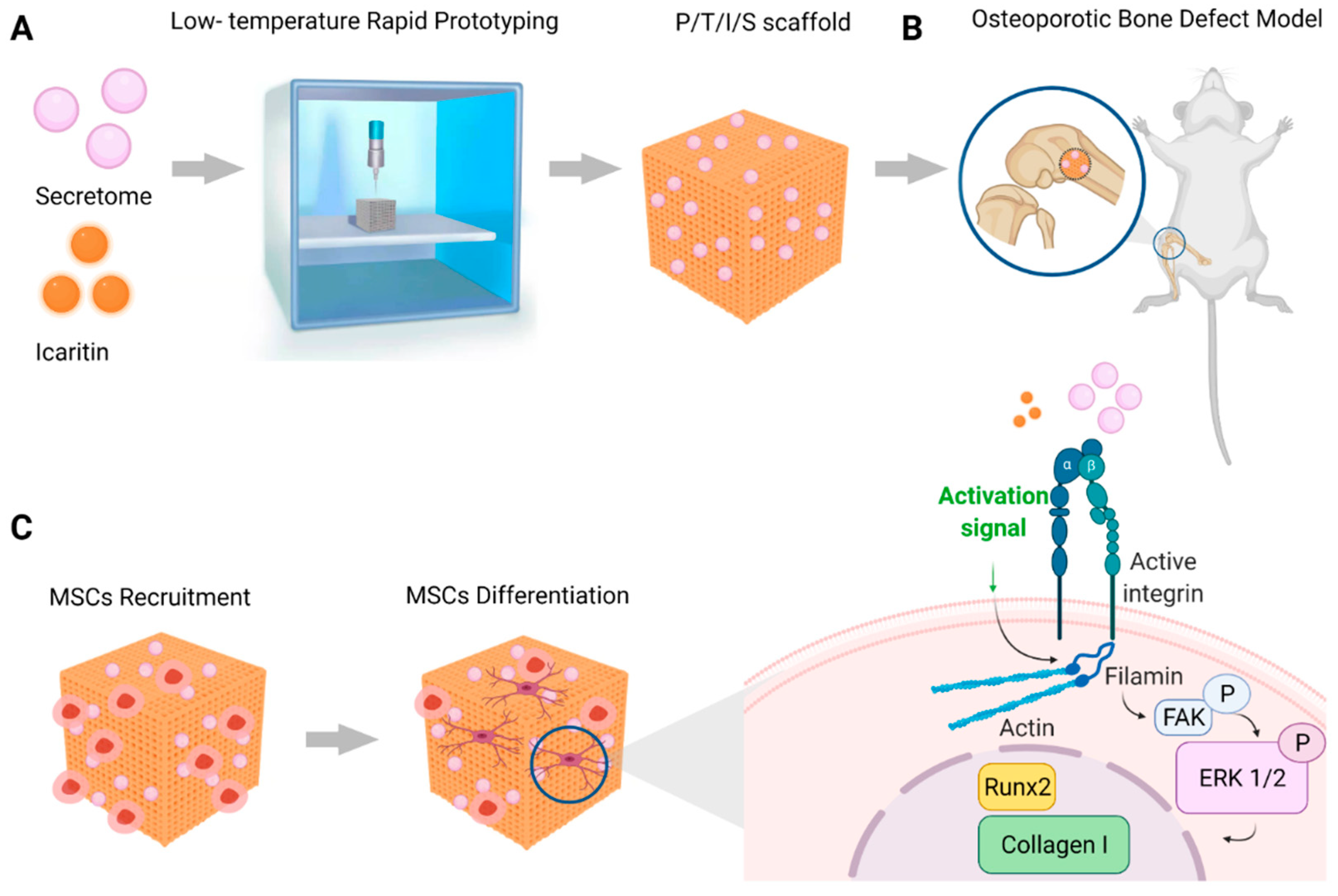
Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterisation of HFS
2.2. Rat MSCs Proliferation Study
2.3. Osteogenic Differentiation
2.4. RNA Extraction and Real-Time PCR
2.5. Cell Spreading
2.6. Western Blot
2.7. P/T and P/T/I Scaffold Fabrication
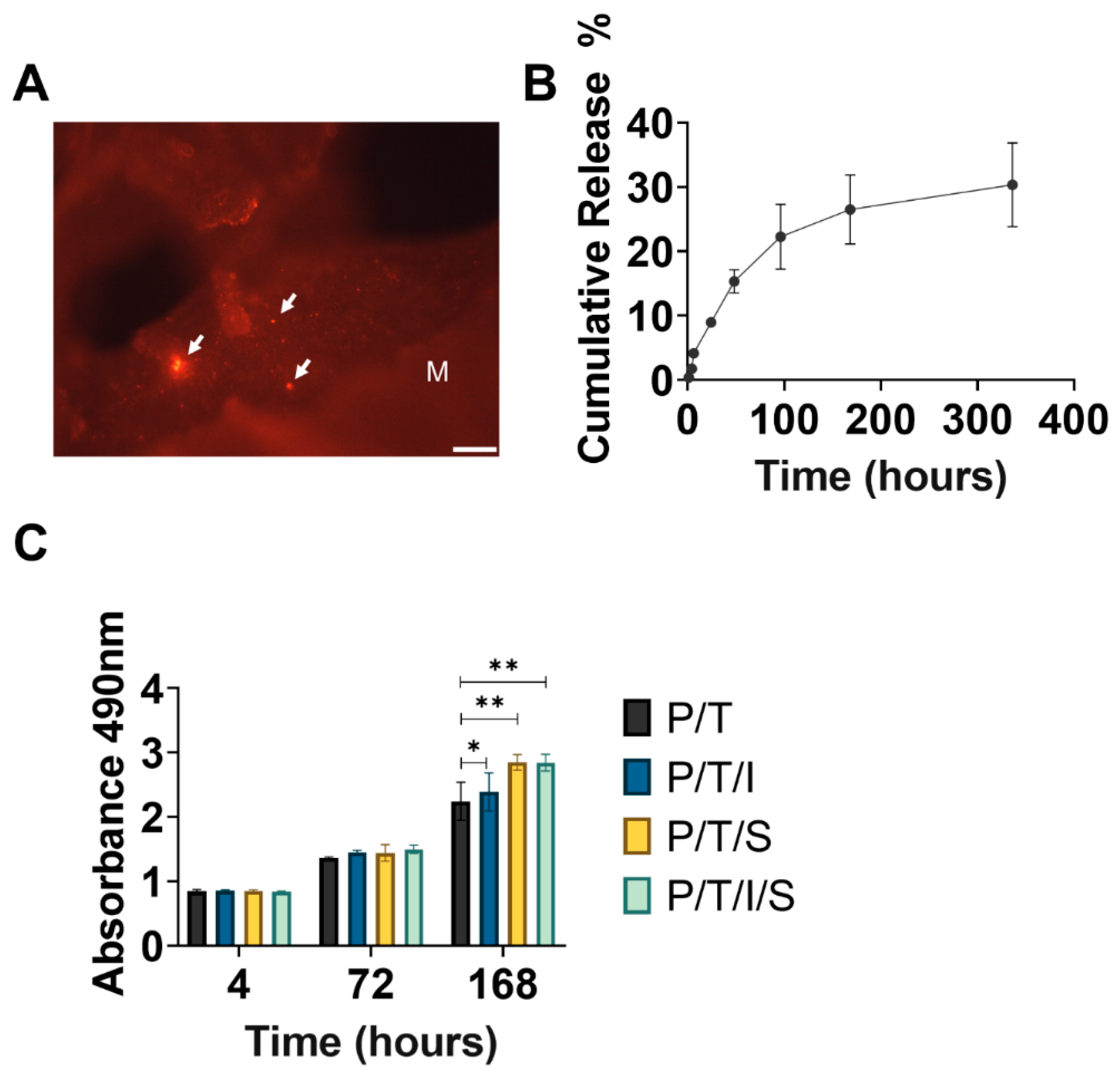
2.8. Preparation of P/T/I Scaffold Loading with HFS and HFS Labelling
2.9. Determination of HFS Released from PLGA/TCP In Vitro
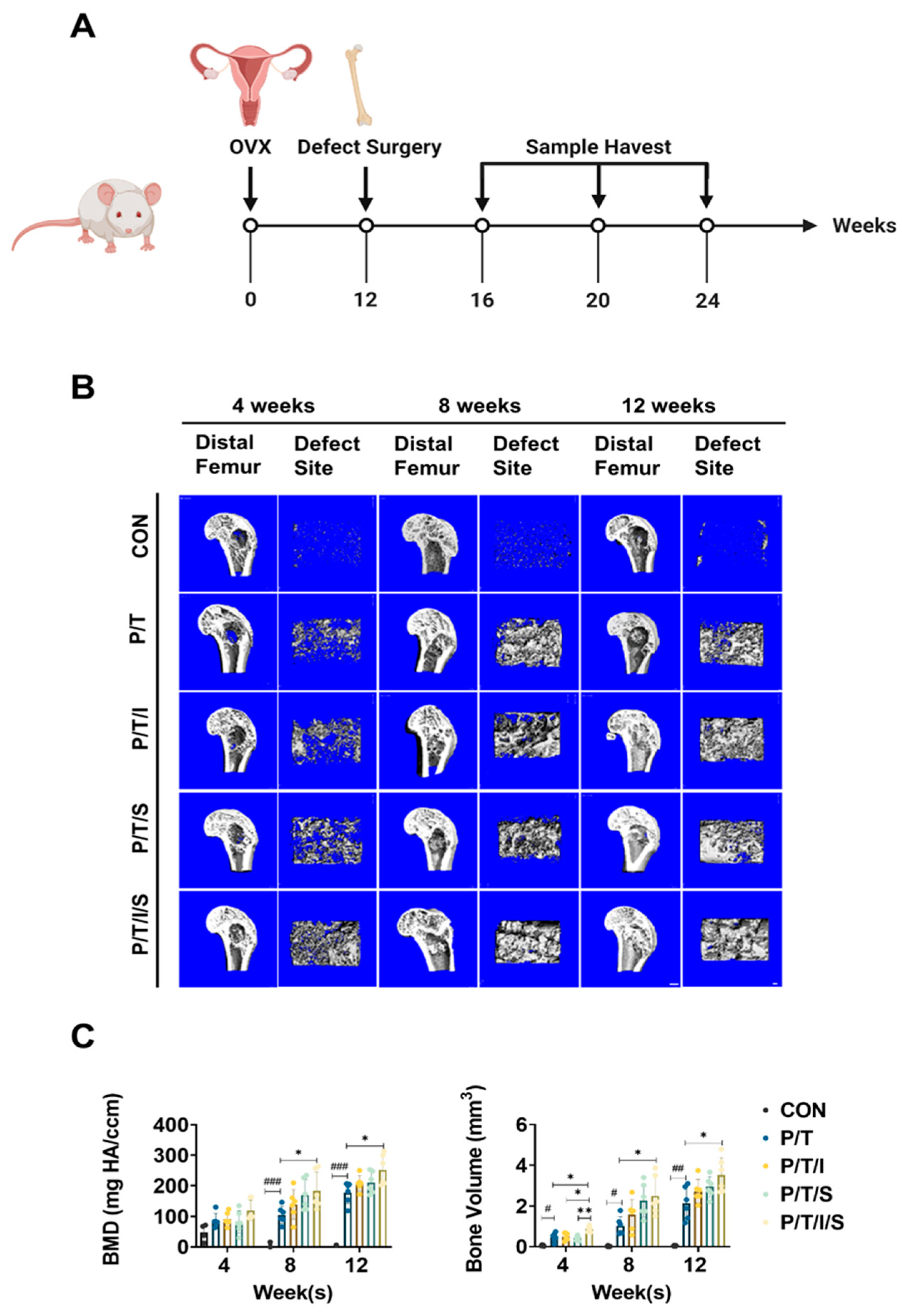
2.10. Animal Study
2.11. Micro-CT Analysis
2.12. Histology and IHC
2.13. Statistical Analysis
3. Results
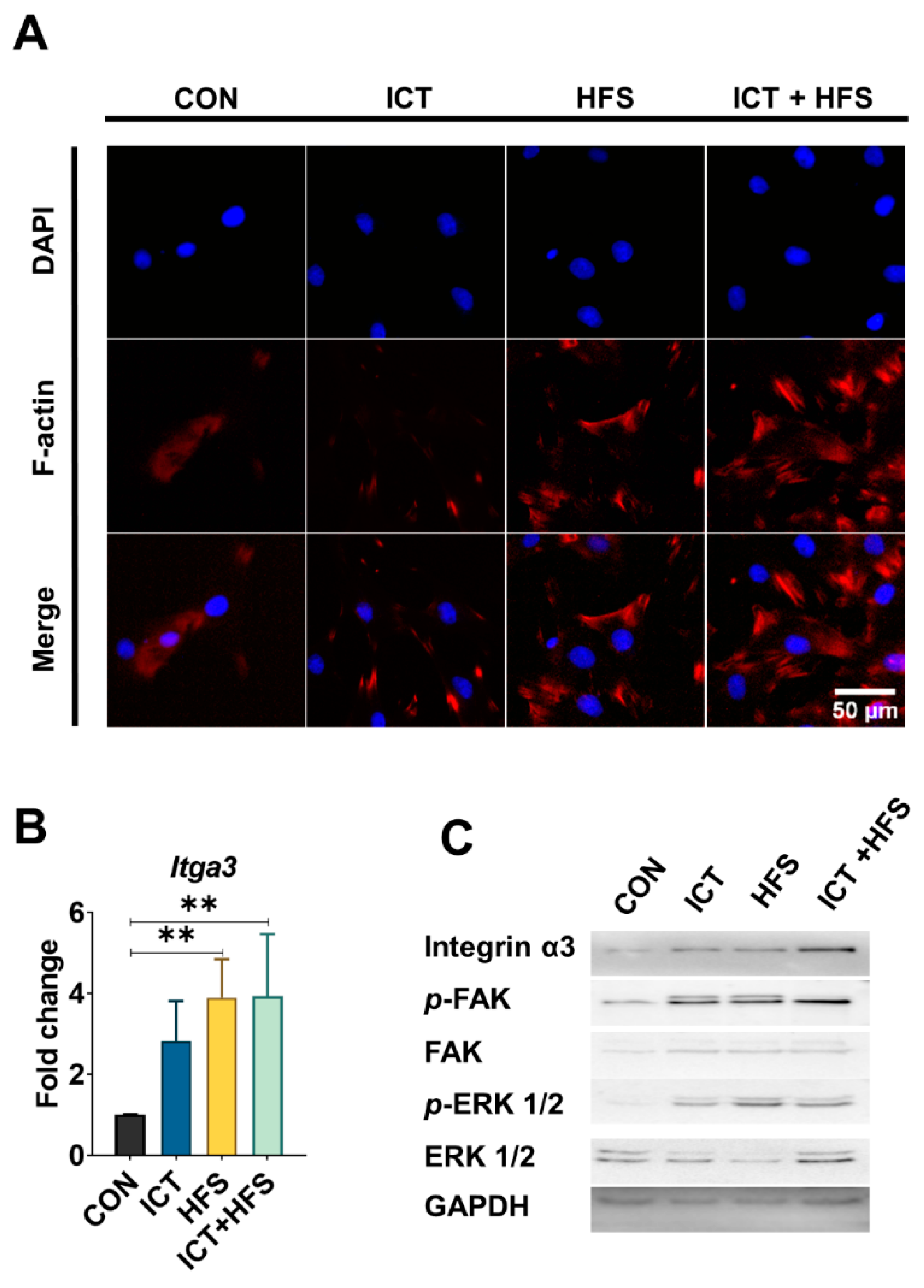
3.1. The Additive Effect of ICT and HFS on the Osteogenesis of rMSCs
3.2. ICT and HFS Additively Promote Osteogenic Differentiation of rMSCs through Integrin-mediated Focal Adhesion Pathway
3.3. Bioactive Scaffold Maintains Sustained Release of Bioactive Factors and Supports the Proliferation of Cells
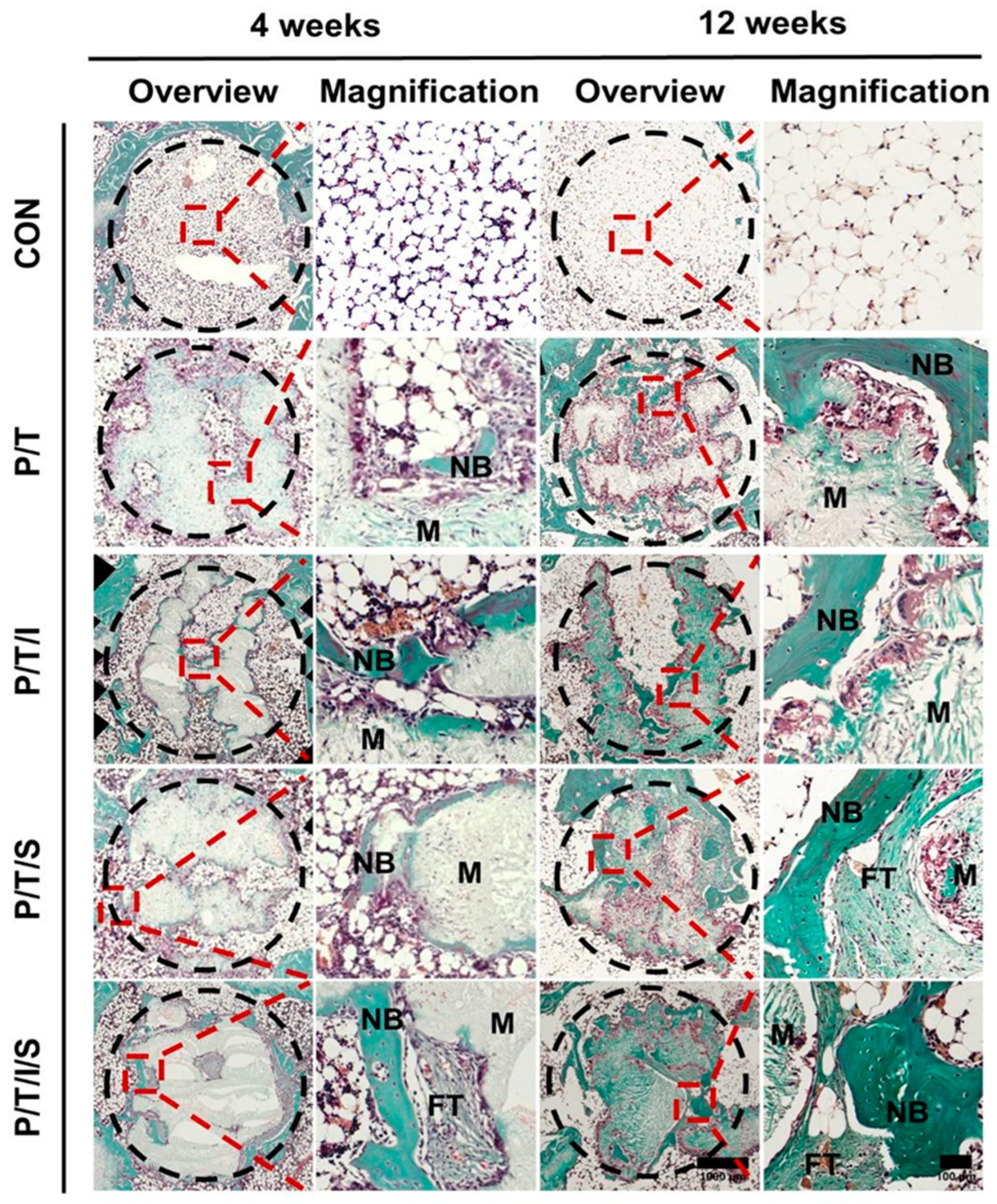
3.4. Bioactive P/T/I/S Scaffold Enhances Osteoporotic Bone Regeneration
3.5. Bioactive P/T/I/S Scaffold Recruits Endogenous MSCs to Boost the Healing of Osteoporotic Bone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Almeida, M.; Manolagas, S. Aging and bone. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–292. [Google Scholar]
- Cheung, W.H.; Miclau, T.; Chow, S.K.-H.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, S21–S26. [Google Scholar] [CrossRef]
- Kyllonen, L.; D’Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.L.; Mazzola, S.; Colombo, A.; Giardina, F.; Romanò, F.; Colombo, M. Non-unions. Clin. Cases Min. Bone Metab. 2017, 14, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Kanakaris, N.K.; Paliobeis, C.; Nlanidakis, N.; Giannoudis, P.V. Biological enhancement of tibial diaphyseal aseptic non-unions: The efficacy of autologous bone grafting, BMPs and reaming by-products. Injury 2007, 38 (Suppl. S2), S65–S75. [Google Scholar] [CrossRef]
- Ashman, O.; Phillips, A.M. Treatment of non-unions with bone defects: Which option and why? Injury 2013, 44 (Suppl. S1), S43–S45. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42 (Suppl. S2), S56–S63. [Google Scholar] [CrossRef]
- Yeong, W.-Y.; Chua, C.-K.; Leong, K.-F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: Challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Lin, S.; Cui, L.; Chen, G.; Huang, J.; Yang, Y.; Zou, K.; Lai, Y.; Wang, X.; Zou, L.; Wu, T.; et al. PLGA/beta-TCP composite scaffold incorporating salvianolic acid B promotes bone fusion by angiogenesis and osteogenesis in a rat spinal fusion model. Biomaterials 2019, 196, 109–121. [Google Scholar] [CrossRef]
- Xie, X.H.; Wang, X.L.; Zhang, G.; He, Y.X.; Leng, Y.; Tang, T.T.; Pan, X.; Qin, L. Biofabrication of a PLGA-TCP-based porous bioactive bone substitute with sustained release of icaritin. J. Tissue Eng. Regen. Med. 2015, 9, 961–972. [Google Scholar] [CrossRef]
- Xie, X.H.; Wang, X.L.; Zhang, G.; He, Y.X.; Wang, X.H.; Liu, Z.; He, K.; Peng, J.; Leng, Y.; Qin, L. Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomed. Mater. 2010, 5, 054109. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yao, D.; Zheng, L.; Liu, W.C.; Liu, Z.; Lei, M.; Huang, L.; Xie, X.; Wang, X.; Chen, Y.; et al. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: Proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials 2015, 59, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Xie, X.H.; Zhang, G.; Chen, S.H.; Yao, D.; He, K.; Wang, X.H.; Yao, X.S.; Leng, Y.; Fung, K.P.; et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J. Orthop. Res. 2013, 31, 164–172. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, J.; Zhang, K.; Yao, H.; Zheng, N.; Zheng, L.; Wang, J.; Wei, K.; Xiao, X.; Qin, L.; et al. Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects. ACS Cent. Sci. 2019, 5, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, S.-Q. Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects. Pharmaceuticals 2022, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: A 24-month randomized, double-blind and placebo-controlled trial. J. Bone Min. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef]
- Yao, D.; Xie, X.H.; Wang, X.L.; Wan, C.; Lee, Y.W.; Chen, S.H.; Pei, D.Q.; Wang, Y.X.; Li, G.; Qin, L. Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis—An in vitro efficacy study. PLoS ONE 2012, 7, e41264. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lee, W.Y.; Huang, B.; Zhang, J.F.; Wu, T.; Jiang, X.; Wang, C.C.; Li, G. Secretome of Human Fetal Mesenchymal Stem Cell Ameliorates Replicative Senescen. Stem Cells Dev. 2016, 25, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, B.; Sun, Y.; Wu, T.; Liu, Y.; Zhang, J.; Lee, W.Y.; Pan, X.; Chai, Y.; Li, G. Human fetal mesenchymal stem cell secretome enhances bone consolidation in distraction osteogenesis. Stem Cell Res. Ther. 2016, 7, 134. [Google Scholar] [CrossRef]
- Wang, B.; Pang, M.; Song, Y.; Wang, H.; Qi, P.; Bai, S.; Lei, X.; Wei, S.; Zong, Z.; Lin, S.; et al. Human fetal mesenchymal stem cells secretome promotes scarless diabetic wound healing through heat-shock protein family. Bioeng. Transl. Med. 2022, e10354. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Q.; Zhang, N.; Wang, B.; Yang, Z.; Ryaby, J.T.; Waldorff, E.I.; Lee, W.Y.-W.; Li, G. A novel pulsed electromagnetic field promotes distraction osteogenesis via enhancing osteogenesis and angiogenesis in a rat model. J. Orthop. Transl. 2020, 25, 87–95. [Google Scholar] [CrossRef]
- Perpétuo, I.P.; Bourne, L.E.; Orriss, I.R. Isolation and Generation of Osteoblasts. In Bone Research Protocols; Idris, A.I., Ed.; Springer: New York, NY, USA, 2019; pp. 21–38. [Google Scholar]
- Chen, S.H.; Lei, M.; Xie, X.H.; Zheng, L.Z.; Yao, D.; Wang, X.L.; Li, W.; Zhao, Z.; Kong, A.; Xiao, D.M.; et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013, 9, 6711–6722. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Huang, M.; Zong, Z.; Wu, X.; Xu, J.; Lan, H.; Zheng, J.; Zhang, X.; Lee, Y.W.; et al. Asiatic Acid Attenuates Bone Loss by Regulating Osteoclastic Differentiation. Calcif. Tissue Int. 2019, 105, 531–545. [Google Scholar] [CrossRef]
- Ning, Z.; Tan, B.; Chen, B.; Lau, D.S.A.; Wong, T.M.; Sun, T.; Peng, S.; Li, Z.; Lu, W.W. Precisely Controlled Delivery of Abaloparatide through Injectable Hydrogel to Promote Bone Regeneration. Macromol. Biosci. 2019, 19, e1900020. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xu, J.; Mi, J.; He, X.; Pan, Q.; Zheng, L.; Zu, H.; Chen, Z.; Dai, B.; Li, X.; et al. Biodegradable magnesium combined with distraction osteogenesis synergistically stimulates bone tissue regeneration via CGRP-FAK-VEGF signaling axis. Biomaterials 2021, 275, 120984. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Y.; Li, Y.; Wang, H.; Kong, L.; Yang, Z.; Zhang, X.; Bai, S.; Zong, Z.; Chen, G.; et al. Local administration of allogeneic or autologous bone marrow-derived mesenchymal stromal cells enhances bone formation similarly in distraction osteogenesis. Cytotherapy 2021, 23, 590–598. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Hof, R.J. Analysis of Bone Architecture in Rodents Using Microcomputed Tomography. In Bone Research Protocols; Helfrich, M.H., Ralston, S.H., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 461–476. [Google Scholar]
- Gruber, H.E. Adaptations of Goldner’s Masson Trichrome Stain for the Study of Undecalcified Plastic Embedded Bone. Biotech. Histochem. 1992, 67, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, J.F.; Shi, L.; Yang, Z.M.; Wu, T.Y.; Wang, H.X.; Lin, W.P.; Lu, Y.F.; Lo, J.H.T.; Zhu, D.H.; et al. MicroRNA-378 Suppressed Osteogenesis of MSCs and Impaired Bone Formation via Inactivating Wnt/β-Catenin Signaling. Mol. Ther. Nucleic Acids 2020, 21, 1017–1028. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Bishop, R.T.; de Ridder, D.; Delgado-Calle, J.; Reagan, M.R. 2D and 3D In Vitro Co-Culture for Cancer and Bone Cell Interaction Studies. In Bone Research Protocols; Idris, A.I., Ed.; Springer: New York, NY, USA, 2019; pp. 71–98. [Google Scholar]
- Sims, N.A.; Martin, T.J. Chapter 4—The osteoblast lineage: Its actions and communication mechanisms. In Principles of Bone Biology, 4th ed.; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–110. [Google Scholar]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Cooper, T.T.; Sherman, S.E.; Bell, G.I.; Ma, J.; Kuljanin, M.; Jose, S.E.; Lajoie, G.A.; Hess, D.A. Characterization of a Vimentinhigh/Nestinhigh proteome and tissue regenerative secretome generated by human pancreas-derived mesenchymal stromal cells. Stem Cells 2020, 38, 666–682. [Google Scholar] [CrossRef]
- Wang, K.; Du, B.; Zhang, Y.; Wu, C.; Wang, X.; Zhang, X.; Wang, L. Vimentin-Rab7a Pathway Mediates the Migration of MSCs and Lead to Therapeutic Effects on ARDS. Stem Cells Int. 2021, 2021, 9992381. [Google Scholar] [CrossRef]
- Tirpáková, M.; Vašíček, J.; Svoradová, A.; Baláži, A.; Tomka, M.; Bauer, M.; Makarevich, A.; Chrenek, P. Phenotypical Characterization and Neurogenic Differentiation of Rabbit Adipose Tissue-Derived Mesenchymal Stem Cells. Genes 2021, 12, 431. [Google Scholar] [CrossRef]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef]
- Karsenty, G. Chapter 7—Transcriptional control of osteoblast differentiation and function. In Principles of Bone Biology, 4th ed.; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–176. [Google Scholar]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Marie, P.J. Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 2013, 9, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Integrin Signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Haÿ, E.; Saidak, Z. Integrin and cadherin signaling in bone: Role and potential therapeutic targets. Trends Endocrinol. Metab. 2014, 25, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Gao, X.; Gao, Y.; Li, Y.; Deng, M.; Tan, J.; Gou, J.; Liu, C.; Dou, C.; Li, Z.; et al. Multiple integrin ligands provide a highly adhesive and osteoinductive surface that improves selective cell retention technology. Acta Biomater. 2019, 85, 106–116. [Google Scholar] [CrossRef]
- Fromigué, O.; Brun, J.; Marty, C.; Da Nascimento, S.; Sonnet, P.; Marie, P.J. Peptide-based activation of alpha5 integrin for promoting osteogenesis. J. Cell. Biochem. 2012, 113, 3029–3038. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Lee, Y.-w.; Feng, L.; Wang, B.; Pan, Q.; Meng, X.; Cao, H.; Li, L.; Wang, H.; et al. Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration. Bioengineering 2022, 9, 525. https://doi.org/10.3390/bioengineering9100525
Zhang X, Wang X, Lee Y-w, Feng L, Wang B, Pan Q, Meng X, Cao H, Li L, Wang H, et al. Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration. Bioengineering. 2022; 9(10):525. https://doi.org/10.3390/bioengineering9100525
Chicago/Turabian StyleZhang, Xiaoting, Xinluan Wang, Yuk-wai Lee, Lu Feng, Bin Wang, Qi Pan, Xiangbo Meng, Huijuan Cao, Linlong Li, Haixing Wang, and et al. 2022. "Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration" Bioengineering 9, no. 10: 525. https://doi.org/10.3390/bioengineering9100525
APA StyleZhang, X., Wang, X., Lee, Y.-w., Feng, L., Wang, B., Pan, Q., Meng, X., Cao, H., Li, L., Wang, H., Bai, S., Kong, L., Chow, D. H. K., Qin, L., Cui, L., Lin, S., & Li, G. (2022). Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration. Bioengineering, 9(10), 525. https://doi.org/10.3390/bioengineering9100525







