PCL/Graphene Scaffolds for the Osteogenesis Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of the Scaffolds
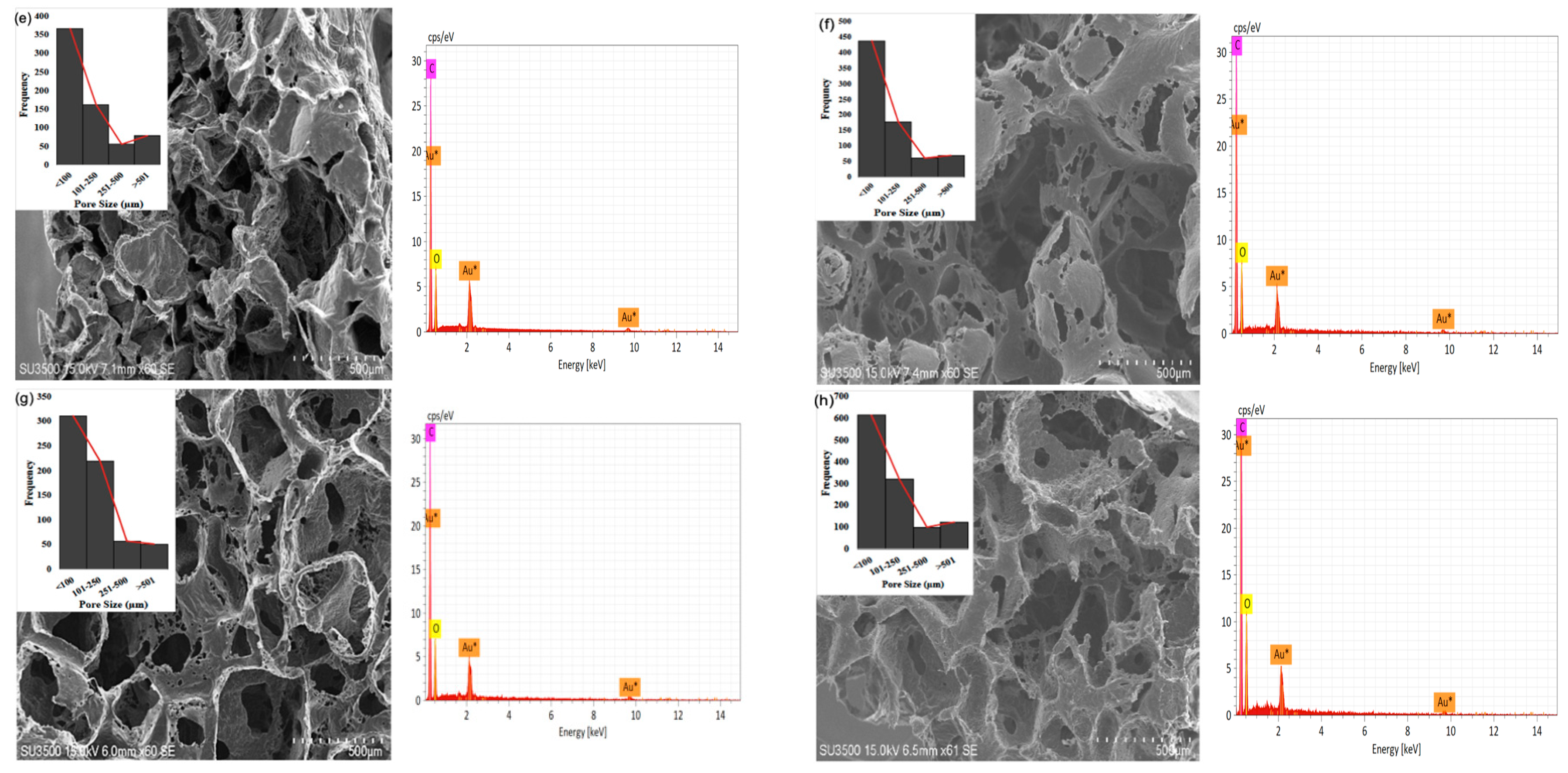
2.2. Characterizations of the Scaffolds
2.2.1. Water Contact Angle (WCA)
2.2.2. Water Absorption Rate
2.2.3. Porosity
2.2.4. Pore Sizes
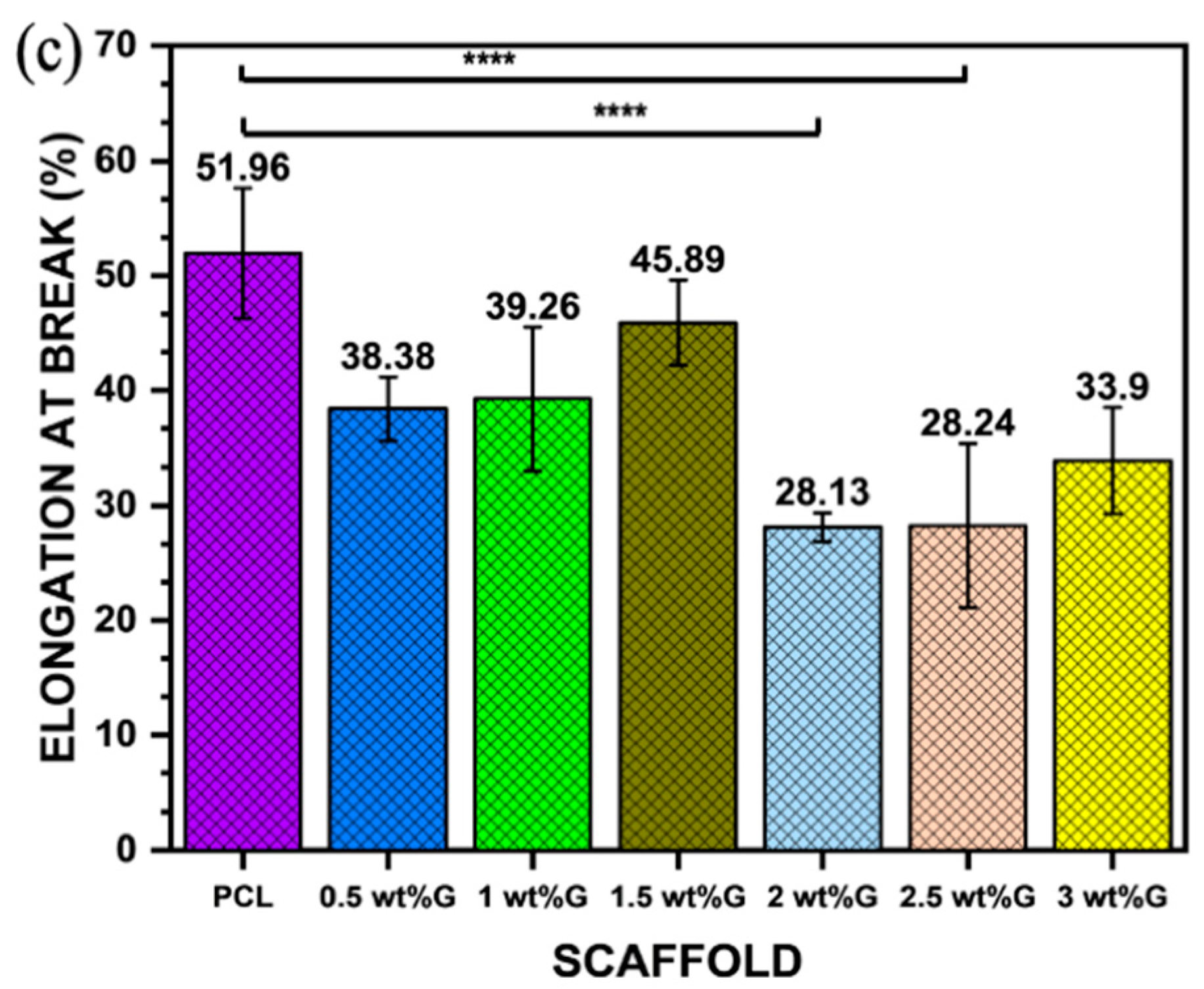
2.2.5. Tensile Test
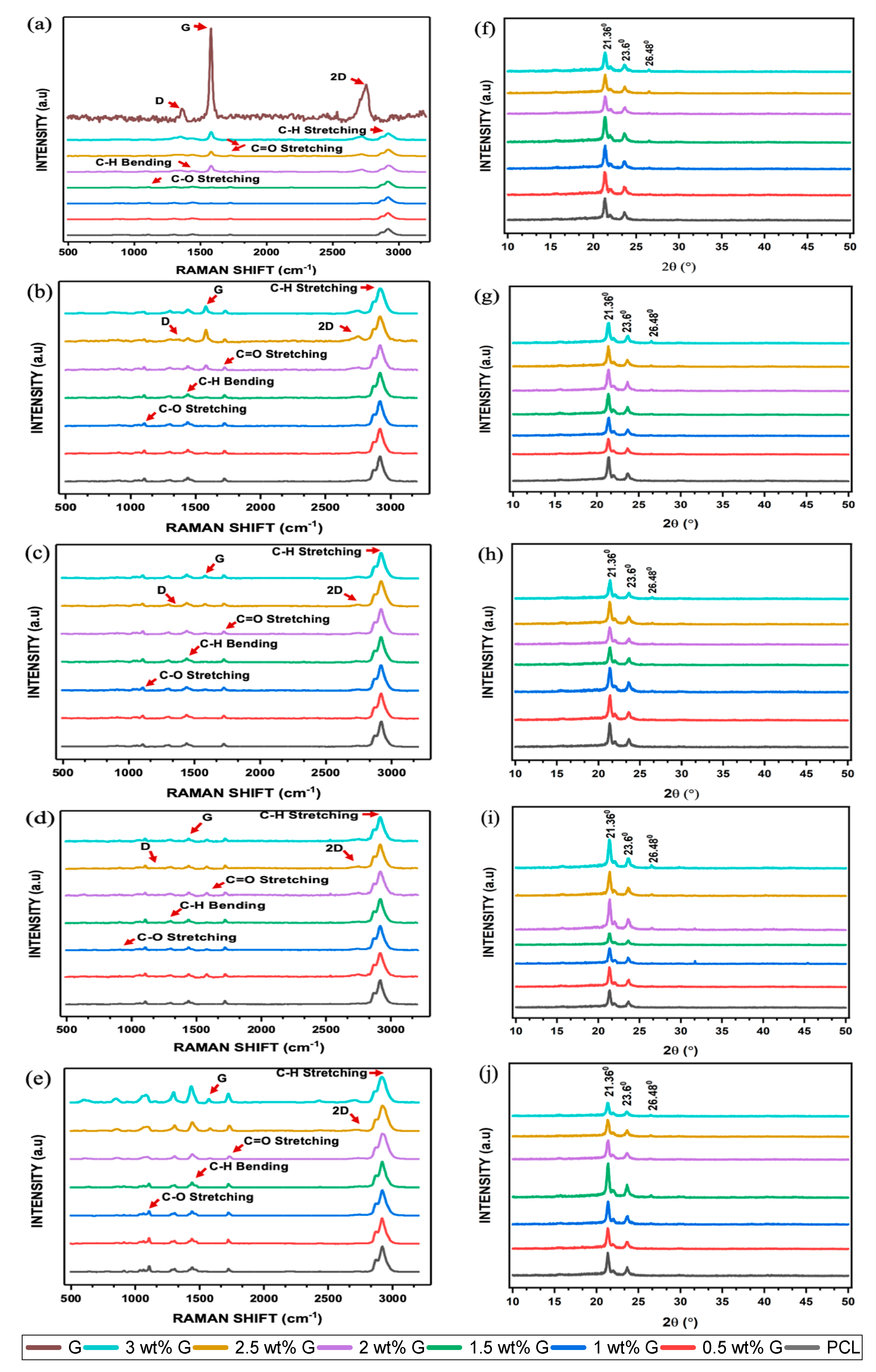
2.2.6. Raman Spectroscopy
2.2.7. X-ray Diffractometer (XRD)
2.3. Biodegradation Time Test
2.4. In Vitro Cell Culture
2.4.1. Scaffold Preparation and Cell Seeding
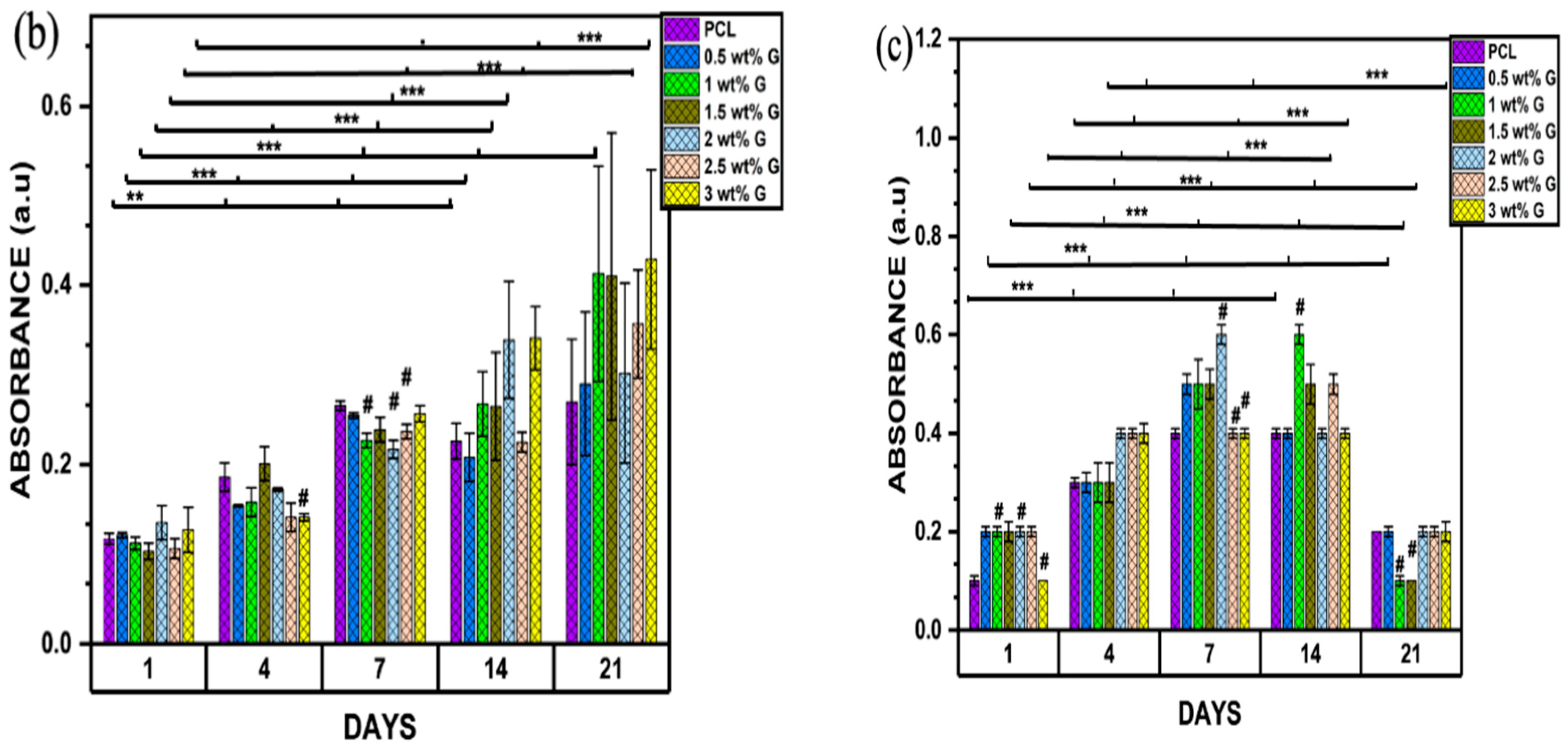
2.4.2. MTT Assay (3-(4,5-Dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide)
Biocompatibility
Proliferation
2.4.3. Alkaline Phosphatase (ALP) Assay (Differentiation Assay)
2.4.4. Cell Morphology and Adhesion
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernardi, S.; Macchiarelli, G.; Bianchi, S. Autologous materials in regenerative dentistry: Harvested bone, platelet concentrates and dentin derivates. Molecules 2020, 25, 5330. [Google Scholar] [CrossRef]
- Wang, W.; Huang, B.; Byun, J.J.; Bartolo, P. Assessment of PCL/carbon material scaffolds for bone regeneration. J. Mech. Behav. Biomed. 2019, 93, 52–60. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Ansari, M.N.M.; Zulkifli, R.M.; Zawawi, N.A.; Arshad, M. Development of biodegradable bio-based composite for bone tissue engineering: Synthesis, characterization, and in vitro biocompatible evaluation. Polymers 2021, 13, 3611. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Rizwan, M.; Razak, S.I.A.; Hassan, A.; Rasheed, T.; Bilal, M. Electroactive polymeric nanocomposite BC-g-(Fe3O4/GO) materials for bone tissue engineering: In vitro evaluations. J. Biomater. Sci. Polym. Ed. 2022, 33, 1349–1368. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Arjan, W.S.A.; Binkadem, M.S.; Mehboob, H.; Haider, A.; Raza, M.A.; Razak, S.I.A.; Hasan, A.; Amin, R. Development of biopolymeric hybrid scaffold-based on AAc/GO/nHAp/TiO2 nanocomposite for bone tissue engineering: In-vitro analysis. Nanomaterials 2021, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, R.; Wang, F.L.; Zhao, X.; Yang, F.; Xu, Y.Q.; Yan, F.; Zhu, Y.; Xia, D.; Liu, Y.S. 3D-Printed PCL/Zn scaffolds for bone regeneration with a dose-dependent effect on osteogenesis and osteoclastogenesis. Mater. Today Bio. 2022, 13, 100202. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Rehman, S.; Hasan, A.; Qureshi, S.; Stojanovi’c, G.M. Bioactive scaffold (sodium alginate)-g-(nHAp@ SiO2@ GO) for bone tissue engineering. Int. J. Biol. Macromol. 2022, 222, 462–472. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Huang, H.Y.; Fan, F.Y.; Shen, Y.K.; Wang, C.H.; Huang, Y.T.; Chern, M.J.; Wang, Y.H.; Wang, Y.H.L. 3D Poly(ε)caprolactone/graphene porous scaffolds for bone tissue engineering. Colloids Surf. A 2020, 606, 1–9. [Google Scholar] [CrossRef]
- Wu, D.T.; Lopez, J.G.M.; Cho, Y.W.; Ma, X.L.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.Y.; Shao, Z.X.; Sun, M.Y.; Ao, Y.F.; Boyan, B.D.; Chen, H.F. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 20, 14–29. [Google Scholar] [CrossRef]
- Tahriri, M.; Monico, M.D.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhi, C.; Lin, Y.; Bao, H.; Wu, G.; Jiang, P.; Mai, Y.W. Thermal Conductivity of Graphene-Based Polymer Nanocomposites. Mater. Sci. Eng. R. Rep. 2020, 142, 100577. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Halbig, C.E.; Sim, Y.F.; Lim, C.T.; Leong, D.T.; Neto, A.H.C.; Garaj, S.; Rosa, V. Cytotoxicity survey of commercial graphene materials from worldwide. Npj 2D Mater. Appl. 2022, 6, 65. [Google Scholar] [CrossRef]
- Chang, T.K.; Chang, Y.C.; Yeh, S.T.; Lin, T.C.; Huang, C.H. In vitro and in vivo biological responses to graphene and graphene oxide: A murine calvarial animal study. Inter. J. Nanomed. 2020, 15, 647–659. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Liu, Y.; Gao, X.; Zhu, T.; Lu, L. Acceleration of bone regeneration in critical-size defect using BMP-9 loaded nHA/Coll/MWCNTs scaffolds seeded with bone marrow mesenchymal stem cells. Biomed Res. Int. 2019, 2019, 7343957. [Google Scholar]
- Furuya, M.; Kikuta, J.; Fujimori, S.; Seno, S.; Maeda, H.; Shirazaki, M.; Yenaka, M.; Mizuno, H.; Iwamoto, Y.; Moromoto, A.; et al. Direct cell-cell contact between mature osteoblast and osteoclast dynamically controls their functions in vivo. Nat. Commun. 2018, 9, 300. [Google Scholar] [CrossRef]
- Bullock, C.J.; Bussy, C. Biocompatibility considerations in the design of graphene biomedical materials. Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar] [CrossRef]
- Cabral, C.S.D.; Miguel, S.P.; Melo-Diogo, D.; Loura, R.O.; Correia, I.J. Green reduced graphene oxide functionalized 3D printed scaffolds for bone tissue engineering. Carbon 2019, 146, 513–523. [Google Scholar] [CrossRef]
- Ezenkawa, O.E.; Hassan, A.; Samsudin, S.A. Comparison of mechanical properties and thermal stability of graphene-based materials and halloysite nanotubes reinforced maleated polymer compatibilized polypropylene nanocomposites. Polym. Compos. 2022, 43, 1852–1863. [Google Scholar] [CrossRef]
- Chadha, N.; Sharma, R.; Saini, P. A new insight into the structural modulation of graphene oxide upon chemical reduction probe by Raman spectroscopy and X-ray diffraction. Carbon Lett. 2021, 31, 1125–1131. [Google Scholar] [CrossRef]
- ISO 10993-12; Minkwitz. Biological Evaluation of Medical Devices-Part 1-2: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- Wei, D.; Charlton, L.; Glidle, A.; Qi, N.; Dobson, P.S.; Dalby, M.J.; Fan, H.; Yin, H. Dynamically modulated core-shell microfibers to study the effect of depth sensing of matrix stiffness on stem cell fate. ACS. Appl. Mater. Interfaces 2021, 13, 37997–38006. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid. Interfaces Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; Van Deursen, P.M.G.; Barbetsea, K.I.; Schneider, G.F. Hydrophilicity of graphene in water through transparency to polar and dispersive interactions. Adv. Mater. 2018, 30, 1703274. [Google Scholar] [CrossRef]
- Alazzam, A.; Alazzam, A. Micropatterning of cells via adjusting surface wettability using plasma treatment and graphene oxide deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar]
- Zhang, K.; Fan, Y.; Dunne; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomatter. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Yi, B.C.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Porelli, D.; Abrami, M.; Pellizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Gassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular bone porosity and pore size distribution in osteoporotic patietns—A low field nuclear magnetic resonance and microcomputed tomography investigation. J. Mech. Behav. Biomed. Mat. 2022, 125, 104933. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Esmail, A.; Pereira, J.R.; Sevrin, C.; Grandfils, C.; Menda, U.D.; Fortunato, E.; Oliva, A.; Freitas, F. Preparation and characterization of porous scaffolds based on poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Life 2021, 11, 935. [Google Scholar] [CrossRef]
- Langridge, B.; Griffin, M.; Butler, P.E. Regenerative medicine for skeletal muscle loss: A review of current tissue engineering approaches. J. Mater. Sci. Mater. Med. 2021, 32, 15. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.R.; Love, M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Dev. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-pore-size Ti6A14V scaffolds with different pore structures for vascularized bone regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef]
- Xue, W.; Du, J.; Li, Q.; Wang, Y.; Lu, Y.; Fan, J.; Yu, S.; Yang, Y. Preparation, properties, and application of graphene-based materials in tissue engineering scaffolds. Tissue Eng. Part B Rev. 2022, 28, 1121–1136. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Pan, Y.; Yao, Y.; Tang, S.; Su, J.; Shin, J.W.; Wei, J.; Zhao, J. Nanoporosity improved water absorption, in vitro degradability, mineralization, osteoblast responses and drug release of poly (butylene succinate)-based composite scaffolds containing nanoporous magnesium silicate compared with magnesium silicate. Int. J. Nanomed. 2017, 12, 3637–3651. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of mechanical properties of biomaterials on degradability, cell behaviors and signalling pathways: Current, progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J. Biomed. Mater. Res A 2014, 102, 3282–3290. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, H.; Gong, Y.; Gong, Y.; Yan, Z.; Zhang, X.; Guo, Y.; Wang, Y. Mechanical strain promotes osteoblastic differentiation through integrin-β1-mediated β-catenin signaling. Int. J. Mol. Med. 2016, 38, 594–600. [Google Scholar] [CrossRef]
- Kashani, H.; Ito, Y.; Han, J.; Liu, P.; Chen, M. Extraordinary tensile strength and ductility of scalable nanoporous graphene. Sci. Adv. 2019, 5, eaat695. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Schorr, N.B.; Jiang, A.G.; Rodriguez-Lopez, J. Probing graphene interfacial reactivity via simultaneous and colocalized Raman-Scanning electrochemical microscopy imaging and interrogation. Anal. Chem. 2018, 90, 7848–7854. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.W. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Meneses, J.; Van de Kemp, T.; Almeida, R.C.; Pereira, R.; Malgahaes, F.D.; Castilho, M. Fabrication of polymer/graphene biocomposites for tissue engineering. Polymers 2022, 14, 1038. [Google Scholar] [CrossRef]
- Chen, X.; Feng, B.; Zhu, D.Q.; Chen, Y.W.; Ji, W.; Ji, T.J.; Li, F. Characteristics and toxicity assessment of electrospun gelatin/PCL nanofibrous scaffold loaded with graphene in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3669–3678. [Google Scholar] [CrossRef]
- Frontinan-Rubio, J.; Gonzalez, V.J.; Vazquez, E.; Duran-Prado, M. Rapid and efficient testing of the toxicity of graphene-related materials in primary human lung cells. Sci. Rep. 2022, 12, 7664. [Google Scholar] [CrossRef]
- Seehra, M.S.; Narang, V.; Geddam, U.K.; Stefaniak, A.B. Correlation between X-ray diffraction and Raman spectra of 16 commercial graphene-based materials and their resulting classification. Carbon 2017, 111, 380–384. [Google Scholar] [CrossRef]
- Patil, R.; Bahadur, P.; Tiwari, S. Dispersed graphene materials of biomedical interest and their toxicological consequences. Adv. Colloid Interface Sci. 2020, 275, 102051. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Sayyar, S.; Wallace, G.G. Effect of graphene addition on polycaprolactone scaffolds fabricated using melt-electrowriting. Polymers 2022, 14, 319. [Google Scholar] [CrossRef]
- Sharma, A.; Goringa, A.; Katherine, A.; Staines, B.; Roger, J.H.; Andrew, A.D.; Pitsillides, O.C.; Richard, O.; Sumeet, M.; Clarkina, C.E. Raman spectroscopy links differentiating osteoblast matrix signatures to pro-angiogenic potential. Matrix Biol. Plus 2020, 5, 100018. [Google Scholar] [CrossRef]
- Sharma, B.; Schuman, T.; De Oliveira, M.H., Jr.; Lopes,, J.M.J. Controlled synthesis and characterization of multilayer graphene films on the C-face of silicon carbide. Phys. Status Solidi 2017, 214, 1600721. [Google Scholar] [CrossRef]
- Ma, Q.; Shi, K.; Su, T.; Wang, Z. Biodegradation of polycaprolactone (PCL) with different molecular weights by Candida antartica lipase. J. Polym. Environ. 2020, 28, 2947–2955. [Google Scholar] [CrossRef]
- Niknam, Z.; Hosseinzadeh, F.; Shams, F.; Fath-Bayati, L.; Nuoroozi, G.; Amirabad, L.M.; Mohebichamkhorami, F.; Naeimi, S.K.; Ghafouri-Fard, S.; Zali, H.; et al. Recent advances and challenges in graphene-based nanocomposite scaffolds for tissue engineering application. J. Biomed. Mater. Res. A 2022, 110, 1695–1721. [Google Scholar] [CrossRef]
- Lasocka, I.; Dabrowska, L.S.; Skibniewski, M.; Kalbacova, M.H. Cytocompatibility of graphene monolayer and its impact on focal cell adhesion mitochondrial morphology and activity in BALB/3T3 fibrobalst. Materials 2021, 14, 643. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic potential of graphene in bone tissue engineering scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef]
- Mansouri, N.; Al-Sarawi, S.F.; Mazumdar, J.; Losic, D. Advancing fabrication and properties of three-dimensional graphene-alginate scaffolds for application in neural tissue engineering. RSC Adv. 2019, 9, 36838–36848. [Google Scholar] [CrossRef]
- Ferreira, H.P.; Moura, D.; Pereira, A.T.; Henriques, P.C.; Barrias, C.C.; Magalhaes, F.D.; Concalves, L.C. Using graphene-based materials for stiff and strong poly (ethylene glycol) hydrogels. Int. J. Mol. Sci. 2022, 23, 2312. [Google Scholar] [CrossRef]
- Suh, H.; Park, J.C.; Han, D.W.; Lee, D.H.; Han, C.D. A bone replaceable artificial bone substitute: Osteoinduction by combining with bone inducing agent. Artif. Organs. 2001, 25, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Wang, Q. Association between bone-alkaline phosphatase and bone mineral density in adults with and without diabetes. Medicine 2018, 97, e0432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, S.X. Fundamental mechanics of cell shape and cell movement. In Cell Movement in Health and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 85–100. [Google Scholar]
- Tewari, M.; Pareek, P.; Kumar, S. Correlating amino acid interaction with graphene-based materials regulating cell function. J. Indian Inst. Sci. 2022, 102, 639–651. [Google Scholar] [CrossRef]
- Matthews, H.K.; Ganguli, S.; Plak, K.; Taubenberger, A.V.; Win, Z.; Williamson, M.; Piel, M.; Guck, J.; Baum, B. Oncogenic signaling alters cell shape and mechanics to facilitate cell division under confinement. Dev. Cell 2020, 52, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Schakenraad, K.; Ernst, J.; Pomp, W.; Danen, E.H.J.; Merks, R.M.H.; Schmidt, T.; Giomi, L. Mechanical interplay netween cell shape and actin cytoskeleton organization. Soft Matter 2020, 16, 6328–6343. [Google Scholar] [CrossRef]
- Panzetta, V.; Fusco, S.; Netti, P.A. Cell mechanosensing is regulated by substrate strain energy rather than stiffness. Proc. Natl. Acad. Sci. USA 2019, 116, 22004–22013. [Google Scholar] [CrossRef]






 = lamellipodia;
= lamellipodia;  = intercellular connections;
= intercellular connections;  = filopodia and micro-vesicles.
= filopodia and micro-vesicles.
 = lamellipodia;
= lamellipodia;  = intercellular connections;
= intercellular connections;  = filopodia and micro-vesicles.
= filopodia and micro-vesicles.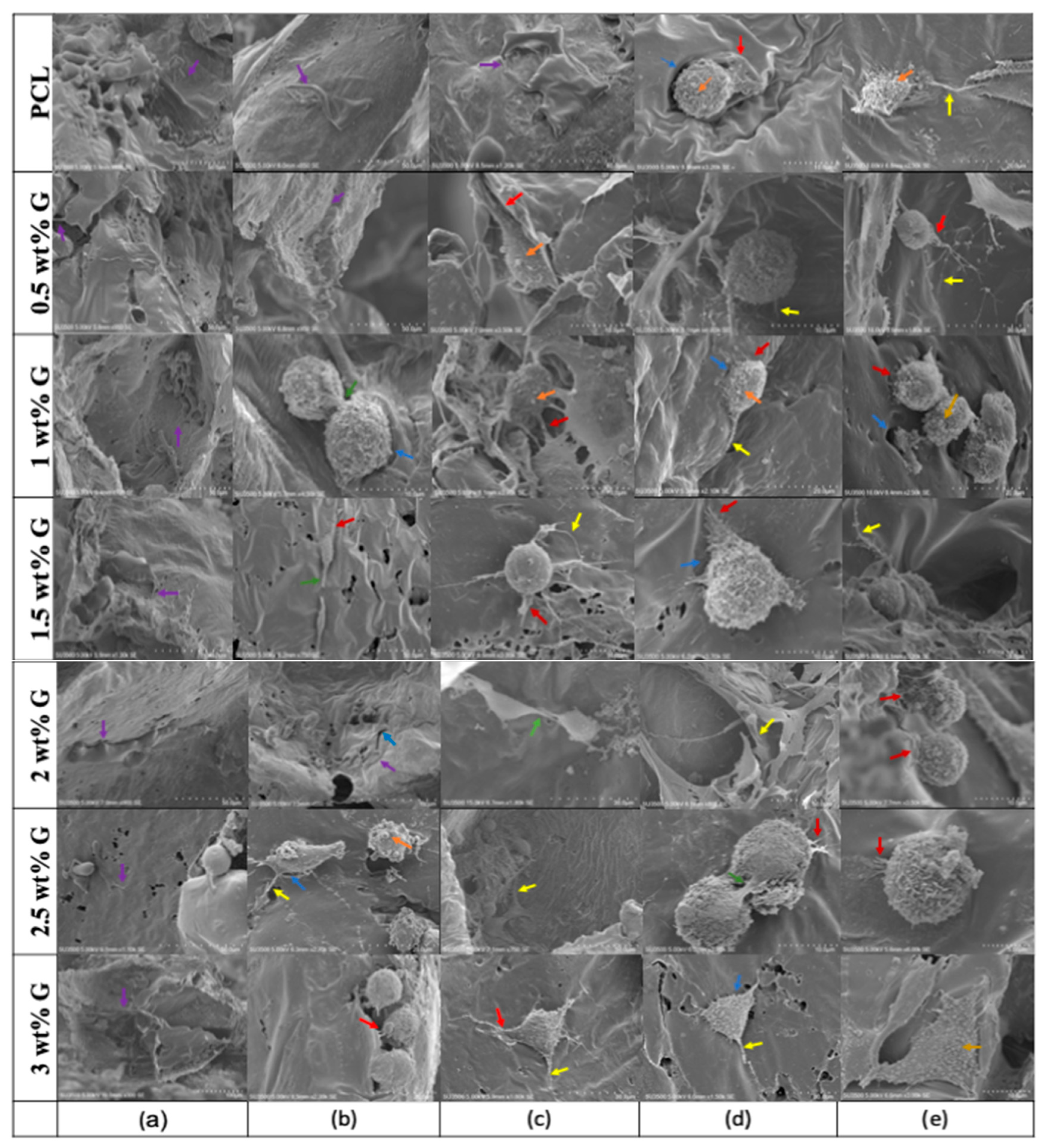
| Scaffold | Ratio ID/IG (COUNTS) | Ratio I2D/IG (COUNTS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial (p < 0.05) | Biodegradation | Initial (p > 0.05) | Biodegradation | |||||||
| 1 Month (p > 0.05) | 2 Months (p > 0.05) | 3 Months (p > 0.05) | 4 Months | 1 Month (p > 0.05) | 2 Months (p> 0.05) | 3 Months (p > 0.05) | 4 Months (p > 0.05) | |||
| 2 wt% G | 0.16 ± 0.02 | 0.15 ± 0.03 | 0.11 ± 0.01 | 0.06 ± 0.03 | - | 0.42 ± 0.03 | 0.63 ± 0.12 | 0.88 ± 0.05 | 1.20 ± 0.37 | 2.43 ± 1.44 |
| 2.5 wt% G | 0.29 ± 0.04 | 0.19 ± 0.04 | 0.19 ± 0.05 | 0.10 ± 0.04 | - | 0.49 ± 0.08 | 0.65 ± 0.14 | 0.81 ± 0.08 | 1.31 ± 0.34 | 1.25 ± 0.87 |
| 3 wt% G | 0.32 ± 0.04 | 0.25 ± 0.05 | 0.19 ± 0.02 | 0.11 ± 0.04 | - | 0.72 ± 0.26 | 1.13 ± 0.51 | 0.76 ± 0.13 | 1.37 ± 0.29 | 5.16 ± 4.58 |
| Scaffold | Intensity of 2θ = 21.36° (a.u) | Intensity of 2θ = 23.6° (a.u) | Intensity of 2θ = 26.48° (a.u) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Biodegradation (Month) | Biodegradation (Month) | Biodegradation (Month) | ||||||||||||
| 1 | 2 | 3 | 4 | Initial | 1 | 2 | 3 | 4 | Initial | 1 | 2 | 3 | 4 | ||
| PCL | 1780 | 2383.3 | 1915 | 1740 | 2035 | 690 | 803.3 | 675 | 640 | 785 | |||||
| 0.5 wt% G | 1880 | 1570 | 2015 | 2000 | 1835 | 675 | 636.7 | 633.1 | 760 | 645 | |||||
| 1 wt% G | 1885 | 1820 | 1888 | 1585 | 2035 | 650 | 665 | 805 | 665 | 765 | |||||
| 1.5 wt% G | 2050 | 2065 | 1389 | 1175 | 3005 | 645 | 790 | 550 | 455 | 1155 | 110 | 255 | |||
| 2 wt% G | 1433.3 | 2018 | 1395 | 3070 | 1696 | 570 | 876 | 515 | 1079.1 | 610 | 120 | 140.5 | 113.9 | 265 | 100.5 |
| 2.5 wt% G | 1516.7 | 2050 | 1794.3 | 2435.9 | 1448.2 | 583.3 | 715 | 645.9 | 850 | 595 | 176.7 | 155.4 | 137.3 | 189.9 | 160 |
| 3 wt% G | 1535 | 2015 | 1495 | 2866.6 | 1170 | 585 | 709 | 497.1 | 1021 | 415 | 195 | 270 | 165 | 308.9 | 135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitasari, S.; Wu, C.-Z.; Shen, Y.-K. PCL/Graphene Scaffolds for the Osteogenesis Process. Bioengineering 2023, 10, 305. https://doi.org/10.3390/bioengineering10030305
Anitasari S, Wu C-Z, Shen Y-K. PCL/Graphene Scaffolds for the Osteogenesis Process. Bioengineering. 2023; 10(3):305. https://doi.org/10.3390/bioengineering10030305
Chicago/Turabian StyleAnitasari, Silvia, Ching-Zong Wu, and Yung-Kang Shen. 2023. "PCL/Graphene Scaffolds for the Osteogenesis Process" Bioengineering 10, no. 3: 305. https://doi.org/10.3390/bioengineering10030305
APA StyleAnitasari, S., Wu, C.-Z., & Shen, Y.-K. (2023). PCL/Graphene Scaffolds for the Osteogenesis Process. Bioengineering, 10(3), 305. https://doi.org/10.3390/bioengineering10030305








