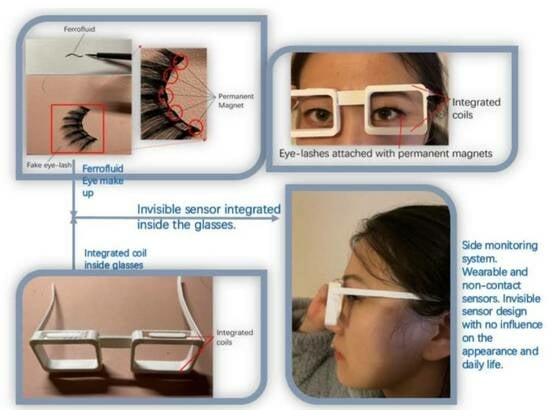
Wearable and Invisible Sensor Design for Eye-Motion Monitoring Based on Ferrofluid and Electromagnetic Sensing Technologies
Abstract
1. Introduction
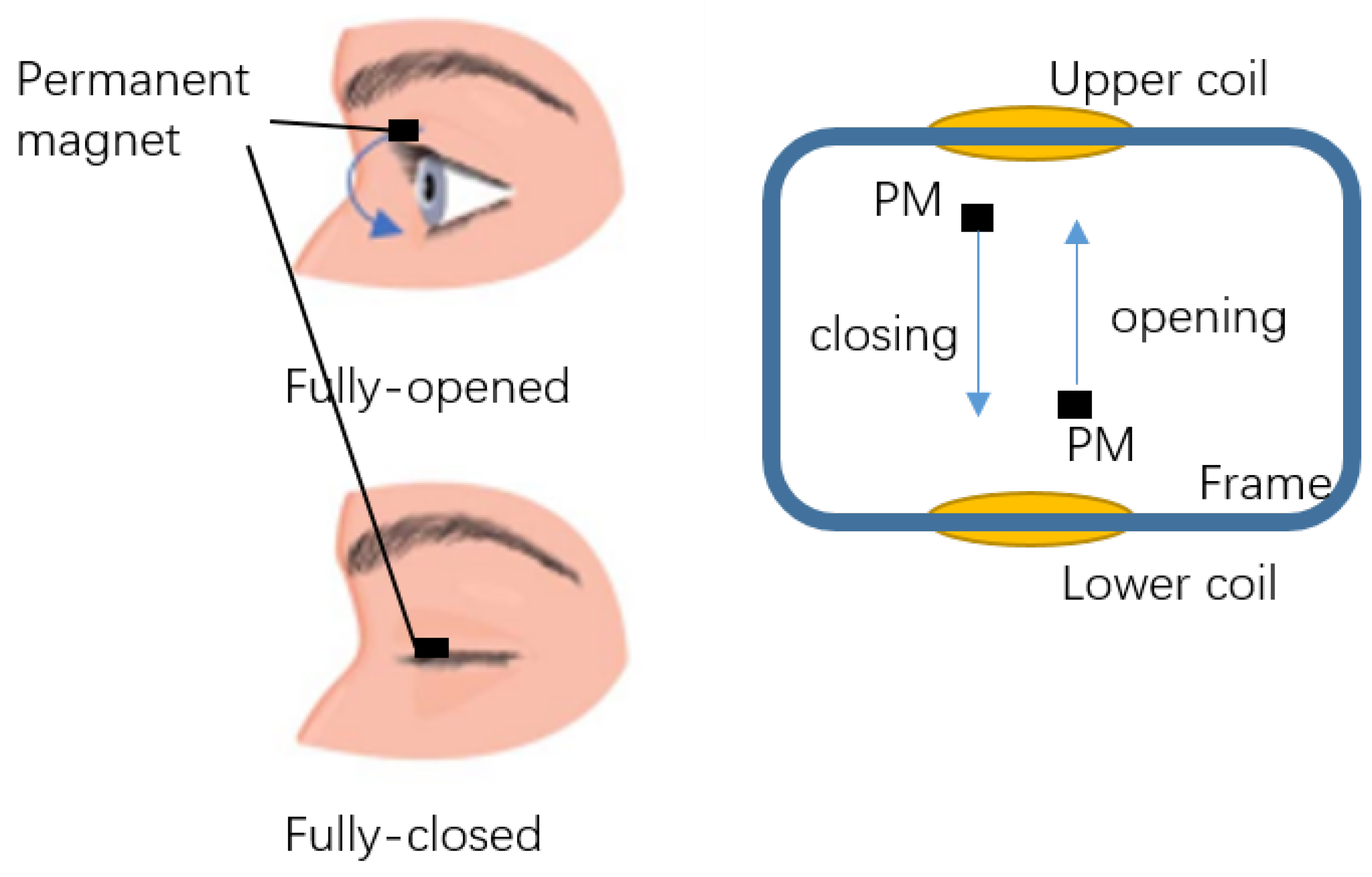
2. Model
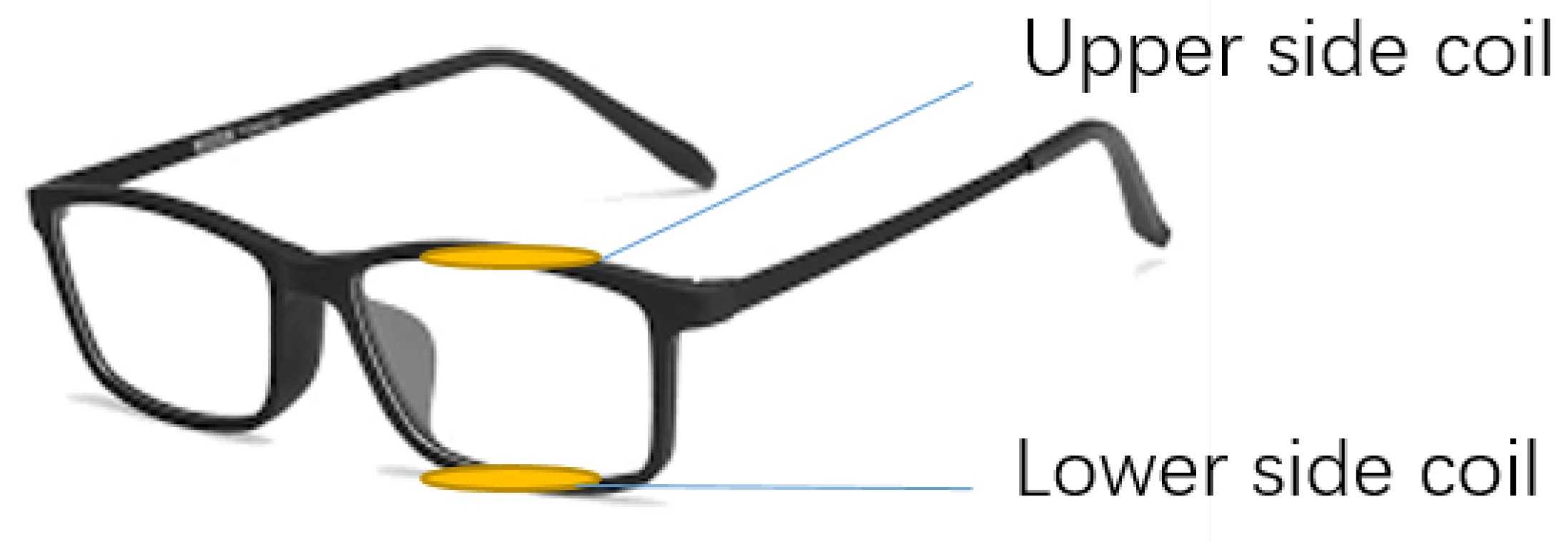
2.1. Design Model
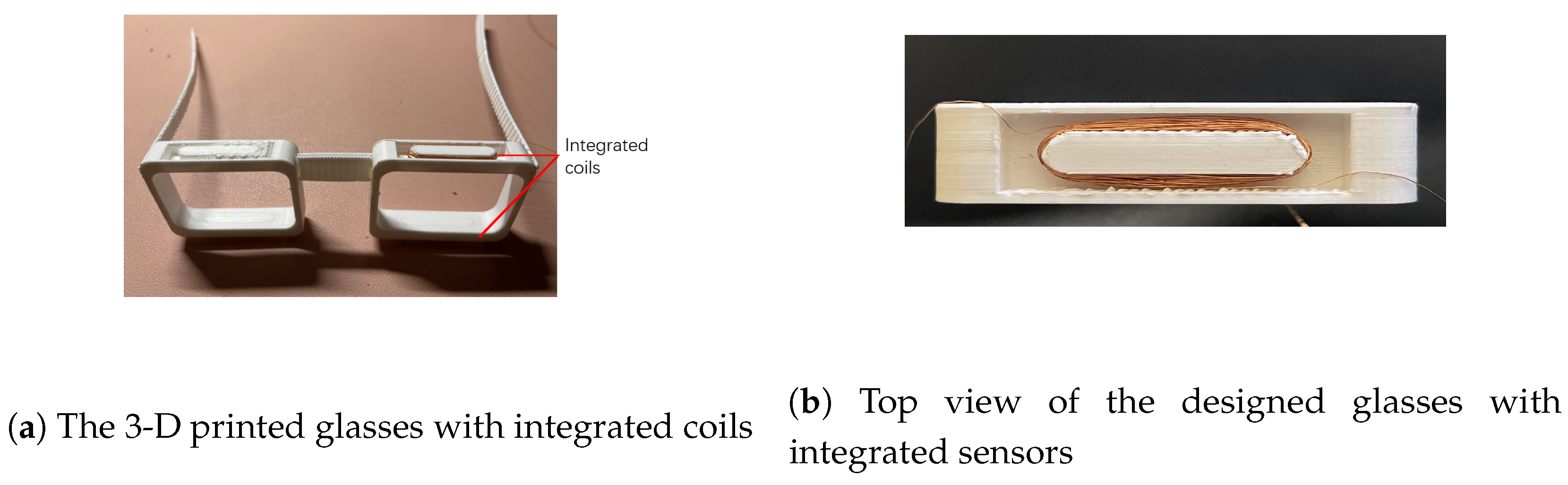
2.2. 3D Print Model
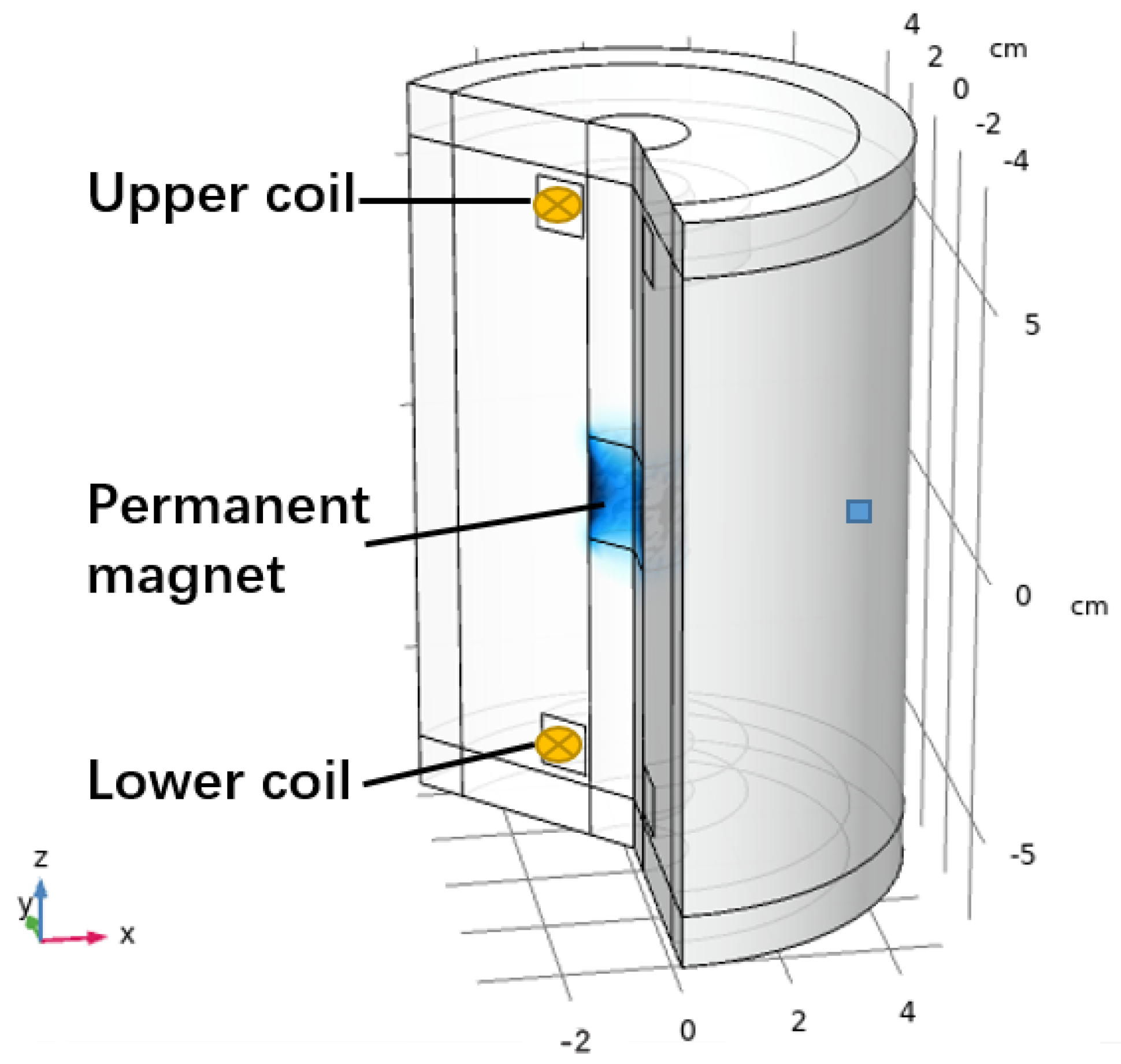
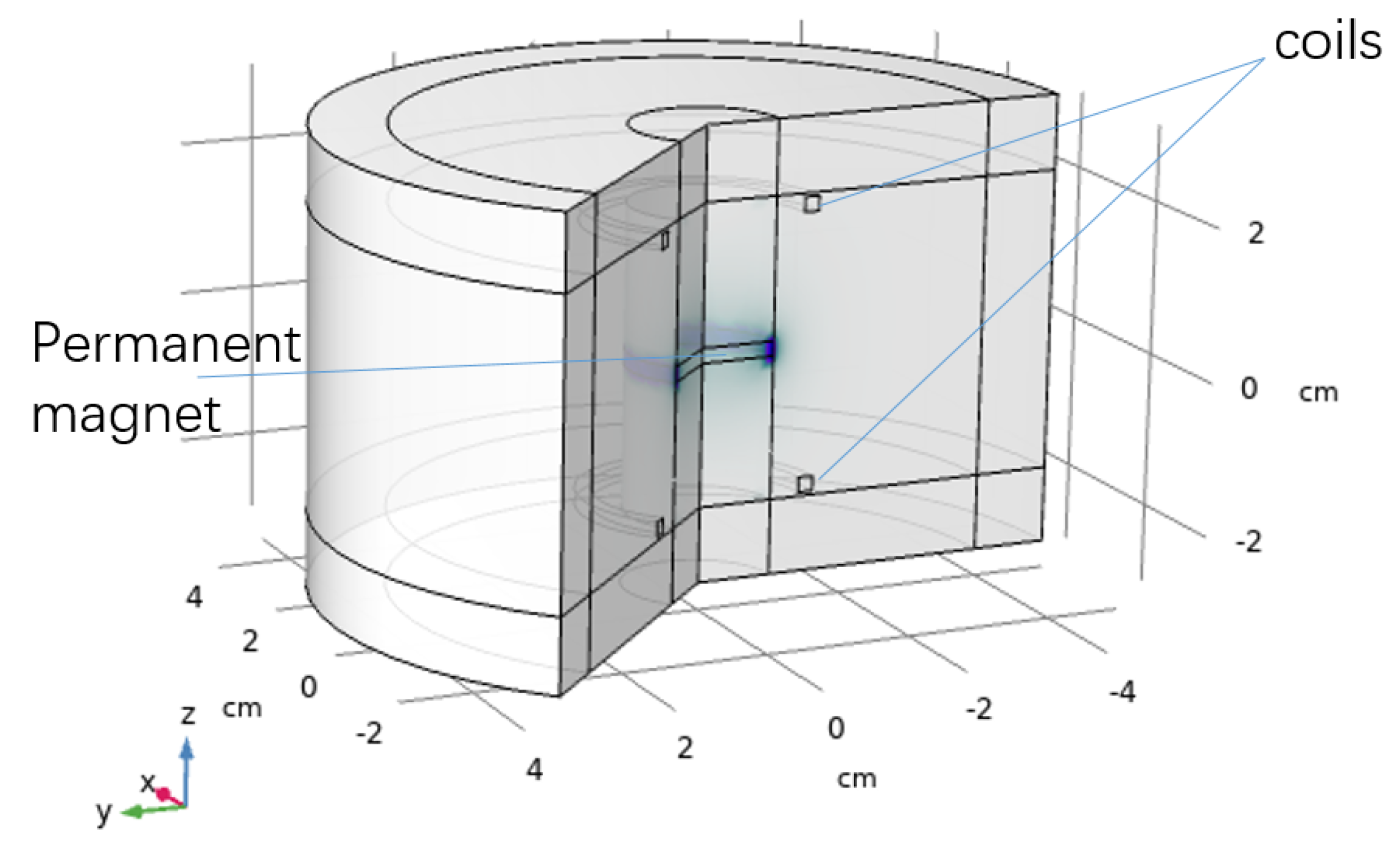
2.3. FEM Simulation Model
3. Method
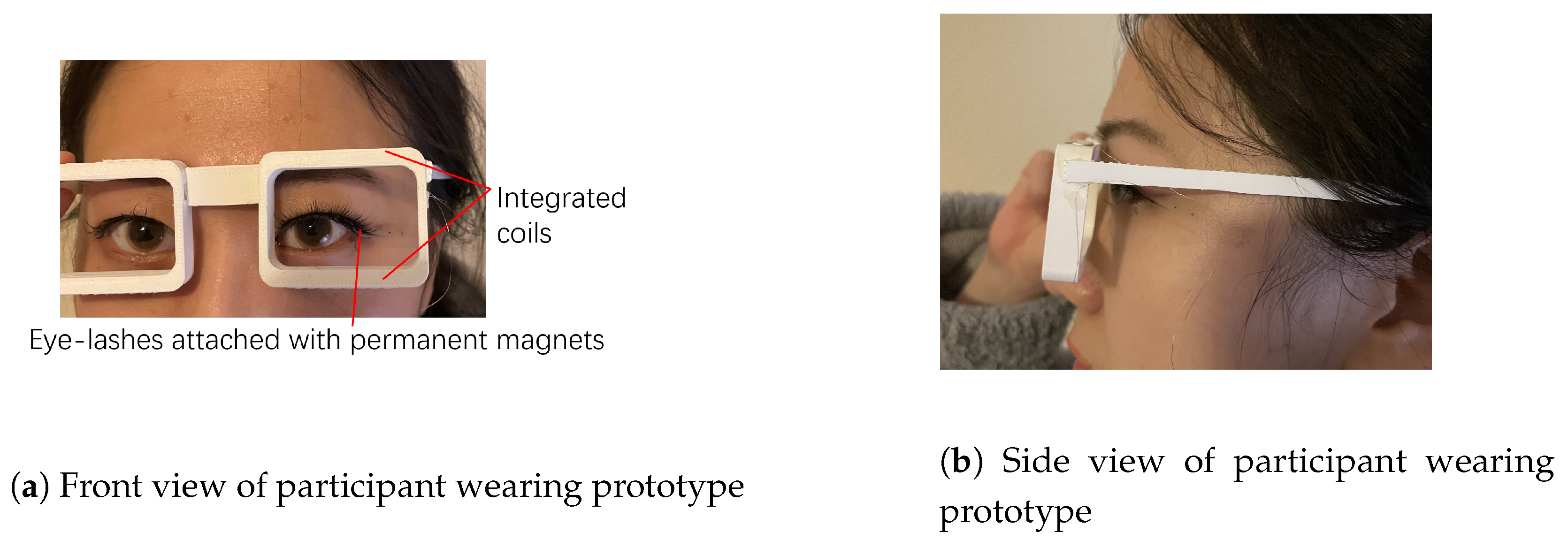
3.1. Experimental Set-Up
3.2. FEM Simulation
4. Result and Discussion
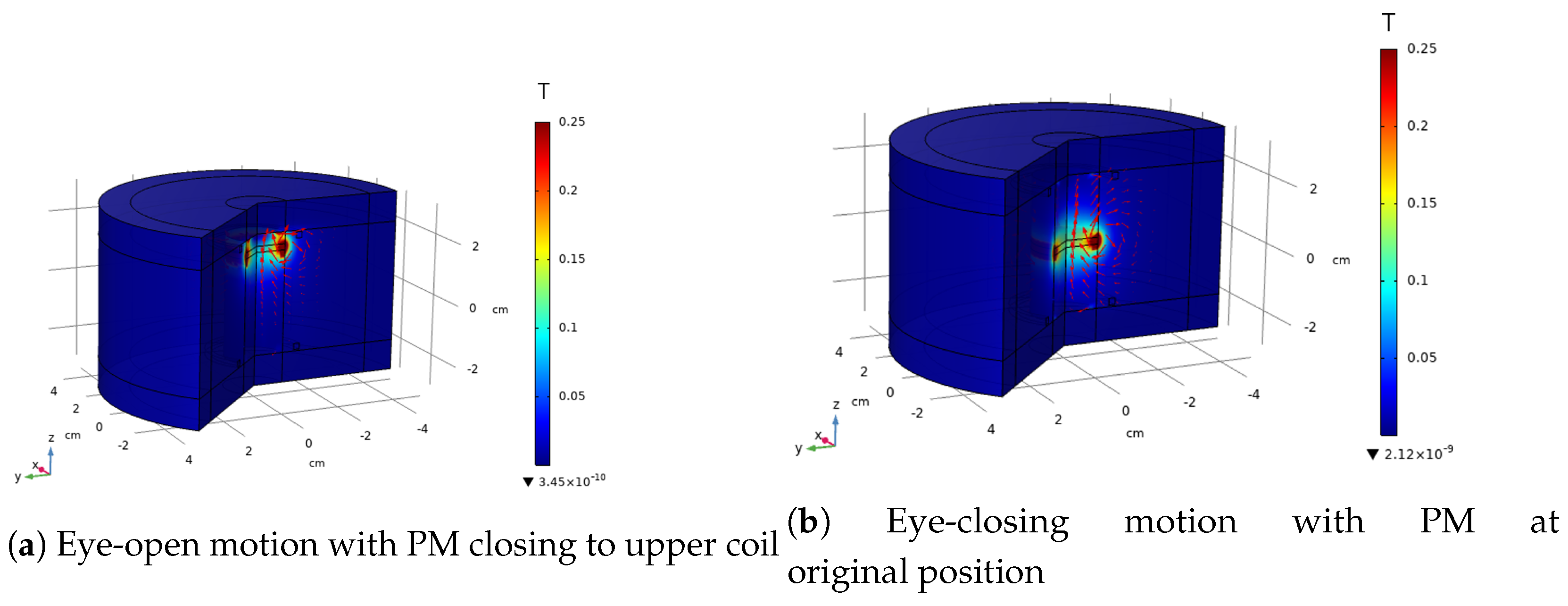
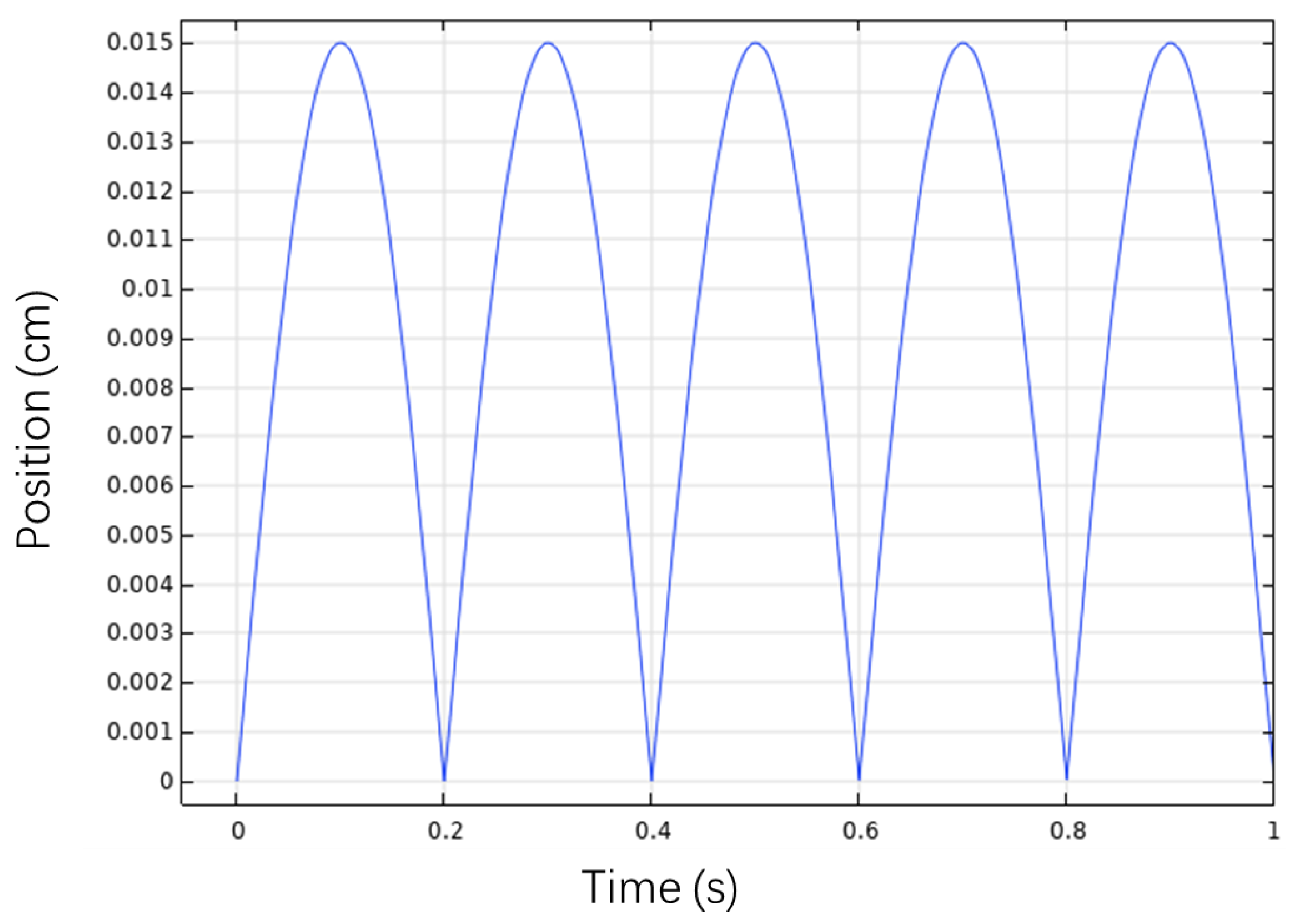
4.1. Simulation Results
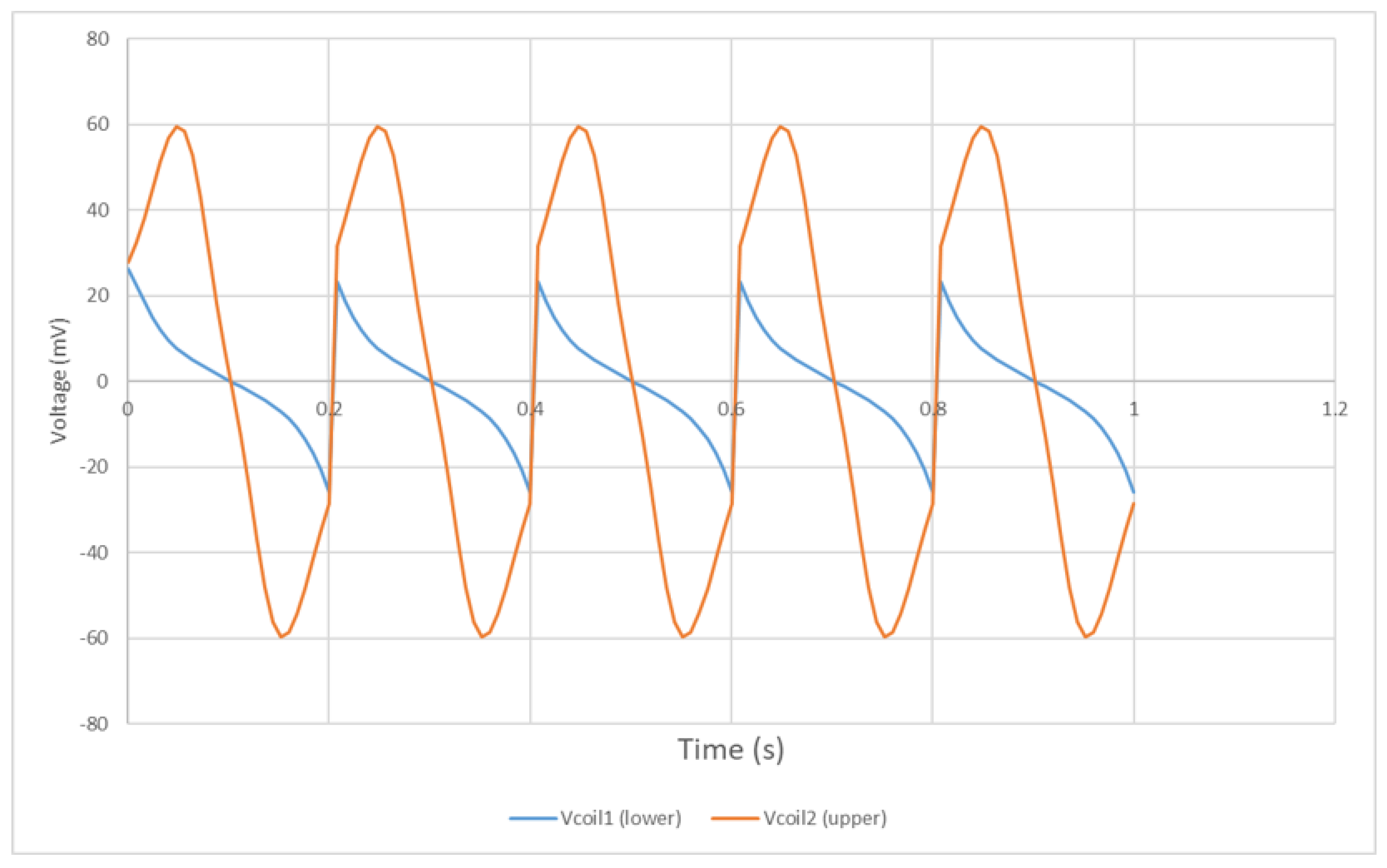
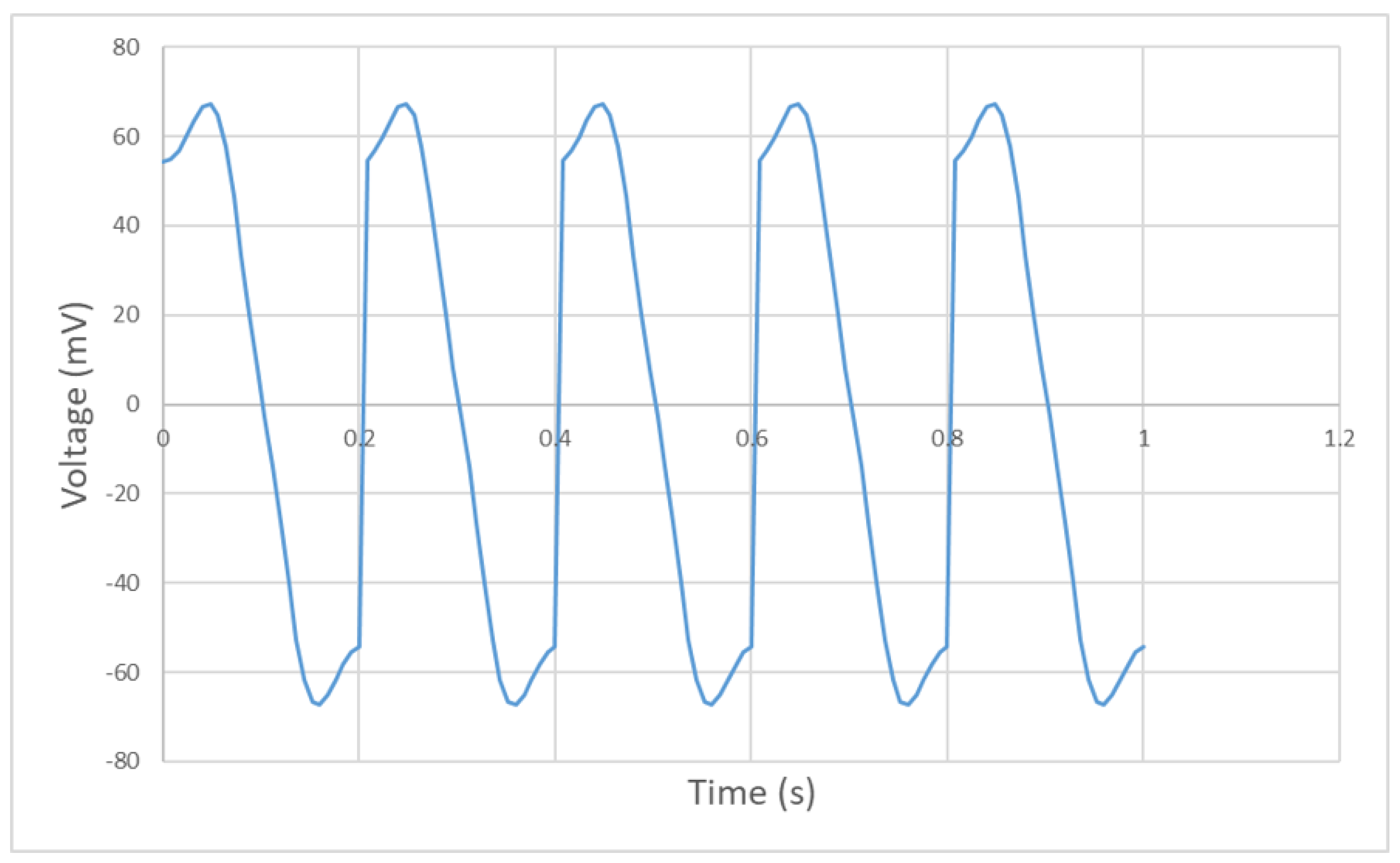
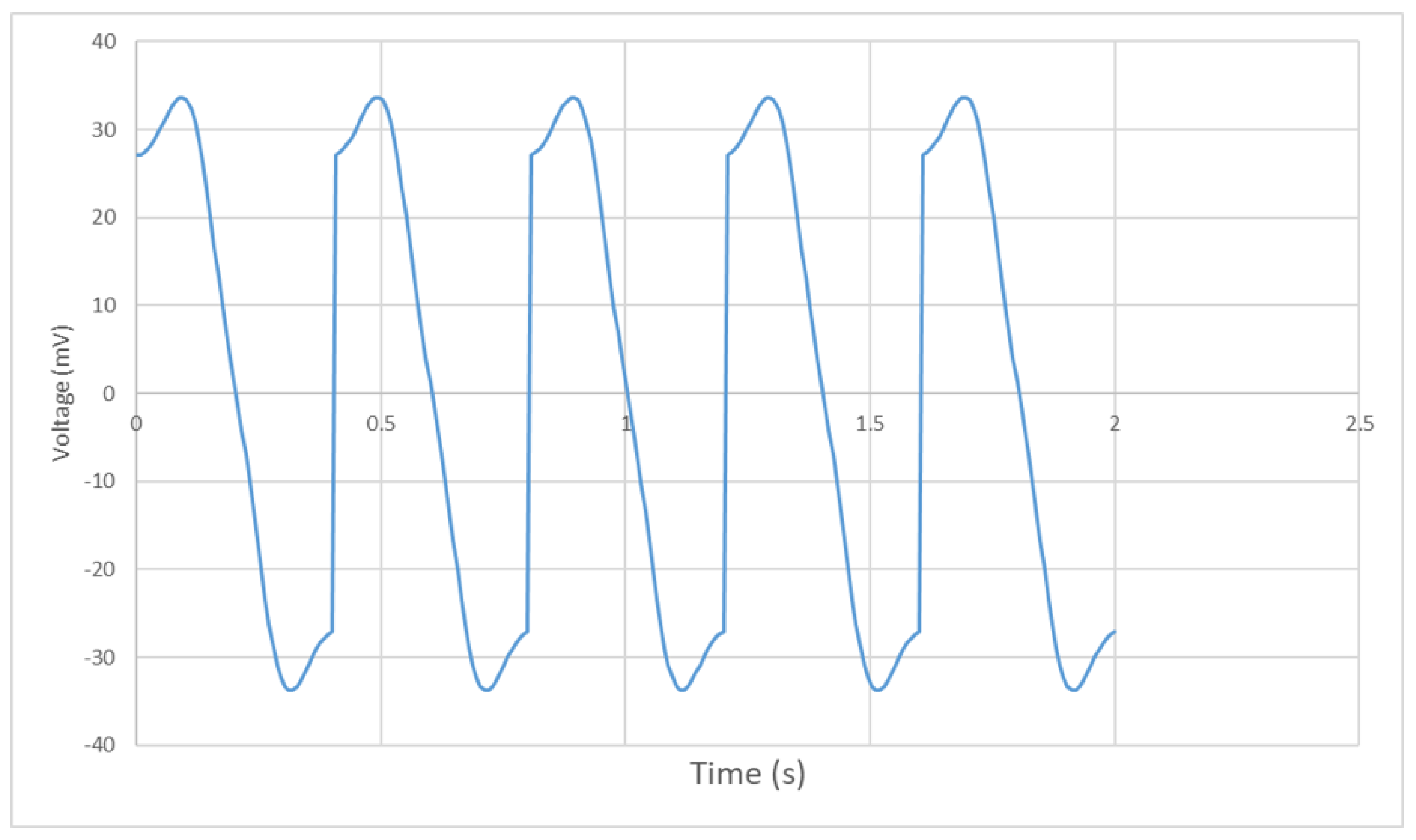
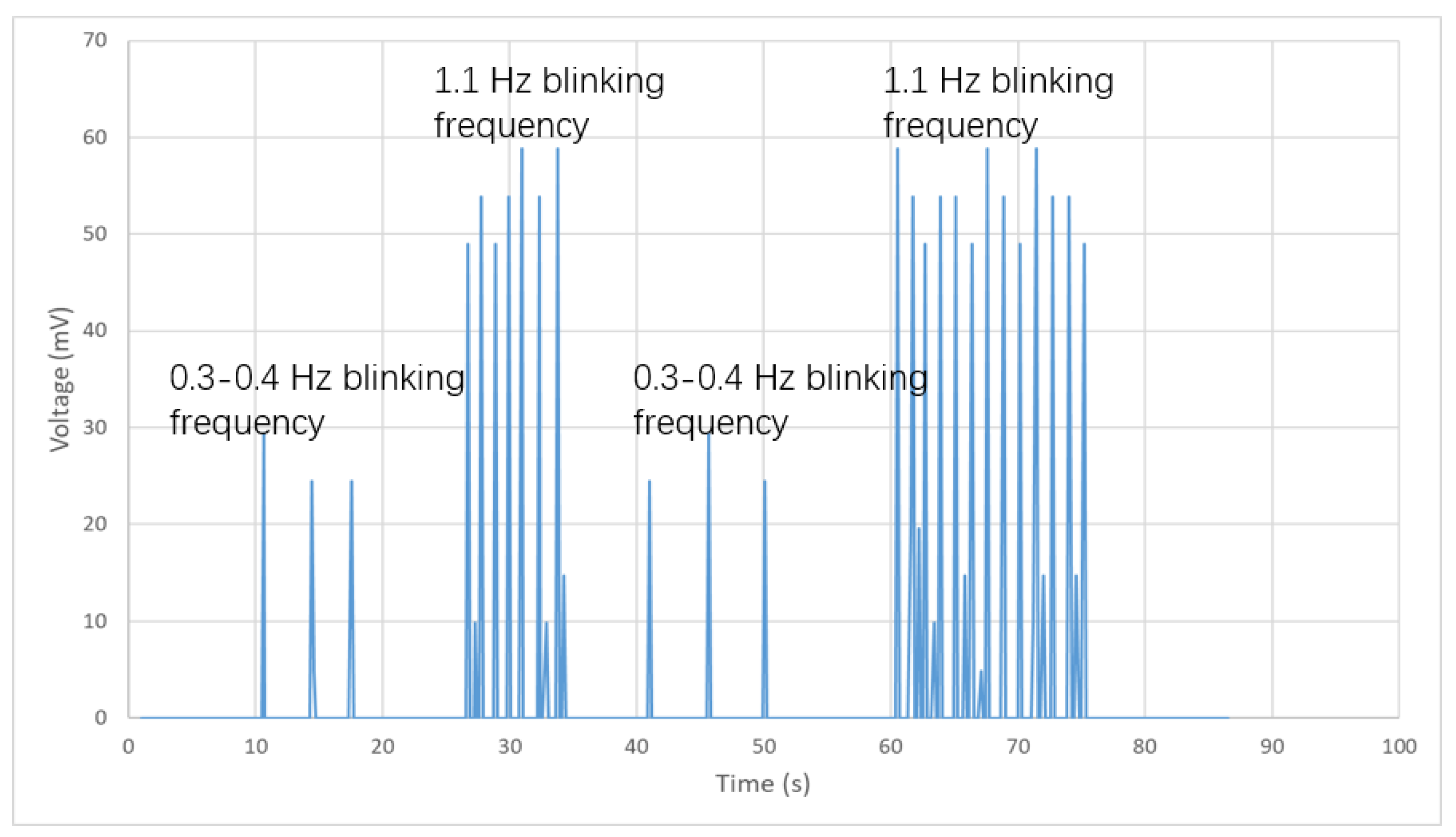
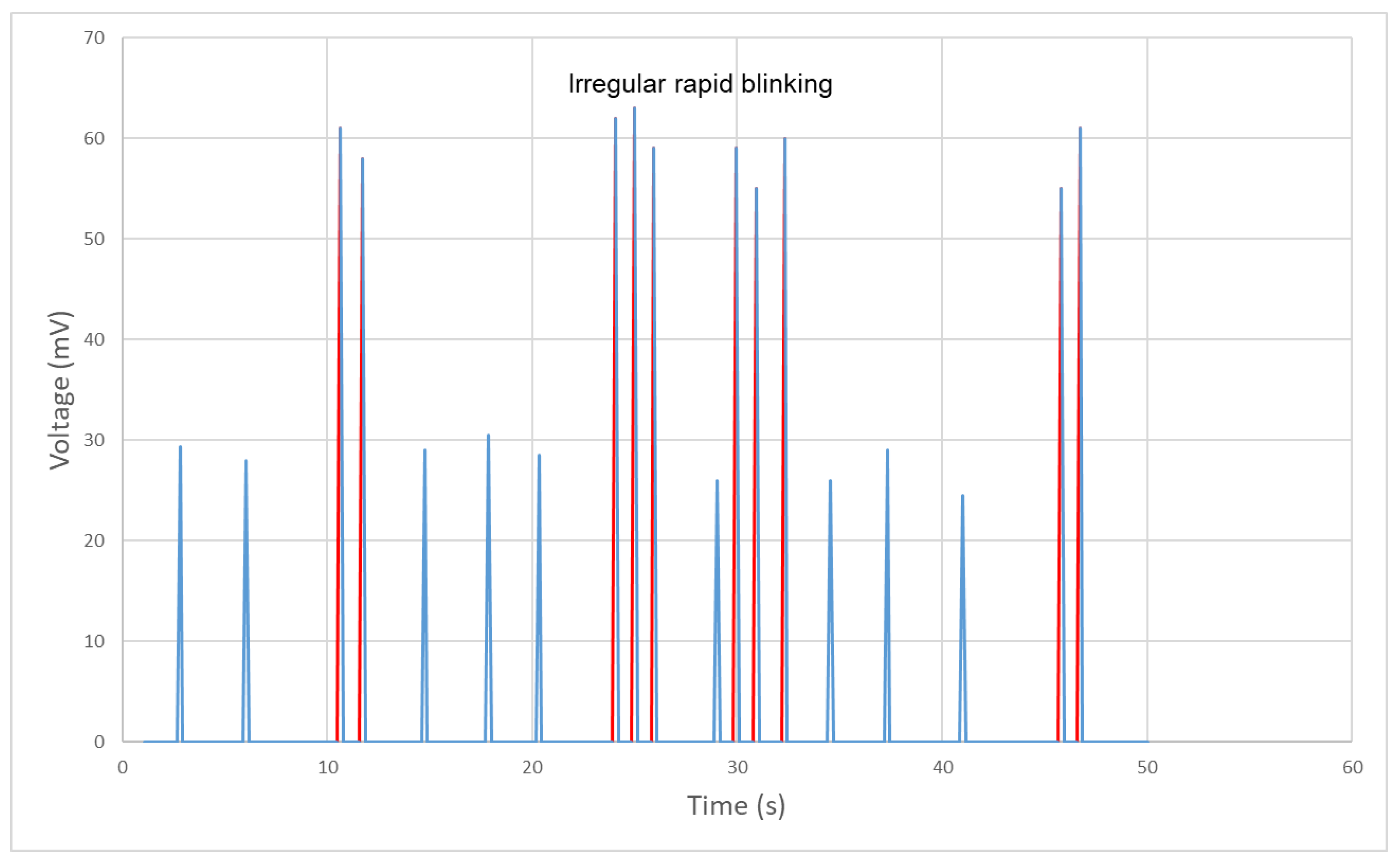
4.2. Experimental Results
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheeler, T.T.; McGorray, S.P.; Dolce, C.; Taylor, M.G.; King, G.J. Effectiveness of early treatment of Class II malocclusion. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Good, W.V.; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 2004, 102, 233. [Google Scholar] [PubMed]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003, 121, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Seftel, D.; Boulware, D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. In Proceedings of the Open Forum Infectious Diseases; Oxford University Press US: Oxford, UK, 2021; Volume 8, p. ofab050. [Google Scholar]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, W.Y.; Kim, Y.J.; Hong, Y.P. A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int. J. Environ. Res. Public Health 2021, 18, 2383. [Google Scholar] [CrossRef]
- Yoo, T.K.; Oh, E. Diabetes mellitus is associated with dry eye syndrome: A meta-analysis. Int. Ophthalmol. 2019, 39, 2611–2620. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Pflugfelder, S.C.; Ng, S.M.; Akpek, E.K. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst. Rev. 2019, 9, CD010051. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, W.; Yan, J.; Noma, N.; Young, A.; Yan, Z. Worldwide prevalence estimates of burning mouth syndrome: A systematic review and meta-analysis. Oral Dis. 2022, 28, 1431–1440. [Google Scholar] [CrossRef]
- Imamura, Y.; Shinozaki, T.; Okada-Ogawa, A.; Noma, N.; Shinoda, M.; Iwata, K.; Wada, A.; Abe, O.; Wang, K.; Svensson, P. An updated review on pathophysiology and management of burning mouth syndrome with endocrinological, psychological and neuropathic perspectives. J. Oral Rehabil. 2019, 46, 574–587. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.S.; Ko, I.; Kim, D.K. Association between burning mouth syndrome and the development of depression, anxiety, dementia, and Parkinson disease. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.; Respondek, G.; Giagkou, N.; Whitwell, J.L.; Kovacs, G.G.; Höglinger, G.U. Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat. Rev. Neurol. 2021, 17, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, D.G.; Litvan, I. Progressive supranuclear palsy: Advances in diagnosis and management. Park. Relat. Disord. 2020, 73, 105–116. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.; Petrides, K.; Mandy, W. The female autism phenotype and camouflaging: A narrative review. Rev. J. Autism Dev. Disord. 2020, 7, 306–317. [Google Scholar] [CrossRef]
- Bottema-Beutel, K.; Kapp, S.K.; Lester, J.N.; Sasson, N.J.; Hand, B.N. Avoiding ableist language: Suggestions for autism researchers. Autism Adulthood 2021, 3, 18–29. [Google Scholar] [CrossRef]
- Wang, J.Y.; Fan, Q.Y.; He, J.H.; Zhu, S.G.; Huang, C.P.; Zhang, X.; Zhu, J.H. SLC6A4 repeat and single-nucleotide polymorphisms are associated with depression and rest tremor in Parkinson’s disease: An exploratory study. Front. Neurol. 2019, 10, 333. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Wolf-Ochoa, M.W.; Hong, T.; Amina, S.; Tassone, F.; Lechpammer, M.; Hagerman, R.; Martínez-Cerdeño, V. Parkinsonism Versus Concomitant Parkinson’s Disease in Fragile X–Associated Tremor/Ataxia Syndrome. Mov. Disord. Clin. Pract. 2020, 7, 413–418. [Google Scholar] [CrossRef]
- Islam, A.; Rahaman, N.; Ahad, M.A.R. A study on tiredness assessment by using eye blink detection. J. Kejuruter. 2019, 31, 209–214. [Google Scholar] [CrossRef]
- Ngo, W.; Situ, P.; Keir, N.; Korb, D.; Blackie, C.; Simpson, T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chiang, T.; Hsu, M.; Liu, J. The validity of eye blink rate in Chinese adults for the diagnosis of Parkinson’s disease. Clin. Neurol. Neurosurg. 2003, 105, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Solomon, S.; Gao, W. Flexible electronics and devices as human–machine interfaces for medical robotics. Adv. Mater. 2022, 34, 2107902. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, devices, and systems of on-skin electrodes for electrophysiological monitoring and human–machine interfaces. Adv. Sci. 2021, 8, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, T.; Luo, Y.; He, K.; Pan, L.; Li, Z.; Cui, Z.; Liu, Z.; Tu, J.; Chen, X. Fusing stretchable sensing technology with machine learning for human–machine interfaces. Adv. Funct. Mater. 2021, 31, 2008807. [Google Scholar] [CrossRef]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, eaaz7946. [Google Scholar] [CrossRef]
- Wang, K.; Yap, L.W.; Gong, S.; Wang, R.; Wang, S.J.; Cheng, W. Nanowire-Based Soft Wearable Human–Machine Interfaces for Future Virtual and Augmented Reality Applications. Adv. Funct. Mater. 2021, 31, 2008347. [Google Scholar] [CrossRef]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) classification tasks: A review. J. Neural Eng. 2019, 16, 031001. [Google Scholar] [CrossRef]
- Thilagaraj, M.; Arunkumar, N.; Ramkumar, S.; Hariharasitaraman, S. Electrooculogram signal identification for elderly disabled using Elman network. Microprocess. Microsyst. 2021, 82, 103811. [Google Scholar] [CrossRef]
- Dubey, A.; Ray, S. Cortical electrocorticogram (ECoG) is a local signal. J. Neurosci. 2019, 39, 4299–4311. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Piscitelli, D.; Norouzi-Gheidari, N.; Batalla, M.A.P.; Archambault, P.; Levin, M.F. Electromyogram-related neuromuscular electrical stimulation for restoring wrist and hand movement in poststroke hemiplegia: A systematic review and meta-analysis. Neurorehabilit. Neural Repair 2019, 33, 96–111. [Google Scholar] [CrossRef]
- Sahay, P.; Kumawat, D.; Gupta, S.; Tripathy, K.; Vohra, R.; Chandra, M.; Venkatesh, P. Detection and monitoring of subclinical ocular siderosis using multifocal electroretinogram. Eye 2019, 33, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Chang, H.; Zhao, B.; Tan, X.; Yang, Y.; Tian, H.; Zhang, S.; Ren, T.L. Electrooculography and Tactile Perception Collaborative Interface for 3D Human–Machine Interaction. Acs Nano 2022, 16, 6687–6699. [Google Scholar] [CrossRef]
- Azari, A.A.; Arabi, A. Conjunctivitis: A systematic review. J. Ophthalmic Vis. Res. 2020, 15, 372. [Google Scholar] [CrossRef] [PubMed]
- Ozturker, Z.K. Conjunctivitis as sole symptom of COVID-19: A case report and review of literature. Eur. J. Ophthalmol. 2021, 31, NP145–NP150. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, M.; Yin, W.; Xu, H.; Zhu, S.; Tang, J.; Chen, L.; Ran, Q.; Zhang, Y.; Qu, Z. Novel wearable sensors for biomechanical movement monitoring based on electromagnetic sensing techniques. IEEE Sens. J. 2019, 20, 1019–1027. [Google Scholar] [CrossRef]
- Kole, M.; Khandekar, S. Engineering applications of ferrofluids: A review. J. Magn. Magn. Mater. 2021, 537, 168222. [Google Scholar] [CrossRef]
- Giwa, S.; Sharifpur, M.; Goodarzi, M.; Alsulami, H.; Meyer, J. Influence of base fluid, temperature, and concentration on the thermophysical properties of hybrid nanofluids of alumina–ferrofluid: Experimental data, modeling through enhanced ANN, ANFIS, and curve fitting. J. Therm. Anal. Calorim. 2021, 143, 4149–4167. [Google Scholar] [CrossRef]
- Sadeghi, M.; Tayebi, T.; Dogonchi, A.; Nayak, M.; Waqas, M. Analysis of thermal behavior of magnetic buoyancy-driven flow in ferrofluid–filled wavy enclosure furnished with two circular cylinders. Int. Commun. Heat Mass Transf. 2021, 120, 104951. [Google Scholar] [CrossRef]
- Anantha Kumar, K.; Sandeep, N.; Sugunamma, V.; Animasaun, I. Effect of irregular heat source/sink on the radiative thin film flow of MHD hybrid ferrofluid. J. Therm. Anal. Calorim. 2020, 139, 2145–2153. [Google Scholar] [CrossRef]



| Symbol | Parameter | Value | Units |
|---|---|---|---|
| Remanence | |||
| Coercivity | 1000 | ||
| Maximum energy product | 300 | ||
| Curie temperature | 310–400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Luk, P.; Zhou, Y. Wearable and Invisible Sensor Design for Eye-Motion Monitoring Based on Ferrofluid and Electromagnetic Sensing Technologies. Bioengineering 2023, 10, 514. https://doi.org/10.3390/bioengineering10050514
Tang J, Luk P, Zhou Y. Wearable and Invisible Sensor Design for Eye-Motion Monitoring Based on Ferrofluid and Electromagnetic Sensing Technologies. Bioengineering. 2023; 10(5):514. https://doi.org/10.3390/bioengineering10050514
Chicago/Turabian StyleTang, Jiawei, Patrick Luk, and Yuyang Zhou. 2023. "Wearable and Invisible Sensor Design for Eye-Motion Monitoring Based on Ferrofluid and Electromagnetic Sensing Technologies" Bioengineering 10, no. 5: 514. https://doi.org/10.3390/bioengineering10050514
APA StyleTang, J., Luk, P., & Zhou, Y. (2023). Wearable and Invisible Sensor Design for Eye-Motion Monitoring Based on Ferrofluid and Electromagnetic Sensing Technologies. Bioengineering, 10(5), 514. https://doi.org/10.3390/bioengineering10050514