Differential Sensitization of Muscle versus Fascia in Individuals with Low Back Pain
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Oversight
2.2. Participants Recruitment and Clinical Evaluation
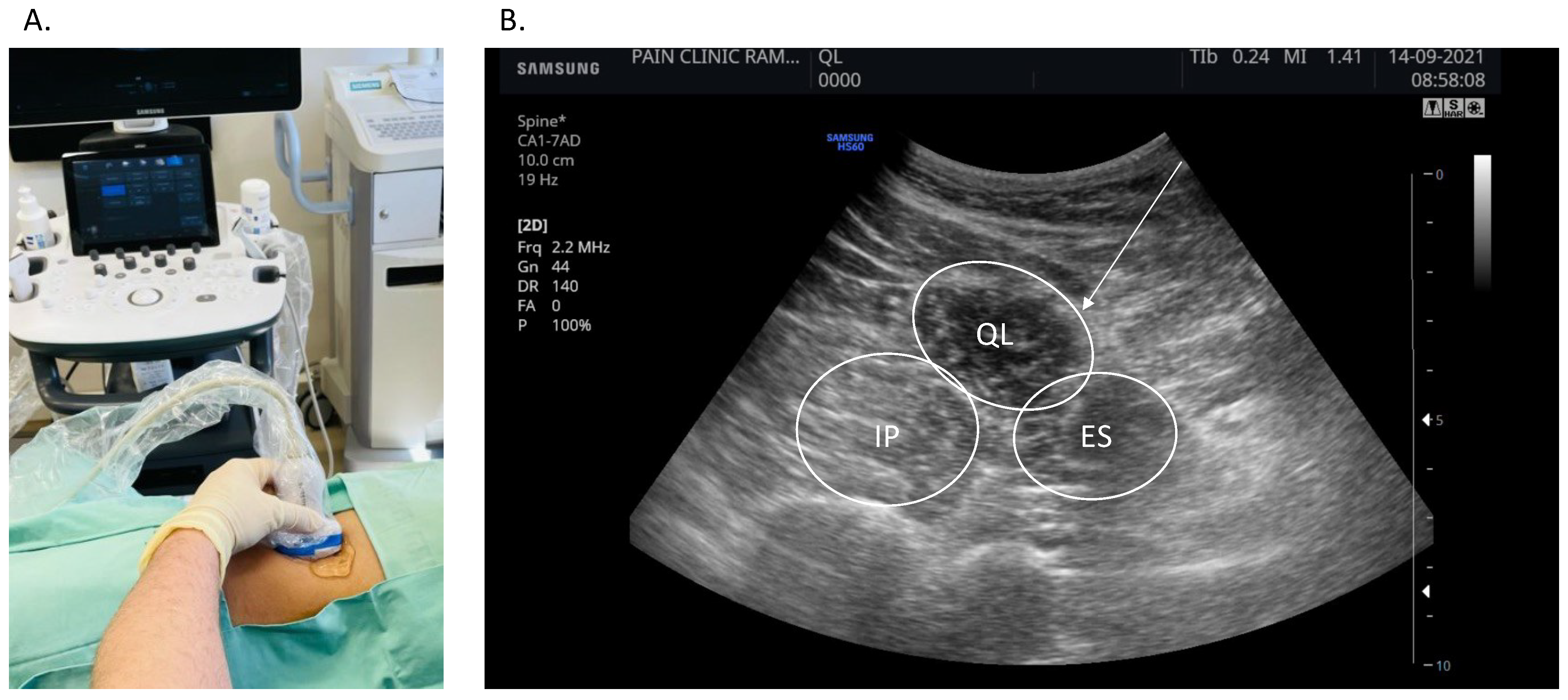
2.3. Ultrasound-Guided Dry Needling of the Muscle, Fascia and Skin
2.4. Data Analysis
3. Results
3.1. Participant Baseline Characteristics
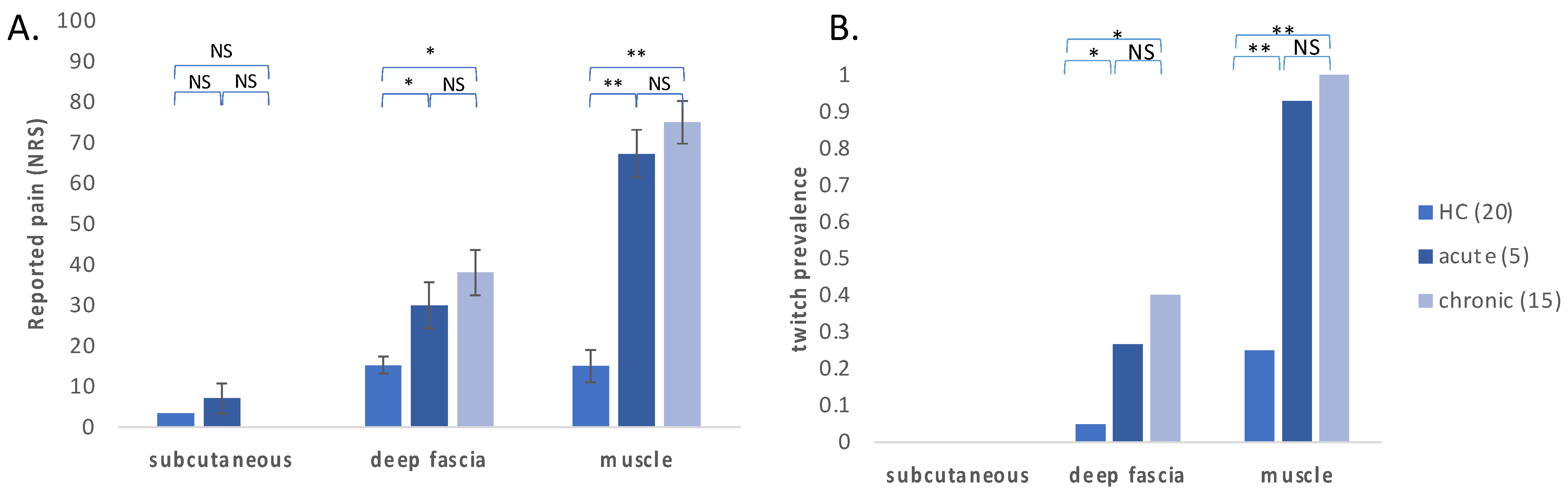
3.2. Evoked Pain and Twitch Response on Stimulation of the Deep Fascia, Muscle and Subcutaneous Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest statement
References
- Gerwin, R.D. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr. Pain Headache Rep. 2001, 5, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Barbero, M.; Schneebeli, A.; Koetsier, E.; Maino, P. Myofascial pain syndrome and trigger points: Evaluation and treatment in patients with musculoskeletal pain. Curr. Opin. Support. Palliat. Care 2019, 13, 270–276. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Dommerholt, J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A delphi study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low back pain, a comprehensive review: Pathophysiology, diagnosis, and treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Ammendolia, A.; Paduano, S.; Calafiore, D.; Marotta, N.; Migliario, M.; Fortunato, L.; Giudice, A.; Michelotti, A.; de Sire, A. Efficacy of rehabilitation on reducing pain in muscle-related temporomandibular disorders: A systematic review and meta-analysis of randomized controlled trials. J. Back Musculoskelet. Rehabil. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Ohrbach, R.; Turk, D.C.; et al. The prevalence of comorbid chronic pain conditions among patients with temporomandibular disorders: A systematic review. J. Am. Dent. Assoc. 2022, 153, 241–250.e10. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C. Myofascial Head Pain. Curr. Pain Headache Rep. 2015, 19, 28. [Google Scholar] [CrossRef]
- Dal Farra, F.; Aquino, A.; Tarantino, A.G.; Origo, D. Effectiveness of myofascial manual therapies in chronic pelvic pain syndrome: A systematic review and meta-analysis. Int. Urogynecol. J. 2022. [Google Scholar] [CrossRef]
- Till, S.R.; Nakamura, R.; Schrepf, A.; As-Sanie, S. Approach to diagnosis and management of chronic pelvic pain in women: Incorporating chronic overlapping pain conditions in assessment and management. Obstet. Gynecol. Clin. N. Am. 2022, 49, 219–239. [Google Scholar] [CrossRef]
- Tim, S.; Mazur-Bialy, A.I. The most common functional disorders and factors affecting female pelvic floor. Life Basel Switz. 2021, 11, 1397. [Google Scholar] [CrossRef]
- Travell, J.; Rinzler, S.H. The myofascial genesis of pain. Postgrad. Med. 1952, 11, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.M.; Fernández-de-Las-Peñas, C.; Finnegan, M.; Freeman, J.L. Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Wolters Kluwer Health: Philadelphia, PA, USA, 2018; ISBN 978-0-7817-5560-3. [Google Scholar]
- Saxena, A.; Chansoria, M.; Tomar, G.; Kumar, A. Myofascial pain syndrome: An overview. J. Pain Palliat. Care Pharmacother. 2015, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Appasamy, M.; Lam, C.; Alm, J.; Chadwick, A.L. Trigger point injections. Phys. Med. Rehabil. Clin. N. Am. 2022, 33, 307–333. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Centre (UK). Evidence Review for Manual Therapy for Chronic Primary Pain: Chronic Pain (Primary and Secondary) in over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain: Evidence Review I; NICE Evidence Reviews Collection; National Institute for Health and Care Excellence (NICE): London, UK, 2021; ISBN 978-1-4731-4066-0. [Google Scholar]
- Fernández-de-Las-Peñas, C.; Nijs, J. Trigger Point dry needling for the treatment of myofascial pain syndrome: Current perspectives within a pain neuroscience paradigm. J. Pain Res. 2019, 12, 1899–1911. [Google Scholar] [CrossRef]
- Unverzagt, C.; Berglund, K.; Thomas, J. Dry needling for myofascial trigger point pain: A clinical commentary. Int. J. Sports Phys. Ther. 2015, 10, 402. [Google Scholar]
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; De Caro, R.; Stecco, C. Fascia and soft tissues innervation in the human hip and their possible role in post-surgical pain. J. Orthop. Res. 2020, 38, 1646–1654. [Google Scholar] [CrossRef]
- Kondrup, F.; Gaudreault, N.; Venne, G. The deep fascia and its role in chronic pain and pathological conditions: A review. Clin. Anat. 2022, 35, 649–659. [Google Scholar] [CrossRef]
- Weiss, K.; Kalichman, L. Deep fascia as a potential source of pain: A narrative review. J. Bodyw. Mov. Ther. 2021, 28, 82–86. [Google Scholar] [CrossRef]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial components of the myofascial pain syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef]
- Lobenhoffer, P.; Biedert, R.; Stauffer, E.; Lattermann, C.; Gerich, T.G.; Müller, W. Occurrence and distribution of free nerve endings in the distal iliotibial tract system of the knee. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 1996, 4, 111–115. [Google Scholar] [CrossRef]
- Schubert, T.E.O.; Weidler, C.; Borisch, N.; Schubert, C.; Hofstädter, F.; Straub, R.H. Dupuytren’s contracture is associated with sprouting of substance P positive nerve fibres and infiltration by mast cells. Ann. Rheum. Dis. 2006, 65, 414–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.-D. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Magerl, W.; Treede, R.-D.; Schilder, A. Dose-dependent pain and pain radiation after chemical stimulation of the thoracolumbar fascia and multifidus muscle: A single-blinded, cross-over study revealing a higher impact of fascia stimulation. Life 2022, 12, 340. [Google Scholar] [CrossRef]
- Hoheisel, U.; Rosner, J.; Mense, S. Innervation changes induced by inflammation of the rat thoracolumbar fascia. Neuroscience 2015, 300, 351–359. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Barry, C.M.; Kestell, G.; Gillan, M.; Haberberger, R.V.; Gibbins, I.L. Sensory nerve fibers containing calcitonin gene-related peptide in gastrocnemius, latissimus dorsi and erector spinae muscles and thoracolumbar fascia in mice. Neuroscience 2015, 291, 106–117. [Google Scholar] [CrossRef]
- Caro-Morán, E.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Cantarero-Villanueva, I.; Madeleine, P.; Arroyo-Morales, M. Pressure pain sensitivity maps of the neck-shoulder region in breast cancer survivors. Pain Med. Malden Mass 2016, 17, 1942–1952. [Google Scholar] [CrossRef]
- Farella, M.; Michelotti, A.; Steenks, M.H.; Romeo, R.; Cimino, R.; Bosman, F. The diagnostic value of pressure algometry in myofascial pain of the jaw muscles. J. Oral Rehabil. 2000, 27, 9–14. [Google Scholar] [CrossRef]
- Petersen, K.L.; Brennum, J.; Olesen, J. Evaluation of pericranial myofascial nociception by pressure algometry. Reproducibility and factors of variation. Cephalalgia Int. J. Headache 1992, 12, 33–37. [Google Scholar] [CrossRef]
- Falla, D.; Arendt-Nielsen, L.; Farina, D. Gender-specific adaptations of upper trapezius muscle activity to acute nociceptive stimulation. Pain 2008, 138, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-T.; Ge, H.-Y.; Yue, S.-W.; Arendt-Nielsen, L. Nociceptive and non-nociceptive hypersensitivity at latent myofascial trigger points. Clin. J. Pain 2009, 25, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Beissner, F.; Brandau, A.; Henke, C.; Felden, L.; Baumgärtner, U.; Treede, R.-D.; Oertel, B.G.; Lötsch, J. Quick discrimination of adelta and C fiber mediated pain based on three verbal descriptors. PLoS ONE 2010, 5, e12944. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, R.J.S.; Battié, M.C. A Comparison of pressure pain detection thresholds in people with chronic low back pain and volunteers without pain. Phys. Ther. 2005, 85, 1085–1092. [Google Scholar] [CrossRef]
- Aspinall, S.L.; Jacques, A.; Leboeuf-Yde, C.; Etherington, S.J.; Walker, B.F. Pressure pain threshold and temporal summation in adults with episodic and persistent low back pain trajectories: A secondary analysis at baseline and after lumbar manipulation or sham. Chiropr. Man. Ther. 2020, 28, 36. [Google Scholar] [CrossRef]
- Nim, C.G.; O’Neill, S.; Geltoft, A.G.; Jensen, L.K.; Schiøttz-Christensen, B.; Kawchuk, G.N. A cross-sectional analysis of persistent low back pain, using correlations between lumbar stiffness, pressure pain threshold, and heat pain threshold. Chiropr. Man. Ther. 2021, 29, 34. [Google Scholar] [CrossRef] [PubMed]
| Healthy Controls (20) | Acute Pain (5) | Chronic Pain (15) | |
|---|---|---|---|
| age (years) | 44.20 (12.01) | 49.87 (13.58) | 39.40 (16.83) |
| body-mass index | 26.00 (3.68) | 28.60 (3.56) | 24.51 (3.45) |
| gender (% males) | 0.95 | 0.93 | 0.80 |
| baseline pain (NRS) | 0.00 (0.00) * | 53.33 (12.20) | 46.00 (5.48) |
| pain duration (days) | 0.00 (0.00) * | 27.13 (23.34) | 468.00 (272.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozacov, R.; Minerbi, A.; Haddad, M.; Vulfsons, S. Differential Sensitization of Muscle versus Fascia in Individuals with Low Back Pain. Bioengineering 2022, 9, 440. https://doi.org/10.3390/bioengineering9090440
Cozacov R, Minerbi A, Haddad M, Vulfsons S. Differential Sensitization of Muscle versus Fascia in Individuals with Low Back Pain. Bioengineering. 2022; 9(9):440. https://doi.org/10.3390/bioengineering9090440
Chicago/Turabian StyleCozacov, Ronen, Amir Minerbi, May Haddad, and Simon Vulfsons. 2022. "Differential Sensitization of Muscle versus Fascia in Individuals with Low Back Pain" Bioengineering 9, no. 9: 440. https://doi.org/10.3390/bioengineering9090440
APA StyleCozacov, R., Minerbi, A., Haddad, M., & Vulfsons, S. (2022). Differential Sensitization of Muscle versus Fascia in Individuals with Low Back Pain. Bioengineering, 9(9), 440. https://doi.org/10.3390/bioengineering9090440







