Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants
Abstract
1. Introduction
1.1. Botanical Properties and Traditional Use
1.2. Chemical Compositions
1.3. Classification of Anti-Bacterial Phytochemicals
1.3.1. Terpenes and Terpenoids
| SN a | Name | Structure | MW | Pathogen | MIC (µg/mL) | Plant | |
|---|---|---|---|---|---|---|---|
| Terpenes (monoterpenes) | |||||||
| 1 | Sabinene |  | 154.3 | B. subtilis, S. epidermidis | >64 [11] | A. indica [11] | |
| P. aeruginosa | >32 [11] | ||||||
| S. aureus | 32 [11] | ||||||
| S. typhi | 128 [11] | ||||||
| S. dyssenteriae | >128 [11] | ||||||
| K. pneumonia | 64 [11] | ||||||
| 2 | Myrcene | 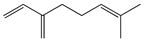 | 136.2 | S. epidermidis | 121 [32] | A. absinthium [7] | |
| B. subtillis | 322.11 [32] | ||||||
| S. dyssenteriae | 325 [32] | ||||||
| K. pneumonia | 400 [32] | ||||||
| 3 | (E)-β-Ocimene | 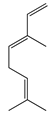 | 136.2 | B. subtillis, S. epidermidis, | 130 [32] | A. dracunculus [33] | |
| P. vulgaris | 220 [32] | ||||||
| S. dyssenteriae | 650 [32] | ||||||
| K. pneumonia | 600 [32] | ||||||
| 4 | (Z)-β-Ocimene | 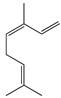 | 136.2 | B. subtillis | 130 [32] | A.dracunculus [33] | |
| S. dyssenteriae | 220 [32] | ||||||
| E. coli, K. pneumonia | 600 [32] | ||||||
| 5 | p-Cymene | 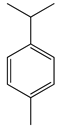 | 134.2 | B. subtilis, S. typhi | >64 [11] | A. indica [11] | |
| S. epidermidis | 128 [11] | ||||||
| P. aeruginosa | 64 [11] | ||||||
| S. aureus | 32 [11] | ||||||
| S. dyssenteriae | >128 [11] | ||||||
| K. pneumonia | >64 [11] | ||||||
| 6 | (+)-Limonene | 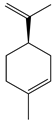 | 136.2 | L. monocytogenes | 20 [34] | A. capillaris [35] | |
| E. colics | 112 [35] | ||||||
| H. influenzae | 128 [35] | ||||||
| Methicillin-resistant S. aureuscs (MRSAcs) | 150 [35] | ||||||
| 7 | (−)-Limonene | 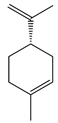 | 136.2 | S. pyogenes, K. pneumoniae | 156 [35] | ||
| S. pneumoniae | 198 [35] | ||||||
| MRSA | 332 [35] | ||||||
| MRSA | 330 [35] | ||||||
| 8 | Camphene |  | 136.2 | V. vulnificus | 400 [36] | A. iwayomogi [36] | |
| S. aureus, S. mutans, E. coli ATCC25922, C. freundii | 1600 [36] | ||||||
| S. epidermidis | 3200 [36] | ||||||
| S. pyogenes, E. faecalis, E. gallinarum, S. typhimurium, E. coli O157:H7, E. cloacae, P. aeruginosa | >12,800 [36] | ||||||
| K. pneumonia | 64 [11] | A. indica [11] | |||||
| P. aeruginosa | 128 [11] | ||||||
| B. subtilis, S. epidermidis, S. typhi | >128 [11] | ||||||
| S. dyssenteriae | 256 [11] | ||||||
| 9 | α-Pinene |  | 136.2 | E. colics | 98 [37] | A. vestita [37] | |
| H. influenzae | 126 [37] | ||||||
| S. pyogenes | 132 [37] | ||||||
| MRSAcs, S. pneumoniae | 172 [37] | ||||||
| K. pneumoniae | 178 [37] | ||||||
| MRSA | 210 [37] | ||||||
| Methicillin and gentamycin-resistant S. aureus (MGRSA), S. aureus | 256 [37] | ||||||
| S. aureus, K. pneumonia | 32 [11] | A. indica [11] | |||||
| P. aeruginosa | 64 [11] | ||||||
| B. subtilis, S. typhi, S. dyssenteriae | 128 [11] | ||||||
| S. epidermidis | >128 [11] | ||||||
| 10 | β-Pinene | 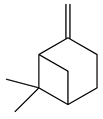 | 136.2 | E. colics | 102 [37] | A. vestita [37] | |
| H. influenzae | 132 [37] | ||||||
| S. pyogenes | 144 [37] | ||||||
| MRSAcs, S. pneumoniae, K. pneumoniae | 170 [37] | ||||||
| MRSA | 210 [37] | ||||||
| MGRSA | 256 [37] | ||||||
| S. aureus | 32 [11] | A. indica [11] | |||||
| K. pneumonia | >32 [11] | ||||||
| P. aeruginosa | 64 [11] | ||||||
| B. subtilis, S. typhi | >64 [11] | ||||||
| S. epidermidis, S. dyssenteriae | 128 [11] | ||||||
| Terpenes (sesquiterpenes) | |||||||
| 11 | α-Elemene | 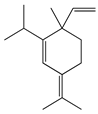 | 204.3 | S. enterica | 0.1 [6] | A. indica [6], A. dracunculus [38] | |
| E. coli | 25 [6] | ||||||
| S. typhimurium | 60 [38] | ||||||
| B. cereus, S. aureus | 250 [38] | ||||||
| L. monocytogenes | 262.5 [6] | ||||||
| 12 | β-Caryophyllene | 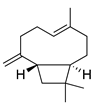 | 204.3 | S. pyogenes | 25 [36] | A. capillaris [35], A. iwayomogi [36], A. feddei [13], A. argyi [26] | |
| S. aureus, E. gallinarum | 50 [36] | ||||||
| H. influenzae, K. pneumoniae | 64 [35] | ||||||
| E. colics | 92 [35] | ||||||
| S. epidermidis | 100 [36] | ||||||
| S. pneumoniae | 122 [35] | ||||||
| S. pyogenes | 126 [35] | ||||||
| MRSAcs | 144 [35] | ||||||
| E. faecalis | 200 [36] | ||||||
| MRSA | 330 [35] | ||||||
| MGRSA | 332 [35] | ||||||
| P. gingivalis | 400 [13] | ||||||
| S. mutans [36], P. intermedia [13] | 800 | ||||||
| S. mutans, S. sanguinis, S. gordonii, A. actinomycetemcomitans | 1600 [13] | ||||||
| V. vulnificus | 6400 [36] | ||||||
| E. coli, S. aureus, S. epidermidis, S. pyogenes, S. sobrinus, S. ratti, S. criceti, S. anginosus, F. nucleatum | 12,800 [13] | ||||||
| S. typhimurium, E. coli ATCC25922, E. coli O157:H7, E. cloacae, P. aeruginosa, C. freundii | >12,800 [36] | ||||||
| 13 | α-Farnesene | 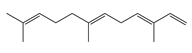 | 204.3 | S. enterica | 3.12 [6] | A. indica [6] | |
| E. coli | 200 [6] | ||||||
| L. monocytogenes | 4000 [6] | ||||||
| 14 | α-Curcumene | 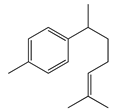 | 202.3 | E. coli | 100 [39] | A. integrifolia L. [39] | |
| S. aureus | 130 [39] | ||||||
| B. cereus | 140 [39] | ||||||
| Y. enterocolitica | 260 [39] | ||||||
| 15 | Dihydro-ar-curcumene | 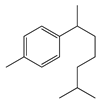 | 204.4 | E. coli | 120 [39] | A. integrifolia L. [39] | |
| E. coli ATCC25922 | 200 [6] | ||||||
| Y. enterocolitica | 280 [39] | ||||||
| L. monocytogenes | 4000 [6] | ||||||
| 16 | Germacrene B |  | 204.4 | S. aureus | 32 [11] | A. indica [11] | |
| P. aeruginosa, S. typhi | >32 [11] | ||||||
| K. pneumonia | 64 [11] | ||||||
| B. subtilis,S. epidermidis | >64 [11] | ||||||
| S. dyssenteriae | >128 [11] | ||||||
| 17 | Germacrene D |  | 204.4 | B. subtillis | 30.3 [32] | A. vulgaris, A. annua, A. herba-alba [28] | |
| S. aureus | 30.3 [32] | ||||||
| P. vulgaris, S. dyssenteriae | 65.1 [32] | ||||||
| K. pneumonia | 90.1 [32] | ||||||
| S. typhi | 90.2 [32] | ||||||
| Terpenoids (monoterpenoids) | |||||||
| 18 | Artemisia ketone |  | 152.2 | S. aureus | >16 [11] | A. indica [11] | |
| P. aeruginosa, S. typhi | 32 [11] | ||||||
| K. pneumonia | >32 [11] | ||||||
| B. subtilis | 64 [11] | ||||||
| S. epidermidis | >64 [11] | ||||||
| S. dyssenteriae | >128 [11] | ||||||
| 19 | Linalool | 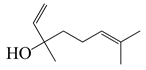 | 154.3 | B. cereus, E. coli, S. aureus, S. typhimurium | 250 [38] | A. annua [10], A. dracunculus [38] | |
| 20 | Nerol |  | 154.3 | S. epidermidis | >16 [11] | A. indica [11] | |
| S. aureus | 32 [11] | ||||||
| P. aeruginosa | 64 [11] | ||||||
| B. subtilis, K. pneumonia, S. typhi | >64 [11] | ||||||
| S. dyssenteriae | 128 [11] | ||||||
| 21 | Grandisol | 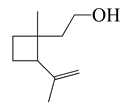 | 154.2 | S. pyogenes, H. influenzae | 130 [37] | A. vestita [37] | |
| S. pneumoniae | 132 [37] | ||||||
| K. pneumoniae | 144 [37] | ||||||
| MRSA | 178 [37] | ||||||
| 22 | Piperitone | 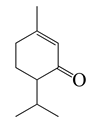 | 152.2 | H. influenzae, E. colics | 72 [37] | A. vestita [37] | |
| K. pneumoniae | 86 [37] | ||||||
| S. pyogenes | 102 [37] | ||||||
| MRSA, S. pneumoniae | 112 [37] | ||||||
| MRSAcs | 122 [37] | ||||||
| MGRSA | 156 [37] | ||||||
| 23 | Terpinen-4-ol |  | 154.2 | S. gordonii | 50 [13] | A. feddei [13] | |
| F. nucleatum, P. intermedia | 200 [13] | ||||||
| P. gingivalis | 400 [13] | ||||||
| E. coli, S. pyogenes, S. aureus, S. ratti, S. anginosus, S. epidermidis, S. mutans, S. sanguinis, S. sobrinus, A. actinomycetemcomitans | 1600 [13] | ||||||
| S. criceti | 3200 [13] | ||||||
| 24 | α-Terpineol |  | 154.2 | S. aureus | 30 [40] | A. feddei [13], A. princeps Pamp. [41] | |
| S. gordonii | 50 [13] | ||||||
| E. coli | 60 [40] | ||||||
| B. cereus, S. Typhimurium | 120 [40] | ||||||
| P. intermedia, P. gingivalis | 200 [13] | ||||||
| F. nucleatum | 400 [13] | ||||||
| G. vaginalis | 560 [41] | ||||||
| S. aureus, S. epidermidis, S. pyogenes, E. coli, S. mutans, S. sobrinus, S. ratti, S. anginosus, S. sanguinis, A. actinomycetemcomitans | 1600 [13] | ||||||
| S. criceti | 3200 [13] | ||||||
| 25 | Thymol | 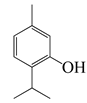 | 150.2 | S. aureus, K. pneumoniae K38cs | 60 [42] | A. haussknechtii [42] | |
| E. coli, P. aeruginosa, A. baumannii A52cs | 80 [42] | ||||||
| 26 | Carveol | 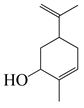 | 152.2 | S. enterica | 0.1 [6] | A. indica [6], A. dracunculus [38] | |
| S. aureus | 15 [38] | ||||||
| E. coli | 25 [6] | ||||||
| S. typhimurium | 30 [38] | ||||||
| E. coli | 60 [38] | ||||||
| L. monocytogenes | 100 [6] | ||||||
| B. cereus | 120 [38] | ||||||
| 27 | (Z)-Chrysanthenyl acetate | 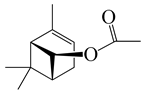 | 194.3 | B. subtilis, S. typhi | 256 [11] | A. indica [11] | |
| S. aureus | 512 [11] | ||||||
| K. pneumonia | >256 [11] | ||||||
| S. dyssenteriae | >128 [11] | ||||||
| 28 | 1,8-Cineole | 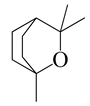 | 154.3 | H. influenzae | 98 [37] | A.feddei [13], A. vestita [37], A. iwayomogi [36] | |
| K. pneumoniae, E. colics | 102 [37] | ||||||
| S. pyogenes | 112 [37] | ||||||
| S. pneumoniae | 132 [37] | ||||||
| MRSAcs | 152 [37] | ||||||
| MRSA | 244 [37] | ||||||
| MGRSA | 256 [37] | ||||||
| S. epidermidis | 800 [13] | ||||||
| P. intermedia | 1600 [13] | ||||||
| S. pyogenes, E. coli, V. vulnificus [36], S. anginosus, F. nucleatum [13] | 3200 [13] | ||||||
| S. aureus, S. mutans, E. faecalis, E. gallinarum, S. typhimurium, S. epidermidis, E. coli O157:H7, E. cloacae, C. freundii [36], S. gordonii, A. actinomycetemcomitans, P. gingivalis [13] | 6400 [13] | ||||||
| S. aureus, S. pyogenes, S. sanguinis, S. mutans, S. ratti, S. sobrinus, S. criceti | 12,800 [13] | ||||||
| P. aeruginosa | >12,800 [36] | ||||||
| S. enterica | 0.1 [6] | A. indica [6] | |||||
| E. coli | 25 [6] | ||||||
| L. monocytogenes | 100 [6] | ||||||
| 29 | α-Thujone | 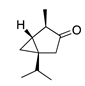 | 152.2 | S. aureus | 32 [11] | A. indica [11], A. absinthium [17] | |
| K. pneumonia | >32 [11] | ||||||
| P. aeruginosa | 64 [11] | ||||||
| B. subtilis, S. epidermidis | >64 [11] | ||||||
| S. typhi, S. dyssenteriae | 128 [11] | ||||||
| 30 | β-Thujone | 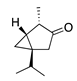 | 152.2 | S. aureus | 90 [43] | A. indica [11], A. absinthium [17] | |
| S. epidermidis | 100 [43] | ||||||
| E. coli | 350 [43] | ||||||
| K. pneumoniae | 650 [43] | ||||||
| P. aeruginosa | 750 [43] | ||||||
| E. cloacae | 830 [43] | ||||||
| 31 | (+)-Borneol |  | 154.3 | S. aureus CCARM3523, S. typhimurium KCCM11862 | 2 [44] | A. iwayomogi [44], A. feddei [13] | |
| S. aureus strains CCARM0027 & CCARM3511, S. typhimurium CCARM 8007 | 4 [44] | ||||||
| S. typhimurium CCARM8009 | 8 [44] | ||||||
| S. enteritidis strains KCCM12201, CCARM8010 & CCARM8011 | >16 [44] | ||||||
| S. aureus | 30 [40] | ||||||
| B. cereus, S. typhimurium | 120 [40] | ||||||
| V. vulnificus | 100 [14] | ||||||
| F. nucleatum, P. intermedia | 200 [13] | ||||||
| E. coli | 250 [40] | ||||||
| S. pyogenes, E. coli O157:H7 [14], E. coli, S. sobrinus [13] | 400 | ||||||
| S. epidermidis, S. pyogenes, S. mutans, S. anginosus, S. gordonii, A. actinomycetemcomitans, P. gingivalis | 800 [13] | ||||||
| E. faecalis, E. gallinarum [14], S. aureus, S. sanguinis [13] | 1600 | ||||||
| S. ratti, S. criceti | 3200 [13] | ||||||
| P. aeruginosa | >12,800 [14] | ||||||
| 32 | (−)-Borneol | 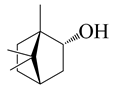 | 154.3 | B. cereus, E. coli, S. aureus | 250 [40] | A. argyi [26], A. indica [11] | |
| S. typhimurium | 800,000 [40] | ||||||
| B. subtilis, | 128 [11] | ||||||
| S. epidermidis | 64 [11] | ||||||
| P. aeruginosa, S. typhi | >64 [11] | ||||||
| S. dyssenteriae | >128 [11] | ||||||
| K. pneumonia | 64 [11] | ||||||
| 33 | Camphor |  | 152.2 | S. aureus CCARM0027 & CCARM3523, S. typhimurium KCCM11862 | 2 [44] | A. annua [10,14] | |
| S. aureus CCARM3511, S. typhimurium CCARM 8007 & CCARM8009 | 4 [44] | ||||||
| S. aureus | 15 [40] | ||||||
| S. enteritidis KCCM12201, CCARM8010 & CCARM8011 | >16 [44] | ||||||
| B. cereus, S. typhimurium, E. coli | 250 [40] | ||||||
| V. vulnificus | 400 [14] | ||||||
| C. perfringens | 500 [10] | ||||||
| S. epidermidis, S. pyogenes, E. coli O157:H7 | 800 [14] | ||||||
| S. aureus, S. mutans, E. coli, E. cloacae, C. freundii | 1600 [14] | ||||||
| E. faecalis, E. gallinarum | 3200 [14] | ||||||
| S. typhimurium | 6400 [14] | ||||||
| P. aeruginosa | >12,800 [14] | ||||||
| Terpenoids (sesquiterpenoids) | |||||||
| 34 | Vulgarone B |  | 218.3 | S. aureus CCARM0027 | 0.5 [44] | A. iwayomogi [44] | |
| S. aureus CCARM3511 & CCARM3523, S. typhimurium KCCM11862 | 1 [44] | ||||||
| S. typhimurium | 2 [44] | ||||||
| S. enteritidis KCCM12201, CCARM8010 & CCARM8011, S. typhimurium CCARM 8007 | >2 [44] | ||||||
| 35 | (+)-(S)-ar-Turmerone | 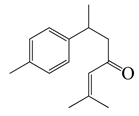 | 217.3 | B. cereus | 100 [39] | A. integrifolia [39] | |
| E. coli | 120 [39] | ||||||
| S. aureus | 150 [39] | ||||||
| Y. enterocolitica | 180 [39] | ||||||
| 36 | (+)-(S)-Dihydro-ar-turmerone | 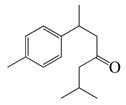 | 219.4 | B. cereus, Y. enterocolitica | 120 [39] | A. integrifolia [39] | |
| E. coli | 140 [39] | ||||||
| S. aureus | 160 [39] | ||||||
| 37 | Zerumbone | 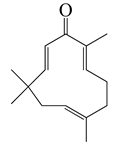 | 218.3 | E. coli | 70 [39] | A. integrifolia [39] | |
| B. cereus | 90 [39] | ||||||
| S. aureus | 110 [39] | ||||||
| Y. enterocolitica | 230 [39] | ||||||
| 38 | Dehydroleucodine |  | 244 | H. pylori HP786cs | 1 [45] | A. douglasiana [45] | |
| H. pylorics, NCTC 11,638 & H796 | 2 [45] | ||||||
| H. pylorics HP781 & HP795 | 4 [45] | ||||||
| H. pylorics HP788 & HP789 | 8 [45] | ||||||
| 39 | 12α,4α-Dihydroxybishopsolicepolide |  | 320.3 | A. naeslundii, A. israelii | 0.5 [9] | A. afra [9] | |
| P. intermedia | 1 [9] | ||||||
| A. actinomycetemcomitans, P. gingivalis | >1 [9] | ||||||
| 40 | 1,3,8-Trihydroxyeudesm-4-en-7α,11βH-12,6α-olide | 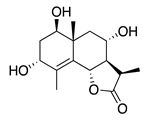 | 282 | B. subtilis | 25 µg/5 µL DMSO/disc [46] | A. herba-alba [46] | |
| S. aureus | 50 µg/5 µL DMSO/disc [46] | ||||||
| 41 | 3α,8β-Dihydroxygermacr-4(15),9(10)-dien-7β,11αH,12,6α-olide | 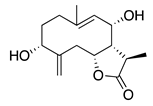 | 266.3 | B. subtilis, S. aureus | 100 µg/5 µL DMSO/disc [46] | A. herba-alba [46] | |
| E. coli | 50 µg/5 µL DMSO/disc [46] | ||||||
| 42 | 1β,8α-Dihydroxy-11α,13-dihydrobalchanin | 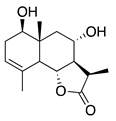 | 264.3 | S. aureus, B. subtilis | 100 µg/5 µL DMSO/disc [46] | A. herba-alba [46] | |
| E. coli | 50 µg/5 µL DMSO/disc [46] | ||||||
| 43 | 11-Epiartapshin | 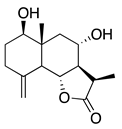 | 264.3 | S. aureus | 25 µg/5 µL DMSO/disc [46] | A. herba-alba [46] | |
| E. coli | 50 µg/5 µL DMSO/disc [46] | ||||||
| B. subtilis | 100 µg/5 µL DMSO/disc [46] | ||||||
| 44 | Artemisinin |  | 282 | S. aureus, B. subtilis, Salmonella sp. | 90 [47] | A. annua [47] | |
| 45 | Artesunate | 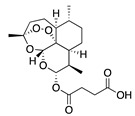 | 384.4 | MRSA | >4096 [48] | A. annua [49] | |
| Terpenoids (diterpenoids) | |||||||
| 46 | Phytol | 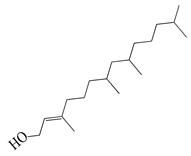 | 296.5 | A. israelii | 0.3 [9] | A. afra [9] | |
| A. naeslundii, P. intermedia | 1 [9] | ||||||
| A. actinomycetemcomitans | >1 [9] | ||||||
| Terpenoids (triterpenoids) | |||||||
| 47 | α-Amyrin | 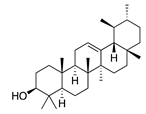 | 426.7 | A. naeslundii | 1 [9] | A. afra [9] | |
| A. israelii, A. actinomycetemcomitans, P. intermedia, P. gingivalis | >1 [9] | ||||||
| 48 | Betulinic acid | 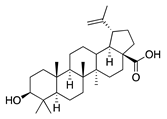 | 456.7 | A. naeslundii | 0.3 [9] | A. afra [9] | |
| A. israelii, P. intermedia, P. gingivalis | 1 [9] | ||||||
| A. actinomycetemcomitans | >1 [9] | ||||||
| 49 | Ursolic acid | 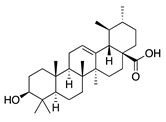 | 456.7 | S. aureus ATCC6538 | 32 [50] | A. annua [49] | |
| E. coli ATCC25922, K. pneumoniae, S. flexneri | 64 [50] | ||||||
| E. coli ATCC27, P. aeruginosa | 512 [50] | ||||||
| S. aureus ATCC12692 & ATCC12624, V. colareae, L. monocytogenes, B. cereus, A. caveae | ≥1024 [50] | ||||||
1.3.2. Polyphenols
| SN a | Name | Structure | MW | Pathogen | MIC (µg/mL) | Plant | ||
|---|---|---|---|---|---|---|---|---|
| Flavonoids | ||||||||
| 50 | Acacetin | 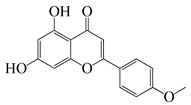 | 284.3 | A. israelii | 0.3 [9] | A. afra [9] | ||
| A. naeslundii, P. intermedia, P. gingivalis | 1 [9] | |||||||
| A. actinomycetemcomitans | >1 [9] | |||||||
| 51 | Casticin | 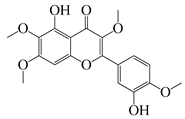 | 374.3 | C. perfringens 200302-1-1-Ba | 800 [10] | A. annua [10] | ||
| 52 | Chrysosplenetin | 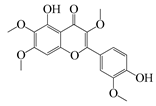 | 374.3 | S. aureus | - | A. rupestris [51] | ||
| 53 | Chrysoeriol | 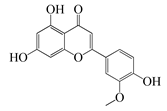 | 300.2 | S. aureus | - | A. rupestris [51] | ||
| 54 | Chrysosplenol B | 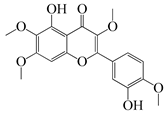 | 374.3 | E. coli WT, E. coli ΔtolC, E. coli ΔtolC (fabl) | >37.4 [52] | A. californica [52] | ||
| 55 | Chrysosplenol D |  | 360.3 | C. perfringens 200302-1-1-Ba | 200–400 [10] | A. annua [10] | ||
| B. subtilis, E. coli, P. fluorescens, and M. tetragenus | 250–500 [10] | |||||||
| 56 | Penduletin | 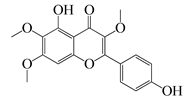 | 344.3 | S. aureus | - | A. rupestris [51] | ||
| 57 | Artemetin | 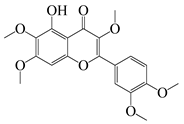 | 388.4 | MRSA | - | A. rupestris [51] | ||
| 58 | Pachypodol |  | 344.3 | MRSA | - | A. rupestris [51] | ||
| 59 | Jaceosidin | 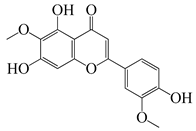 | 330 | E. coli ΔtolC, E. coli ΔtolC (fabl) | 3.3 [52] | A. californica [52], A. argyi [53] | ||
| E. coli WT | >33 [52] | |||||||
| 60 | Jaceidin |  | 360 | E. coli ΔtolC, E. coli ΔtolC (fabl) | 18 [52] | A. californica [52] | ||
| E. coli WT | >36 [52] | |||||||
| Coumarins | ||||||||
| 61 | Scopoletin | 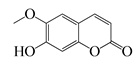 | 192.2 | A. israelii | 0.3 [9] | A. afra [9] | ||
| P. intermedia | 0.5 [9] | |||||||
| A. naeslundii | 1 [9] | |||||||
| A. actinomycetemcomitans, P. gingivalis | >1 [9] | |||||||
| 62 | 5-β-D-Glucopyranosyloxy-7-methoxy-6H-benzopyran-2-one | 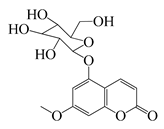 | 354 | B. subtilis | 25 µg/5 µL DMSO/disc [46] | A. herba-alba [46] | ||
| S. aureus, E. coli | 100 µg/5 µL DMSO/disc [46] | |||||||
1.3.3. Miscellaneous Group
| SN a | Name | Structure | MW | Pathogen | MIC (µg/mL) | Plant |
|---|---|---|---|---|---|---|
| 63 | 2,4-Dihydroxy-6-methoxyacetophenone |  | 182.2 | C. perfringens 200302-1-1-Ba | 800 [10] | A. annua [10] |
| 64 | Capillin |  | 168.2 | S. pneumoniae, H. influenzae, E. colics | 64 [35] | A. capillaris [35] |
| K. pneumoniae | 72 [35] | |||||
| S. pyogenes | 98 [35] | |||||
| MRSAcs | 112 [35] | |||||
| MRSA, MGRSA | 156 [35] | |||||
| 65 | Benzoic acid p-(β-D-glucopyranosyloxy)-methyl ester | 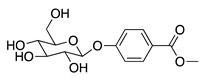 | 312.3 | B. subtilis | 50 µg/5 µL DMSO/disc [46] | A. herba-alba [46] |
| S. aureus | 25 µg/5 µL DMSO/disc [46] | |||||
| 66 | Diisooctyl phthalate | 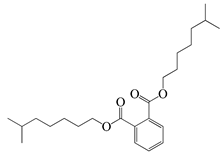 | 390.6 | S. enterica | 1.56 [6] | A. indica [6] |
| E. coli | 200 [6] | |||||
| L. monocytogenes | 30,000 [6] | |||||
| 67 | Integracid |  | 221 | S. aureus | 60 [54] | A. integrifolia [39] |
| B. cereus | 80 [39] | |||||
| E. coli | 100 [39] | |||||
| Y. enterocolitica | 120 [39] | |||||
| 68 | (Z)-2-(Hexa-2,4-diyn-1-ylidene)-1,6-dioxaspiro [4.5]dec-3-ene | 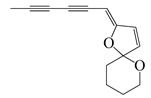 | 124.3 | S. aureus | 4.7 [55] | A. pallens [55] |
| B. subtilis | 7.8 [55] | |||||
| E. coli | 8.4 [55] | |||||
| P. aeruginosa | 10 [55] | |||||
| 69 | (E)-2-(Hexa-2,4-diyn-1-ylidene)-1,6-dioxaspiro [4.5]dec-3-ene | 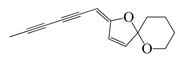 | 124.3 | S. aureus | 1.6 [55] | A. pallens [55] |
| E. coli | 2.1 [55] | |||||
| B. subtilis | 2.4 [55] | |||||
| P. aeruginosa | 2.7 [55] | |||||
| 70 | Ponticaepoxide | 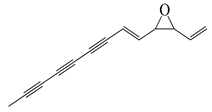 | 182.2 | C. perfringens 200302-1-1-Ba | 100–200 [10] | A. annua [10] |
| 71 | (+)-threo-(5E)-Trideca-1,5-dien-7,9,11-triyne-3,4-diol |  | 200.3 | C. perfringens 200302-1-1-Ba | 400–800 [10] | A. annua [10] |
| 72 | Methyl linolenate | 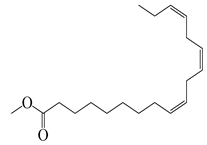 | 292.5 | S. enterica | 0.19 [6] | A. indica [6] |
| E. coli | 25 [6] | |||||
| L. monocytogenes | 6400 [6] | |||||
| 73 | Estragole |  | 148.2 | S. aureus 1199 | 101.6 [56] | A. annua [10] |
| S. aureus 1199B | 128 [56] | |||||
| 74 | Rosmarinic acid (RA) |  | 360 | S. aureus, E. coli | 500 [57] | A. absinthium, A. annua, A. alba, etc. [58] |
| S. aureus | 800 [59] | |||||
| MRSAcs | 10,000 [59] | |||||
| 75 | Eugenol |  | 164.2 | carbapenem-resistant Klebsiella pneumoniae (CRKP) | 200 [60] | A. annua [10] |
2. Anti-Bacterial Properties of the Artemisia Plants
3. Mechanisms of Action of Artemisia Plants and Their Compounds
3.1. Targeting Cell Membrane/Cell Wall
3.2. Targeting DNA
3.3. Target Protein and Enzymes
3.4. Others
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A. Tackling Antimicrobial Resistance in the Shadow of COVID-19. mBio 2021, 12, e00473-21. [Google Scholar] [CrossRef]
- Ukuhor, H.O. The interrelationships between antimicrobial resistance, COVID-19, past, and future pandemics. J. Infect. Public Health 2021, 14, 53–60. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Apaya, M.K.; Kuo, T.-F.; Yang, M.-T.; Yang, G.; Hsiao, C.-L.; Chang, S.-B.; Lin, Y.; Yang, W.-C. Phytochemicals as modulators of β-cells and immunity for the therapy of type 1 diabetes: Recent discoveries in pharmacological mechanisms and clinical potential. Pharmacol. Res. 2020, 156, 104754. [Google Scholar] [CrossRef]
- Dictionary of Natural Products 31.2. CRC Press, Taylor & Francis Group. 2023. Available online: https://dnp.chemnetbase.com/chemical/ChemicalSearch.xhtml?dswid=-72 (accessed on 10 March 2023).
- Yang, M.-T.; Kuo, T.-F.; Chung, K.-F.; Liang, Y.-C.; Yang, C.-W.; Lin, C.-Y.; Feng, C.-S.; Chen, Z.-W.; Lee, T.-H.; Hsiao, C.-L. Authentication, phytochemical characterization and anti-bacterial activity of two Artemisia species. Food Chem. 2020, 333, 127458. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, S.; Liu, L.; Toshmatov, Z.; Huang, L.; Shi, K.; Zhang, C.; Shao, H. Evaluation of the phytotoxic effect of the essential oil from Artemisia absinthium. Ecotoxicol. Environ. Saf. 2021, 226, 112856. [Google Scholar] [CrossRef]
- Shi, X.-S.; Song, Y.-P.; Meng, L.-H.; Yang, S.-Q.; Wang, D.-J.; Zhou, X.-W.; Ji, N.-Y.; Wang, B.-G.; Li, X.-M. Isolation and Characterization of antibacterial carotane sesquiterpenes from Artemisia argyi associated endophytic Trichoderma virens QA-8. Antibiotics 2021, 10, 213. [Google Scholar] [CrossRef]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial constituents of Artemisia afra Jacq. ex Willd. against periodontal pathogens. Evid.-Based Complement. Altern. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef]
- Ivarsen, E.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Engberg, R.M.; Grevsen, K.; Kjaer, A. Bioassay-guided chromatographic isolation and identification of antibacterial compounds from Artemisia annua L. that inhibit Clostridium perfringens growth. J. AOAC Int. 2014, 97, 1282–1290. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary metabolites from Artemisia genus as biopesticides and innovative nano-based application strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-D.; Jung, E.-K.; Kil, B.-S.; Lee, K.-Y. Chemical composition and antibacterial activity of essential oil from Artemisia feddei. J. Microbiol. Biotechnol. 2007, 17, 2061–2065. [Google Scholar] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B., Jr.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef]
- Gou, Y.; Zhang, F.; Tang, Y.; Jiang, C.; Bai, G.; Xie, H.; Chen, M.; Liao, Z. Engineering nootkatone biosynthesis in Artemisia annua. ACS Synth. Biol. 2021, 10, 957–963. [Google Scholar] [CrossRef]
- Muto, T.; Watanabe, T.; Okamura, M.; Moto, M.; Kashida, Y.; Mitsumori, K. Thirteen-week repeated dose toxicity study of wormwood (Artemisia absinthium) extract in rats. J. Toxicol. Sci. 2003, 28, 471–478. [Google Scholar] [CrossRef]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.; Seca, A.M. Research advances on health effects of edible Artemisia species and some sesquiterpene lactones constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef]
- Xia, M.; Liu, D.; Liu, Y.; Liu, H. The therapeutic effect of artemisinin and its derivatives in kidney disease. Front. Pharmacol. 2020, 11, 380. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, genome evolution, biotechnological issues and research including applied perspectives in Artemisia (Asteraceae). Adv. Bot. Res. 2011, 60, 349–419. [Google Scholar]
- Mathlouthi, A.; Saadaoui, N.; Ben-Attia, M. Essential oils from Artemisia species inhibit biofilm formation and the virulence of Escherichia coli EPEC 2348/69. Biofouling 2021, 37, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-T.; Tan, J.; Tan, J.; Li, R. Chemical components and molecular microcapsules of Folium Artemisia argyi essential oil with β-cyclodextrin derivatives. J. Essent. Oil Bear. Plants 2016, 19, 1155–1169. [Google Scholar] [CrossRef]
- Guan, X.; Ge, D.; Li, S.; Huang, K.; Liu, J.; Li, F. Chemical composition and antimicrobial activities of Artemisia argyi Lévl. et Vant essential oils extracted by simultaneous distillation-extraction, subcritical extraction and hydrodistillation. Molecules 2019, 24, 483. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Garjonyte, R. Compositional variability and toxic activity of Mugwort (Artemisia vulgaris) essential oils. Nat. Prod. Commun. 2016, 11, 1353–1356. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The genus Artemisia: A 2012–2017 literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Altaf-Ul-Amin, M.; Darusman, L.K. KNApSAcK family databases: Integrated metabolite–plant species databases for multifaceted plant research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef]
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical composition of essential oils. In Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 119–171. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antigermination activity of Artemisia dracunculus (Tarragon). Nat. Prod. Commun. 2015, 10, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, D.-H.; Feng, Y. Antibacterial activity and mode of action of the Artemisia capillaris essential oil and its constituents against respiratory tract infection-causing pathogens. Mol. Med. Rep. 2015, 11, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H.; Kim, Y.-H.; Kil, B.-S.; Kim, K.-J.; Jeong, S.-I.; You, Y.-O. Chemical composition and antibacterial activity of essential oil of Artemisia iwayomogi. Planta Med. 2003, 69, 1159–1162. [Google Scholar]
- Yang, C.; Hu, D.H.; Feng, Y. Essential oil of Artemisia vestita exhibits potent in vitro and in vivo antibacterial activity: Investigation of the effect of oil on biofilm formation, leakage of potassium ions and survival curve measurement. Mol. Med. Rep. 2015, 12, 5762–5770. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Xu, Y.-H.; Bao, W.-Q.; Hao, J.-S. Structure elucidation and antimicrobial activities of five compounds from Artemisia integrifolia L. Z. Für Nat. C 2019, 74, 275–278. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Trinh, H.-T.; Lee, I.-A.; Hyun, Y.-J.; Kim, D.-H. Artemisia princeps Pamp. Essential oil and its constituents eucalyptol and α-terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF-κB activation. Planta Med. 2011, 77, 1996–2002. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N. Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. Int. J. Biol. Macromol. 2019, 128, 893–901. [Google Scholar] [CrossRef]
- Tsiri, D.; Graikou, K.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Spyropoulos, C.; Chinou, I. Chemosystematic value of the essential oil composition of Thuja species cultivated in Poland—Antimicrobial activity. Molecules 2009, 14, 4707–4715. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Byun, Y.H.; Shin, E.J.; Chung, H.S.; Lee, Y.H.; Shin, S. Antibacterial effects of vulgarone B from Artemisia iwayomogi alone and in combination with oxacillin. Arch. Pharmacal Res. 2009, 32, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Wendel, G.; Maria, A.; Pelzer, L. Antimicrobial activity of Artemisia douglasiana and dehydroleucodine against Helicobacter pylori. J. Ethnopharmacol. 2009, 124, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Abd El Aty, A.A.; Shahat, A.A.; Abdel-Azim, N.S.; Shams, K.A.; Elshamy, A.A.; Ahmed, M.M.; Youns, S.H.; El-Wassimy, T.M.; El-Toumy, S.A. New antimicrobial metabolites from the medicinal herb Artemisia herba-Alba. Nat. Prod. Res. 2021, 35, 1959–1967. [Google Scholar] [CrossRef]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.-K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed. Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef]
- Jiang, W.; Li, B.; Zheng, X.; Liu, X.; Pan, X.; Qing, R.; Cen, Y.; Zheng, J.; Zhou, H. Artesunate has its enhancement on antibacterial activity of β-lactams via increasing the antibiotic accumulation within methicillin-resistant Staphylococcus aureus (MRSA). J. Antibiot. 2013, 66, 339–345. [Google Scholar] [CrossRef]
- Yuliang, W.; Zejian, W.; Hanlin, S.; Ming, Y.; Kexuan, T. The hypolipidemic effect of artesunate and ursolic acid in rats. Pak. J. Pharm. Sci. 2015, 28, 871–874. [Google Scholar]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, Â.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Lan, J.-E.; Li, X.-J.; Zhu, X.-F.; Sun, Z.-L.; He, J.-M.; Zloh, M.; Gibbons, S.; Mu, Q. Flavonoids from Artemisia rupestris and their synergistic antibacterial effects on drug-resistant Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 1881–1886. [Google Scholar] [CrossRef]
- Allison, B.J.; Allenby, M.C.; Bryant, S.S.; Min, J.E.; Hieromnimon, M.; Joyner, P.M. Antibacterial activity of fractions from three Chumash medicinal plant extracts and in vitro inhibition of the enzyme enoyl reductase by the flavonoid jaceosidin. Nat. Prod. Res. 2017, 31, 707–712. [Google Scholar] [CrossRef]
- Zhang, J.J.; Qu, L.B.; Bi, Y.F.; Pan, C.X.; Yang, R.; Zeng, H.J. Antibacterial activity and mechanism of chloroform fraction from aqueous extract of mugwort leaves (Artemisia argyi L.) against Staphylococcus aureus. Lett. Appl. Microbiol. 2022, 74, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, S.R.; Bezerra, A.H.; de Sousa Silveira, Z.; Macedo, N.S.; dos Santos Barbosa, C.R.; Muniz, D.F.; Dos Santos, J.F.S.; Coutinho, H.D.M.; da Cunha, F.A.B. Antibacterial activity of eugenol on the IS-58 strain of Staphylococcus aureus resistant to tetracycline and toxicity in Drosophila melanogaster. Microb. Pathog. 2022, 164, 105456. [Google Scholar] [CrossRef] [PubMed]
- Honmore, V.S.; Natu, A.D.; Khedkar, V.M.; Arkile, M.A.; Sarkar, D.; Rojatkar, S.R. Two antibacterial spiro compounds from the roots of Artemisia pallens wall: Evidence from molecular docking. Nat. Prod. Res. 2022, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Muniz, D.F.; dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.M.; da Costa, R.H.; de Morais Oliveira-Tintino, C.D. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Gohari, A.R.; Saeidnia, S.; Shahverdi, A.R.; Yassa, N.; Malmir, M.; Mollazade, K.; Naghinejad, A.R. Phytochemistry and antimicrobial compounds of Hymenocrater calycinus. EurAsian J. BioSci. 2009, 3, 64–68. [Google Scholar] [CrossRef]
- Stanković, M.; Ickovski, J.D.; Ljupković, R.B.; Stojanović, G.S. The effects of Artemisia methanol extracts and ferulic acid, rutin, rosmarinic acid, and quercetin on micronucleus distribution on human lymphocytes. Nat. Prod. Res. 2022, 36, 4530–4533. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2020, 139, 103924. [Google Scholar] [CrossRef]
- Adamu, M.; Naidoo, V.; Eloff, J.N.J.B.C.; Medicine, A. Some southern African plant species used to treat helminth infections in ethnoveterinary medicine have excellent antifungal activities. BMC Complement. Altern. Med. 2012, 12, 213. [Google Scholar] [CrossRef]
- Poiată, A.; Tuchiluş, C.; Ivănescu, B.; Ionescu, A.; Lazăr, M. Antibacterial activity of some Artemisia species extract. Rev. Med.-Chir. Soc. Med. Nat. IASI 2009, 113, 911–914. [Google Scholar]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Engberg, R.M.; Grevsen, K.; Ivarsen, E.; Fretté, X.; Christensen, L.P.; Højberg, O.; Jensen, B.B.; Canibe, N. The effect of Artemisia annua on broiler performance, on intestinal microbiota and on the course of a Clostridium perfringens infection applying a necrotic enteritis disease model. Avian Pathol. 2012, 41, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Essential oils from the Asteraceae family active against multidrug-resistant bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Elsevier: San Diego, CA, USA, 2013; pp. 205–221. [Google Scholar]
- Sha’Afi, R.; Gary-Bobo, C.; Solomon, A. Permeability of red cell membranes to small hydrophilic and lipophilic solutes. J. Gen. Physiol. 1971, 58, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vörös-Horváth, B.; Bencsik, T.; Micalizzi, G.; Mondello, L.; Horváth, G.; Kőszegi, T.; Széchenyi, A. Antimicrobial activity of different Artemisia essential oil formulations. Molecules 2020, 25, 2390. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellvi, S.; Pagán, R.; Garcia-Gonzalo, D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C.-I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.-P.; Bégin, A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37, 155–162. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Hu, D.-P.; Gu, Y.-Z.; Xu, H.-X.; Li, X.-Q. Antibacterial and membrane-damaging activities of rosmarinic acid against pathogenic organisms of acne. Lat. Am. J. Pharm. 2015, 34, 1866–1870. [Google Scholar]
- Shahidi, F.; Naczk, M. Nutritional and Pharmacological Effects of Food Phenolics. In Food Phenolics: Sources, Chemistry, Effects, Applications, 1st ed.; Technomic Pub. Co.: Lancaster, PA, USA, 1995; pp. 171–191. [Google Scholar]
- Melkina, O.E.; Plyuta, V.A.; Khmel, I.A.; Zavilgelsky, G.B. The mode of action of cyclic monoterpenes (−)-limonene and (+)-α-pinene on bacterial cells. Biomolecules 2021, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Forgac, M. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002, 3, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Mamone, G.; Ferranti, P.; Ercolini, D.; Mauriello, G. Changes in the proteome of Salmonella enterica serovar Thompson as stress adaptation to sublethal concentrations of thymol. Proteomics 2010, 10, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-L.; Grinius, L.; Hooper, D.C. NorA functions as a multidrug efflux protein in both cytoplasmic membrane vesicles and reconstituted proteoliposomes. J. Bacteriol. 2002, 184, 1370–1377. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Is bacterial fatty acid synthesis a valid target for antibacterial drug discovery? Curr. Opin. Microbiol. 2011, 14, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, S. Recent advances in inhibitors of bacterial fatty acid synthesis type II (FASII) system enzymes as potential antibacterial agents. ChemMedChem 2013, 8, 1589–1608. [Google Scholar] [CrossRef] [PubMed]
- Reece, R.J.; Maxwell, A. DNA gyrase: Structure and function. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 335–375. [Google Scholar] [CrossRef]
- Raei, P.; Pourlak, T.; Memar, M.Y.; Alizadeh, N.; Aghamali, M.; Zeinalzadeh, E.; Asgharzadeh, M.; Kafil, H. Thymol and carvacrol strongly inhibit biofilm formation and growth of carbapenemase-producing Gram negative bacilli. Cell. Mol. Biol. 2017, 63, 108–112. [Google Scholar] [CrossRef]
- Jafri, H.; Ansari, F.A.; Ahmad, I. Prospects of essential oils in controlling pathogenic biofilm. In New Look to Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–236. [Google Scholar]
- da Costa, R.H.S.; Rocha, J.E.; de Freitas, T.S.; Pereira, R.L.S.; Junior, F.N.P.; de Oliveira, M.R.C.; Batista, F.L.A.; Coutinho, H.D.M.; de Menezes, I.R.A. Evaluation of antibacterial activity and reversal of the NorA and MepA efflux pump of estragole against Staphylococcus aureus bacteria. Arch. Microbiol. 2021, 203, 3551–3555. [Google Scholar] [CrossRef]
- Jiang, W.; Li, B.; Zheng, X.; Liu, X.; Cen, Y.; Li, J.; Pan, X.; Cao, H.; Zheng, J.; Zhou, H. Artesunate in combination with oxacillin protect sepsis model mice challenged with lethal live methicillin-resistant Staphylococcus aureus (MRSA) via its inhibition on proinflammatory cytokines release and enhancement on antibacterial activity of oxacillin. Int. Immunopharmacol. 2011, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Nik Mohamad Nek Rahimi, N.; Natrah, I.; Loh, J.-Y.; Ervin Ranzil, F.K.; Gina, M.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Phytocompounds as an Alternative Antimicrobial Approach in Aquaculture. Antibiotics 2022, 11, 469. [Google Scholar] [CrossRef]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems pharmacology for investigation of the mechanisms of action of traditional Chinese medicine in drug discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, isolation and characterization of bioactive compounds from Artemisia and their biological significance: A review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P. Artemisia annua, a traditional plant brought to light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]



| Kingdom | Plantae |
|---|---|
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Order | Asterales |
| Family | Asteraceae |
| Genus | Artemisia |
| Species | >500 species |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umam, K.; Feng, C.-S.; Yang, G.; Tu, P.-C.; Lin, C.-Y.; Yang, M.-T.; Kuo, T.-F.; Yang, W.-C.; Tran Nguyen Minh, H. Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants. Bioengineering 2023, 10, 633. https://doi.org/10.3390/bioengineering10060633
Umam K, Feng C-S, Yang G, Tu P-C, Lin C-Y, Yang M-T, Kuo T-F, Yang W-C, Tran Nguyen Minh H. Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants. Bioengineering. 2023; 10(6):633. https://doi.org/10.3390/bioengineering10060633
Chicago/Turabian StyleUmam, Khotibul, Ching-Shan Feng, Greta Yang, Ping-Chen Tu, Chih-Yu Lin, Meng-Ting Yang, Tien-Fen Kuo, Wen-Chin Yang, and Hieu Tran Nguyen Minh. 2023. "Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants" Bioengineering 10, no. 6: 633. https://doi.org/10.3390/bioengineering10060633
APA StyleUmam, K., Feng, C.-S., Yang, G., Tu, P.-C., Lin, C.-Y., Yang, M.-T., Kuo, T.-F., Yang, W.-C., & Tran Nguyen Minh, H. (2023). Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants. Bioengineering, 10(6), 633. https://doi.org/10.3390/bioengineering10060633









