Radiomics for the Prediction of Pathological Complete Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Prospective Observational Trial
Abstract
1. Introduction
2. Materials and Methods
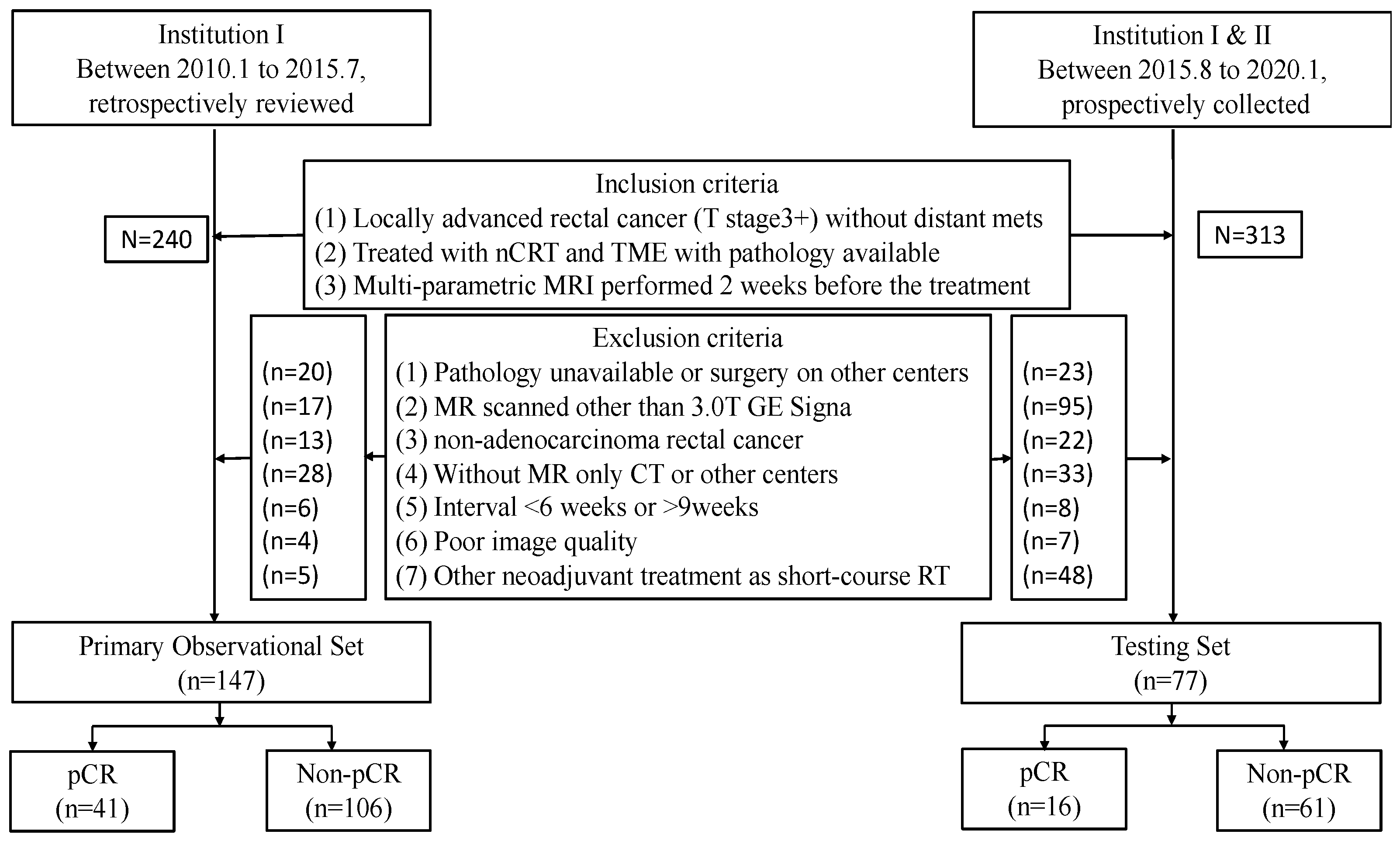
2.1. Study Population
2.2. Radiomics Model Evaluation
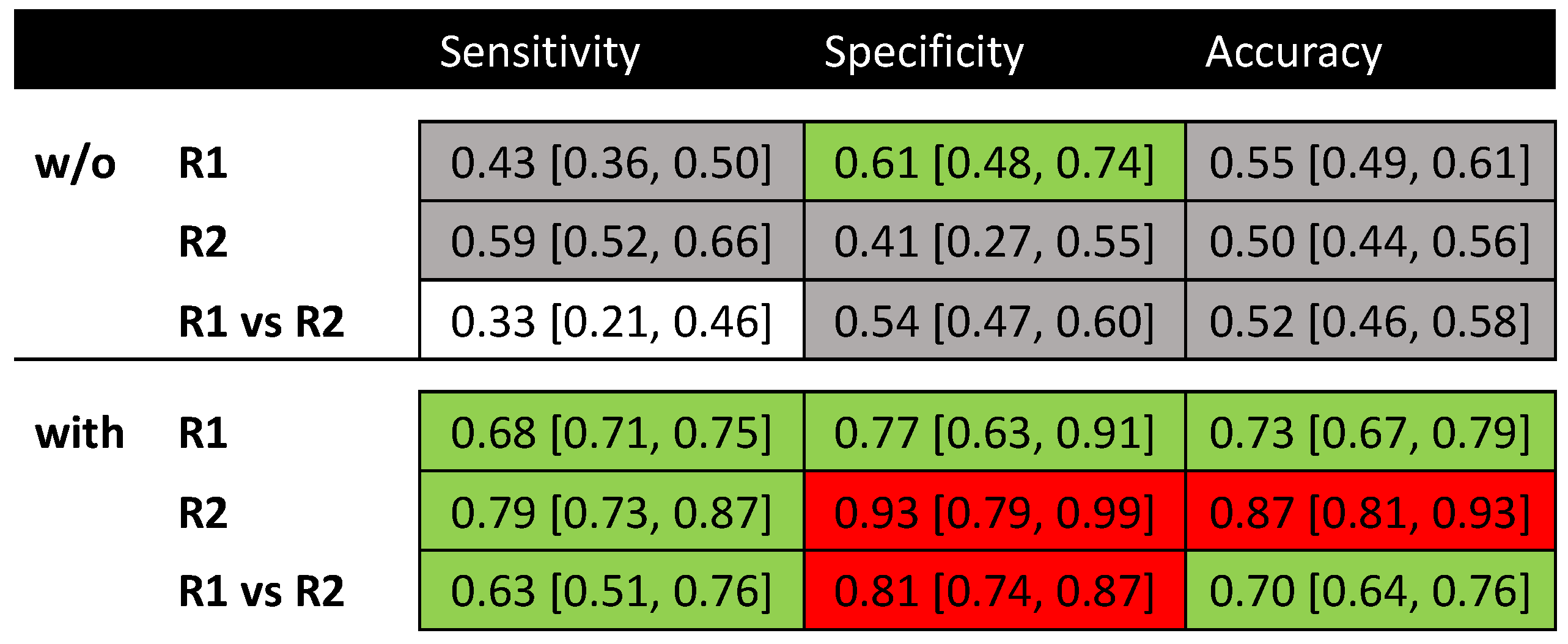
2.3. Qualitative Evaluation
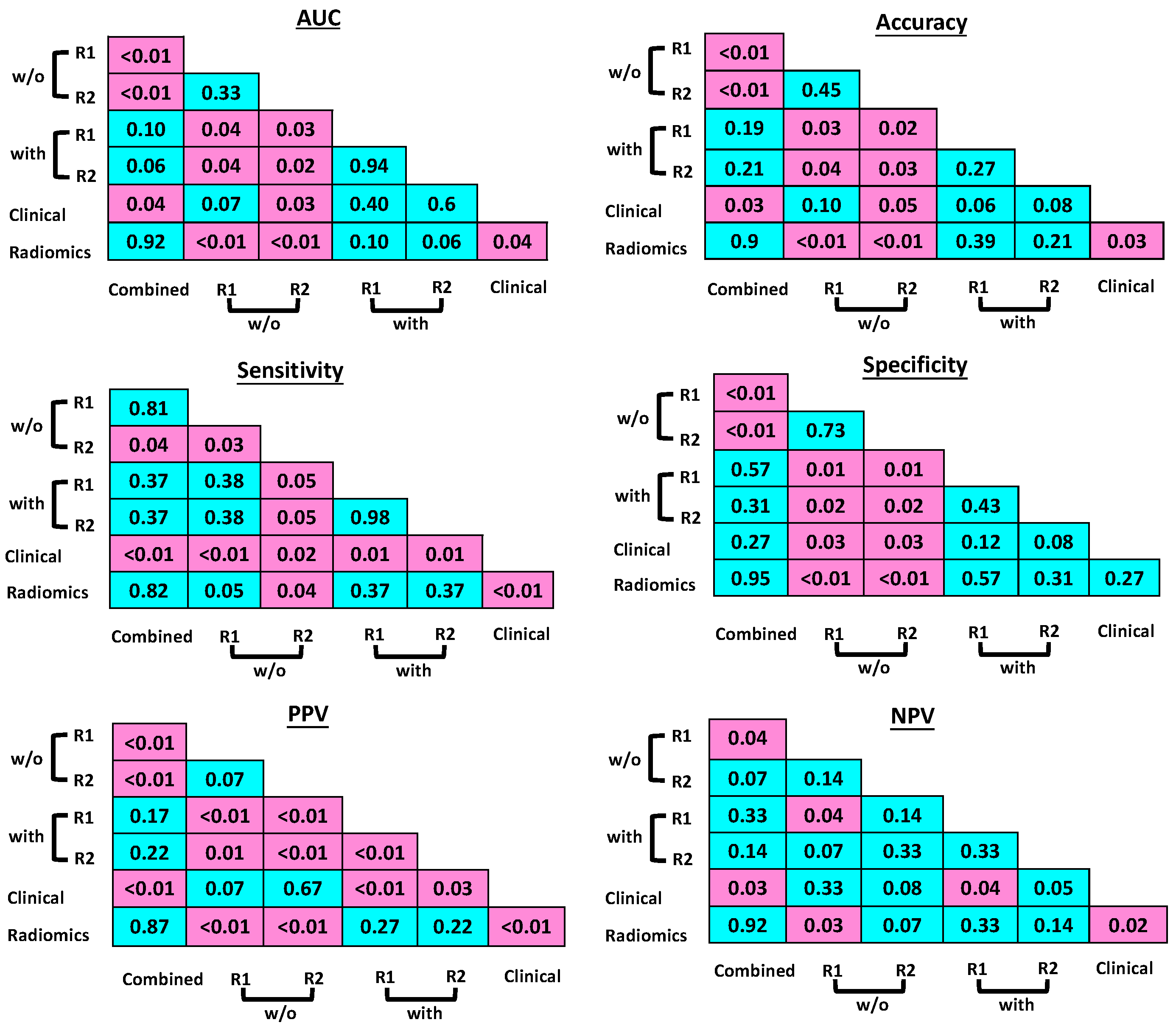
2.4. Statistical Analysis
3. Results
3.1. Radiomics Model Evaluation
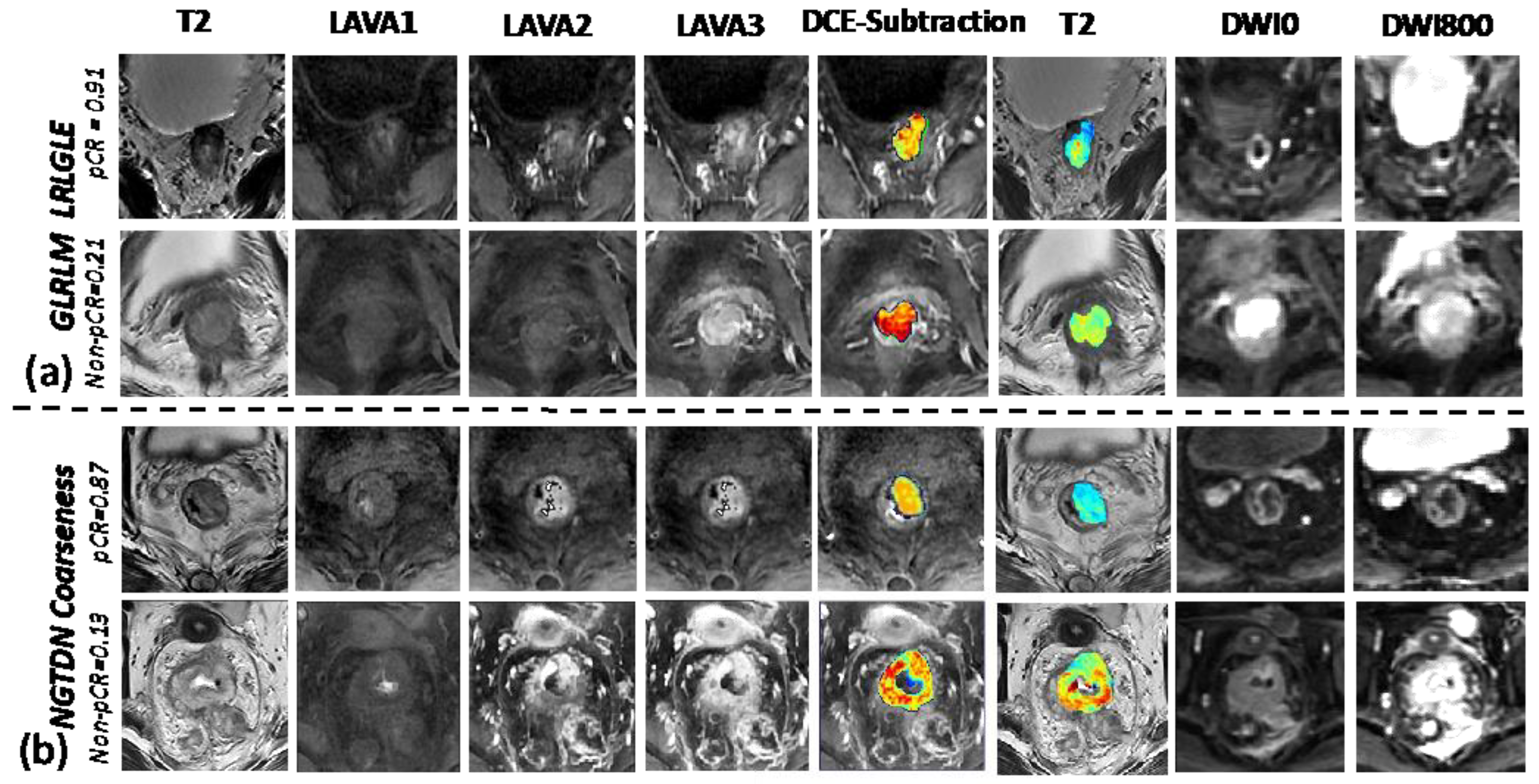
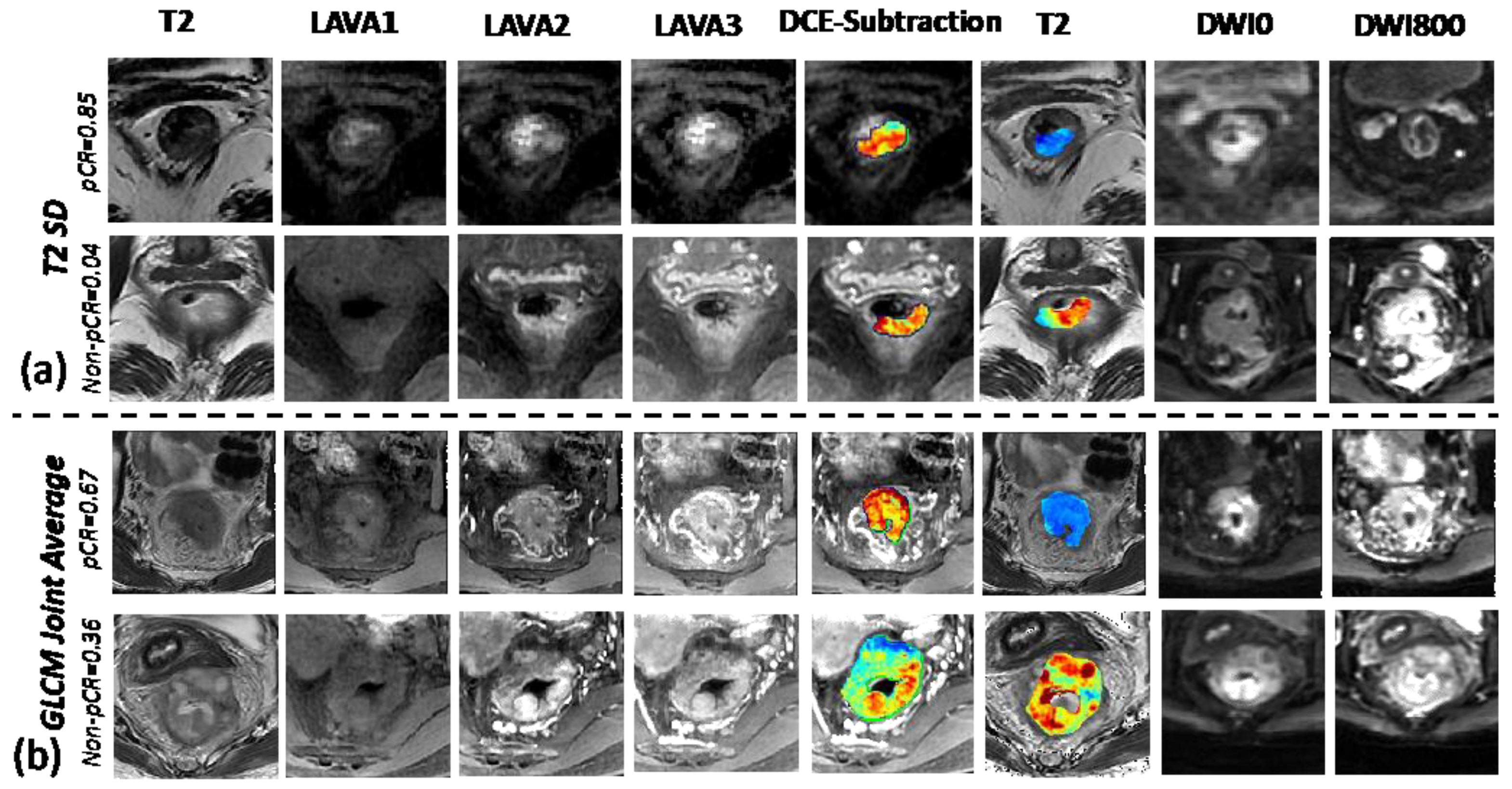
3.2. Understanding the Selected Radiomic Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Inclusion and Exclusion Criteria
Appendix B. MRI Protocols
| Hospital | Scanner | Pt. No. | Sequence | TR/TE (ms) | FOV (mm) | Matrix | Slice Thickness (mm) | Slice Gap (mm) | Slices | Flip Angle |
|---|---|---|---|---|---|---|---|---|---|---|
| Sir Run Run Shaw | GE 3.0 Signa | 147 + 33 | T2w | 3200/134 | 250 | 512 × 512 | 5 | 6 | 180 | 90 |
| DWI | 5900/66 | 250 | 256 × 256 | 6 | 6 | 30 | 90 | |||
| CE-T1w | 3.2/1.5 | 420 | 512 × 512 | 2.2 | 2.2 | 184 | 2 | |||
| Xia Sha | GE 3.0 Signa | 43 | T2w | 2900/131 | 250 | 512 × 512 | 5 | 6 | 180 | 90 |
| DWI | 5900/66 | 250 | 256 × 256 | 6 | 6 | 28 | 90 | |||
| CE-T1w | 4.3/22 | 360 | 512 × 512 | 2.2 | 2 | 192 | 2 |
Appendix C. Neoadjuvant Chemoradiotherapy Treatment Protocol and Pathological Treatment Response Evaluation
Appendix D. MRI Annotation
Appendix E. Radiomics Processing
Appendix F. Results to Show Detailed Radiomic Feature Results beetween the pCR and Non-pCR Groups
| Features | PCR | Non-PCR |
| GLRLM LRLGLE (L2-L1) | 0.77 ± 0.31 (median 0.72, IQR [0.59, 0.9]) | 0.31 ± 0.45 (median 0.38, IQR [0.11, 0.7]) |
| GLCM joint average (T2) | 0.69 ± 0.29 (median 0.65, IQR [0.49, 0.81]) | 0.79 ± 0.49 (median 0.71, IQR [0.42, 0.87]) |
| NGTDM coarseness (L3-L1) | 0.79 ± 0.22 (median 0.77, IQR [0.58, 0.88]) | 0.45 ± 0.52 (median 0.41, IQR [0.13, 0.85]) |
| GLCM ID (L) | 0.37 ± 0.22 (median 0.45, IQR [0.31, 0.68]) | 0.67 ± 0.44 (median 0.65, IQR [0.47, 0.85]) |
| Std (T2) | 0.69 ± 0.51 (median 0.65, IQR [0.19, 0.88]) | 0.87 ± 0.52 (median 0.82, IQR [0.61, 0.95]) |
| Sphericity (ADC) | 0.47 ± 0.4 (median 0.52, IQR [0.07, 0.72]) | 0.79 ± 0.37 (median 0.64, IQR [0.34, 0.88]) |
| GLCM RV (L3-L1) | 0.39 ± 0.21 (median 0.32, IQR [0.17, 0.62]) | 0.62 ± 0.45 (median 0.56, IQR [0.2, 0.89]) |
| GLDM LDE (L2-L1) | 0.76 ± 0.47 (median 0.79, IQR [0.61, 0.92]) | 0.67 ± 0.42 (median 0.71, IQR [0.31, 0.85]) |
| GLDM GLNN (L3-L1) | 0.82 ± 0.29 (median 0.75, IQR [0.65, 0.9]) | 0.7 ± 0.39 (median 0.69, IQR [0.32, 0.82]) |
| GLDM GLV (ADC) | 0.64 ± 0.21 (median 0.67, IQR [0.41, 0.81]) | 0.57 ± 0.22 (median 0.56, IQR [0.29, 0.78]) |
| GLRLM GLN (ADC) | 0.71 ± 0.46 (median 0.62, IQR [0.41, 0.88]) | 0.47 ± 0.51 (median 0.43, IQR [0.11, 0.81]) |
| GLCM cluster prominence (L2-L1) | 0.77 ± 0.32 (median 0.77, IQR [0.41, 0.88]) | 0.35 ± 0.42 (median 0.42, IQR [0.11, 0.72]) |
| NGTDM strength (T2) | 0.69 ± 0.52 (median 0.66, IQR [0.29, 0.86]) | 0.59 ± 0.39 (median 0.52, IQR [0.13, 0.88]) |
| GLRLM RLN (L2-L1) | 0.57 ± 0.39 (median 0.61, IQR [0.39, 0.81]) | 0.67 ± 0.21 (median 0.66, IQR [0.47, 0.78]) |
| Radiomics score | 0.77 ± 0.21 (median: 0.69, IQR [0.55, 0.92]) | 0.32 ± 0.24 (median 0.35, IQR [0.18, 0.62]) |
References
- Breugom, A.J.; Swets, M.; Bosset, J.F.; Collette, L.; Sainato, A.; Cionini, L.; Glynne-Jones, R.; Counsell, N.; Bastiaannet, E.; van den Broek, C.B.; et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rodel, C.; Kuo, L.J.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Grossmann, P.; Tan, Y.; Oxnard, G.G.; Rizvi, N.; Schwartz, L.H.; Zhao, B. Defining a Radiomic Response Phenotype: A Pilot Study using targeted therapy in NSCLC. Sci. Rep. 2016, 6, 33860. [Google Scholar] [CrossRef]
- LeBlanc, J.K. Imaging and management of rectal cancer. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 665–676. [Google Scholar] [CrossRef]
- van der Paardt, M.P.; Zagers, M.B.; Beets-Tan, R.G.; Stoker, J.; Bipat, S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: A systematic review and meta-analysis. Radiology 2013, 269, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.C.; Andrews, E.J.; Power, D.G. Multicenter Evaluation of Rectal cancer ReImaging POst Neoadjuvant (MERRION) Therapy. Ann. Surg. 2015, 262, e53. [Google Scholar] [CrossRef] [PubMed]
- Dinapoli, N.; Barbaro, B.; Gatta, R.; Chiloiro, G.; Casa, C.; Masciocchi, C.; Damiani, A.; Boldrini, L.; Gambacorta, M.A.; Dezio, M.; et al. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 765–774. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.Y.; Shi, Y.J.; Wang, L.; Zhu, H.T.; Tang, Z.; Wang, S.; Li, X.T.; Tian, J.; Sun, Y.S. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 7253–7262. [Google Scholar] [CrossRef]
- Nie, K.; Shi, L.; Chen, Q.; Hu, X.; Jabbour, S.K.; Yue, N.; Niu, T.; Sun, X. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5256–5264. [Google Scholar] [CrossRef]
- Horvat, N.; Veeraraghavan, H.; Khan, M.; Blazic, I.; Zheng, J.; Capanu, M.; Sala, E.; Garcia-Aguilar, J.; Gollub, M.J.; Petkovska, I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology 2018, 287, 833–843. [Google Scholar] [CrossRef]
- Shin, J.; Seo, N.; Baek, S.E.; Son, N.H.; Lim, J.S.; Kim, N.K.; Koom, W.S.; Kim, S. MRI Radiomics Model Predicts Pathologic Complete Response of Rectal Cancer Following Chemoradiotherapy. Radiology 2022, 303, 351–358. [Google Scholar] [CrossRef]
- Yi, X.; Pei, Q.; Zhang, Y.; Zhu, H.; Wang, Z.; Chen, C.; Li, Q.; Long, X.; Tan, F.; Zhou, Z.; et al. MRI-Based Radiomics Predicts Tumor Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Front. Oncol. 2019, 9, 552. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, Y.; Liu, Z.; Zhou, Z.; Lai, B.; Sun, K.; Li, L.; Huang, L.; Feng, Y.; Cao, W.; et al. Radiomics-Based Preoperative Prediction of Lymph Node Status Following Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Front. Oncol. 2020, 10, 604. [Google Scholar] [CrossRef]
- Cruz Rivera, S.; Liu, X.; Chan, A.W.; Denniston, A.K.; Calvert, M.J.; Spirit, A.I.; Group, C.-A.W.; Spirit, A.I.; Group, C.-A.S.; Spirit, A.I.; et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Nat. Med. 2020, 26, 1351–1363. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Stipa, F.; Picchio, M.; Burza, A.; Soricelli, E.; Vitelli, C.E. Long-term outcome of local excision after preoperative chemoradiation for ypT0 rectal cancer. Dis. Colon Rectum. 2014, 57, 1245–1252. [Google Scholar] [CrossRef]
- Aklilu, M.; Eng, C. The current landscape of locally advanced rectal cancer. Nat. Rev. Clin. Oncol. 2011, 8, 649–659. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U.; Silva e Sousa, A.H.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Carten, R.V.; Babiker, A.; Abulafi, M.; Lord, A.C.; Brown, G. Prognostic Importance of MRI-Detected Extramural Venous Invasion in Rectal Cancer: A Literature Review and Systematic Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, N.; Tang, Y.; Shi, J.; Zhao, Y.; Ma, H.; Wang, S.; Li, Y.X.; Jin, J. The prognostic value of MRI-detected extramural vascular invasion (mrEMVI) for rectal cancer patients treated with neoadjuvant therapy: A meta-analysis. Eur. Radiol. 2021, 31, 8827–8837. [Google Scholar] [CrossRef] [PubMed]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef]
- Khwaja, S.A.; Thipphavong, S.; Kirsch, R.; Menezes, R.J.; Kennedy, E.D.; Brierley, J.D.; Jhaveri, K.S. Evaluation of a multiparametric MRI scoring system for histopathologic treatment response following preoperative chemoradiotherapy for rectal cancer. Eur. J. Radiol. 2021, 138, 109628. [Google Scholar] [CrossRef] [PubMed]
- Hotker, A.M.; Tarlinton, L.; Mazaheri, Y.; Woo, K.M.; Gonen, M.; Saltz, L.B.; Goodman, K.A.; Garcia-Aguilar, J.; Gollub, M.J. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters. Eur. Radiol. 2016, 26, 4303–4312. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, X.; Shi, Z.; Yang, Z.; Du, X.; Zhao, Z.; Cheng, X. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur. Radiol. 2019, 29, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.R.; Gillies, R.J. The Biological Meaning of Radiomic Features. Radiology 2021, 299, E256. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Wang, Y.; Bryant, S.H. An efficient algorithm coupled with synthetic minority over-sampling technique to classify imbalanced PubChem BioAssay data. Anal. Chim. Acta 2014, 806, 117–127. [Google Scholar] [CrossRef]
- Bammer, R.; Keeling, S.L.; Augustin, M.; Pruessmann, K.P.; Wolf, R.; Stollberger, R.; Hartung, H.P.; Fazekas, F. Improved diffusion-weighted single-shot echo-planar imaging (EPI) in stroke using sensitivity encoding (SENSE). Magn. Reson. Med. 2001, 46, 548–554. [Google Scholar] [CrossRef]
- Borkowski, K.; Klodowski, K.; Figiel, H.; Krzyzak, A.T. A theoretical validation of the B-matrix spatial distribution approach to diffusion tensor imaging. Magn. Reson. Imaging 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Kamal, A.M.; Wasfey, E.F.; Elghamry, W.R.; Sabry, O.M.; Elghobary, H.A.; Radwan, S.M. Genetic signature of CTLA-4, BTLA, TIM-3 and LAG-3 molecular expression in colorectal cancer patients: Implications in diagnosis and survival outcomes. Clin. Biochem. 2021, 96, 13–18. [Google Scholar] [CrossRef]
- Ghorbaninezhad, F.; Masoumi, J.; Bakhshivand, M.; Baghbanzadeh, A.; Mokhtarzadeh, A.; Kazemi, T.; Aghebati-Maleki, L.; Shotorbani, S.S.; Jafarlou, M.; Brunetti, O.; et al. CTLA-4 silencing in dendritic cells loaded with colorectal cancer cell lysate improves autologous T cell responses in vitro. Front. Immunol. 2022, 13, 931316. [Google Scholar] [CrossRef]


| Clinical Characteristic | Retrospective Observational Cohort (n = 147) | Prospective Testing Cohort (n = 77) | p-Value |
|---|---|---|---|
| Age (y) * | 58.43 ± 10 [27–80] | 59.37 ± 9.52 [27–75] | 0.49 § |
| Gender | |||
| Male | 104 | 55 | 0.98 + |
| Female | 43 | 22 | |
| Tumor location | |||
| Upper | 14 | 4 | 0.21 ¶ |
| Middle | 93 | 52 | |
| Lower | 40 | 21 | |
| cT-stage | |||
| 3a | 80 | 28 | 0.05 ¶ |
| 3b | 44 | 21 | |
| 3c | 12 | 17 | |
| 3d | 5 | 5 | |
| 4 | 6 | 6 | |
| cN-stage | |||
| 0 | 33 | 6 | 0.06 ¶ |
| 1 | 74 | 42 | |
| 2 | 40 | 29 | |
| Concurrent chemo | |||
| Capecitabine | 22 | 19 | 0.11 + |
| Oxaliplatin | 125 | 58 | |
| Adjuvant chemo | |||
| Folfox6 | 108 | 67 | 0.07 ¶ |
| Xelox | 27 | 7 | |
| Capecitabine | 8 | 1 | |
| None | 4 | 2 | |
| Pathology result | |||
| pCR | 41 | 17 | 0.41 ¶ |
| Non-pCR | 106 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Zhang, Y.; Hu, J.; Zhou, W.; Hu, X.; Cui, T.; Yue, N.J.; Sun, X.; Nie, K. Radiomics for the Prediction of Pathological Complete Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Prospective Observational Trial. Bioengineering 2023, 10, 634. https://doi.org/10.3390/bioengineering10060634
Shi L, Zhang Y, Hu J, Zhou W, Hu X, Cui T, Yue NJ, Sun X, Nie K. Radiomics for the Prediction of Pathological Complete Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Prospective Observational Trial. Bioengineering. 2023; 10(6):634. https://doi.org/10.3390/bioengineering10060634
Chicago/Turabian StyleShi, Liming, Yang Zhang, Jiamiao Hu, Weiwen Zhou, Xi Hu, Taoran Cui, Ning J. Yue, Xiaonan Sun, and Ke Nie. 2023. "Radiomics for the Prediction of Pathological Complete Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Prospective Observational Trial" Bioengineering 10, no. 6: 634. https://doi.org/10.3390/bioengineering10060634
APA StyleShi, L., Zhang, Y., Hu, J., Zhou, W., Hu, X., Cui, T., Yue, N. J., Sun, X., & Nie, K. (2023). Radiomics for the Prediction of Pathological Complete Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer: A Prospective Observational Trial. Bioengineering, 10(6), 634. https://doi.org/10.3390/bioengineering10060634







