Mechanism of Pulp Regeneration Based on Concentrated Growth Factors Regulating Cell Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of hDPCs
2.2. Conditioned Medium Preparation
2.2.1. Preparation of CGF Extract
2.2.2. Preparation of CGF Conditional Medium
2.3. Scanning Electron Microscopy (SEM)
2.4. Cell Proliferation Assay
2.5. Cell Migration Assay
2.6. Alizarin Red S Staining
2.7. Detection of ALP Activity
2.8. Real-Time Quantitative Polymerase Chain Reaction
2.9. Growth Factor Detection by Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. In Vivo Transplantation Assay of hDPCs and Concentrated Growth Complex in Nude Mice
2.11. Micro-CT Analysis
2.12. Hematoxylin-Eosin Staining
2.13. IHC
2.14. Statistical Analysis
3. Results
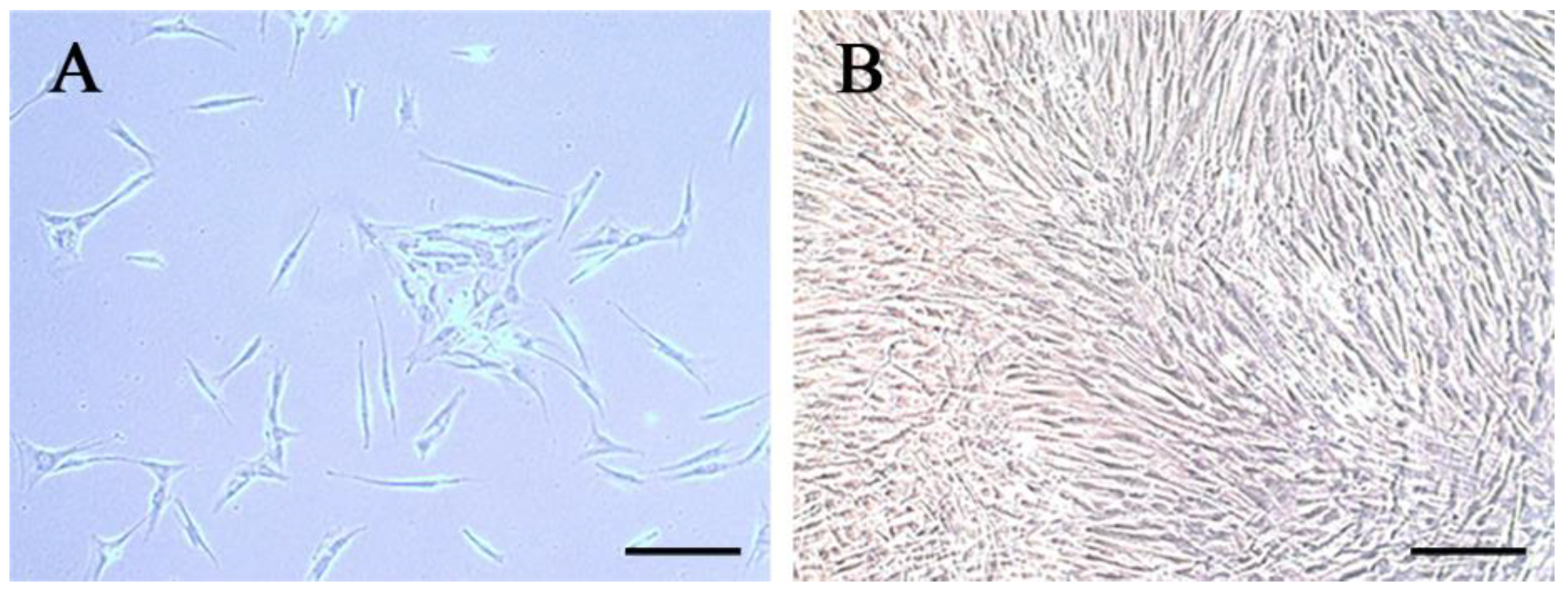
3.1. Characteristics of hDPCs
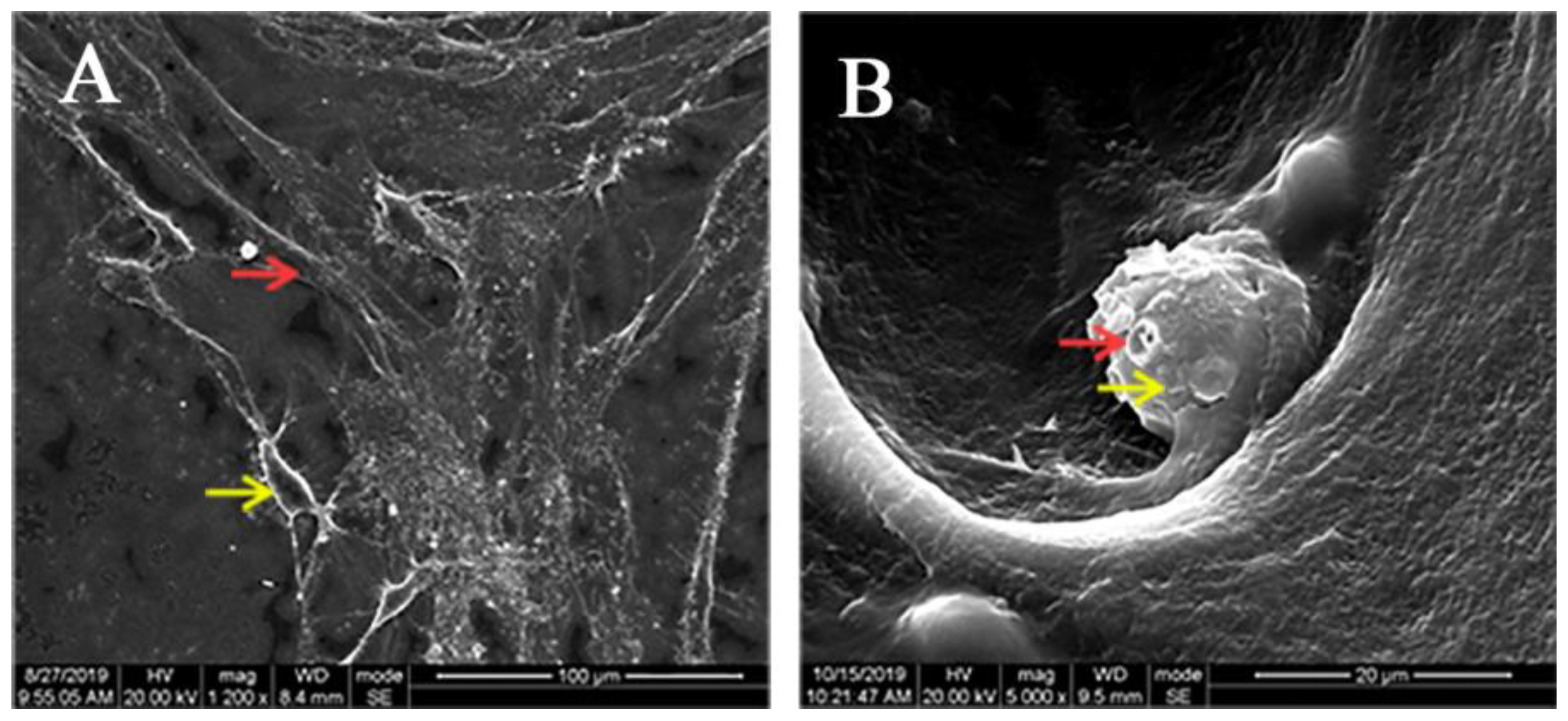
3.2. Adhesion of hDPCs to LPCGF Fibrin Network
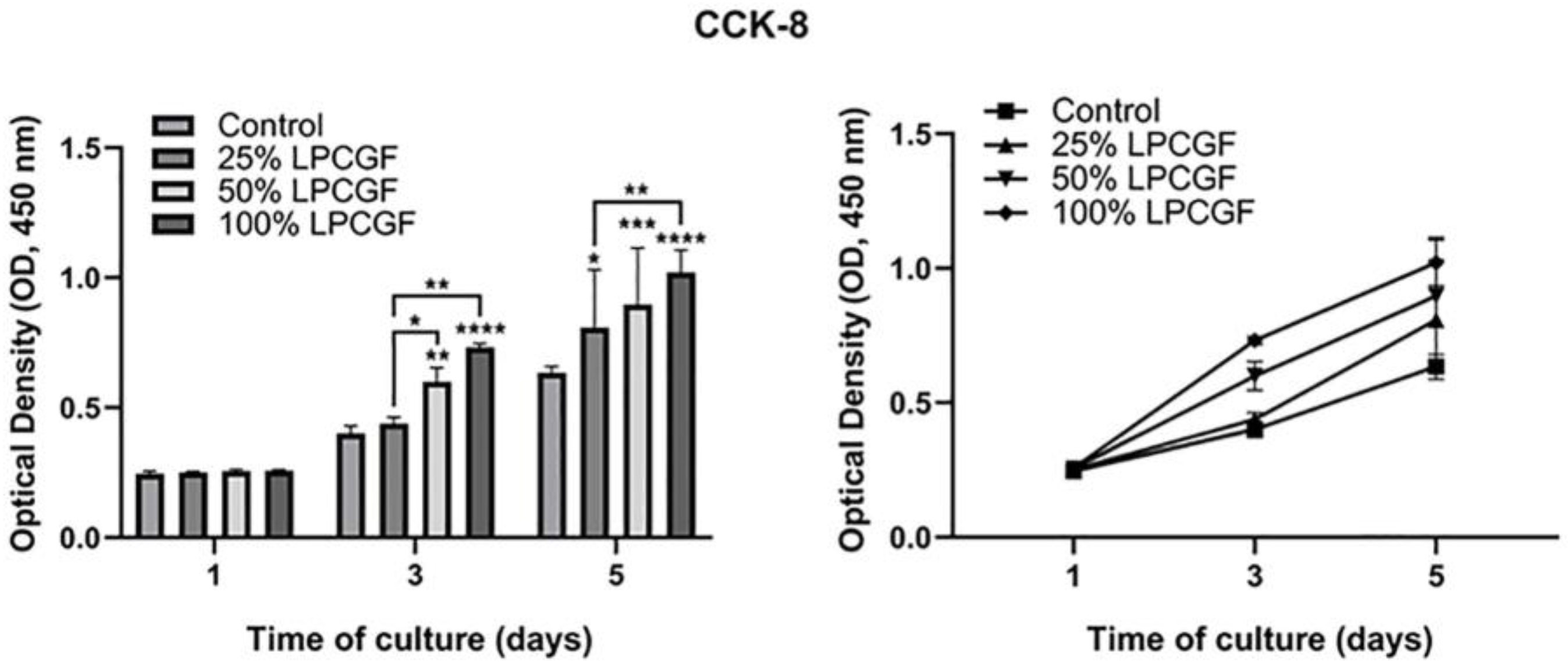
3.3. Effect of LPCGF on hDPC Proliferation
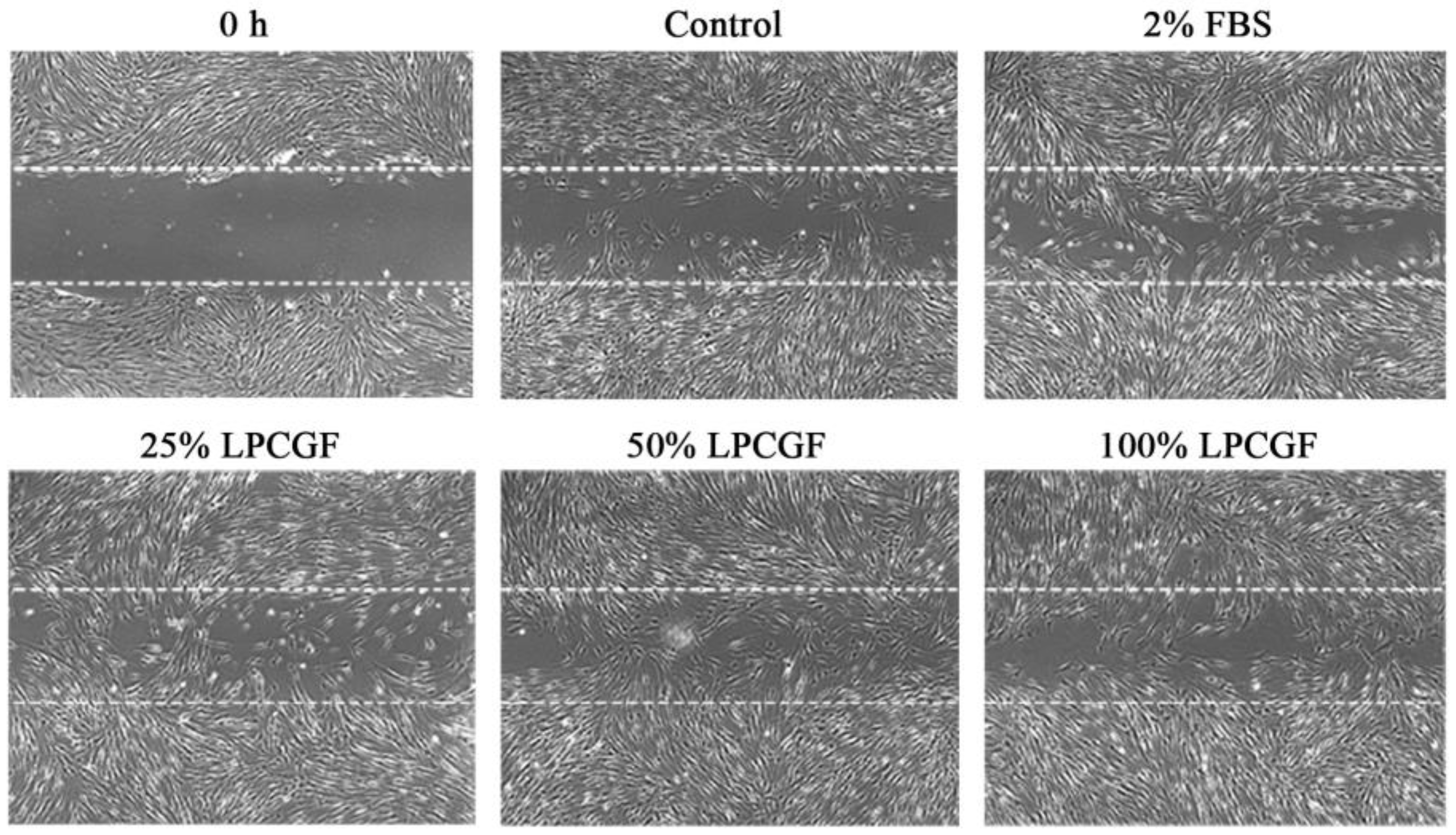
3.4. Effect of LPCGF on hDPC Migration
3.5. The Effect of LPCGF on hDPC Differentiation
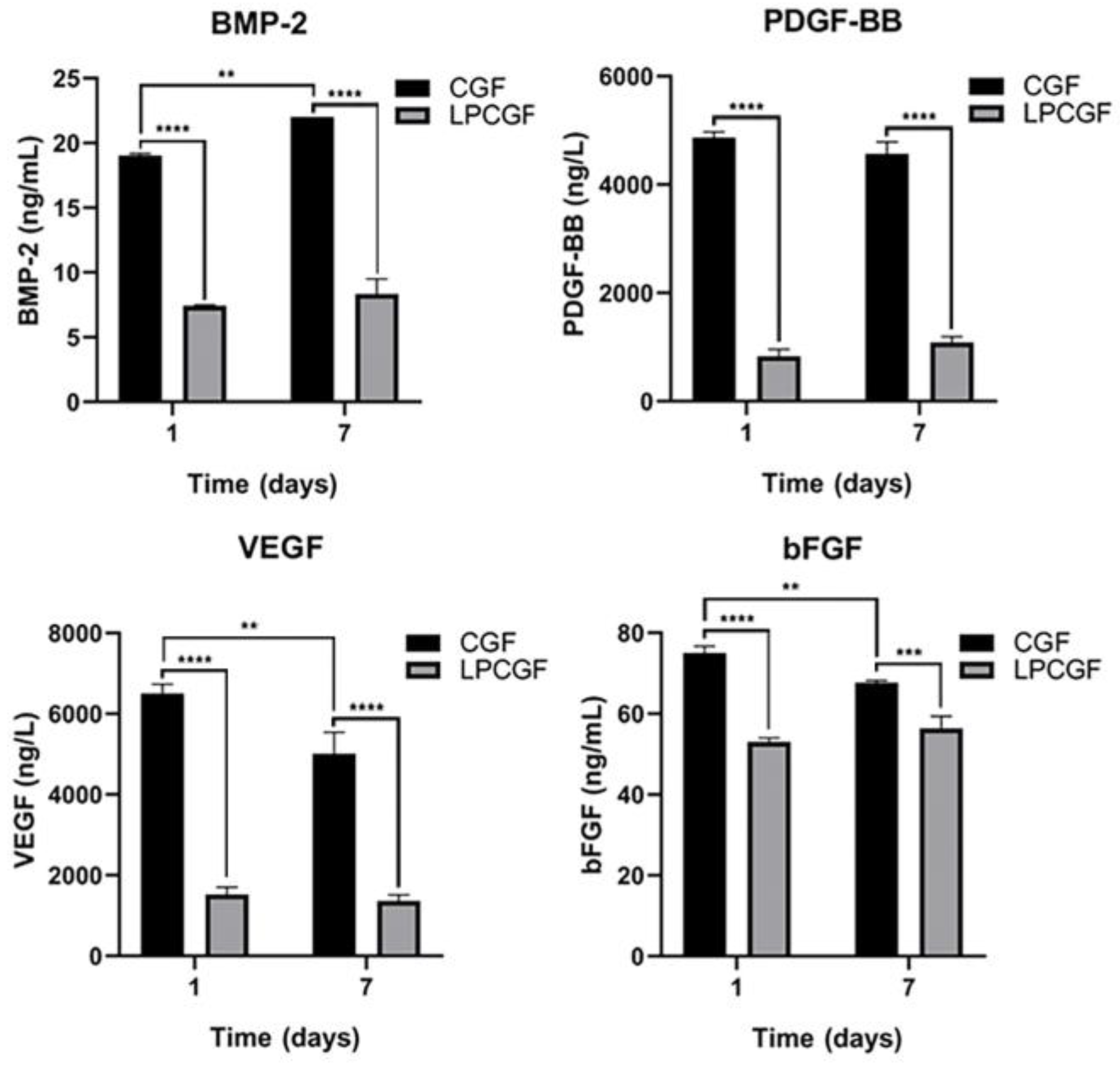
3.6. Changes of Growth Factor Content in CGF and LPCGF
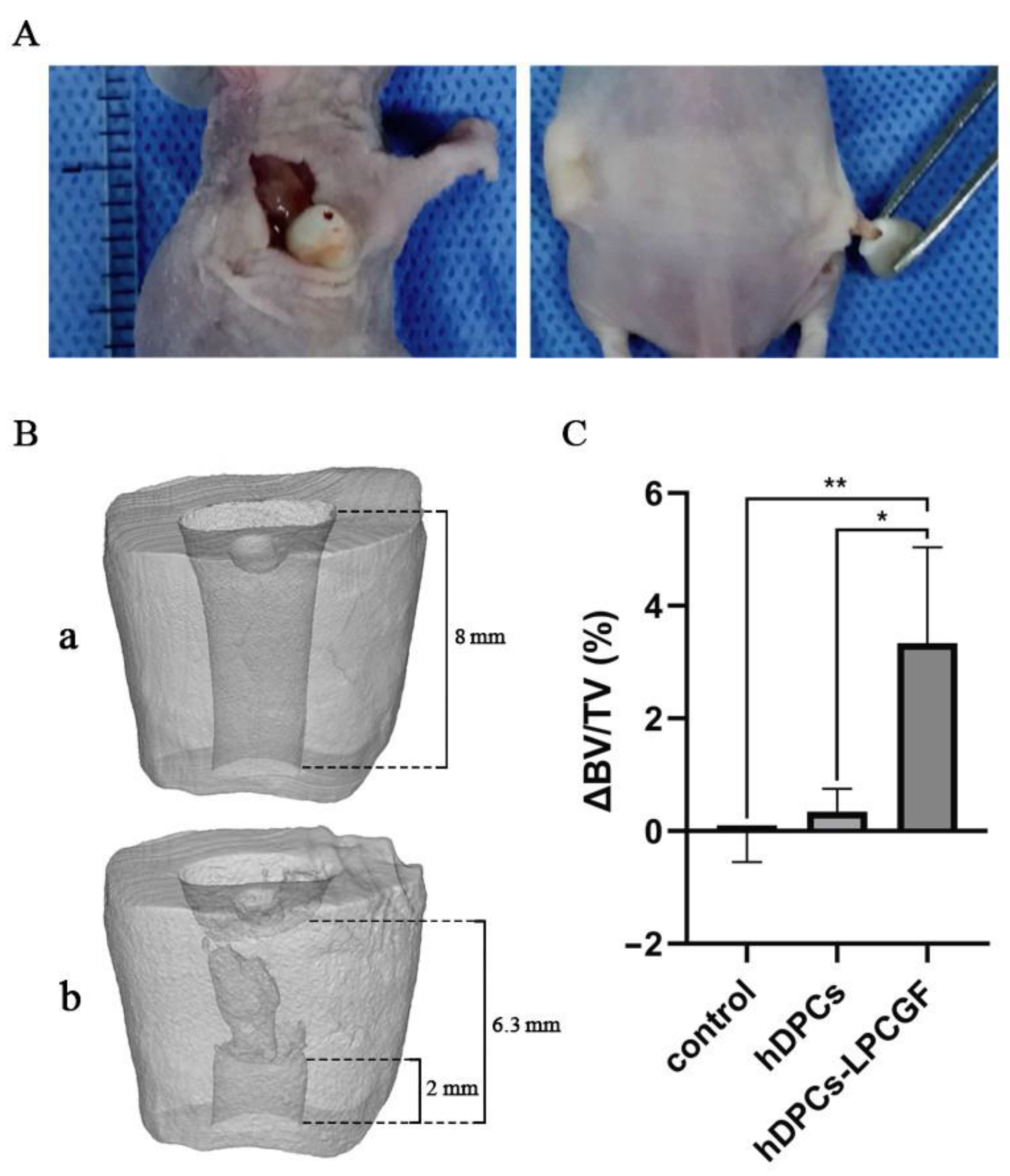
3.7. The Effect of hDPCs-LPCGF Complex on the Pulp Tissue Regeneration and the Root/Apical Foramen Development In Vivo
3.7.1. Micro-CT Results
3.7.2. Histologic Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diogenes, A.; Ruparel, N.B.; Shiloah, Y.; Hargreaves, K.M. Regenerative endodontics: A way forward. J. Am. Dent. Assoc. 2016, 147, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef]
- Huang, D.M.; Yang, M.B.; Zhou, X.D. Clinical management and prognosis evaluation of pulp regeneration therapy. Zhonghua Kou Qiang Yi Xue Za Zhi 2019, 54, 584–590. [Google Scholar]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef]
- Nakayama, H.; Iohara, K.; Hayashi, Y.; Okuwa, Y.; Kurita, K.; Nakashima, M. Enhanced regeneration potential of mobilized dental pulp stem cells from immature teeth. Oral Dis. 2017, 23, 620–628. [Google Scholar] [CrossRef]
- Murakami, M.; Horibe, H.; Iohara, K.; Hayashi, Y.; Osako, Y.; Takei, Y.; Nakata, K.; Motoyama, N.; Kurita, K.; Nakashima, M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 2013, 34, 9036–9047. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Shimizu, E.; Ricucci, D.; Albert, J.; Alobaid, A.S.; Gibbs, J.L.; Huang, G.T.-J.; Lin, L.M. Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J. Endod. 2013, 39, 1078–1083. [Google Scholar] [CrossRef]
- Becerra, P.; Ricucci, D.; Loghin, S.; Gibbs, J.L.; Lin, L.M. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J. Endod. 2014, 40, 133–139. [Google Scholar] [CrossRef]
- Martin, G.; Ricucci, D.; Gibbs, J.L.; Lin, L.M. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J. Endod. 2013, 39, 138–144. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, L.; Arbildo-Vega, H.I.; Lapinska, B.; Lukomska-Szymanska, M.; Khijmatgar, S.; Parolia, A.; Bucchi, C.; Del Fabbro, M. Effectiveness of Autologous Platelet Concentrates in Management of Young Immature Necrotic Permanent Teeth—A Systematic Review and Meta-Analysis. Cells 2020, 9, 2241. [Google Scholar] [CrossRef]
- Arshad, S.; Tehreem, F.; Khan, M.R.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-Rich Fibrin Used in Regenerative Endodontics and Dentistry: Current Uses, Limitations, and Future Recommendations for Application. Int. J. Dent. 2021, 2021, 4514598. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Lolato, A.; Bucchi, C.; Taschieri, S.; Weinstein, R.L. Autologous Platelet Concentrates for Pulp and Dentin Regeneration: A Literature Review of Animal Studies. J. Endod. 2016, 42, 250–257. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Fang, D.; Long, Z.; Hou, J. Clinical Application of Concentrated Growth Factor Fibrin Combined with Bone Repair Materials in Jaw Defects. J. Oral Maxillofac. Surg. 2020, 78, 882–892. [Google Scholar] [CrossRef]
- Harrison, S.; Vavken, P.; Kevy, S.; Jacobson, M.; Zurakowski, D.; Murray, M.M. Platelet activation by collagen provides sustained release of anabolic cytokines. Am. J. Sports Med. 2011, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Masuki, H.; Okudera, T.; Watanabe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Aghamohammadi, Z.; Tayebi, L. In vitro and in vivo effects of concentrated growth factor on cells and tissues. J. Biomed. Mater. Res. A 2020, 108, 1338–1350. [Google Scholar] [CrossRef]
- Takeda, Y.; Katsutoshi, K.; Matsuzaka, K.; Inoue, T. The Effect of Concentrated Growth Factor on Rat Bone Marrow Cells In Vitro and on Calvarial Bone Healing In Vivo. Int. J. Oral Maxillofac. Implants 2015, 30, 1187–1196. [Google Scholar] [CrossRef]
- Jun, H.; Lei, D.; Qifang, Y.; Yuan, X.; Deqin, Y. Effects of concentrated growth factors on the angiogenic properties of dental pulp cells and endothelial cells: An in vitro study. Braz. Oral Res. 2018, 32, e48. [Google Scholar] [CrossRef]
- Pirpir, C.; Yilmaz, O.; Candirli, C.; Balaban, E. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int. J. Implant Dent. 2017, 3, 7. [Google Scholar] [CrossRef]
- Xu, F.; Qiao, L.; Zhao, Y.; Chen, W.; Hong, S.; Pan, J.; Jiang, B. The potential application of concentrated growth factor in pulp regeneration: An in vitro and in vivo study. Stem Cell Res. Ther. 2019, 10, 134. [Google Scholar] [CrossRef]
- Sun, H.-H.; Chen, B.; Zhu, Q.-L.; Kong, H.; Li, Q.-H.; Gao, L.-N.; Xiao, M.; Chen, F.-M.; Yu, Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 2014, 35, 9459–9472. [Google Scholar] [CrossRef]
- Honda, H.; Tamai, N.; Naka, N.; Yoshikawa, H.; Myoui, A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J. Artif. Organs 2013, 16, 305–315. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, S.G.; Oh, J.S.; You, J.S.; Kim, J.S.; Lim, S.C.; Jeong, M.-A.; Kim, J.-S.; Jung, C.; Kwon, Y.-S.; et al. Early Bone Formation at a Femur Defect Using CGF and PRF Grafts in Adult Dogs: A Comparative Study. Implant Dent. 2016, 25, 387–393. [Google Scholar] [CrossRef]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 17. [Google Scholar] [CrossRef]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039–1048. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef]
- Mullane, E.M.; Dong, Z.; Sedgley, C.M.; Hu, J.C.; Botero, T.M.; Holland, G.R.; Nör, J.E. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J. Dent. Res. 2008, 87, 1144–1148. [Google Scholar] [CrossRef]
- Li, X.W.; Sun, H.C.; Liu, X.H. Vascular endothelial growth factor-loaded microspheres promote dental pulp regeneration and vascularization. Zhonghua Kou Qiang Yi Xue Za Zhi 2018, 53, 42–48. [Google Scholar]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef]
- Conde, M.C.; Chisini, L.A.; Demarco, F.F.; Nor, J.E.; Casagrande, L.; Tarquinio, S.B. Stem cell-based pulp tissue engineering: Variables enrolled in translation from the bench to the bedside, a systematic review of literature. Int. Endod. J. 2016, 49, 543–550. [Google Scholar] [CrossRef]
- Akasaka, Y.; Ono, I.; Kamiya, T.; Ishikawa, Y.; Kinoshita, T.; Ishiguro, S.; Ishiguro, S.; Yokoo, T.; Imaizumi, R.; Inomata, N.; et al. The mechanisms underlying fibroblast apoptosis regulated by growth factors during wound healing. J. Pathol. 2010, 221, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, F.; Avellana-Adalid, V.; Nait-Oumesmar, B.; Baron-Van Evercooren, A. Fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor AB (PDGF AB) promote adult SVZ-derived oligodendrogenesis in vivo. Mol. Cell Neurosci. 2002, 20, 390–403. [Google Scholar] [CrossRef]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J. Periodontol. 2003, 74, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, F.; Zhang, X.; Wang, S.; Jin, Y.; Zhang, W.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Human Dental Pulp Stem Cells Mediated Dentin-Pulp Complex Regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Dobie, K.; Smith, G.; Sloan, A.J.; Smith, A.J. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect. Tissue Res. 2002, 43, 387–390. [Google Scholar] [CrossRef]
- Nie, X.; Tian, W.; Zhang, Y.; Chen, X.; Dong, R.; Jiang, M.; Chen, F.; Jin, Y. Induction of transforming growth factor-beta 1 on dentine pulp cells in different culture patterns. Cell Biol. Int. 2006, 30, 295–300. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, J.; Mu, R.; Zhu, R.; Chen, F.; Wei, X.; Shi, X.; Zang, S.; Jin, L. Bone morphogenetic protein 7 promotes odontogenic differentiation of dental pulp stem cells in vitro. Life Sci. 2018, 202, 175–181. [Google Scholar] [CrossRef]
- Qin, C.; Brunn, J.; Cadena, E.; Ridall, A.; Tsujigiwa, H.; Nagatsuka, H.; Nagai, N.; Butler, W. The expression of dentin sialophosphoprotein gene in bone. J. Dent. Res. 2002, 81, 392–394. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Xiao, G. Regulation of the osteoblast-specific transcription factor, Runx2: Responsiveness to multiple signal transduction pathways. J. Cell Biochem. 2003, 88, 446–454. [Google Scholar] [CrossRef]
- Brazelton, T.R.; Blau, H.M. Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells 2005, 23, 1251–1265. [Google Scholar] [CrossRef]


| Gene | Primer Sequences |
|---|---|
| GAPDH-HUMAN-F | GGAGCGAGATCCCTCCAAAAT |
| GAPDH-HUMAN-R | GGCTGTTGTCATACTTCTCATGG |
| DSPP-F | TTTGGGCAGTAGCATGGGC |
| DSPP-R | CCATCTTGGGTATTCTCTTGCCT |
| RUNX2-F | CCTTTACTTACACCCCGCCA |
| RUNX2-R | GGATCCTGACGAAGTGCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Zheng, Y.; Guo, Q.; Li, W.; Ye, L.; Gao, B. Mechanism of Pulp Regeneration Based on Concentrated Growth Factors Regulating Cell Differentiation. Bioengineering 2023, 10, 513. https://doi.org/10.3390/bioengineering10050513
Yu S, Zheng Y, Guo Q, Li W, Ye L, Gao B. Mechanism of Pulp Regeneration Based on Concentrated Growth Factors Regulating Cell Differentiation. Bioengineering. 2023; 10(5):513. https://doi.org/10.3390/bioengineering10050513
Chicago/Turabian StyleYu, Sijing, Yi Zheng, Qiang Guo, Wenxu Li, Ling Ye, and Bo Gao. 2023. "Mechanism of Pulp Regeneration Based on Concentrated Growth Factors Regulating Cell Differentiation" Bioengineering 10, no. 5: 513. https://doi.org/10.3390/bioengineering10050513
APA StyleYu, S., Zheng, Y., Guo, Q., Li, W., Ye, L., & Gao, B. (2023). Mechanism of Pulp Regeneration Based on Concentrated Growth Factors Regulating Cell Differentiation. Bioengineering, 10(5), 513. https://doi.org/10.3390/bioengineering10050513





