Advances in Zebrafish for Diabetes Mellitus with Wound Model
Abstract
1. Introduction
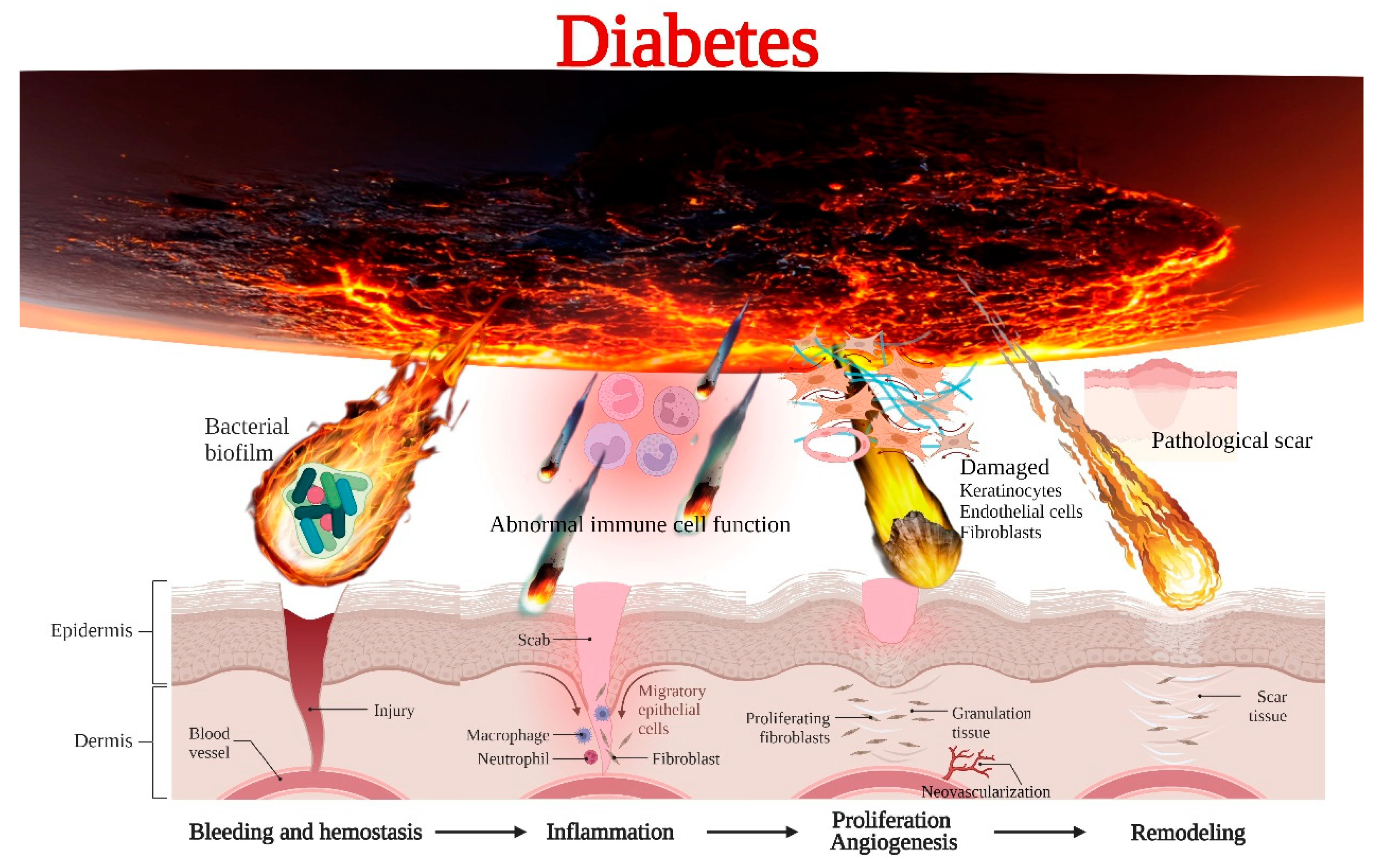
2. Mechanism of Delayed Healing of Diabetic Wounds
2.1. Immune Dysfunction
2.2. Microbial Invasion
2.3. Impaired Cell Proliferation and Angiogenesis
2.4. Pathological Scar
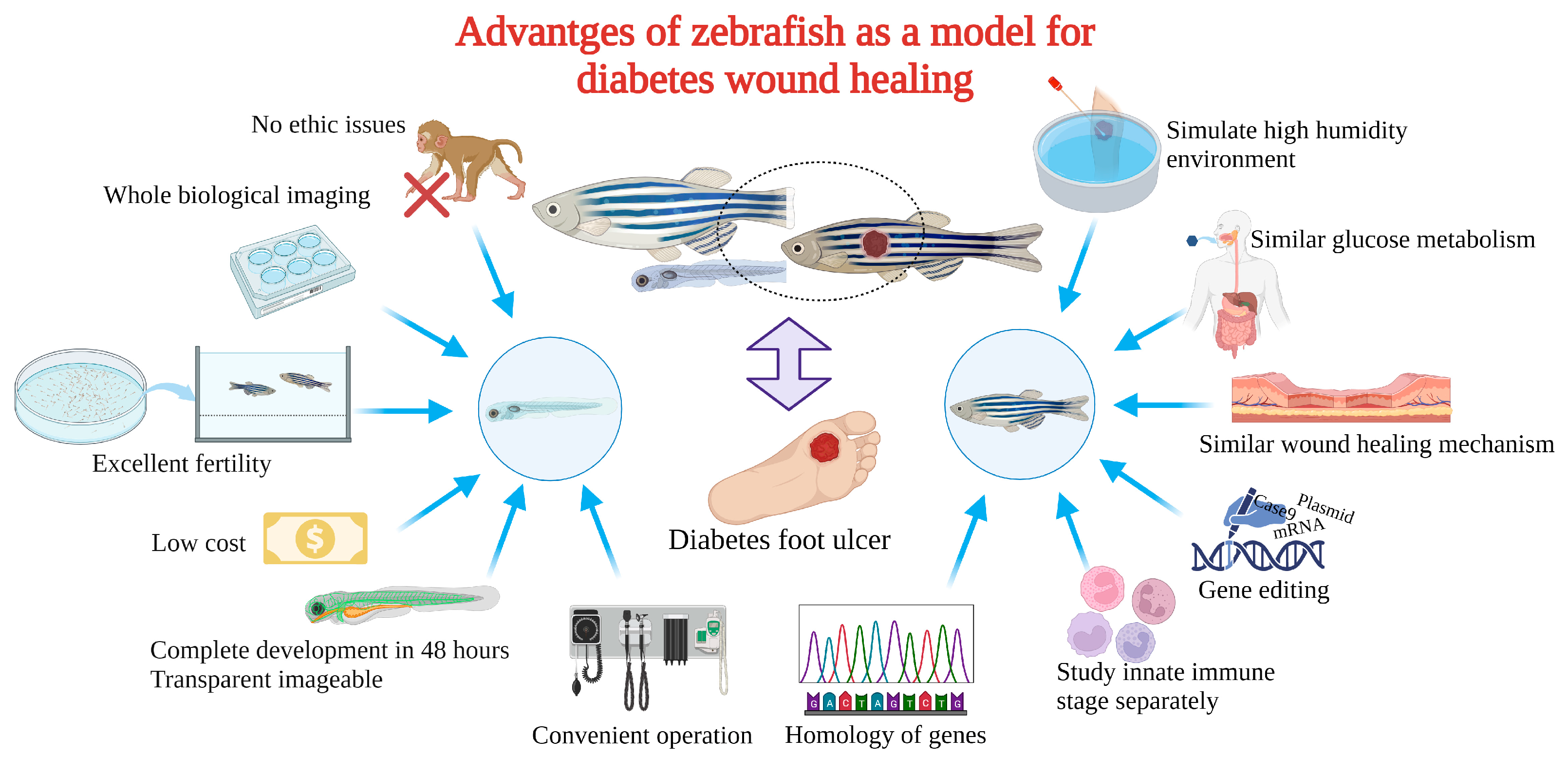
3. The Advantages of Zebrafish for Diabetic Wound Healing
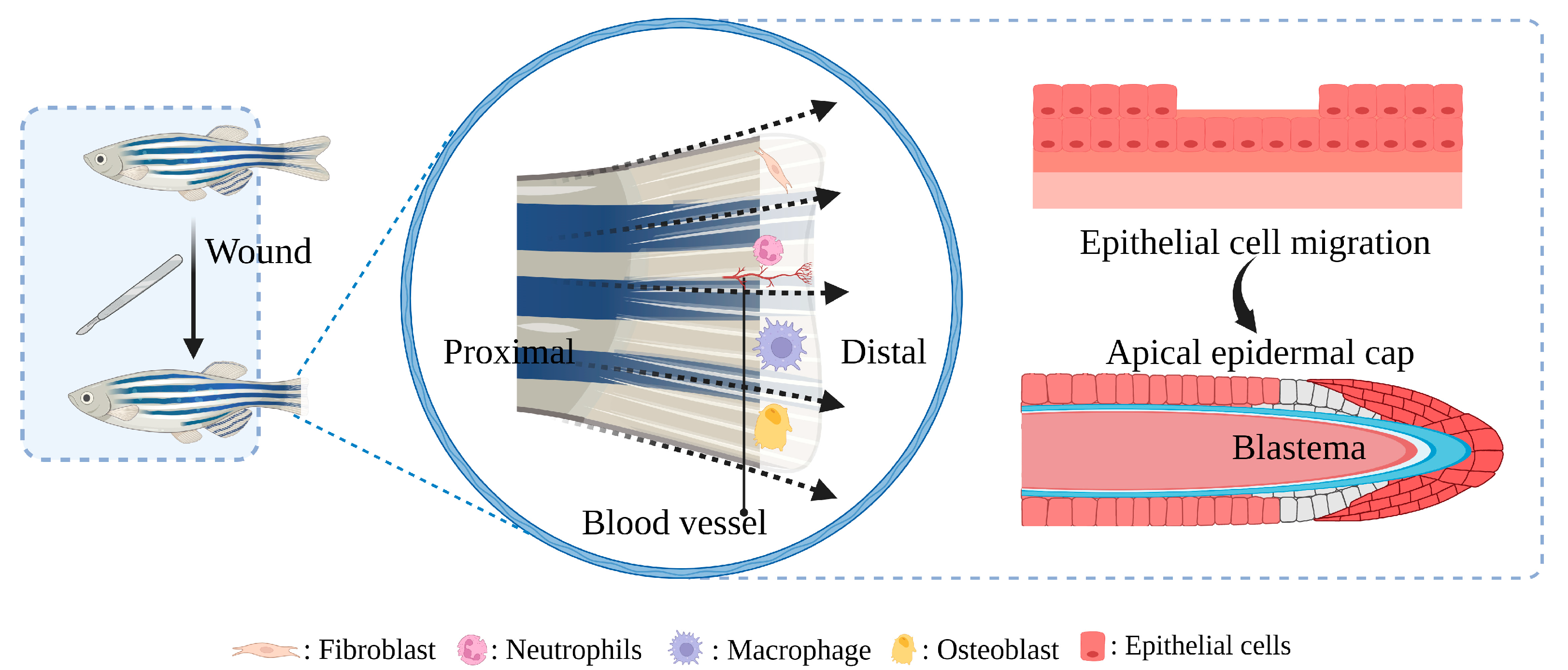
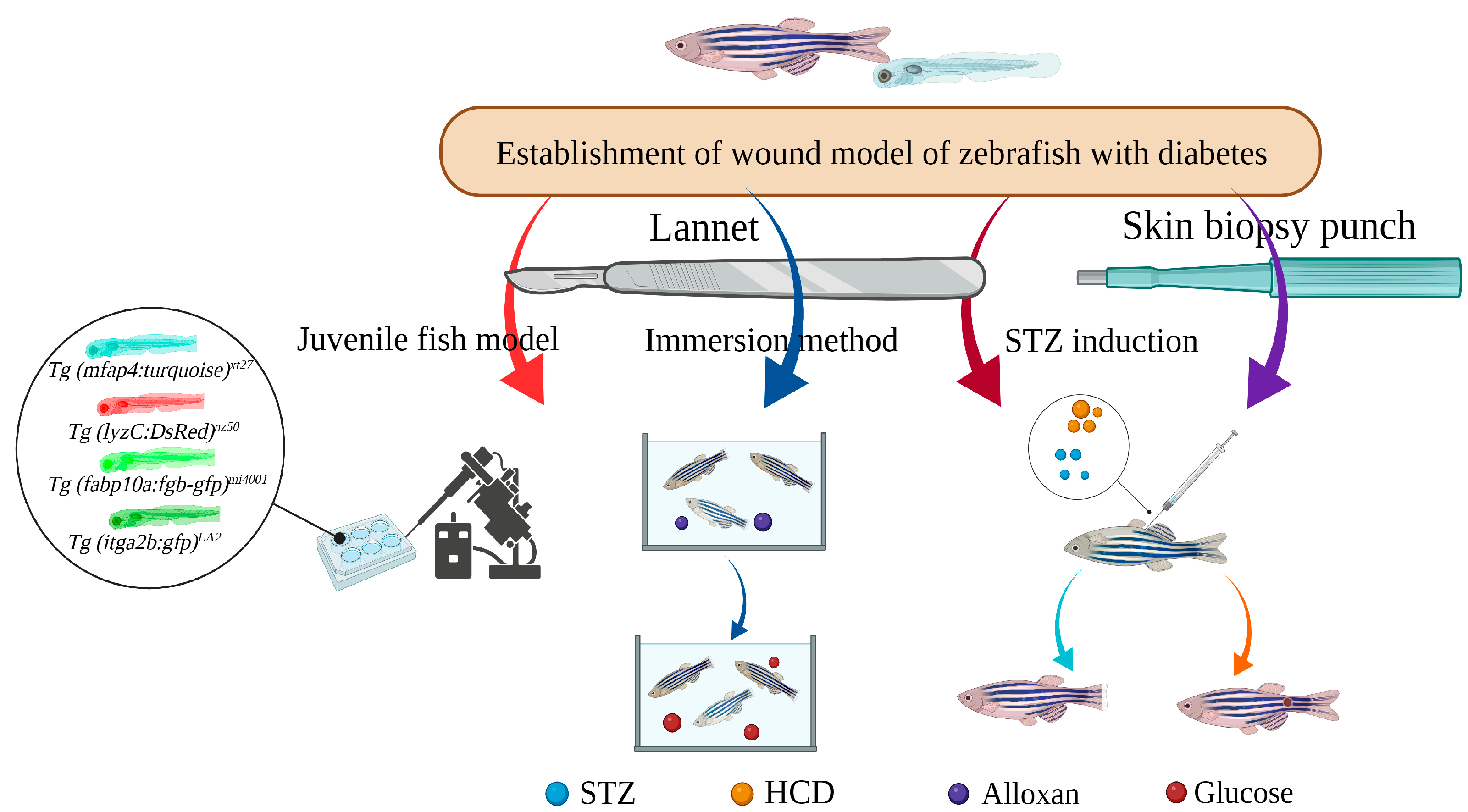
4. Construction and Application of Zebrafish Diabetic Wound Model
4.1. STZ-Induced Caudal Fin Regeneration Model of Zebrafish with Type 1 Diabetes
4.2. Caudal Fin Model of Type II Diabetes in Adult Zebrafish Induced by Alloxan and Glucose Combined with Aqueous Solution Exposure
4.3. Caudal Fin Regeneration Model of Zebrafish Juvenile Type II Diabetes Induced by Single Immersion or Injection of Glucose
4.4. Skin Wound Model of Adult Zebrafish Type I Diabetes Induced by STZ Injection
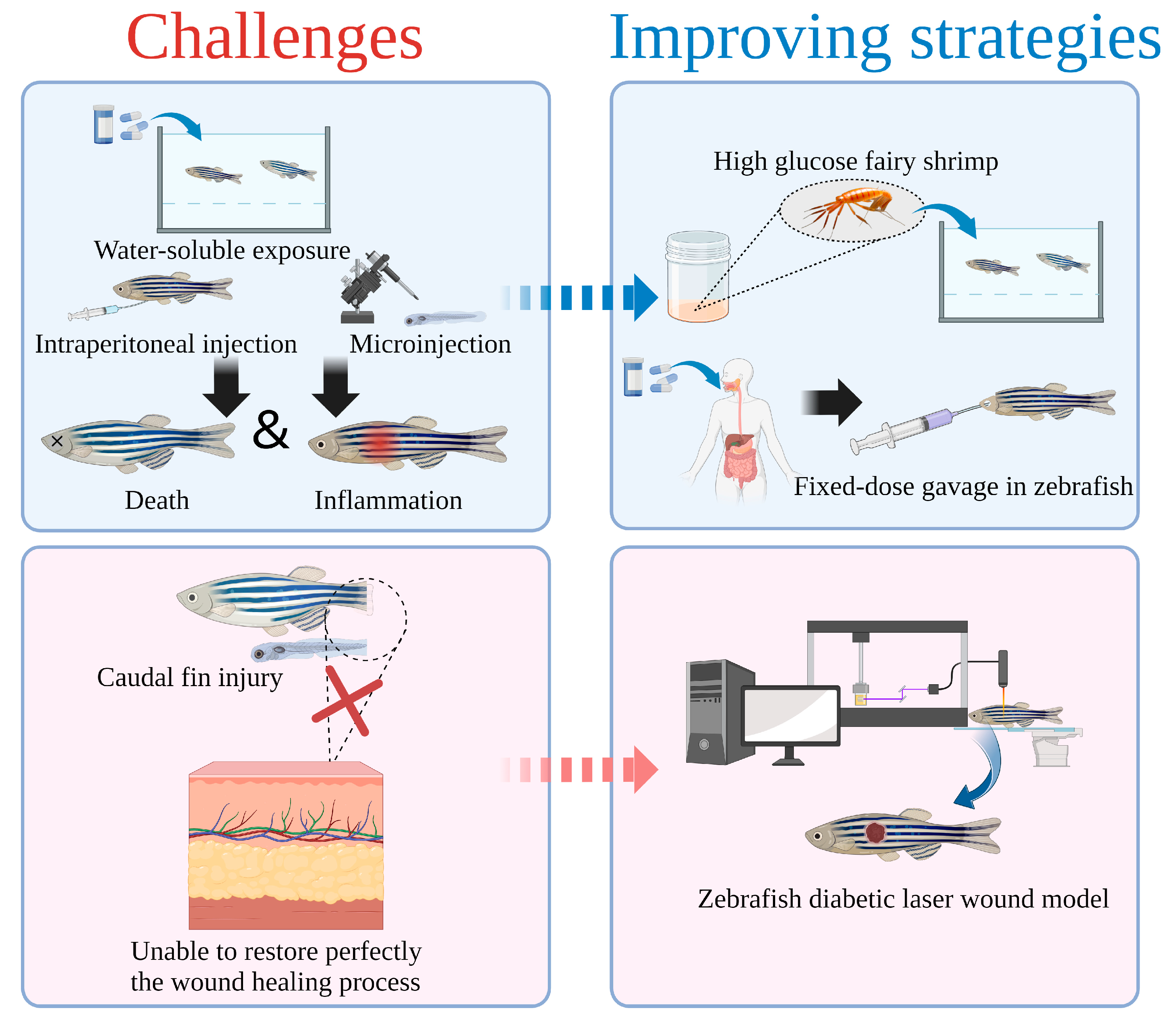
5. Challenge and Improving Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation endproducts |
| AQP | Aquaporin |
| BMI | Body mass index |
| DFUs | Diabetic foot ulcers |
| hpa | Hours-post-amputation |
| dpf | Day post fertilization |
| DM | Diabetes mellitus |
| DNMTs | DNA methyl transferases |
| EGF | Epidermal growth factor |
| ECM | Extracellular matrix |
| FGFs | Fibroblast growth factors |
| HSPs | Heat shock proteins |
| HCD | High cholesterol diet |
| HFD | High fat diet |
| iNOS | Inducible nitricoxidesynthase |
| IGF-1 | Insulin-like growth factors-1 |
| MIF | Macrophagemigration inhibitory factor |
| MIP | Macrophage inflammatory protein |
| MMPs | Matrix metallo proteinases |
| MM | Metabolic memory |
| NETs | Neutrophil extracellular traps |
| ND | Normal diet |
| PARP | Poly (ADP-ribose) polymerase |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
| TIMP-1 | Tissue inhibitor of matrixmetallo proteinase-1 |
| TLRs | Toll-like receptors |
| TGF-β | Transforming growth factor-beta |
| VEGF | Vascular endothelial growth factor |
| VEGFR-2 | VEGF receptor-2 |
References
- Chen, W.; Mao, M.; Fang, J.; Xie, Y.; Rui, Y. Fracture Risk Assessment in Diabetes Mellitus. Front. Endocrinol. 2022, 13, 961761. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Samadi, A.; Burade, S.; Mahmud, T. Complete Biosynthetic Pathway to the Antidiabetic Drug Acarbose. Nat. Commun. 2022, 13, 3455. [Google Scholar] [CrossRef]
- Giugliano, D.; Longo, M.; Caruso, P.; Di Fraia, R.; Scappaticcio, L.; Gicchino, M.; Petrizzo, M.; Bellastella, G.; Maiorino, M.I.; Esposito, K. Feasibility of Simplification From a Basal-Bolus Insulin Regimen to a Fixed-Ratio Formulation of Basal Insulin Plus a GLP-1RA or to Basal Insulin Plus an SGLT2 Inhibitor: BEYOND, a Randomized, Pragmatic Trial. Diabetes Care 2021, 44, 1353–1360. [Google Scholar] [CrossRef]
- Li, S.; Mohamedi, A.H.; Senkowsky, J.; Nair, A.; Tang, L. Imaging in Chronic Wound Diagnostics. Adv. Wound Care New Rochelle 2020, 9, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in Acute and Chronic Wound Healing: Oxygen in Wound Healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Zheng, Z.; Wan, Y.; Liu, Y.; Yang, Y.; Tang, J.; Huang, W.; Cheng, B. Sympathetic Denervation Accelerates Wound Contraction but Inhibits Reepithelialization and Pericyte Proliferation in Diabetic Mice. J. Diabetes Res. 2017, 2017, 7614685. [Google Scholar] [CrossRef]
- Randi, A.M.; Laffan, M.A. Von Willebrand Factor and Angiogenesis: Basic and Applied Issues. J. Thromb. Haemost. 2017, 15, 13–20. [Google Scholar] [CrossRef]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P. A Zebrafish Model of Diabetes Mellitus and Metabolic Memory. J. Vis. Exp. 2013, 72, e50232. [Google Scholar] [CrossRef]
- Graves, N.; Phillips, C.J.; Harding, K. A Narrative Review of the Epidemiology and Economics of Chronic Wounds. Br. J. Dermatol. 2021, 187, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Lin, X.; Mo, C. Integrated Analysis of CircRNA-MiRNA-MRNA Regulatory Network Identifies Potential Diagnostic Biomarkers in Diabetic Foot Ulcer. Noncoding RNA Res. 2020, 5, 116–124. [Google Scholar] [CrossRef]
- Tang, N.; Zheng, Y.; Jiang, X.; Zhou, C.; Jin, H.; Jin, K.; Wu, W.; Haick, H. Wearable Sensors and Systems for Wound Healing-Related PH and Temperature Detection. Micromachines 2021, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Rodrigues, J.; Gonçalves, M.; Amaral, C.; Lima, M.; Carvalho, E. Impaired T-Cell Differentiation in Diabetic Foot Ulceration. Cell Mol. Immunol. 2017, 14, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Eichelberger, K.R.; Goldman, W.E. Manipulating Neutrophil Degranulation as a Bacterial Virulence Strategy. PLoS Pathog. 2020, 16, e1009054. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as Protagonists and Targets in Chronic Inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of Macrophage Polarization in Autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An Unrestrained Proinflammatory M1 Macrophage Population Induced by Iron Impairs Wound Healing in Humans and Mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, H.; Khamaisi, M. Skin Well-Being in Diabetes: Role of Macrophages. Cell. Immunol. 2020, 356, 104154. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Lan, C.-C.E. High-Glucose Environment Disturbs the Physiologic Functions of Keratinocytes: Focusing on Diabetic Wound Healing. J. Dermatol. Sci. 2016, 84, 121–127. [Google Scholar] [CrossRef]
- Khan, R.; Kadamkode, V.; Kesharwani, D.; Purkayastha, S.; Banerjee, G.; Datta, M. Circulatory MiR-98-5p Levels Are Deregulated during Diabetes and It Inhibits Proliferation and Promotes Apoptosis by Targeting PPP1R15B in Keratinocytes. RNA Biol. 2020, 17, 188–201. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Brem, H.; Stojadinovic, O.; Diegelmann, R.F.; Entero, H.; Lee, B.; Pastar, I.; Golinko, M.; Rosenberg, H.; Tomic-Canic, M. Molecular Markers in Patients with Chronic Wounds to Guide Surgical Debridement. Mol. Med. 2007, 13, 30–39. [Google Scholar] [CrossRef]
- Zindle, J.K.; Wolinsky, E.; Bogie, K.M. A Review of Animal Models from 2015 to 2020 for Preclinical Chronic Wounds Relevant to Human Health. J. Tissue Viability 2021, 30, 291–300. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Bristol, D.G.; Manning, T.O.; Rogers, R.A.; Riviere, J.E. Interspecies and Interregional Analysis of the Comparative Histologic Thickness and Laser Doppler Blood Flow Measurements at Five Cutaneous Sites in Nine Species. J. Investig. Dermatol. 1990, 95, 582–586. [Google Scholar] [CrossRef]
- Yang, C.-H.; Liang, C.-T.; Jiang, S.-T.; Chen, K.-H.; Yang, C.-C.; Cheng, M.-L.; Ho, H.-Y. A Novel Murine Model Expressing a Chimeric MSCARB2/HSCARB2 Receptor Is Highly Susceptible to Oral Infection with Clinical Isolates of Enterovirus 71. J. Virol. 2019, 93, e00183-19. [Google Scholar] [CrossRef]
- Galiano, R.D.; Michaels, V.J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and Reproducible Murine Model of Excisional Wound Healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Chen, C.; Gu, Y.; Philippe, J.; Zhang, P.; Bachman, H.; Zhang, J.; Mai, J.; Rufo, J.; Rawls, J.F.; Davis, E.E.; et al. Acoustofluidic Rotational Tweezing Enables High-Speed Contactless Morphological Phenotyping of Zebrafish Larvae. Nat. Commun. 2021, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Chen, H.; Stauffer, A.M.; Giger, K.E.; Sinha, S.; Horstick, E.J.; Humbert, J.E.; Hansen, C.A.; Robishaw, J.D. Zebrafish G Protein Gamma2 Is Required for VEGF Signaling during Angiogenesis. Blood 2006, 108, 160–166. [Google Scholar] [CrossRef]
- Li, Y.-W.; Chiang, K.-Y.; Li, Y.-H.; Wu, S.-Y.; Liu, W.; Lin, C.-R.; Wu, J.-L. MiR-145 Mediates Zebrafish Hepatic Outgrowth through Progranulin A Signaling. PLoS ONE 2017, 12, e0177887. [Google Scholar] [CrossRef]
- Zhao, S.; Xia, J.; Wu, X.; Zhang, L.; Wang, P.; Wang, H.; Li, H.; Wang, X.; Chen, Y.; Agnetti, J.; et al. Deficiency in Class III PI3-Kinase Confers Postnatal Lethality with IBD-like Features in Zebrafish. Nat. Commun. 2018, 9, 2639. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.N.; Koudijs, M.J.; Patient, R.K.; Ingham, P.W.; Schulte-Merker, S.; van Eeden, F.J.M. Hedgehog Signalling via a Calcitonin Receptor-like Receptor Can Induce Arterial Differentiation Independently of VEGF Signalling in Zebrafish. Blood 2012, 120, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Li, M.; Berger, S.; Meilak, M.; Rientjes, J.; Currie, P.D. Effect of Ataluren on Dystrophin Mutations. J. Cell Mol. Med. 2020, 24, 6680–6689. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Hohn, C.; Petrie-Hanson, L. Rag1−/− Mutant Zebrafish Demonstrate Specific Protection Following Bacterial Re-Exposure. PLoS ONE 2012, 7, e44451. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Grotek, B.; Jacinto, A.; Weidinger, G.; Saúde, L. The Regenerative Capacity of the Zebrafish Caudal Fin Is Not Affected by Repeated Amputations. PLoS ONE 2011, 6, e22820. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.-Y.; Feng, Z.-X.; Zhang, L.-Y.; Zhong, Y.-F.; Jin, M.-Y.; Xu, F.-W.; Feng, C.-Y.; Du, Y.-Z.; et al. Mussel-Inspired Collagen-Hyaluronic Acid Composite Scaffold with Excellent Antioxidant Properties and Sustained Release of a Growth Factor for Enhancing Diabetic Wound Healing. Mater. Today Bio 2022, 15, 100320. [Google Scholar] [CrossRef]
- Wang, D.; Chen, H.; Lei, L.; Chen, J.; Gao, J.; Liu, J.; Li, Q.; Xie, Y.; Hu, Y.; Ni, Y. Biofabricated Macrophage and Fibroblast Membranes Synergistically Promote Skin Wound Healing. Bioeng. Transl. Med. 2022, 7, e10344. [Google Scholar] [CrossRef]
- Ma, J.; Yong, L.; Lei, P.; Li, H.; Fang, Y.; Wang, L.; Chen, H.; Zhou, Q.; Wu, W.; Jin, L.; et al. Advances in MicroRNA from Adipose-Derived Mesenchymal Stem Cell-Derived Exosome: Focusing on Wound Healing. J. Mater. Chem. B 2022, 10, 9565–9577. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carnero-Montoro, E.; van Dongen, J.; Lent, S.; Nedeljkovic, I.; Ligthart, S.; Tsai, P.-C.; Martin, T.C.; Mandaviya, P.R.; Jansen, R.; et al. An Integrative Cross-Omics Analysis of DNA Methylation Sites of Glucose and Insulin Homeostasis. Nat. Commun. 2019, 10, 2581. [Google Scholar] [CrossRef]
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.S.; de Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.M.; Jackson, C.J.; de Azevedo Maia, G.L.; et al. Lupeol, a Dietary Triterpene, Enhances Wound Healing in Streptozotocin-Induced Hyperglycemic Rats with Modulatory Effects on Inflammation, Oxidative Stress, and Angiogenesis. Oxid. Med. Cell Longev. 2019, 2019, 3182627. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, C.; Yu, T.; Zhu, D. Quantitative Evaluation of Skin Disorders in Type 1 Diabetic Mice by in vivo Optical Imaging. Biomed. Opt. Express 2019, 10, 2996–3008. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Brazel, C.B.; Simon, J.C.; Tuckermann, J.P.; Saalbach, A. Inhibition of 11β-HSD1 Expression by Insulin in Skin: Impact for Diabetic Wound Healing. J. Clin. Med. 2020, 9, 3878. [Google Scholar] [CrossRef]
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Anuar, M.R.; Karim, S.; Ong, S.K.; Yusof, F.A.M.; Tan, W.-N.; Sulaiman, B.; Ooi, M.L.; et al. Homalomena Pineodora Essential Oil Nanoparticle Inhibits Diabetic Wound Pathogens. Sci. Rep. 2020, 10, 3307. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF Loaded Electrospun Dual Porous Core-Shell Membrane for Diabetic Wound Healing. Int. J. Nanomed. 2019, 14, 8573–8588. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, P.; Chen, H.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Jin, L.; Sun, D. Advances in LncRNAs from Stem Cell-Derived Exosome for the Treatment of Cardiovascular Diseases. Front. Pharmacol. 2022, 13, 986683. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, S.; Wu, Z.; Li, Q.; Ren, S.; Chen, J.; Xu, X.; Wang, C.; Lu, C.; Yang, X.; et al. ADSC-Exo@MMP-PEG Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio 2022, 16, 100365. [Google Scholar] [CrossRef] [PubMed]
- Tilves, C.M.; Zmuda, J.M.; Kuipers, A.L.; Nestlerode, C.S.; Evans, R.W.; Bunker, C.H.; Patrick, A.L.; Miljkovic, I. Association of Lipopolysaccharide-Binding Protein with Aging-Related Adiposity Change and Prediabetes Among African Ancestry Men. Diabetes Care 2016, 39, 385. [Google Scholar] [CrossRef]
- Ji, H.; Peng, R.; Jin, L.; Ma, J.; Yang, Q.; Sun, D.; Wu, W. Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications. Pharmaceutics 2021, 13, 1452. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Y.; Wei, C.; Zhao, X.; Zhou, Q.; Xie, L. DNase I Improves Corneal Epithelial and Nerve Regeneration in Diabetic Mice. J. Cell Mol. Med. 2020, 24, 4547–4556. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ogawa, R. Role of Inflammasomes in Keloids and Hypertrophic Scars—Lessons Learned from Chronic Diabetic Wounds and Skin Fibrosis. Int. J. Mol. Sci. 2022, 23, 6820. [Google Scholar] [CrossRef] [PubMed]
- Bodman, M.A.; Varacallo, M. Peripheral Diabetic Neuropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chong, H.C.; Chan, J.S.K.; Goh, C.Q.; Gounko, N.V.; Luo, B.; Wang, X.; Foo, S.; Wong, M.T.C.; Choong, C.; Kersten, S.; et al. Angiopoietin-like 4 Stimulates STAT3-Mediated INOS Expression and Enhances Angiogenesis to Accelerate Wound Healing in Diabetic Mice. Mol. Ther. 2014, 22, 1593–1604. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and Antiseptic Strategies in Wound Management. Int. Wound J. 2013, 10 (Suppl. 1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Urao, N.; Koh, T.J. Proliferation of Ly6C+ Monocytes/Macrophages Contributes to Their Accumulation in Mouse Skin Wounds. J. Leukoc. Biol. 2020, 107, 551–560. [Google Scholar] [CrossRef]
- Icli, B.; Nabzdyk, C.S.; Lujan-Hernandez, J.; Cahill, M.; Auster, M.E.; Wara, A.; Sun, X.; Ozdemir, D.; Giatsidis, G.; Orgill, D.P.; et al. Regulation of Impaired Angiogenesis in Diabetic Dermal Wound Healing by MicroRNA-26a. J. Mol. Cell Cardiol. 2016, 91, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yin, H.; Chen, X.; Chen, T.-H.; Liu, H.-M.; Rao, S.-S.; Tan, Y.-J.; Qian, Y.-X.; Liu, Y.-W.; Hu, X.-K.; et al. Ångstrom-Scale Silver Particle–Embedded Carbomer Gel Promotes Wound Healing by Inhibiting Bacterial Colonization and Inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Rao, S.-S.; Ren, L.; Hu, X.-K.; Tan, Y.-J.; Hu, Y.; Luo, J.; Liu, Y.-W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from Human Urine-Derived Stem Cells Facilitates Diabetic Wound Repair by Promoting Angiogenesis. Theranostics 2018, 8, 1607–1623. [Google Scholar] [CrossRef]
- Seo, G.Y.; Ho, M.T.; Bui, N.T.; Kim, Y.M.; Koh, D.; Lim, Y.; Hyun, C.; Cho, M. Novel Naphthochalcone Derivative Accelerate Dermal Wound Healing through Induction of Epithelial-Mesenchymal Transition of Keratinocyte. J. Biomed. Sci. 2015, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential Glucose Requirement in Skin Homeostasis and Injury Identifies a Therapeutic Target for Psoriasis. Nat. Med. 2018, 24, 617–627. [Google Scholar] [CrossRef]
- Pedowitz, N.J.; Batt, A.R.; Darabedian, N.; Pratt, M.R. MYPT1 O-GlcNAc Modification Regulates Sphingosine-1-Phosphate Mediated Contraction. Nat. Chem. Biol. 2021, 17, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nishikori, Y.; Shiota, N.; Okunishi, H. The Role of Mast Cells in Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Mice. Arch. Dermatol. Res. 2014, 306, 823–835. [Google Scholar] [CrossRef]
- Park, K.H.; Han, S.H.; Hong, J.P.; Han, S.-K.; Lee, D.-H.; Kim, B.S.; Ahn, J.H.; Lee, J.W. Topical Epidermal Growth Factor Spray for the Treatment of Chronic Diabetic Foot Ulcers: A Phase III Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Diabetes Res. Clin. Pract. 2018, 142, 335–344. [Google Scholar] [CrossRef]
- Olsen, A.S.; Sarras, M.P.; Leontovich, A.; Intine, R.V. Heritable Transmission of Diabetic Metabolic Memory in Zebrafish Correlates with DNA Hypomethylation and Aberrant Gene Expression. Diabetes 2012, 61, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.K.; Zammit, N.W.; Walters, S.N.; Koay, Y.C.; Wu, J.; Tan, B.M.; Villanueva, J.E.; Brink, R.; Loudovaris, T.; Cantley, J.; et al. Nuclear Factor ΚB-Inducing Kinase Activation as a Mechanism of Pancreatic β Cell Failure in Obesity. J. Exp. Med. 2015, 212, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, A.; Roy, N.; Bajwa, R.; Gut, P.; Lipson, K.; Yang, C.; Covassin, L.; Racki, W.J.; Rossini, A.A.; Phillips, N.; et al. Dynamic Glucoregulation and Mammalian-like Responses to Metabolic and Developmental Disruption in Zebrafish. Gen. Comp. Endocrinol. 2011, 170, 334–345. [Google Scholar] [CrossRef]
- Lai, A.K.W.; Lo, A.C.Y. Animal Models of Diabetic Retinopathy: Summary and Comparison. J. Diabetes Res. 2013, 2013, 106594. [Google Scholar] [CrossRef] [PubMed]
- Maierdiyali, A.; Wang, L.; Luo, Y.; Li, Z. Effect of Tank Size on Zebrafish Behavior and Physiology. Animals 2020, 10, 2353. [Google Scholar] [CrossRef]
- Andersson, O.; Adams, B.A.; Yoo, D.; Ellis, G.C.; Gut, P.; Anderson, R.M.; German, M.S.; Stainier, D.Y.R. Adenosine Signaling Promotes Regeneration of Pancreatic β-Cells in vivo. Cell Metab. 2012, 15, 885–894. [Google Scholar] [CrossRef]
- Moustaqil, M.; Fontaine, F.; Overman, J.; McCann, A.; Bailey, T.L.; Soto, P.R.; Bhumkar, A.; Giles, N.; Hunter, D.J.B.; Gambin, Y.; et al. Homodimerization Regulates an Endothelial Specific Signature of the SOX18 Transcription Factor. Nucleic Acids Res. 2018, 46, 11381–11395. [Google Scholar] [CrossRef]
- Soman, S.; Keatinge, M.; Moein, M.; Da Costa, M.; Mortiboys, H.; Skupin, A.; Sugunan, S.; Bazala, M.; Kuznicki, J.; Bandmann, O. Inhibition of the Mitochondrial Calcium Uniporter Rescues Dopaminergic Neurons in pink1−/− Zebrafish. Eur. J. Neurosci. 2017, 45, 528–535. [Google Scholar] [CrossRef]
- LeBert, D.C.; Huttenlocher, A. Inflammation and Wound Repair. Semin. Immunol. 2014, 26, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Jörgens, K.; Hillebrands, J.-L.; Hammes, H.-P.; Kroll, J. Zebrafish: A Model for Understanding Diabetic Complications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, F.J.; Martínez-Morcillo, F.J.; de Oliveira, S.; Candel, S.; Cabas, I.; García-Ayala, A.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Mesa-del-Castillo, P.; Cayuela, M.L.; et al. Hydrogen Peroxide in Neutrophil Inflammation: Lesson from the Zebrafish. Dev. Comp. Immunol. 2020, 105, 103583. [Google Scholar] [CrossRef]
- Yamamoto, D.; Sato, D.; Nakayama, H.; Nakagawa, Y.; Shimada, Y. ZF-Mapper: Simple and Complete Freeware for Fluorescence Quantification in Zebrafish Images. Zebrafish 2019, 16, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.; Matty, M.A.; Jurczyszak, D.; Gabor, K.A.; Millard, P.J.; Tobin, D.M.; Kim, C.H. Infectious Disease Models in Zebrafish. Methods Cell Biol. 2017, 138, 101–136. [Google Scholar] [CrossRef]
- Meng, H.; Shang, Y.; Cheng, Y.; Wang, K.; Yu, J.; Cao, P.; Fan, S.; Li, Y.; Cui, J. Knockout of Zebrafish Colony-Stimulating Factor 1 Receptor by CRISPR/Cas9 Affects Metabolism and Locomotion Capacity. Biochem. Biophys. Res. Commun. 2021, 551, 93–99. [Google Scholar] [CrossRef]
- Albadri, S.; Del Bene, F.; Revenu, C. Genome Editing Using CRISPR/Cas9-Based Knock-in Approaches in Zebrafish. Methods 2017, 121–122, 77–85. [Google Scholar] [CrossRef]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-Throughput Gene Targeting and Phenotyping in Zebrafish Using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef]
- van de Venter, M.; Didloff, J.; Reddy, S.; Swanepoel, B.; Govender, S.; Dambuza, N.S.; Williams, S.; Koekemoer, T.C.; Venables, L. Wild-Type Zebrafish (Danio rerio) Larvae as a Vertebrate Model for Diabetes and Comorbidities: A Review. Animals 2020, 11, 54. [Google Scholar] [CrossRef]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S. Skin Considerations for Older Adults with Wounds. Br. J. Community Nurs. 2019, 24, S15–S19. [Google Scholar] [CrossRef]
- Hyun, S.; Moffatt-Bruce, S.; Cooper, C.; Hixon, B.; Kaewprag, P. Prediction Model for Hospital-Acquired Pressure Ulcer Development: Retrospective Cohort Study. JMIR Med. Inf. 2019, 7, e13785. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-H.; Nam, H.-S.; Kang, D.-J. Efficacy Comparison Study of Human Epidermal Growth Factor (EGF) between Heberprot-P® and Easyef® in Adult Zebrafish and Embryo under Presence or Absence Combination of Diabetic Condition and Hyperlipidemia to Mimic Elderly Patients. Geriatrics 2022, 7, 45. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Cho, K.-H. A Point Mutant of Apolipoprotein A-I (V156K) Showed Enhancement of Cellular Insulin Secretion and Potent Activity of Facultative Regeneration in Zebrafish. Rejuvenation Res. 2012, 15, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sarras, M.P.; Leontovich, A.A.; Olsen, A.S.; Intine, R.V. Impaired Tissue Regeneration Corresponds with Altered Expression of Developmental Genes That Persists in the Metabolic Memory State of Diabetic Zebrafish. Wound Repair Regen 2013, 21, 320–328. [Google Scholar] [CrossRef]
- Sarras, M.P.; Mason, S.; McAllister, G.; Intine, R.V. Inhibition of Poly-ADP Ribose Polymerase Enzyme Activity Prevents Hyperglycemia-Induced Impairment of Angiogenesis during Wound Healing: Angiogenesis Restored by Parp Inhibition. Wound Repair Regen 2014, 22, 666–670. [Google Scholar] [CrossRef]
- Olsen, A.S.; Sarras, M.P.; Intine, R.V. Limb Regeneration Is Impaired in an Adult Zebrafish Model of Diabetes Mellitus. Wound Repair Regen 2010, 18, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.Y.G.; Hui, M.N.Y.; Li, L.; Lee, S.M.Y.; Leung, A.Y.H.; Cheng, S.H. Angiogenic Efficacy of Simplified 2-Herb Formula (NF3) in Zebrafish Embryos in vivo and Rat Aortic Ring in vitro. J. Ethnopharmacol. 2012, 139, 447–453. [Google Scholar] [CrossRef]
- Wibowo, I.; Utami, N.; Anggraeni, T.; Barlian, A.; Putra, R.E.; Indriani, A.D.; Masadah, R.; Ekawardhani, S. Propolis Can Improve Caudal Fin Regeneration in Zebrafish (Danio rerio) Induced by The Combined Administration of Alloxan and Glucose. Zebrafish 2021, 18, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Cholan, P.M.; Britton, W.J.; Oehlers, S.H. Glucose Inhibits Haemostasis and Accelerates Diet-Induced Hyperlipidaemia in Zebrafish Larvae. Sci. Rep. 2021, 11, 19049. [Google Scholar] [CrossRef]
- Ennerfelt, H.; Voithofer, G.; Tibbo, M.; Miller, D.; Warfield, R.; Allen, S.; Kennett Clark, J. Disruption of Peripheral Nerve Development in a Zebrafish Model of Hyperglycemia. J. Neurophysiol. 2019, 122, 862–871. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, X.; Chen, B.; Zhang, Y.; Zu, Y.; Li, W. Zebrafish as a Useful Model for Zoonotic Vibrio Parahaemolyticus Pathogenicity in Fish and Human. Dev. Comp. Immunol. 2016, 55, 159–168. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Su, Y.; Wang, D.; Yin, B.; Shu, B.; Zhang, J.; Zhu, X.; Jia, C. Characterization of Mycobacterium Marinum Infections in Zebrafish Wounds and Sinus Tracts. Wound Repair Regen 2017, 25, 536–540. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.-A.; Kang, D.-H.; De Zoysa, M. Spirulina Maxima Derived Marine Pectin Promotes the in vitro and in vivo Regeneration and Wound Healing in Zebrafish. Fish Shellfish Immunol. 2020, 107, 414–425. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W. Reinforcement of Polycaprolactone/Chitosan with Nanoclay and Controlled Release of Curcumin for Wound Dressing. ACS Omega 2019, 4, 22292–22301. [Google Scholar] [CrossRef] [PubMed]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Che Mohamad, C.A.; Wan Sulaiman, W.M.A.; Abdul Wahab, R.; Ahmed, Q.U.; Abdul Ghaffar, M.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria Malayana Jack Leaf Extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of Hyperglycaemia in Zebrafish (Danio rerio) Leads to Morphological Changes in the Retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Wu, W.; Fang, Y.; Liu, F.; Yang, Q.; Hu, X.; Gu, X.; He, Z.; Sun, D.; et al. Effect of Aerobic Exercise as a Treatment on Type 2 Diabetes Mellitus with Depression-like Behavior Zebrafish. Life Sci. 2022, 300, 120578. [Google Scholar] [CrossRef]
- Goto, S.; Setoguchi, S.; Yamakawa, H.; Watase, D.; Terada, K.; Matsunaga, K.; Karube, Y.; Takata, J. Prodrugs for Skin Delivery of Menahydroquinone-4, an Active Form of Vitamin K2(20), Could Overcome the Photoinstability and Phototoxicity of Vitamin K2(20). Int. J. Mol. Sci. 2019, 20, 2548. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Yang, W.-Y.; Cheng, C.-C.; Hsiao, M.-C.; Tsai, S.-L.; Lin, H.-K.; Lin, K.-H.; Yuh, C.-H. Low Molecular Weight Fucoidan Prevents Radiation-Induced Fibrosis and Secondary Tumors in a Zebrafish Model. Cancers 2020, 12, 1608. [Google Scholar] [CrossRef]
- Seo, S.B.; Dananjaya, S.H.S.; Nikapitiya, C.; Park, B.K.; Gooneratne, R.; Kim, T.-Y.; Lee, J.; Kim, C.-H.; De Zoysa, M. Silver Nanoparticles Enhance Wound Healing in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2017, 68, 536–545. [Google Scholar] [CrossRef]
- Georgantzoglou, A.; Poplimont, H.; Walker, H.A.; Lämmermann, T.; Sarris, M. A Two-Step Search and Run Response to Gradients Shapes Leukocyte Navigation in vivo. J. Cell Biol. 2022, 221, e202103207. [Google Scholar] [CrossRef] [PubMed]
| Inducement | Occurrence Stage | Mechanism | References |
|---|---|---|---|
| Persistent bacterial infection and biofilm | The whole process | Low CD4+ T cell counts; | [6] |
| insufficient blood perfusion; | [14] | ||
| abnormal pH (slightly alkaline); | [15] | ||
| Abnormal function of mast cells. | [16] | ||
| Massive infiltration of neutrophils | Inflammation | Excessive neutrophil extracellular traps (NETs); | [7] |
| Weak scavenging effect of macrophages; | [17] | ||
| high pro-inflammatory factors levels; reactive oxygen species (ROS) vicious circle. | [18] | ||
| More pro-inflammatory macrophages and insufficient activity of anti-inflammatory macrophages | Inflammation | Impaired phagocytosis; decreased ability to polarize to anti-inflammatory state; excessive accumulation of AGEs. | [19,20,21,22] |
| Hyperkeratosis and insufficiency of wound margin; Stagnant reepithelialization | Proliferation | Weak migration ability of keratinocytes; Weak induction of regulated growth factors; Weak keratinocyte growth factors and fibroblast growth factors (FGFs) in wound; abnormal expression of miRNA. | [23], [24] |
| Defect of angiogenesis and local oxygen deficiency | Proliferation | Low expression of insulin-like growth factors-1 (IGF-1) and abnormal function of vascular endothelial cells; | [7] |
| low vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2) levels; | [25] | ||
| fibrin restricts angiogenesis. | [26] | ||
| Delayed production of ECM and limited wound contraction | Proliferation and remodeling | Fibroblasts senescence; no response to growth factors such as FGF; less transformation to myofibroblasts. | [7], [27] |
| Delayed maturation of scar tissue | Remodeling | High level of matrix metalloproteinases (MMPs); low level of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1); influence of microbial community. | [5], [7], [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Ma, J.; Fang, Y.; Lei, P.; Wang, L.; Qu, L.; Wu, W.; Jin, L.; Sun, D. Advances in Zebrafish for Diabetes Mellitus with Wound Model. Bioengineering 2023, 10, 330. https://doi.org/10.3390/bioengineering10030330
Lin B, Ma J, Fang Y, Lei P, Wang L, Qu L, Wu W, Jin L, Sun D. Advances in Zebrafish for Diabetes Mellitus with Wound Model. Bioengineering. 2023; 10(3):330. https://doi.org/10.3390/bioengineering10030330
Chicago/Turabian StyleLin, Bangchang, Jiahui Ma, Yimeng Fang, Pengyu Lei, Lei Wang, Linkai Qu, Wei Wu, Libo Jin, and Da Sun. 2023. "Advances in Zebrafish for Diabetes Mellitus with Wound Model" Bioengineering 10, no. 3: 330. https://doi.org/10.3390/bioengineering10030330
APA StyleLin, B., Ma, J., Fang, Y., Lei, P., Wang, L., Qu, L., Wu, W., Jin, L., & Sun, D. (2023). Advances in Zebrafish for Diabetes Mellitus with Wound Model. Bioengineering, 10(3), 330. https://doi.org/10.3390/bioengineering10030330









