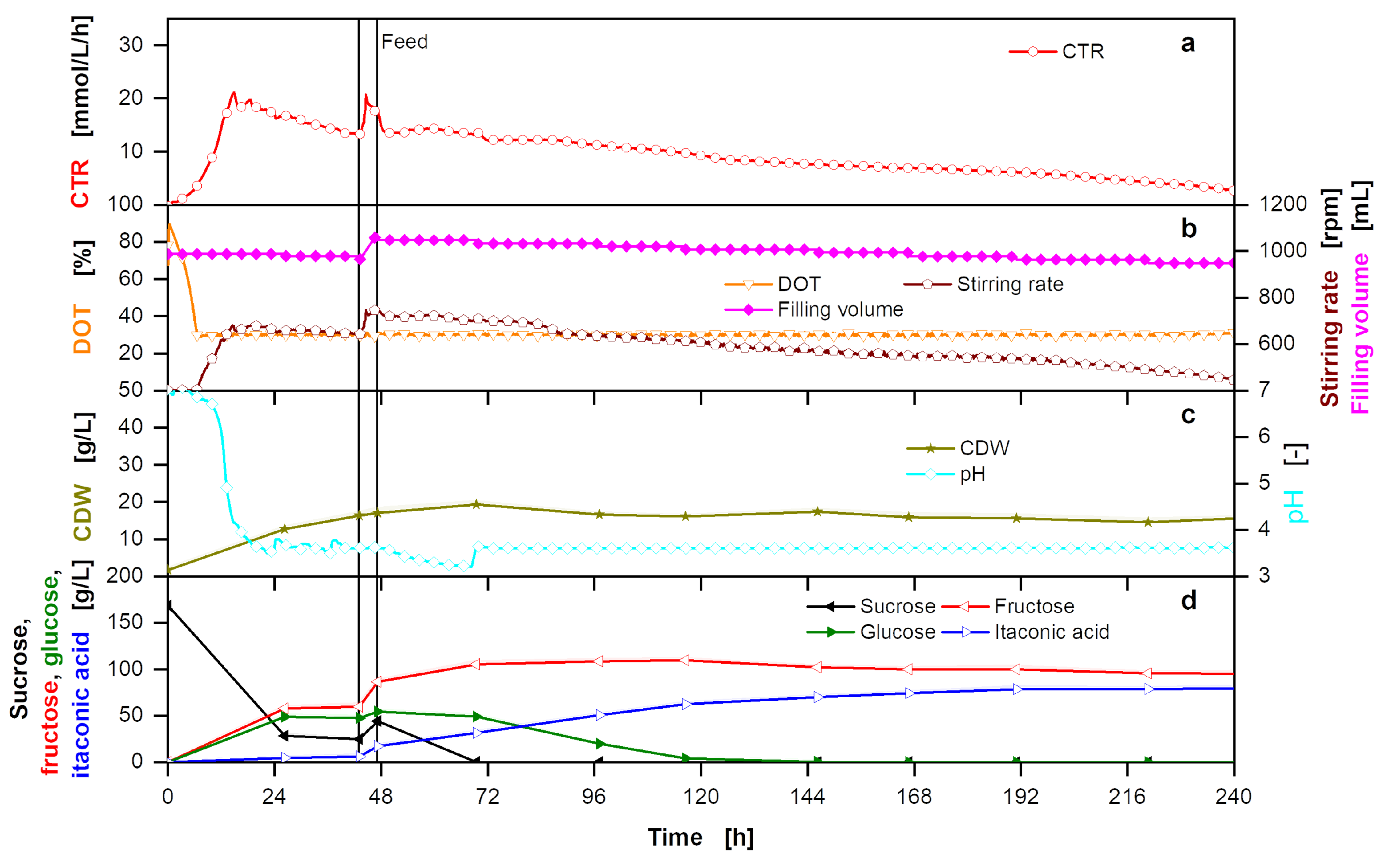
Figure A9.
Overfed extended-batch fermentation of U. cynodontis ITA Max pH with pH shift and thick juice as carbon source. (a) OTR, CTR and RQ. The horizontal dashed line shows RQ = 1. RQ values are only shown for OTR values > 5 mmol/L/h. (b) DOT, stirring rate and filling volume. (c) CDW and pH. (d) Sucrose, glucose, fructose and ITA concentration. For the batch phase, 215 g/L sucrose from thick juice are initially added to the medium. During the feed phase (between the vertical solid lines), 215 g of additional sucrose from thick juice are added into the fermentation vessel. Cultivation is performed in a 2 L stirred tank reactor with an initial filling volume of 1 L at 30 °C with a constant aeration and stirring rate of 1 L/min and 1200 rpm, respectively. For clarity, only every 20th measured online data point is shown.
Figure A9.
Overfed extended-batch fermentation of U. cynodontis ITA Max pH with pH shift and thick juice as carbon source. (a) OTR, CTR and RQ. The horizontal dashed line shows RQ = 1. RQ values are only shown for OTR values > 5 mmol/L/h. (b) DOT, stirring rate and filling volume. (c) CDW and pH. (d) Sucrose, glucose, fructose and ITA concentration. For the batch phase, 215 g/L sucrose from thick juice are initially added to the medium. During the feed phase (between the vertical solid lines), 215 g of additional sucrose from thick juice are added into the fermentation vessel. Cultivation is performed in a 2 L stirred tank reactor with an initial filling volume of 1 L at 30 °C with a constant aeration and stirring rate of 1 L/min and 1200 rpm, respectively. For clarity, only every 20th measured online data point is shown.
Appendix H. Overfed Fermentation of U. cynodontis ITA Max pH with Mg(OH)2
To evaluate the potential of Mg(OH)
2 as a base in fermentation (
Section 3.1.2), a fermentation is performed with Mg(OH)
2 instead of NaOH (
Figure A10). While ITA production is similar to fermentations with NaOH, cell growth stagnates after 69.4 h of the fermentation. In combination with a similar ITA productivity, compared to fermentations with NaOH, the resulting low CDW leads to an overall process yield of 0.48 g
ITA/g
glucose eq., without including leftover sugar in the calculations. MgOH
2 is fed as a suspension, which can result in blocking of tubing and pipes, as is visible between 48 h and 69.4 h. The blocking is removed and pH control is reestablished after 69.4 h. For future process design, where the base of the fermentation and the acid for crystallization are aligned for a maximum process yield, further research into the influence of different cations resulting from base consumption on growth and productivity of
U. cynodontis ITA Max pH is necessary.
Figure A10.
Overfed extended-batch fermentation of U. cynodontis ITA Max pH with Mg(OH)2 as pH-adjusting agent and thick juice as carbon source. (a) CTR. (b) DOT, stirring rate and filling volume. (c) CDW and pH. (d) Sucrose, glucose, fructose and ITA concentration. For the batch phase, 160 g/L sucrose from thick juice are initially added to the medium. During the feed phase (between the vertical solid lines) 100 g of additional sucrose from thick juice are added into the fermentation vessel. Cultivation is performed in a 2 L stirred tank reactor with an initial filling volume of 1 L at 30 °C with a constant aeration rate of 1 L/min. For clarity, only every 20th measured online data point is shown.
Figure A10.
Overfed extended-batch fermentation of U. cynodontis ITA Max pH with Mg(OH)2 as pH-adjusting agent and thick juice as carbon source. (a) CTR. (b) DOT, stirring rate and filling volume. (c) CDW and pH. (d) Sucrose, glucose, fructose and ITA concentration. For the batch phase, 160 g/L sucrose from thick juice are initially added to the medium. During the feed phase (between the vertical solid lines) 100 g of additional sucrose from thick juice are added into the fermentation vessel. Cultivation is performed in a 2 L stirred tank reactor with an initial filling volume of 1 L at 30 °C with a constant aeration rate of 1 L/min. For clarity, only every 20th measured online data point is shown.
Appendix N. Raman Spectroscopy of Sugars
For pure component models (PCM) of glucose, fructose and sucrose, 10 wt% sugar in water is used. For constructing the ITA PCMs, spectra of ITAH
2 and ITA
2− at pH 1.9 and pH 13.0 are used. For ITAH
− a binary spectrum with H
2O was determined using Multivariate Curve Resolution with Alternating Least Squares (MCR-ALS) [
103,
104,
105,
106] as it could not be recorded by itself due to being present in mixture with the other two ITA species at any given pH value. The total mixture hard model is calibrated with mixtures of sugar and ITA in water between 1 and 10 wt% and 3 and 8 wt%, respectively. Raman spectra of sucrose, fructose and glucose for PCM can be found in
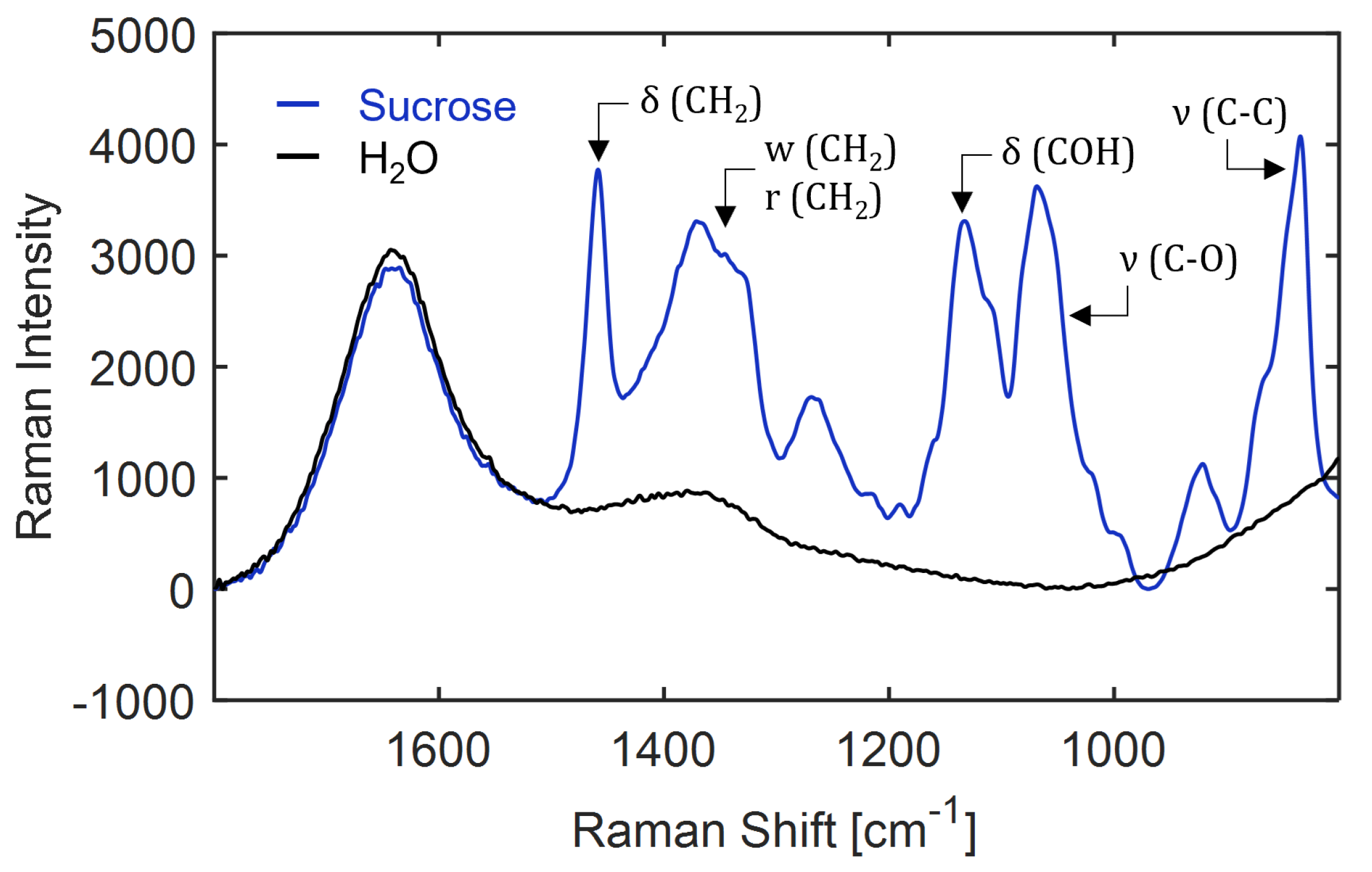
Figure A16–
Figure A18. Prominent peaks are identified in the region of 1200-1500 cm
−1, where mainly Raman bands caused by -CH
2 are located. A strong band at 1458 cm
−1 due to the
-CH2 bending mode, a broader set of merged peaks around 1300–1440 cm
−1 due to
r-CH2 rocking and
w-CH2 wagging modes, and a medium to strong peak around 1264–1268 cm
−1 due to
-CH2 twisting vibrations can be found. Further bands are visible between 1000 and 1200 cm
−1, where
C-O-H bending vibrations and
C-O stretching modes occur. Below 800 cm
−1, mostly
C-H bending vibrations and
C-C stretching modes are present [
107,
108,
109,
110].
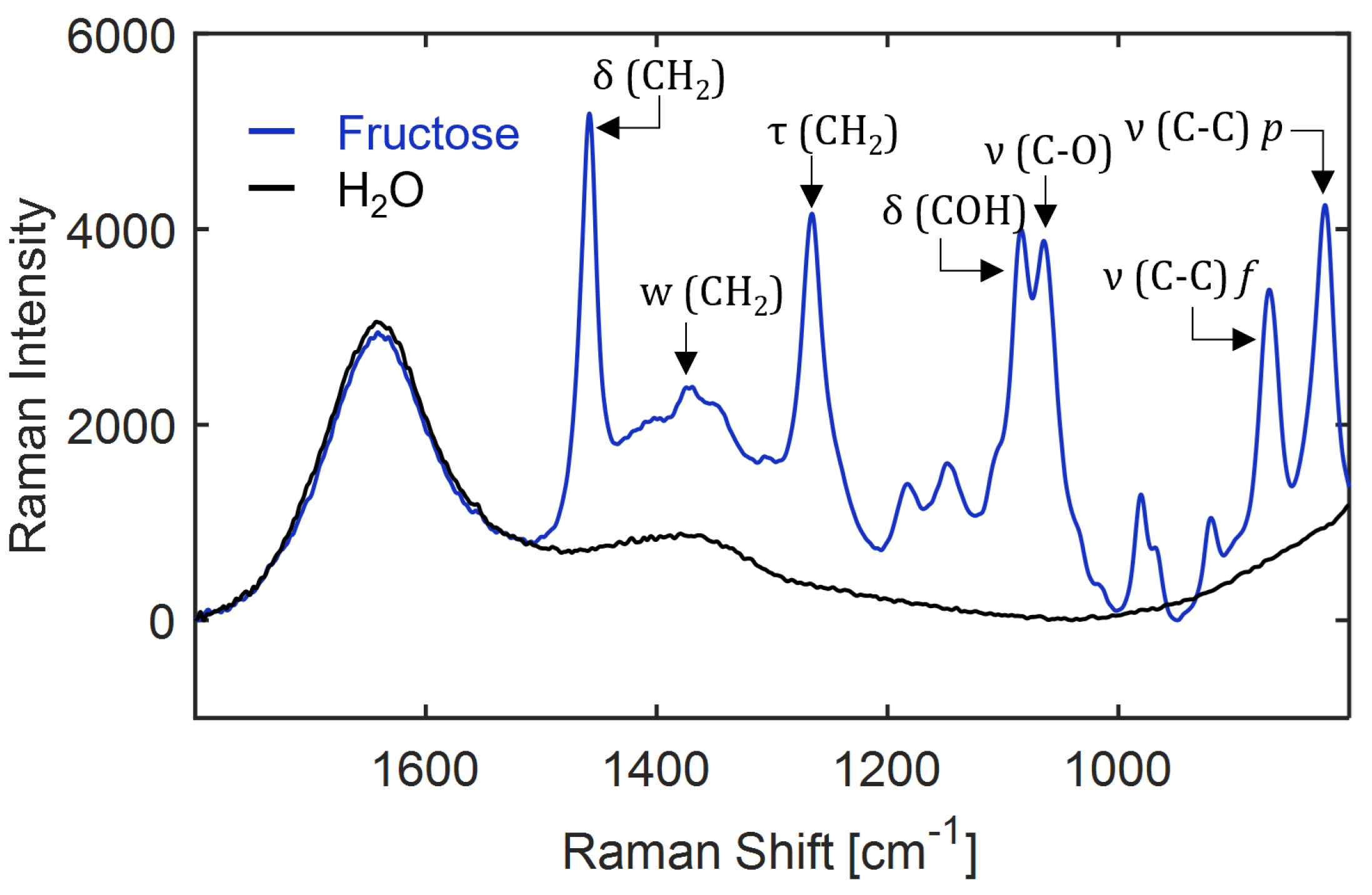
Additionally, for glucose and fructose, bands are present that are distinctly caused by its isomers.
-D-glucose has a C-O stretching mode at 1040 cm
−1, while
-D-glucose has a band blue-shifted compared to
-D-glucose at 1063 cm
−1 [
109]. While structurally different, these compounds co-exist in approximate constant ratio when dissolved, due to equilibrium in ring opening and closing behavior at a certain temperature and pH. Hence, for glucose a single PCM is constructed that represents the Raman bands of both the
and
form of glucose in given ratios. Similarly, the fructose spectrum is a mixture of bands of
-D-fructopyranose and D-fructofuranose, which are also represented by a single PCM assuming a constant ratio of these isomers in solution [
109,
111].
Figure A16.
Assignment of molecular vibrations to the Raman spectrum of 10 wt% sucrose in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : bending, w: wagging, r: rocking and : deformation modes.
Figure A16.
Assignment of molecular vibrations to the Raman spectrum of 10 wt% sucrose in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : bending, w: wagging, r: rocking and : deformation modes.
Figure A17.
Assignment of molecular vibrations to the Raman spectrum of 10 wt% glucose in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : bending and w: wagging modes, : alpha-D-glucose, : beta-D-glucose.
Figure A17.
Assignment of molecular vibrations to the Raman spectrum of 10 wt% glucose in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : bending and w: wagging modes, : alpha-D-glucose, : beta-D-glucose.
Figure A18.
Assignment of molecular vibrations to the Raman spectrum of 10 wt% fructose in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : bending, w: wagging and : twisting modes, f: D-fructofuranose, p: D-fructopyranose.
Figure A18.
Assignment of molecular vibrations to the Raman spectrum of 10 wt% fructose in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : bending, w: wagging and : twisting modes, f: D-fructofuranose, p: D-fructopyranose.
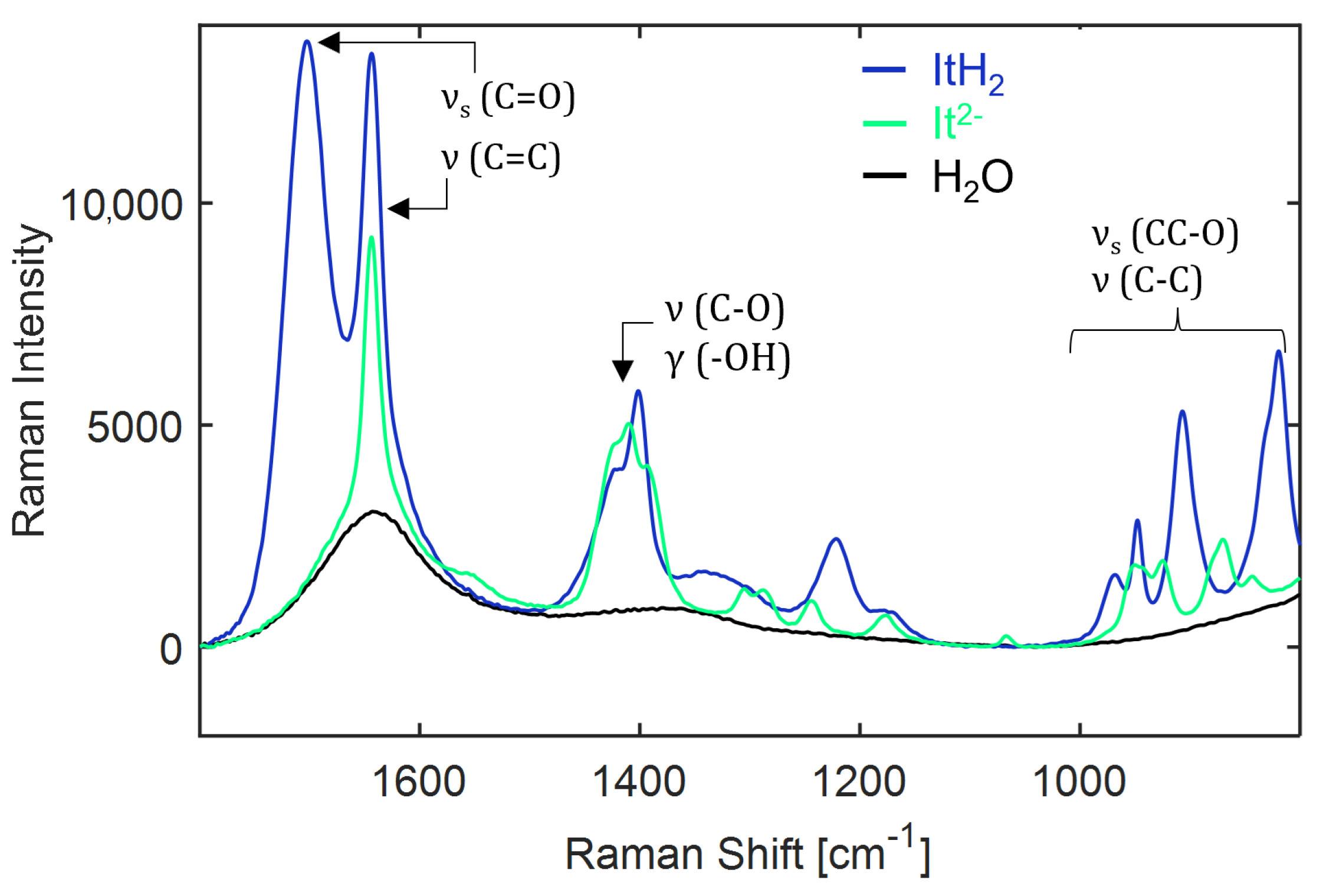
The Raman spectra of ITA can be found in
Figure A19. To be able to include the sugars in the IHM approach from Echtermeyer et al. (2021) [
93], the corresponding model for ITA is extended to a range of 800 to 1800 cm
−1. Additional prominent bands for ITA found in the region below 1025 cm
−1 are
C-C stretching modes and
s,(C-O) symmetric stretching vibrations related to the carboxylic acid groups [
112,
113]. Some of these bands shift to higher wavenumbers at higher pH values due to a higher degree of dissociation of the carboxylic acid groups and presence of ions [
112].
Figure A19.
Raman spectra recorded during titration of 8 wt% ITA with 1 M NaOH solution at 25
C. Spectra are treated with a linear baseline. Inset shows the concentration profiles of the ITA species, calculated using non-ideal thermodynamics, as in Echtermeyer et al. (2021) [
93]. ITAH
2: undissociated, ITAH
−: first dissociation state and ITA
2−: fully dissociated ITA.
Figure A19.
Raman spectra recorded during titration of 8 wt% ITA with 1 M NaOH solution at 25
C. Spectra are treated with a linear baseline. Inset shows the concentration profiles of the ITA species, calculated using non-ideal thermodynamics, as in Echtermeyer et al. (2021) [
93]. ITAH
2: undissociated, ITAH
−: first dissociation state and ITA
2−: fully dissociated ITA.
Figure A20.
Assignment of molecular vibrations to the Raman spectrum of 8 wt% (ITAH2) and 3 wt% (ITA2−) ITA in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : antisymmetric stretching and : deformation modes. ITAH2: undissociated and ITA2−: fully dissociated ITA.
Figure A20.
Assignment of molecular vibrations to the Raman spectrum of 8 wt% (ITAH2) and 3 wt% (ITA2−) ITA in water recorded at 25 C. The pure water spectrum (black curve) is shown for reference. : stretching, : antisymmetric stretching and : deformation modes. ITAH2: undissociated and ITA2−: fully dissociated ITA.
Appendix O. Indirect Hard Modelling and Figures of Merit
The mixture hard model created with IHM is set up in PEAXACT and shown in
Figure A21. It is comprised of a total of seven main components represented by PCM: water, three ITA species (ITAH
2, ITAH
−, ITA
2−), sucrose, glucose and fructose. Prior to fitting a Raman spectrum, a pretreatment model is first applied which excludes the region 1545– 1565 cm
−1 attributed to the oxygen gas peak and the spectral range is reduced to 800–1800 cm
−1. Secondly a linear baseline model is applied, fitted through the lowest points in the spectrum. One auxiliary PCM is included to compensate for a sloped baseline below 1000 cm
−1, as shown in magenta (
Figure A21).
Figure A21.
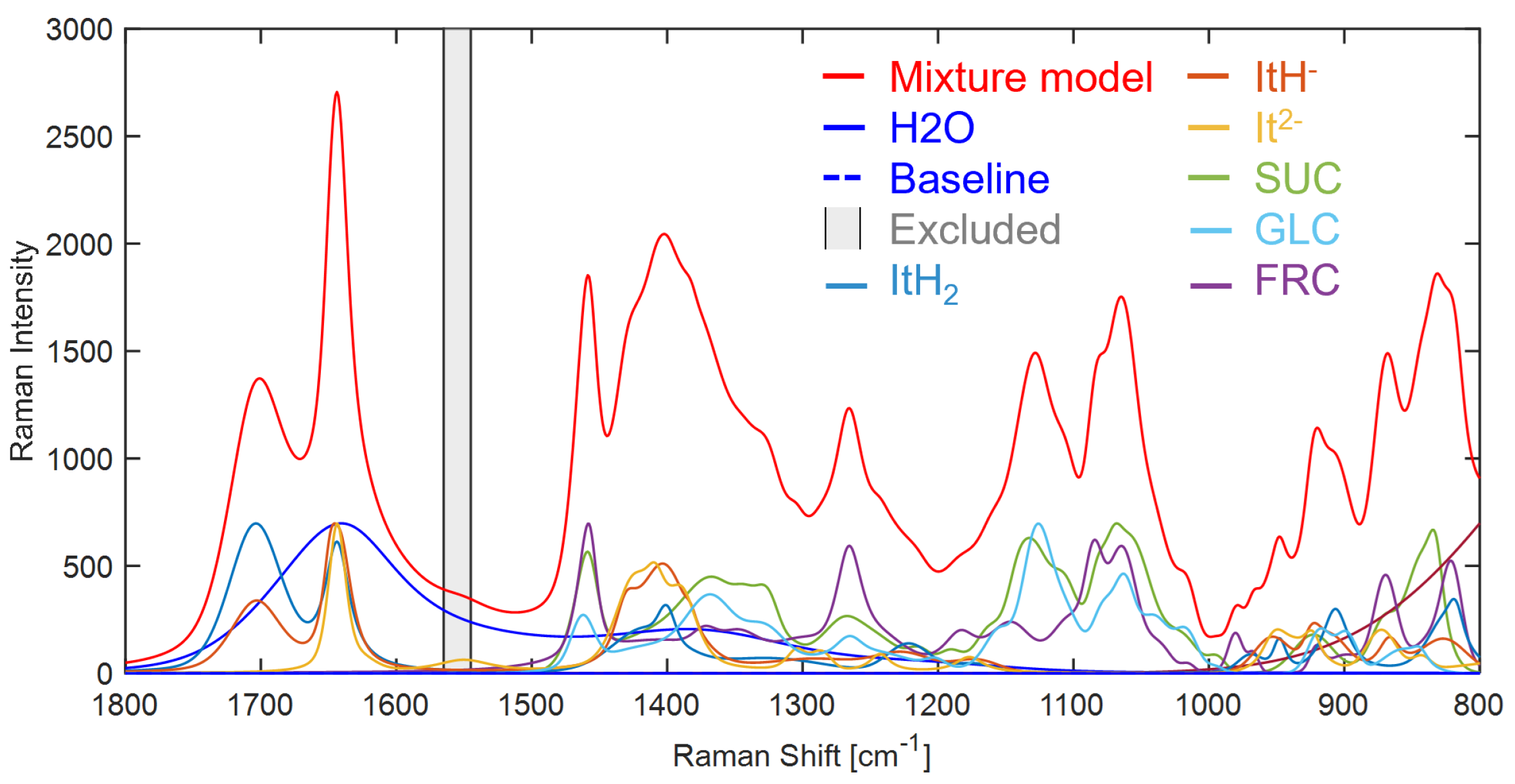
IHM constructed for ITA dissociation with sucrose, glucose and fructose and water solvent. ITAH2: undissociated, ITAH−: first dissociation state and ITA2−: fully dissociated ITA, SUC: sucrose, GLC: glucose, FRC: fructose.
Figure A21.
IHM constructed for ITA dissociation with sucrose, glucose and fructose and water solvent. ITAH2: undissociated, ITAH−: first dissociation state and ITA2−: fully dissociated ITA, SUC: sucrose, GLC: glucose, FRC: fructose.
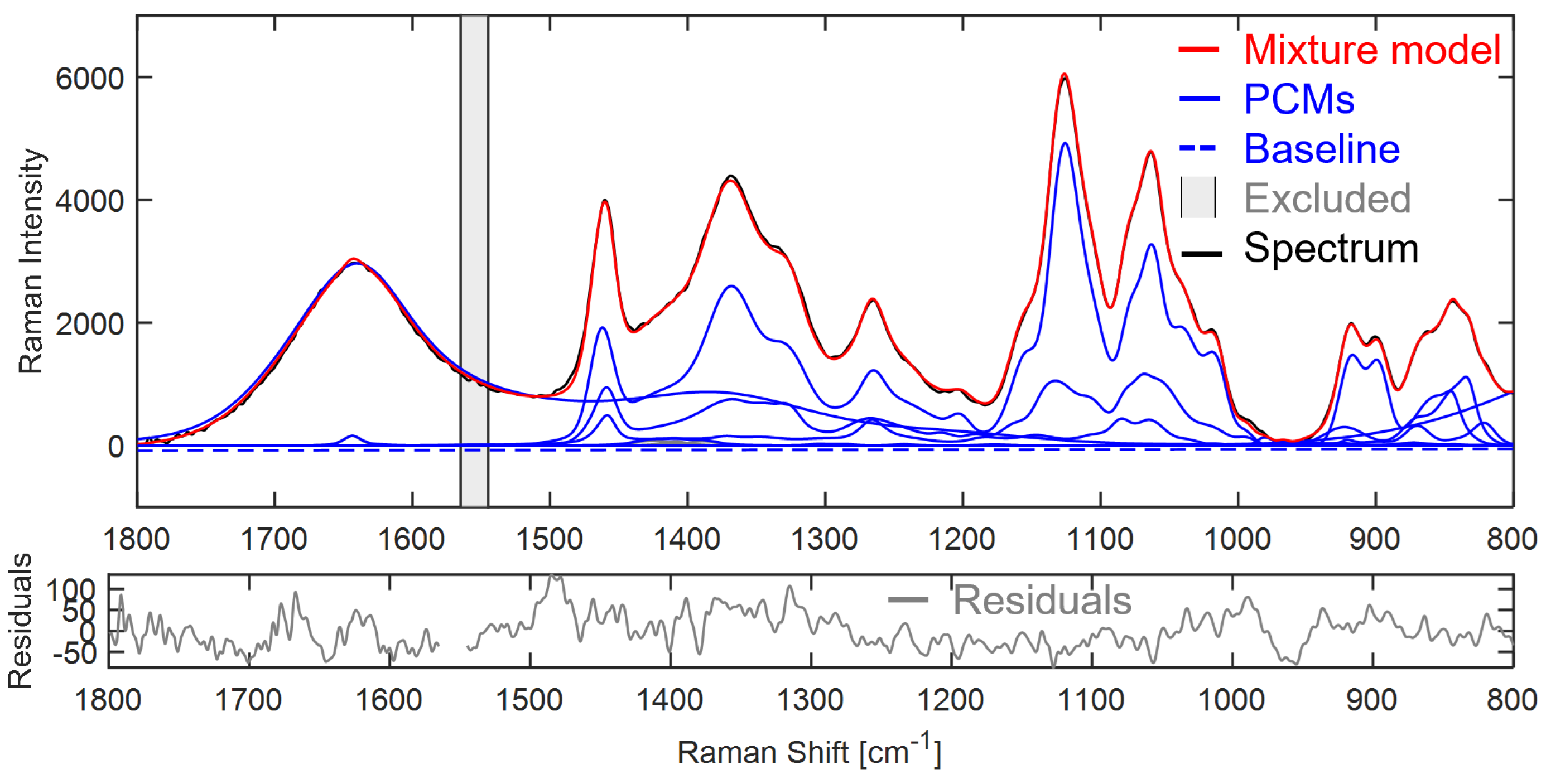
After the mixture hard model is constructed, its goodness of fit is determined to assess the model quality and accuracy. First of all, the model is fitted to a spectrum known to contain the components present in the model as PCMs.
Figure A22 shows the model fitted to a mixture of sucrose, glucose and fructose. The residuals show an absolute maximum value of 131, which is more than an order of magnitude smaller than the intensity (5981) of the maximum peak in the spectrum at 1126 cm
−1 for the alcohol (
COH) bending vibration of the sugars. Additionally, the RMS residual is calculated (Equation (
A15)).
N is the number of data points in the spectrum and
y are the Raman intensities at each point
n, is calculated as 39.8, which is comparably low compared to the peak intensities in the spectrum.
Figure A22.
The mixture hard model consisting of sucrose, glucose, fructose and ITA dissociation PCMs fitted to a Raman spectrum of 1 wt% fructose, 3 wt% sucrose and 8 wt% glucose. It has a linear baseline and excluded range between 1545 and 1565 cm−1.
Figure A22.
The mixture hard model consisting of sucrose, glucose, fructose and ITA dissociation PCMs fitted to a Raman spectrum of 1 wt% fructose, 3 wt% sucrose and 8 wt% glucose. It has a linear baseline and excluded range between 1545 and 1565 cm−1.
The following figures of merit are also calculated for the mixture hard model to assess further quality and properties of the constructed model. The calibration performance for a component
i is determined by the root mean squared error of calibration (RMSEC
i) [g/g] and the root mean squared error of leave-10%-out cross-validation (RMSECV
i) [g/g], each calculated as shown in Equation (
A16).
K is the total number of data points,
k included in the calibration and
w the concentration in g/g of the sample measured. The leave-10%-out cross-validation is achieved by repeating the calibration several times and at every iteration 10% of the data is left out of the calibration for calculating the RMSECV, until each datapoint has been left out at least once. Additionally, also the coefficient of determination, R
2i is calculated. Furthermore, the bias of the achieved calibration for each component is assessed using the stability
i [-], as depicted in Equation (
A17).
The detection limit [g/g] is calculated in Equation
A18 by evaluating 10 measurements of a blank sample consisting of deionized water. The weight fraction,
and standard deviation,
are determined using the mixture model predictions of the blank samples. With a confidence factor,
of 3, a confidence of 99.86% is obtained when a normal distribution of the measurement errors is assumed [
114].
The resulting PCMs constructed with IHM consist of 24, 19 and 24 component peaks for sucrose, glucose and fructose, respectively, in the range of 800 to 1800 cm
−1. To match this range, the existing IHM from Echtermeyer et al. (2021) [
93] for ITAH
2, ITAH
− and ITA
2− is extended from 1025 to 1800 cm
−1 to 800 to 1800 cm
−1. From there, ITA PCMs are constructed with 13, 14 and 16 component peaks, respectively, for ITAH
2, ITAH
− and ITA
2−.
The PCMs for ITA dissociation, sucrose, glucose, fructose and water are combined in a total mixture hard model (
Appendix O). Except for water and ITAH
−, the RMSEC
i and RMSECV
i values are below 1.218 × 10
−3 g/g, which indicates that the model has a good accuracy. The ITAH
− has lower accuracy, as there is a less accurate model fit in the region between pH 3 and 5 (
Figure A19) compared to Echtermeyer et al. (2021) [
93]. This also affects the water accuracy, as the calibration has an additional closure constraint, stating that the total sum of the weight fractions should add up to 1. As a result, ITAH
− accuracy also lowers the H
2O accuracy. The
i is below 3% for all components and thus indicates an unbiased and stable calibration result. Finally, the Limit of Detection (LOD
i) shows values that are an order of magnitude lower than the RMSECV
i’s, indicating a precise model. In conclusion, although similar, the overlapping spectral bands of the three sugars and ITA do not hinder an accurate and precise result in a mixture spectral hard model.
Table A4.
Figures of merit for the mixture hard model.
Table A4.
Figures of merit for the mixture hard model.
| Component | R2i [-] | RMSECi [×10−3 g/g] | RMSECVi [×10−3 g/g] | LODi [×10−3 g/g] | i [%] |
|---|
| ITAH2 | 0.997 | 0.985 | 1.018 | 0.118 | 3 |
| ITAH− | 0.985 | 1.481 | 1.490 | 0.020 | 1 |
| ITA−2 | 0.993 | 1.056 | 1.070 | 0.148 | 1 |
| Sucrose | 0.998 | 1.208 | 1.218 | <0.01 | 1 |
| Glucose | 0.999 | 0.551 | 0.558 | <0.01 | 1 |
| Fructose | 0.9997 | 0.392 | 0.398 | <0.01 | 2 |
| H2O | 0.986 | 2.805 | 2.842 | n.d.1 | 1 |