The Role of Fascial Tissue Layer in Electric Signal Transmission from the Forearm Musculature to the Cutaneous Layer as a Possibility for Increased Signal Strength in Myoelectric Forearm Exoprosthesis Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Subject Selection
2.2. sEMG Measurements
- (a)
- Measurement of anthropometric and kinematic data—the largest circumference in the proximal third of the forearm, as well as in the middle and distal third, adipose layer thickness in the proximal, middle and distal third of the distal forearm, elbow flexion deficit, elbow extension deficit and the degree of prono-supination. The adipose layer was measured using standard calipers, and the flexion, extension, pronation and supination angles were determined using a standard medical goniometer.
- (b)
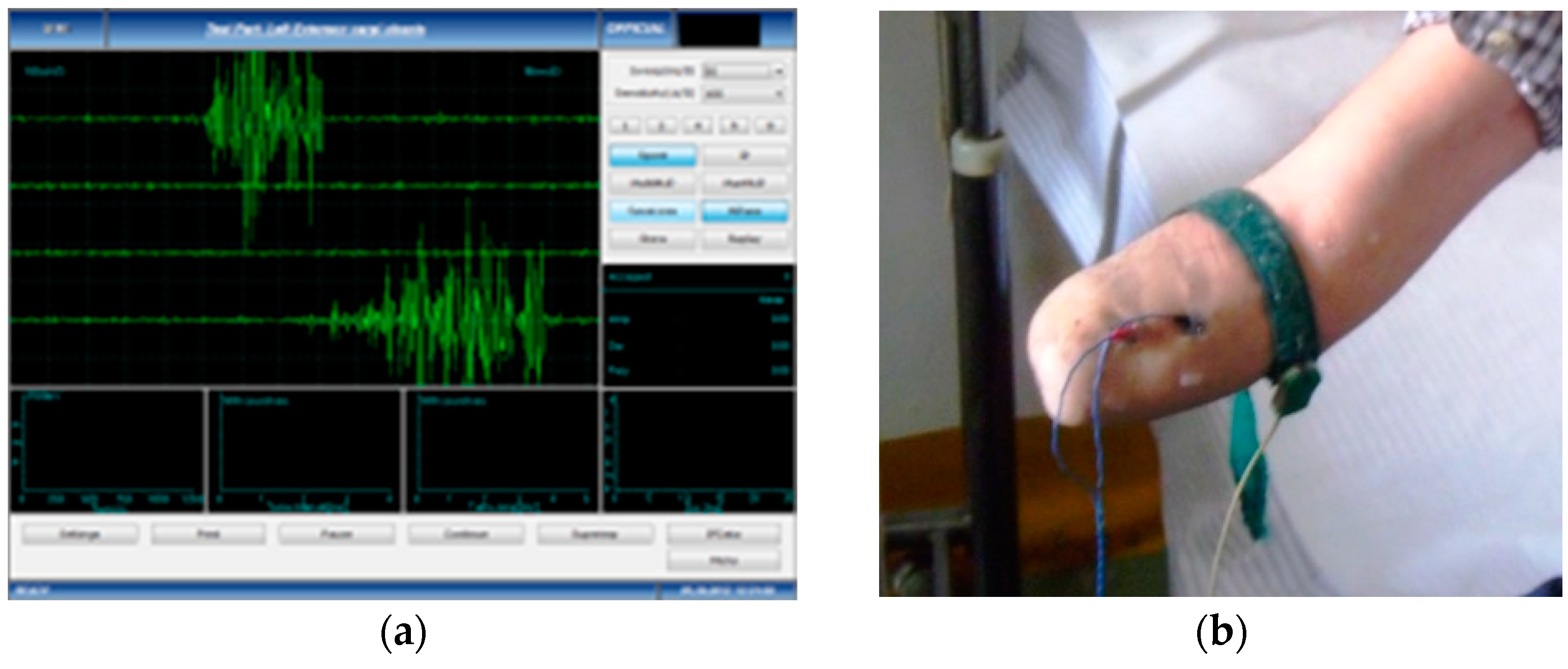
- Recording of EMG data—both the amputation stumps as well as the healthy forearms were tested using a standard EMG for the retrieval of surface-level myoelectric signals; two models were used: Alpin Biomed Keypoint 4 and Anjue CMS-6600. The initial stage, that of measuring the EMG signals, was conducted for all 6 participants in the study, both the amputated forearms as well as the healthy ones. The muscles that contribute to the major movement of the forearm and of the hand were selected for recording of the EMG data, one muscle representing each of the three compartments of the forearm: the flexor carpi radialis muscle from the anterior compartment, in the superficial plane and innervated by the radial nerve; the brahioradialis muscle from the lateral compartment, in the superficial plane and innervated by the radial nerve; and the extensor digitorum communis muscle, in the superficial plane, innervated by the radial nerve.
- (c)
- Skin preparation for both the amputated and non-amputated limbs—where it was needed, the amputation stump was cleaned of excessive hair follicles, if these prevented the surface EMG electrode from adhering to the skin, then a 72% alcoholic solution was used locally to remove excess sebum. The electrodes were applied to the surface of the amputation stumps, as well as to the unaffected forearms, in corresponding areas, to pick up the signals emitted by the same muscle. For the test subject with the bilateral amputation, both stumps were tested, while the healthy subject was tested for both unaffected forearms.
- (d)
- Data acquisition and processing of the EMG signal—the subjects performed voluntary isometric contractions, with 1 s intervals, for 30 to 60 s, each in accordance with their individual capacity for effort (Figure 1). The signals were imported in MathWorks MatLab software for processing (https://www.mathworks.com/products/matlab.html (accessed on 5 January 2023)) to eliminate the acquisition errors and to highlight the differences between the first set of data (Group 1, the amputation stumps) and the second set of data (Group 2, the unaffected forearms). Using the Biopac Systems AcqKnowledge software (https://www.biopac.com/product/acqknowledge-software/ (accessed on 5 January 2023)), the mean value of the amplitudes in effective contraction intervals were calculated, where an effective contraction interval is defined as the interval of voluntary muscle contraction that produces a distinct signal that is strong enough to be picked up by surface EMG sensors.
2.3. Signal Processing
2.4. The Virtual Reality Training Protocol
2.5. sEMG Interface
2.6. The Experimental Myoprosthesis
2.7. Virtual Reality Components
2.8. Signal Modulation
2.9. The Individual Training Sessions
3. Results
3.1. General Subject Description
3.2. Anatomical Characteristics
3.3. Mean sEMG Amplitude Values
3.4. The CONM Patient Data and Procedure
4. Discussion
Limitations of the Current Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zlotolow, D.A.; Kozin, S.H. Advances in Upper Extremity Prosthetics. Hand Clin. 2012, 28, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.L.; Lura, D.J.; Highsmith, M.J. Differences in myoelectric and body-powered upper-limb prostheses: Systematic literature review. J. Rehabil. Res. Dev. 2015, 52, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Aszmann, O.C.; Dietl, H.; Frey, M. Selective nerve transfers to improve the control of myoelectrical arm prostheses. Handchir. Mikrochir. Plast. Chir. 2008, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Lipschutz, R.D.; Stubblefield, K.A.; Lock, B.A.; Huang, H.; Williams, T.W., 3rd; Weir, R.F.; Kuiken, T.A. Control of a Six Degree of Freedom Prosthetic Arm After Targeted Muscle Reinnervation Surgery. Arch. Phys. Med. Rehabil. 2008, 89, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Daley, H.; Englehart, K.; Hargrove, L.; Kuruganti, U. High density electromyography data of normally limbed and transradial amputee subjects for multifunction prosthetic control. J. Electromyogr. Kinesiol. 2012, 22, 478–484. [Google Scholar] [CrossRef]
- Stock, M.S.; Thompson, B.J. Adipose tissue thickness does not affect the electromechanical delay. Physiol. Meas. 2016, 37, 418–428. [Google Scholar] [CrossRef]
- Register GCT. The Circumferential Osteoneuromyoplasty Method of Forearm Amputation or Stump Retouch. 2022. Available online: https://drks.de/search/en/trial/DRKS00004868 (accessed on 5 January 2023).
- Kuiken, T.A.; Lowery, M.M.; Stoykov, N.S. The effect of subcutaneous fat on myoelectric signal amplitude and cross-talk. Prosthet. Orthot. Int. 2003, 27, 48–54. [Google Scholar] [CrossRef]
- Lanza, M.B.; Balshaw, T.G.; Massey, G.J.; Folland, J.P. Does normalization of voluntary EMG amplitude to MMAX account for the influence of electrode location and adiposity? Scand. J. Med. Sci. Sports 2018, 28, 2558–2566. [Google Scholar] [CrossRef]
- Teklemariam, A.; Hodson-Tole, E.F.; Reeves, N.D.; Costen, N.P.; Cooper, G. A Finite Element Model Approach to Determine the Influence of Electrode Design and Muscle Architecture on Myoelectric Signal Properties. PLoS ONE 2016, 11, e0148275. [Google Scholar] [CrossRef]
- Minetto, M.A.; Botter, A.; Šprager, S.; Agosti, F.; Patrizi, A.; Lanfranco, F.; Sartorio, A. Feasibility study of detecting surface electromyograms in severely obese patients. J. Electromyogr. Kinesiol. 2013, 23, 285–295. [Google Scholar] [CrossRef]
- Pan, L.; Liu, K.; Li, J. Effect of Subcutaneous Muscle Displacement of Flexor Carpi Radialis on Surface Electromyography. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Guidolin, D.; Fede, C.; Macchi, V.; De Caro, R.; Stecco, C. Ultrasound Imaging of Brachial and Antebrachial Fasciae. Diagnostics 2021, 11, 2261. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Pirri, N.; Porzionato, A.; Boscolo-Berto, R.; De Caro, R.; Stecco, C. Inter- and Intra-Rater Reliability of Ultrasound Measurements of Superficial and Deep Fasciae Thickness in Upper Limb. Diagnostics 2022, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Yu, L.; Geng, Y. Conditioning and Sampling Issues of EMG Signals in Motion Recognition of Multifunctional Myoelectric Prostheses. Ann. Biomed. Eng. 2011, 39, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; De Caro, R.; Stecco, C. Ultrasound Imaging of the Superficial Fascia in the Upper Limb: Arm and Forearm. Diagnostics 2022, 12, 1884. [Google Scholar] [CrossRef] [PubMed]
- Merrill, D.R.; Lockhart, J.; Troyk, P.R.; Weir, R.F.; Hankin, D.L. Development of an Implantable Myoelectric Sensor for Advanced Prosthesis Control. Artif. Organs 2011, 35, 249–252. [Google Scholar] [CrossRef]
- Baker, J.J.; Scheme, E.; Englehart, K.; Hutchinson, D.T.; Greger, B. Continuous Detection and Decoding of Dexterous Finger Flexions with Implantable MyoElectric Sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 424–432. [Google Scholar] [CrossRef]
- Cipriani, C.; Segil, J.L.; Birdwell, J.A.; Weir, R.F.F. Dexterous Control of a Prosthetic Hand Using Fine-Wire Intramuscular Electrodes in Targeted Extrinsic Muscles. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 828–836. [Google Scholar] [CrossRef]
- Ruff, R.; Poppendieck, W.; Gail, A.; Westendorff, S.; Russold, M.; Lewis, S.; Meiners, T.; Hoffmann, K.-P. Acquisition of myoelectric signals to control a hand prosthesis with implantable epimysial electrodes. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5070–5073. [Google Scholar] [CrossRef]
- Hahne, J.M.; Farina, D.; Jiang, N.; Liebetanz, D. A Novel Percutaneous Electrode Implant for Improving Robustness in Advanced Myoelectric Control. Front. Neurosci. 2016, 10, 114. [Google Scholar] [CrossRef]
- Culjat, M.O.; Son, J.; Fan, R.E.; Wottawa, C.; Bisley, J.W.; Grundfest, W.S.; Dutson, E.P. Remote tactile sensing glove-based system. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1550–1554. [Google Scholar] [CrossRef]
- Pasquina, P.F.; Evangelista, M.; Carvalho, A.; Lockhart, J.; Griffin, S.; Nanos, G.; McKay, P.; Hansen, M.; Ipsen, D.; Vandersea, J.; et al. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand. J. Neurosci. Methods 2015, 244, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Franti, E.; Milea, L.; Dascalu, M.; Moga, M.; Florea, C.; Barbilian, A.; Teodorescu, M.; Schiopu, P.; Lazo, F.; Pogarasteanu, M. Personalized Support System for the Patients with Forearm Amputations. Rom. J. Inf. Sci. Technol. 2012, 15, 368–376. [Google Scholar]
- Franti, E.; Milea, L.; Buţu, V.; Cismas, S.; Lungu, M.; Şchiopu, P.; Barbilian, A.; Plavitu, A. Methods of Acquisition and Signal Processing for Myoelectric Control of Artificial Arms. Rom. J. Inf. Sci. Technol. 2012, 15, 91–105. [Google Scholar]
- Jiang, N.; Vest-Nielsen, J.L.; Muceli, S.; Farina, D. EMG-based simultaneous and proportional estimation of wrist/hand kinematics in uni-lateral trans-radial amputees. J. Neuroeng. Rehabil. 2012, 9, 42. [Google Scholar] [CrossRef]
- Ranger, B.J.; Feigin, M.; Zhang, X.; Moerman, K.M.; Herr, H.; Anthony, B.W. 3D Ultrasound Imaging of Residual Limbs with Camera-Based Motion Compensation. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.R.; Al-Timemy, A.H.; Nguyen, H.T. Transradial Amputee Gesture Classification Using an Optimal Number of sEMG Sensors: An Approach Using ICA Clustering. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 837–846. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, F.; Yang, L.; Zhang, Y.; Li, G. Reduction of the effect of arm position variation on real-time performance of motion classification. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2772–2775. [Google Scholar] [CrossRef]
- Adewuyi, A.A.; Hargrove, L.J.; Kuiken, T.A. Resolving the effect of wrist position on myoelectric pattern recognition control. J. Neuroeng. Rehabil. 2017, 14, 39. [Google Scholar] [CrossRef]
- Muceli, S.; Farina, D. Simultaneous and Proportional Estimation of Hand Kinematics from EMG during Mirrored Movements at Multiple Degrees-of-Freedom. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 20, 371–378. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Zhang, Z.; Geng, Y.; Zhou, R. Selection of sampling rate for EMG pattern recognition based prosthesis control. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5058–5061. [Google Scholar] [CrossRef]
- Li, X.; Samuel, O.W.; Zhang, X.; Wang, H.; Fang, P.; Li, G. A motion-classification strategy based on sEMG-EEG signal combination for upper-limb amputees. J. Neuroeng. Rehabil. 2017, 14, 2. [Google Scholar] [CrossRef]
- Li, G.; Schultz, A.E.; Kuiken, T.A. Quantifying Pattern Recognition-Based Myoelectric Control of Multifunctional Transradial Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 185–192. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Li, G.; Englehart, K.B.; Hudgins, B.S. Principal components analysis preprocessing for improved classification accuracies in pattern-recognition-based myoelectric control. IEEE Trans. Biomed. Eng. 2009, 56, 1407–1414. [Google Scholar] [CrossRef]
- McClanahan, A.; Moench, M.; Fu, Q. Dimensionality analysis of forearm muscle activation for myoelectric control in transradial amputees. PLoS ONE 2020, 15, e0242921. [Google Scholar] [CrossRef]
- Bellingegni, A.D.; Gruppioni, E.; Colazzo, G.; Davalli, A.; Sacchetti, R.; Guglielmelli, E.; Zollo, L. NLR, MLP, SVM, and LDA: A comparative analysis on EMG data from people with trans-radial amputation. J. Neuroeng. Rehabil. 2017, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Amsüss, S. Reflections on the present and future of upper limb prostheses. Expert Rev. Med. Devices 2016, 13, 321–324. [Google Scholar] [CrossRef]
- Vujaklija, I.; Farina, D.; Aszmann, O. New developments in prosthetic arm systems. Orthop. Res. Rev. 2016, 8, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, K.A.; Shin, A.Y.; Kaufman, K.R. Interfaces with the peripheral nervous system for the control of a neuroprosthetic limb: A review. J. Neuroeng. Rehabil. 2020, 17, 43. [Google Scholar] [CrossRef]
- Mereu, F.; Leone, F.; Gentile, C.; Cordella, F.; Gruppioni, E.; Zollo, L. Control Strategies and Performance Assessment of Upper-Limb TMR Prostheses: A Review. Sensors 2021, 21, 1953. [Google Scholar] [CrossRef] [PubMed]
- Myers, H.; Lu, D.; Gray, S.J.; Bruscino-Raiola, F. Targeted muscle reinnervation to improve electromyography signals for advanced myoelectric prosthetic limbs: A series of seven patients. ANZ J. Surg. 2020, 90, 591–596. [Google Scholar] [CrossRef]
- Geng, Y.; Zhou, P.; Li, G. Toward attenuating the impact of arm positions on electromyography pattern-recognition based motion classification in transradial amputees. J. Neuroeng. Rehabil. 2012, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Samuel, O.W.; Wei, Y.; Li, G. Improving the Robustness of Real-Time Myoelectric Pattern Recognition against Arm Position Changes in Transradial Amputees. BioMed Res. Int. 2017, 2017, 5090454. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Samuel, O.W.; Lin, C.; Asogbon, M.G.; Fang, P.; Idowu, P.O. Realizing Efficient EMG-Based Prosthetic Control Strategy. In Neural Interface: Frontiers and Applications Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1101. [Google Scholar]
- Parajuli, N.; Sreenivasan, N.; Bifulco, P.; Cesarelli, M.; Savino, S.; Niola, V.; Esposito, D.; Hamilton, T.J.; Naik, G.R.; Gunawardana, U.; et al. Real-Time EMG Based Pattern Recognition Control for Hand Prostheses: A Review on Existing Methods, Challenges and Future Implementation. Sensors 2019, 19, 4596. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; A Kuiken, T. EMG pattern recognition control of multifunctional prostheses by transradial amputees. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6914–6917. [Google Scholar] [CrossRef]
- Vidovic, M.M.-C.; Hwang, H.-J.; Amsuss, S.; Hahne, J.M.; Farina, D.; Muller, K.-R. Improving the Robustness of Myoelectric Pattern Recognition for Upper Limb Prostheses by Covariate Shift Adaptation. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Tkach, D.C.; Young, A.J.; Smith, L.H.; Rouse, E.J.; Hargrove, L.J. Real-Time and Offline Performance of Pattern Recognition Myoelectric Control Using a Generic Electrode Grid with Targeted Muscle Reinnervation Patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Samuel, O.W.; Li, X.; Geng, Y.; Asogbon, M.G.; Fang, P.; Huang, Z.; Li, G. Resolving the adverse impact of mobility on myoelectric pattern recognition in upper-limb multifunctional prostheses. Comput. Biol. Med. 2017, 90, 76–87. [Google Scholar] [CrossRef]
- Resnik, L.; Huang, H.H.; Winslow, A.; Crouch, D.L.; Zhang, F.; Wolk, N. Evaluation of EMG pattern recognition for upper limb prosthesis control: A case study in comparison with direct myoelectric control. J. Neuroeng. Rehabil. 2018, 15, 23. [Google Scholar] [CrossRef]
- Cipriani, C.; Antfolk, C.; Controzzi, M.; Lundborg, G.; Rosen, B.; Carrozza, M.C.; Sebelius, F. Online Myoelectric Control of a Dexterous Hand Prosthesis by Transradial Amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 260–270. [Google Scholar] [CrossRef]
- Hashim, N.A.; Abd Razak, N.A.; Gholizadeh, H.; Abu Osman, N.A. Video Game-Based Rehabilitation Approach for Individuals Who Have Undergone Upper Limb Amputation: Case-Control Study. JMIR Serious Games 2021, 9, e17017. [Google Scholar] [CrossRef]
- Das, N.; Nagpal, N.; Bankura, S.S. A review on the advancements in the field of upper limb prosthesis. J. Med. Eng. Technol. 2018, 42, 532–545. [Google Scholar] [CrossRef]
- Dawson, M.; Carey, J.; Fahimi, F. Myoelectric training systems. Expert Rev. Med. Devices 2011, 8, 581–589. [Google Scholar] [CrossRef]



| Stump No. | Subject | Amputation Level | Amputation Side | Time from the Amputation | Surgical Indication | Type of Amputation | Posttraumatic Scar |
|---|---|---|---|---|---|---|---|
| 1 | F.B. | forearm proximal 1/3 | right | 5 years | trauma | standard | no |
| 2 | F.B. | forearm middle 1/3 | left | 5 years | trauma | standard | no |
| 3 | M.G. | forearm middle 1/3 | right | 22 years | trauma | standard | no |
| 4 | E.C. | forearm distal 1/3 | right | 3 years | infection | standard/CONM | no |
| 5 | S.B. | forearm distal 1/3 | right | 8 months | trauma | standard | yes |
| 6 | V.R. | midcarpal | right | 12 years | trauma | standard | no |
| Subject No. | Subject | Age (Years) | Sex | Height (cm) | Weight | Amputation Level | Unilateral/Bilateral/No Amputation |
|---|---|---|---|---|---|---|---|
| 1 | F.B. | 32 | M | 179 | 86 | forearm/forearm | bilateral |
| 2 | M.G. | 59 | M | 171 | 78 | forearm | unilateral |
| 3 | S.B. | 35 | M | 178 | 82 | forearm | unilateral |
| 4 | V.R. | 47 | M | 174 | 77 | midcarpal | unilateral |
| 5 | E.C. | 37 | M | 176 | 74 | forearm | unilateral |
| 6 | R.I. | 32 | M | 174 | 69 | no amputation | no amputation |
| Criteria | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Circumference proximal 1/3 in mm | 240 | 267 | 253 | 250 | 248 | 249 |
| Circumference middle 1/3 in mm | 137 | 176 | 173 | 165 | - | 198 |
| Circumference distal 1/3 in mm | - | 120 | 113 | - | - | 114 |
| Fat layer thickness proximal 1/3 (mm) | 11 | 12 | 9 | 10 | 12 | 12 |
| Fat layer thickness middle 1/3 (mm) | 11 | 8 | 10 | 9 | - | 10 |
| Fat layer thickness distal 1/3 (mm) | - | 4 | 3 | - | - | 3 |
| Flexion deficit—elbow | <5° | <5° | <5° | <5° | <5° | <5° |
| Extension deficit—elbow | <5° | <5° | <5° | <5° | <5° | <5° |
| Prono-supination deficit | <5° | <5° | <5° | <5° | <5° | <5° |
| Criteria | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Circumference proximal 1/3 in mm | 280 | 323 | 277 | 275 | 312 | 256 |
| Circumference middle 1/3 in mm | 224 | 240 | 214 | 210 | 254 | 210 |
| Circumference distal 1/3 in mm | 161 | 165 | 142 | 141 | 173 | 154 |
| Fat layer thickness proximal 1/3 (mm) | 12 | 14 | 12 | 11 | 13 | 14 |
| Fat layer thickness middle 1/3 (mm) | 12 | 10 | 11 | 10 | - | 12 |
| Fat layer thickness distal 1/3 (mm) | - | 3 | 4 | - | - | 3 |
| Flexion deficit—elbow (degrees) | <5° | <5° | <5° | <5° | <5° | <5° |
| Extension deficit—elbow (degrees) | <5° | <5° | <5° | <5° | <5° | <5° |
| Prono-supination deficit (degrees) | <5° | <5° | <5° | <5° | <5° | <5° |
| Case No. | Healthy Forearm | Amputation Stump | |
|---|---|---|---|
| Mean amplitude values for sEMG signals emitted by the flexor carpi radialis muscle | 1 | 4,6 nW | 0.043 nW |
| 2 | 4.0 nW | 0.037 nW | |
| 3 | 3.9 nW | 0.047 nW | |
| 4 | 4.3 nW | 0.062 nW | |
| 5 | 3.6 nW | 0.034 nW | |
| 6 | 4.8 nW | 0.046 nW | |
| Mean amplitude values for sEMG signals emitted by the brahioradialis muscle | 1 | 3.8 nW | 0.048 nW |
| 2 | 3.5 nW | 0.027 nW | |
| 3 | 4.5 nW | 0.043 nW | |
| 4 | 2.8 nW | 0.038 nW | |
| 5 | 3.4 nW | 0.042 nW | |
| 6 | 2.9 nW | 0.047 nW | |
| Mean amplitude values for sEMG signals emitted by the extensor digitorum communis muscle. | 1 | 4.3 nW | 0.043 nW |
| 2 | 4.8 nW | 0.047 nW | |
| 3 | 3.2 nW | 0.039 nW | |
| 4 | 4.6 nW | 0.055 nW | |
| 5 | 3.6 nW | 0.041 nW | |
| 6 | 4.5 nW | 0.038 nW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogarasteanu, M.-E.; Moga, M.; Barbilian, A.; Avram, G.; Dascalu, M.; Franti, E.; Gheorghiu, N.; Moldovan, C.; Rusu, E.; Adam, R.; et al. The Role of Fascial Tissue Layer in Electric Signal Transmission from the Forearm Musculature to the Cutaneous Layer as a Possibility for Increased Signal Strength in Myoelectric Forearm Exoprosthesis Development. Bioengineering 2023, 10, 319. https://doi.org/10.3390/bioengineering10030319
Pogarasteanu M-E, Moga M, Barbilian A, Avram G, Dascalu M, Franti E, Gheorghiu N, Moldovan C, Rusu E, Adam R, et al. The Role of Fascial Tissue Layer in Electric Signal Transmission from the Forearm Musculature to the Cutaneous Layer as a Possibility for Increased Signal Strength in Myoelectric Forearm Exoprosthesis Development. Bioengineering. 2023; 10(3):319. https://doi.org/10.3390/bioengineering10030319
Chicago/Turabian StylePogarasteanu, Mark-Edward, Marius Moga, Adrian Barbilian, George Avram, Monica Dascalu, Eduard Franti, Nicolae Gheorghiu, Cosmin Moldovan, Elena Rusu, Razvan Adam, and et al. 2023. "The Role of Fascial Tissue Layer in Electric Signal Transmission from the Forearm Musculature to the Cutaneous Layer as a Possibility for Increased Signal Strength in Myoelectric Forearm Exoprosthesis Development" Bioengineering 10, no. 3: 319. https://doi.org/10.3390/bioengineering10030319
APA StylePogarasteanu, M.-E., Moga, M., Barbilian, A., Avram, G., Dascalu, M., Franti, E., Gheorghiu, N., Moldovan, C., Rusu, E., Adam, R., & Orban, C. (2023). The Role of Fascial Tissue Layer in Electric Signal Transmission from the Forearm Musculature to the Cutaneous Layer as a Possibility for Increased Signal Strength in Myoelectric Forearm Exoprosthesis Development. Bioengineering, 10(3), 319. https://doi.org/10.3390/bioengineering10030319








