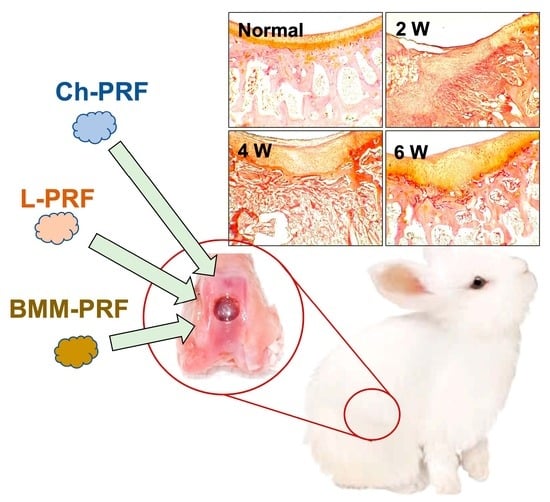
Osteochondral Regeneration Ability of Uncultured Bone Marrow Mononuclear Cells and Platelet-Rich Fibrin Scaffold
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Preparation of Fibrin Scaffold and Bone Marrow Mononuclear Cells
2.2.1. Preparation of Choukroun’s Platelet-Rich Fibrin Scaffold from Peripheral Blood
2.2.2. Preparation of Leukocyte Platelet-Rich Fibrin Scaffold from Peripheral Blood
2.2.3. Preparation of Scaffolds Combining Uncultured Bone Marrow Mononuclear Cells and Platelets-Rich Fibrin from Bone Marrow
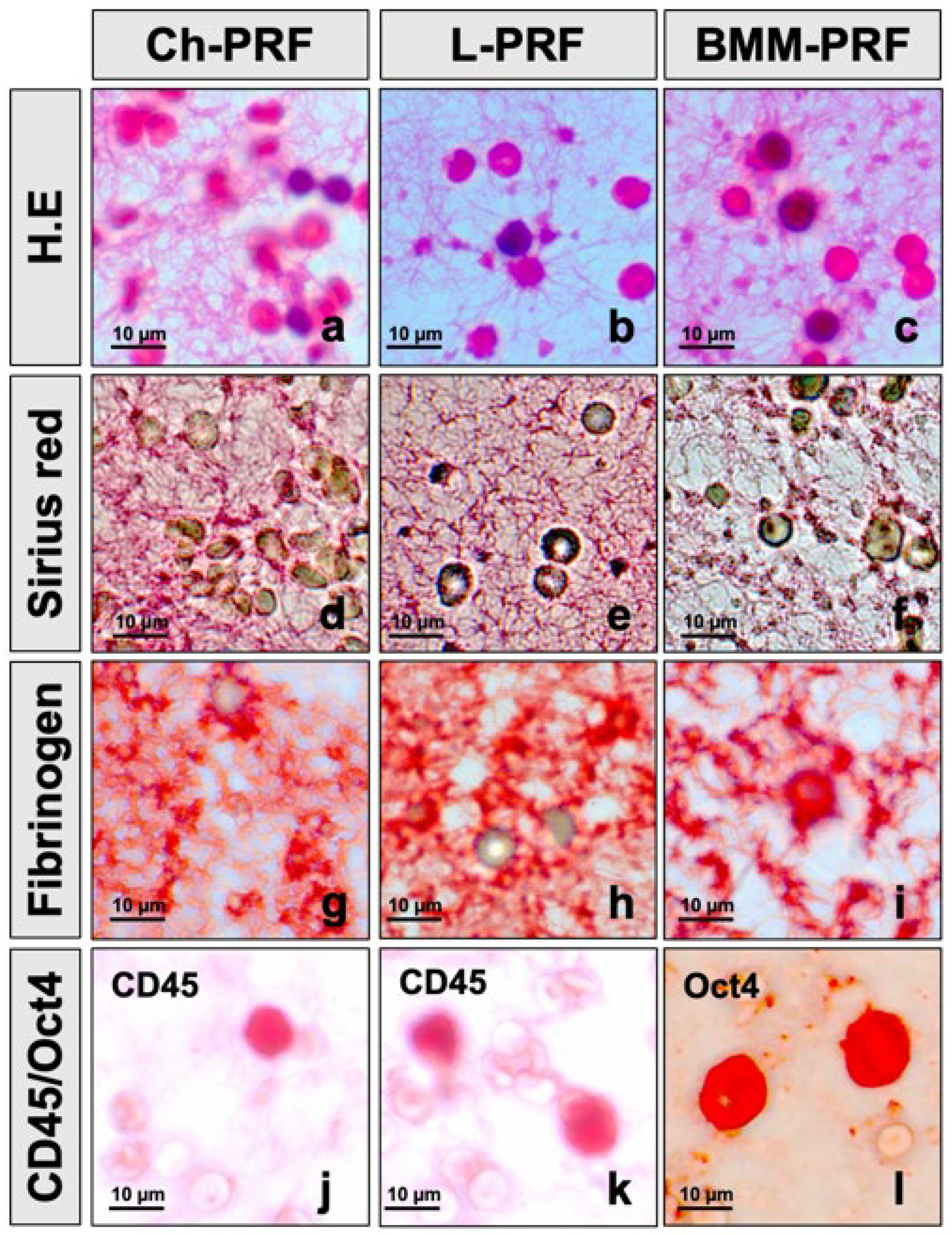
2.2.4. PicroSirius Red Staining for Assessing the Fibrin Structure of the PRF Scaffold
2.2.5. Immunohistochemical Staining for Detecting Cells Distributing in PRF Scaffold
2.3. Gene Expression Analysis
2.4. Surgical Procedures for Inducing Rabbit’s Knee Osteochondral Defects
2.5. Macroscopic Assessment
2.6. Microscopic Assessment
2.7. Statistical Analysis
3. Results
3.1. Histological Characteristics of PRF Scaffold from Peripheral Blood and Bone Marrow Aspirate
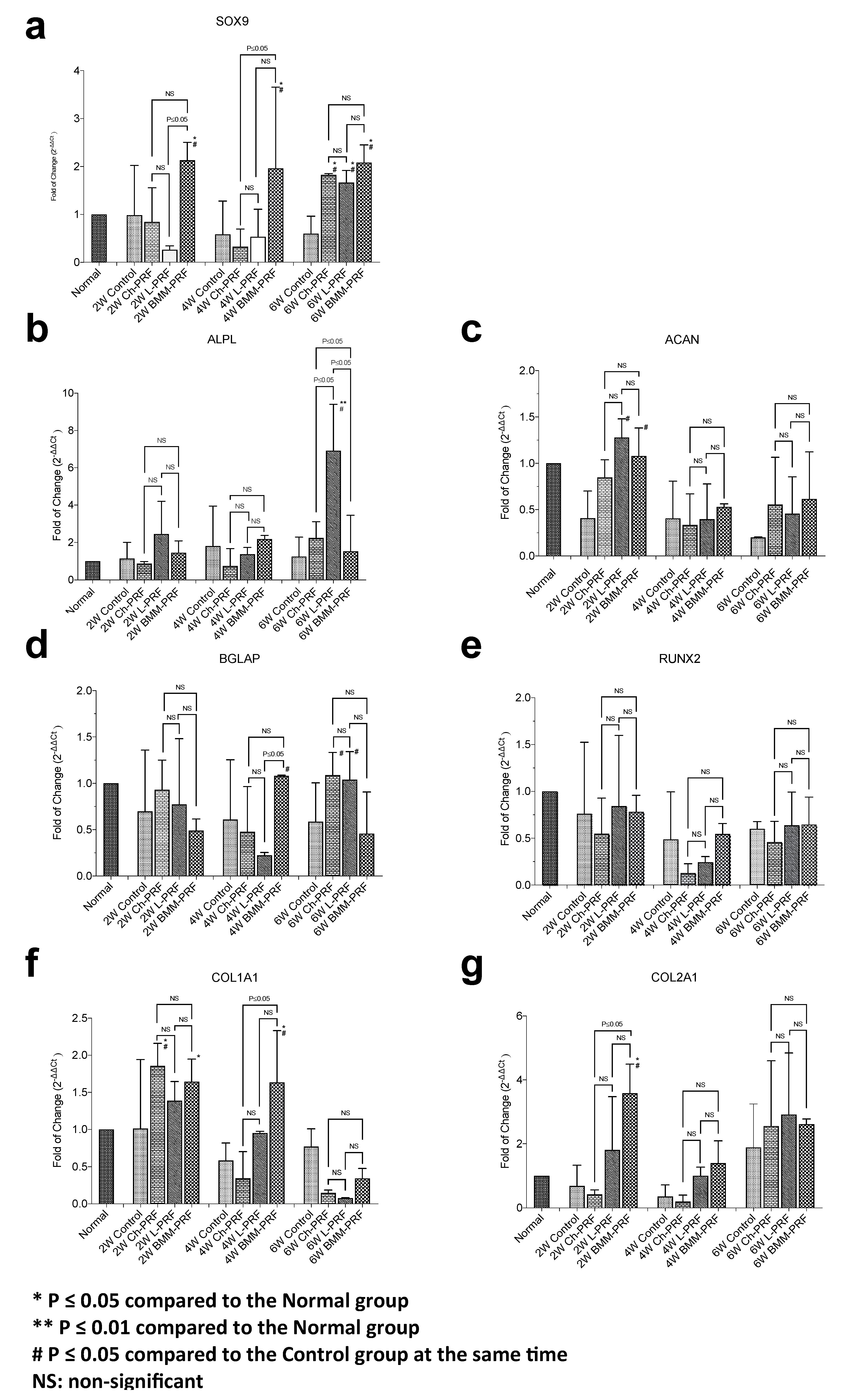
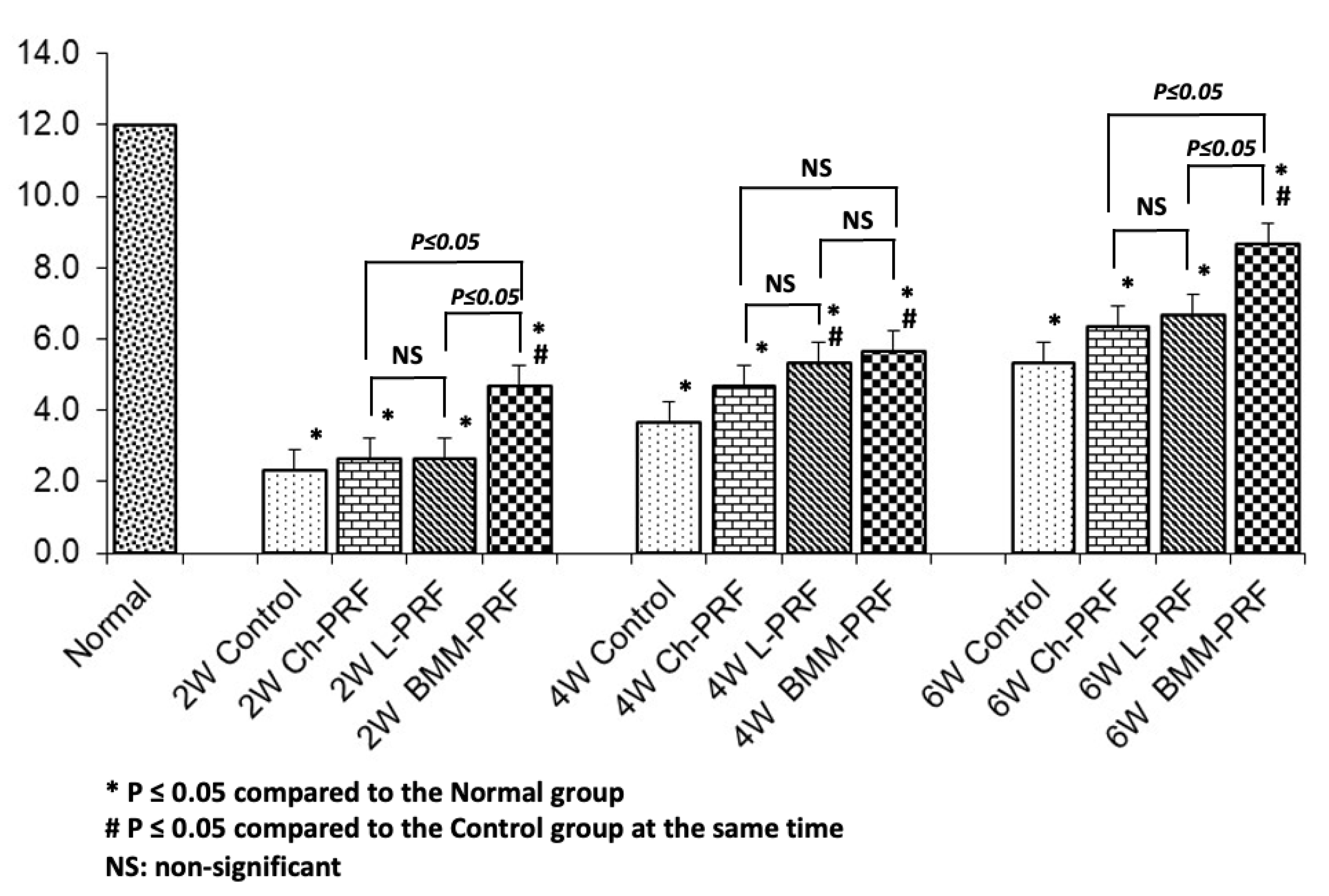
3.2. Gene Expression Analysis of Osteochondral Repair
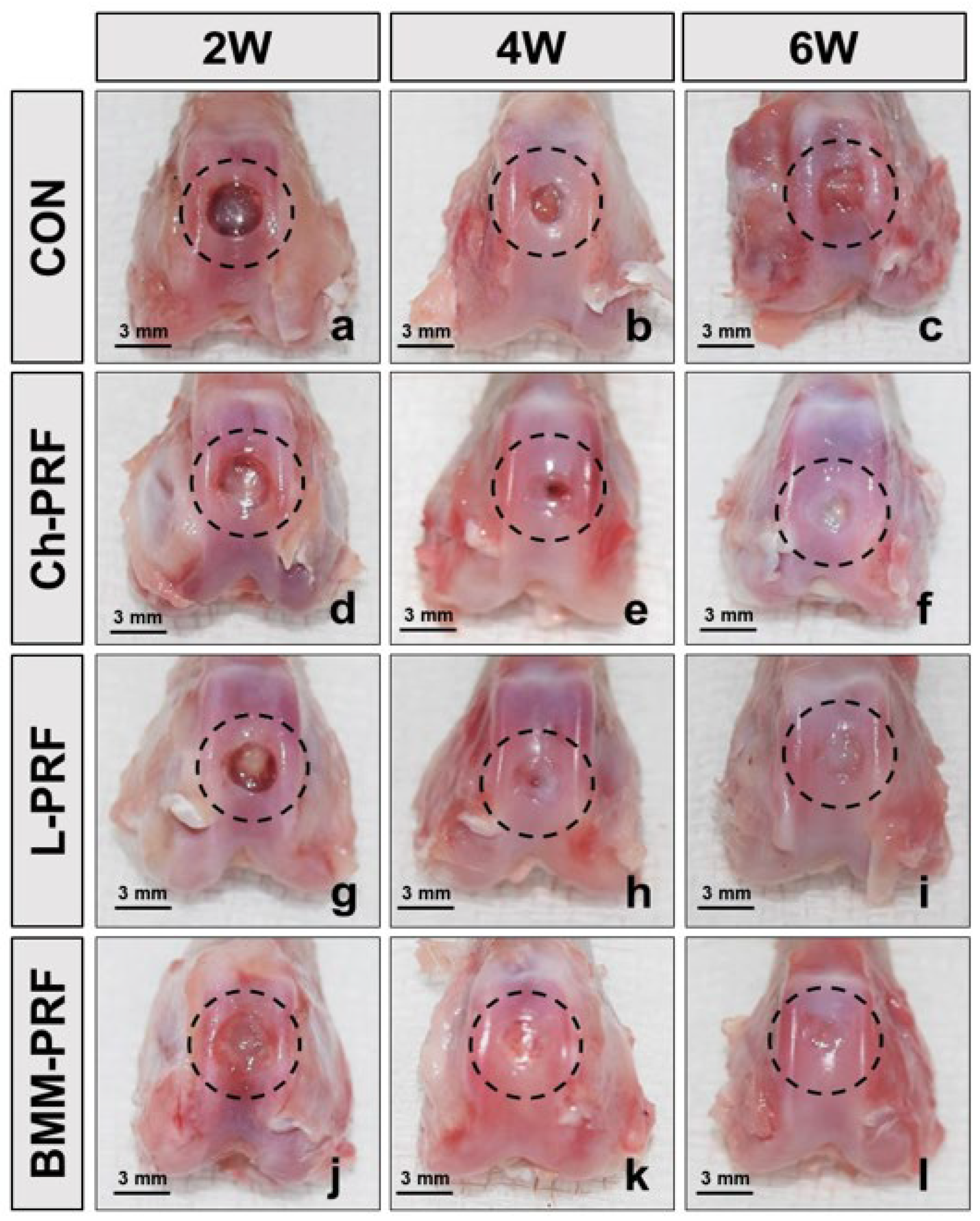
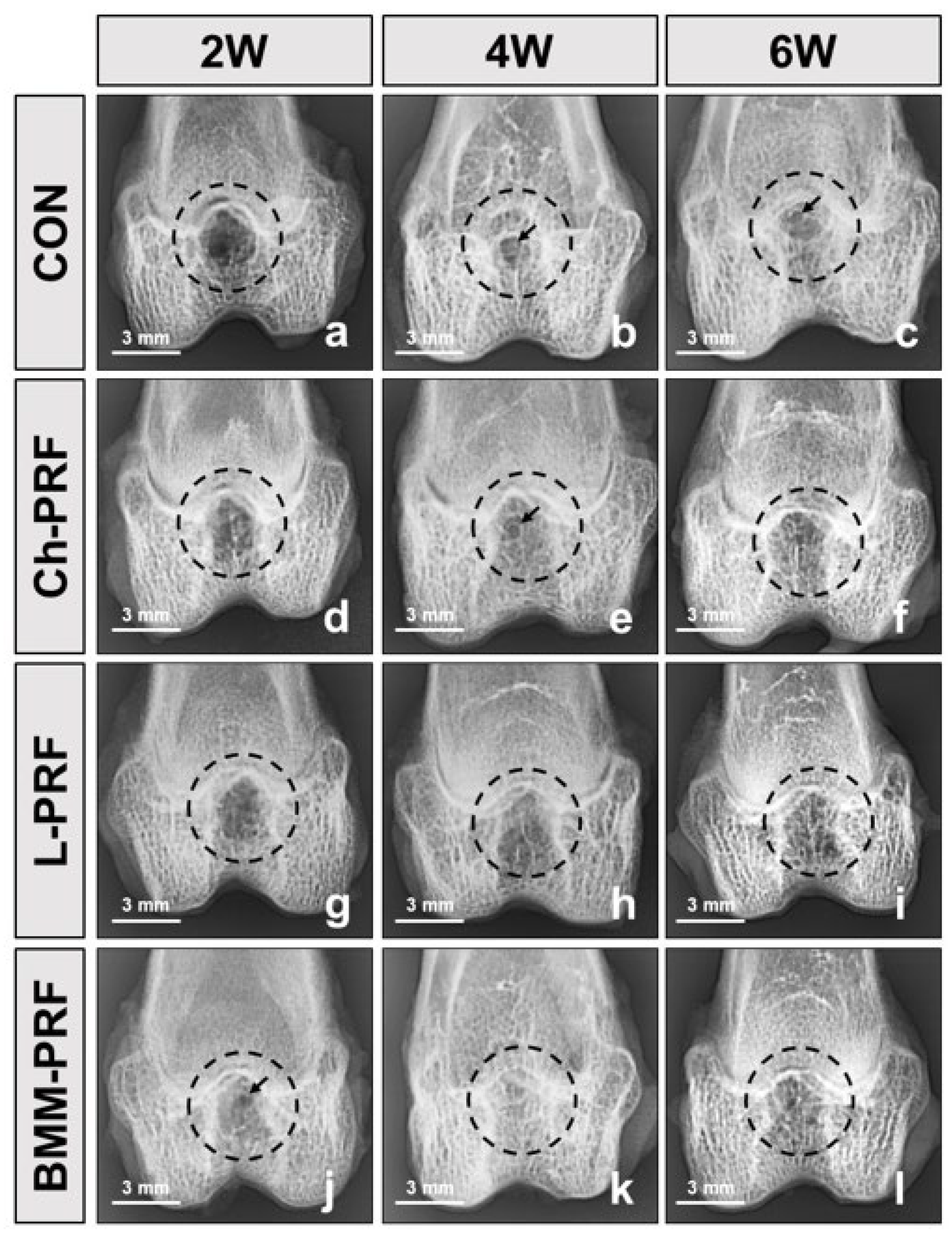
3.3. Macroscopic Assessment of Osteochondral Regeneration
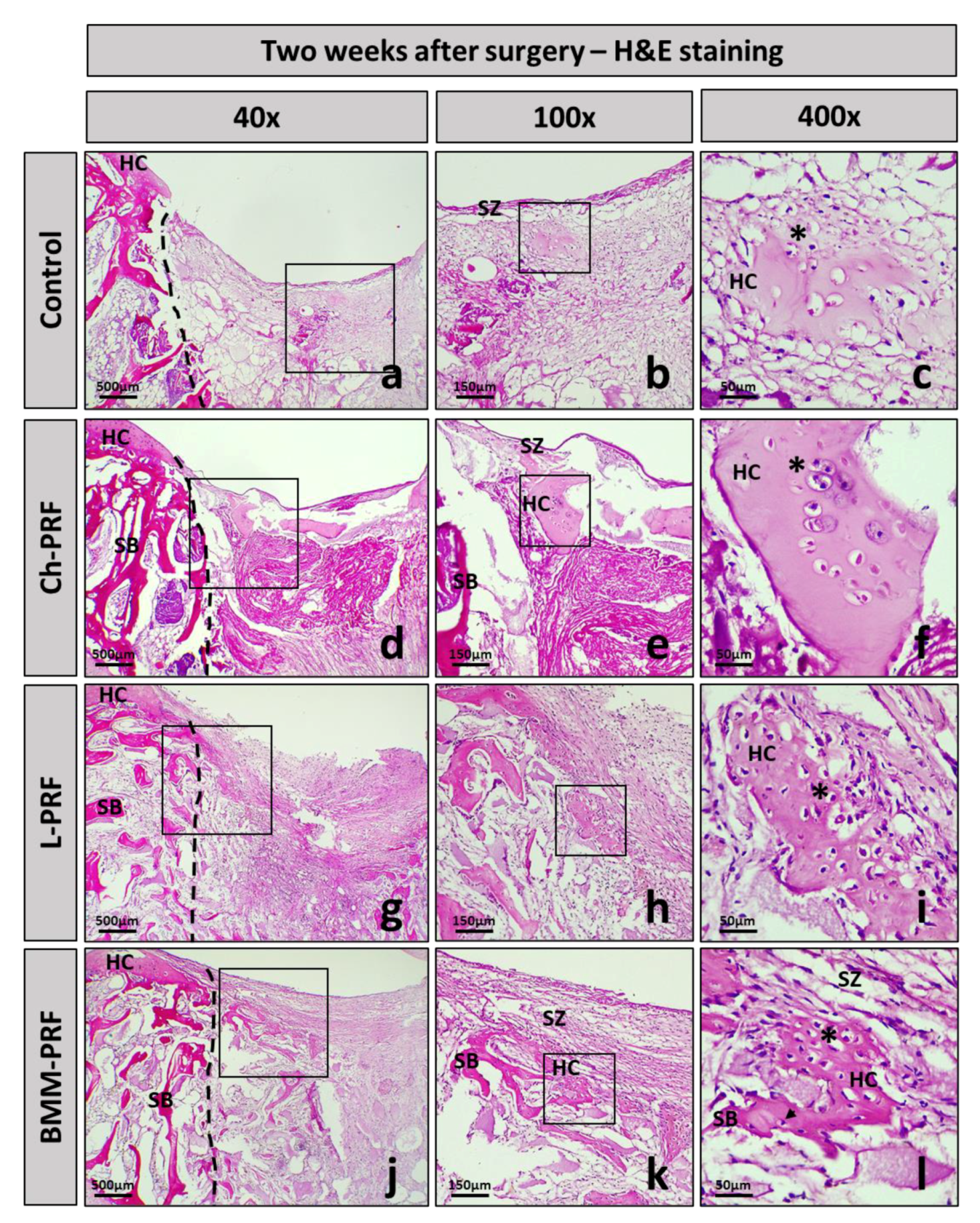
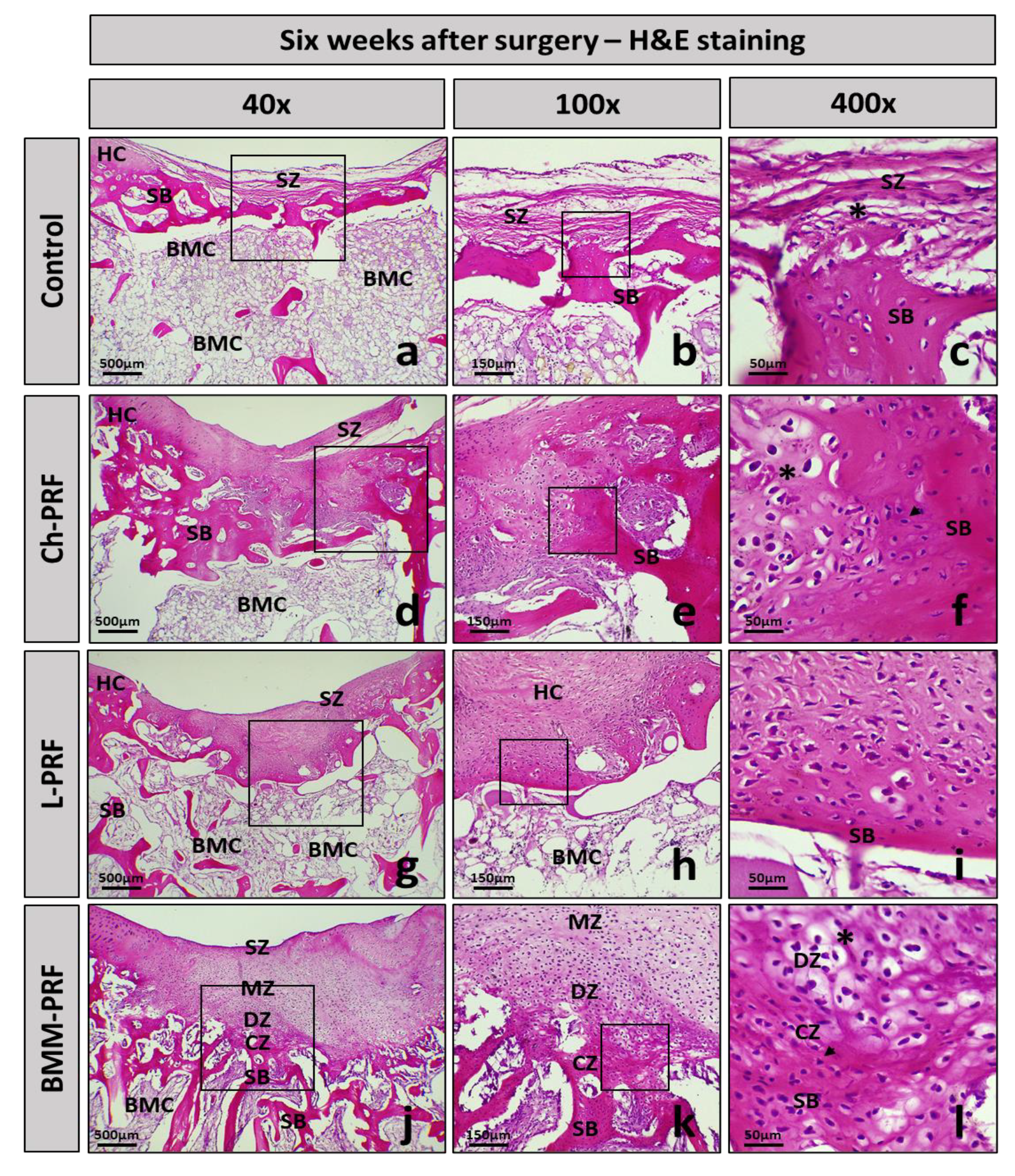
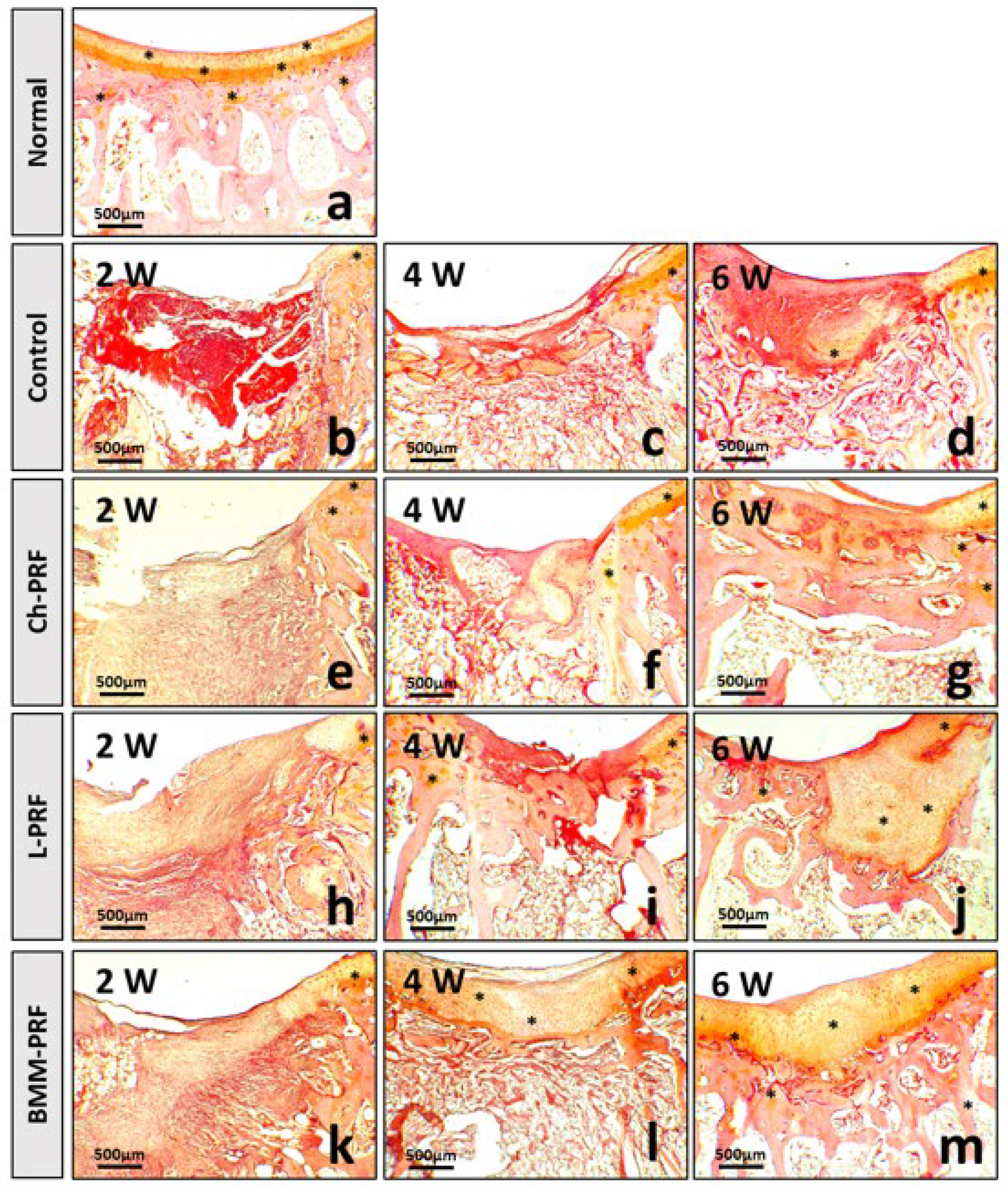
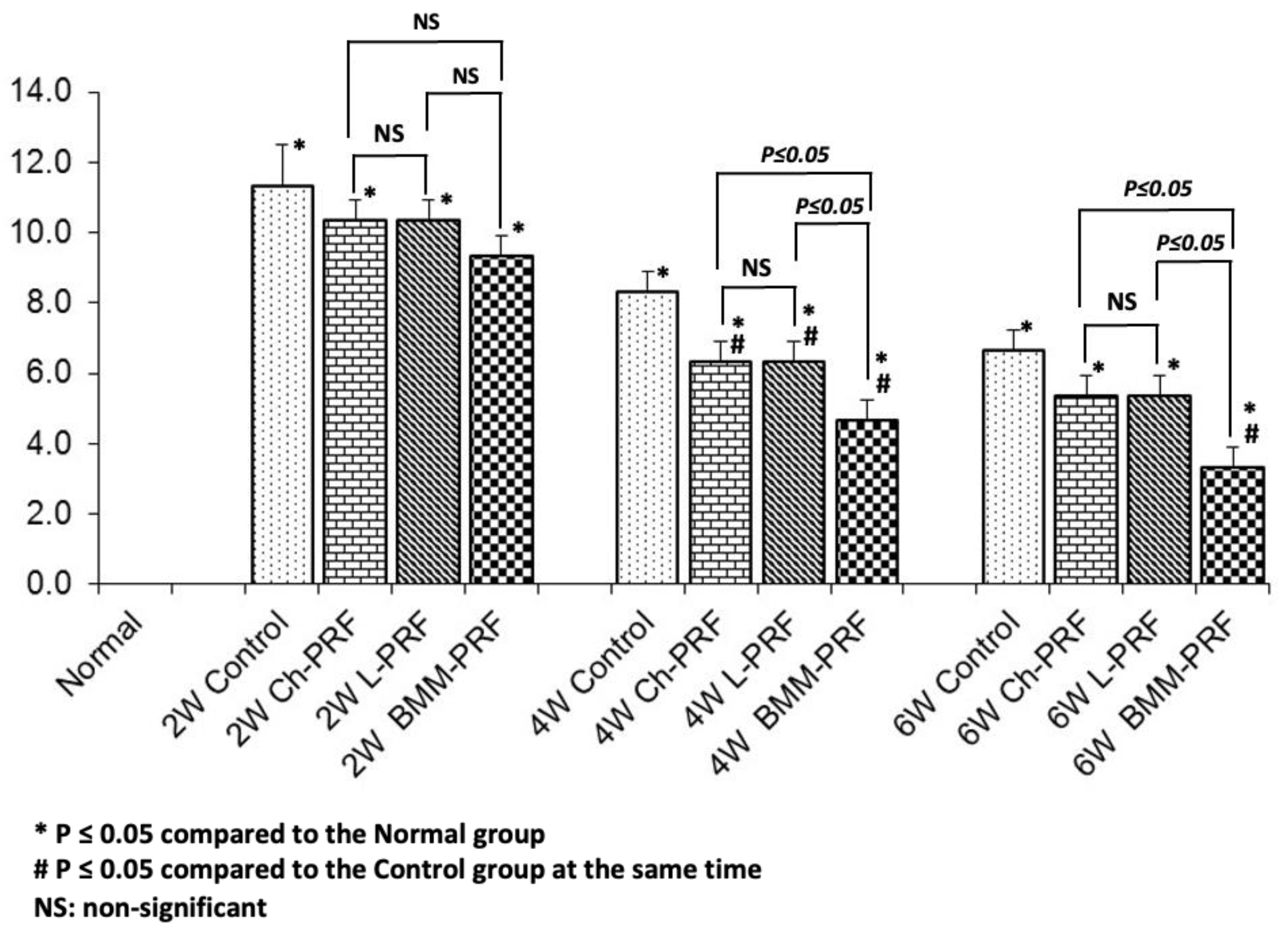
3.4. Microscopic Evaluation of the Osteochondral Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Sun, C.; Wang, Q.; Yang, W.; Zhang, Z.; Xia, Z.; Shao, Z.; Wang, B. Endogenous Repair and Regeneration of Injured Articular Cartilage: A Challenging but Promising Therapeutic Strategy. Aging Dis. 2021, 12, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Rousche, K.T.; Tuan, R.S. Technology Insight: Adult stem cells in cartilage regeneration and tissue engineering. Nat. Clin. Pract. Rheumatol. 2006, 2, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Inui, A.; Iwakura, T.; Reddi, A.H. Human stem cells and articular cartilage regeneration. Cells 2012, 1, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lee, S.J.; Lee, T.J.; Yoon, T.H.; Choi, C.H. Results of microfracture in the osteoarthritic knee with focal full-thickness articular cartilage defects and concomitant medial meniscal tears. Knee Surg. Relat. Res. 2013, 25, 71–76. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef]
- Wakitani, S.; Goto, T.; Pineda, S.J.; Young, R.G.; Mansour, J.M.; Caplan, A.I.; Goldberg, V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Jt. Surg. 1994, 76, 579–592. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Tiwary, R.; Amarpal; Aithal, H.P.; Kinjavdekar, P.; Pawde, A.M.; Singh, R. Effect of IGF-1 and Uncultured Autologous Bone-Marrow-Derived Mononuclear Cells on Repair of Osteochondral Defect in Rabbits. Cartilage 2014, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Arturo, J.; Perez, C.; Segura, O.; Segura Guerrero, O.; Bastidas, Y.; Vivas Sandoval, A.L.; Ruiz Ballesteros, C. Bone and cartilage regeneration with intra-articular injection of autologous bone marrow mononuclear cells (aBM-MNC) in different cases of artrhrosis. Clinical trial (phase I/II). Cytotherapy 2017, 19, S227. [Google Scholar] [CrossRef]
- Rajput, B.S.; Kulkarni, R.N.; Bopardikar, A.; Somalapur, P.; Kumar, R. Retrospective analysis of role of autologous bone marrow derived mononuclear stem cells in the management of degenerative arthritis of knee. J. Stem Cell Res. Ther. 2018, 4, 22–28. [Google Scholar] [CrossRef]
- Salem, M.; Rizk, A.; Mosbah, E.; Hamed, M.; Karrouf, G.; Zaghloul, A. Effect of Platlate Riched Fibrin and mononuclear cells on regeneration of osteochondral defect in rabbits. Mansoura Vet. Med. J. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stähli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef]
- Göral, A.; Aslan, C.; Bolat Küçükzeybek, B.; Işık, D.; Hoşnuter, M.; Durgun, M. Platelet-Rich Fibrin Improves the Viability of Diced Cartilage Grafts in a Rabbit Model. Aesthetic Surg. J. 2016, 36, NP153–NP162. [Google Scholar] [CrossRef]
- Abd El Raouf, M.; Wang, X.; Miusi, S.; Chai, J.; Mohamed AbdEl-Aal, A.B.; Nefissa Helmy, M.M.; Ghanaati, S.; Choukroun, J.; Choukroun, E.; Zhang, Y.; et al. Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets 2019, 30, 213–221. [Google Scholar] [CrossRef]
- Chun, Y.S.; Kim, S.A.; Kim, Y.H.; Lee, J.H.; Shetty, A.A.; Kim, S.J. Autologous Collagen-Induced Chondrogenesis: From Bench to Clinical Development. Medicina 2023, 59, 530. [Google Scholar] [CrossRef] [PubMed]
- Thorp, H.; Kim, K.; Bou-Ghannam, S.; Kondo, M.; Maak, T.; Grainger, D.W.; Okano, T. Enhancing chondrogenic potential via mesenchymal stem cell sheet multilayering. Regen. Ther. 2021, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Vining, B.; Bagheri-Fam, S.; Harley, V. SOX9 in organogenesis: Shared and unique transcriptional functions. Cell. Mol. Life Sci. 2022, 79, 522. [Google Scholar] [CrossRef]
- Narai, T.; Watase, R.; Nakayama, Y.; Kodani, I.; Inoue, T.; Kokura, K. Establishment of human immortalized mesenchymal stem cells lines for the monitoring and analysis of osteogenic differentiation in living cells. Heliyon 2020, 6, e05398. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Umapathy, A.; Srinivasan, S.; Barker, C.N.; Brooks, A.; Hearn, J.; Chhana, A.; Williams, E.; Sheppard, H.; McGlashan, S.R. The Chondrogenic Potential of First-Trimester and Term Placental Mesenchymal Stem/Stromal Cells. Cartilage 2021, 13, 544S–558S. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Sub-Cell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef]
- Nguyen-Thanh, T.; Kim, D.; Lee, S.; Kim, W.; Park, S.K.; Kang, K.P. Inhibition of histone deacetylase 1 ameliorates renal tubulointerstitial fibrosis via modulation of inflammation and extracellular matrix gene transcription in mice. Int. J. Mol. Med. 2018, 41, 95–106. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, T.H.; Hsu, C.C.; Yeh, M.L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells 2021, 10, 3536. [Google Scholar] [CrossRef]
- Meng, X.; Grad, S.; Wen, C.; Lai, Y.; Alini, M.; Qin, L.; Wang, X. An impaired healing model of osteochondral defect in papain-induced arthritis. J. Orthop. Transl. 2021, 26, 101–110. [Google Scholar] [CrossRef]
- Van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr. Cartil. 2007, 15, 1397–1402. [Google Scholar] [CrossRef]
- Kazemi, D.; Fakhrjou, A.; Dizaji, V.M.; Alishahi, M.K. Effect of autologous platelet rich fibrin on the healing of experimental articular cartilage defects of the knee in an animal model. BioMed Res. Int. 2014, 2014, 486436. [Google Scholar] [CrossRef] [PubMed]
- Goljanian Tabrizi, A.; Hashemi, H.; Mohsenifar, Z.; Bohlouli, M. Long-term Evaluation of the Effect of Platelet-rich Fibrin on Cartilage Tissue Regeneration: An Animal Model Study. J. Regen. Reconstr. Restor. (Triple R) 2021, 5, e27. [Google Scholar] [CrossRef]
- Dejnek, M.; Moreira, H.; Płaczkowska, S.; Barg, E.; Reichert, P.; Królikowska, A. Leukocyte-Rich Platelet-Rich Plasma as an Effective Source of Molecules That Modulate Local Immune and Inflammatory Cell Responses. Oxidative Med. Cell. Longev. 2022, 2022, 8059622. [Google Scholar] [CrossRef] [PubMed]
- Baca-Gonzalez, L.; Serrano Zamora, R.; Rancan, L.; Gonzalez Fernandez-Tresguerres, F.; Fernandez-Tresguerres, I.; Lopez-Pintor, R.M.; Lopez-Quiles, J.; Leco, I.; Torres, J. Plasma rich in growth factors (PRGF) and leukocyte-platelet rich fibrin (L-PRF): Comparative release of growth factors and biological effect on osteoblasts. Int. J. Implant Dent. 2022, 8, 39. [Google Scholar] [CrossRef]
- Beitia, M.; Delgado, D.; Mercader, J.; Sánchez, P.; López de Dicastillo, L.; Sánchez, M. Action of Platelet-Rich Plasma on In Vitro Cellular Bioactivity: More than Platelets. Int. J. Mol. Sci. 2023, 24, 5367. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, L.; Yan, B.; Zhang, L.; Du, W.; Liu, F.; Yuan, Q.; Tong, P.; Shan, L.; Efferth, T. Growth factors-based beneficial effects of platelet lysate on umbilical cord-derived stem cells and their synergistic use in osteoarthritis treatment. Cell Death Dis. 2020, 11, 857. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhang, B.; Zhao, Q.; Liu, Y.; Qi, W. Effect of Activated Platelet-Rich Plasma on Chondrogenic Differentiation of Rabbit Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Int. 2021, 2021, 9947187. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.M.; Everts, P.A.; Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef]
- Wise, J.K.; Alford, A.I.; Goldstein, S.A.; Stegemann, J.P. Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopedic tissue engineering. Tissue Eng. Part A 2014, 20, 210–224. [Google Scholar] [CrossRef]
- Hernández, P. Use of bone marrow-derived cells for regenerative medicine in Cuba. Bone Marrow Transplant. 2016, 51, 134. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wu, H.; Li, H.; Cai, L.; Wang, Q.; Liu, X.; Xiao, R.; Yin, N.; Cao, Y. Bone Marrow Mononuclear Cells Combined with Beta-Tricalcium Phosphate Granules for Alveolar Cleft Repair: A 12-Month Clinical Study. Sci. Rep. 2017, 7, 13773. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, J.E.J.; Creemers, L.B.; Tsuchida, A.I.; van Rijen, M.H.P.; Custers, R.J.H.; Dhert, W.J.A.; Saris, D.B.F. One-stage focal cartilage defect treatment with bone marrow mononuclear cells and chondrocytes leads to better macroscopic cartilage regeneration compared to microfracture in goats. Osteoarthr. Cartil. 2013, 21, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Tang, J.; Geng, R.; Hu, H.; Zhu, C.; Cui, W.; Fan, W. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int. J. Clin. Exp. Pathol. 2014, 7, 1415–1426. [Google Scholar]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of Bone Marrow Mononuclear Cells on Biomaterials for Bone Tissue Engineering In Vitro. BioMed Res. Int. 2015, 2015, 762407. [Google Scholar] [CrossRef]


| Gene | Primer Name | Forward | Reverse |
|---|---|---|---|
| 18S-rRNA | r18S | ATCAGATACCGTCGTAGTTC | TTCCGTCAATTCCTTTAAG |
| SRY-box transcription factor 9 | rSOX9 | GCTCCGACACCGAGAATACA | TTGACGTGGGGCTTGTTCTT |
| Alkaline Phosphatase | rALPL | ACTGTGGACTACCTCTTG | GGTCAGTGATGTTGTTCC |
| Aggrecan | rACAN | TGGAGAAGCCCTTGCATCTG | TGGGACGGAGGATGCTTCTA |
| Bone Gamma-Carboxyglutamate Protein | rBGLAP | ACTCTTGTCGCCCTGCTG | CTGCCCTCCCTCTTGGAC |
| RUNX family transcription factor 2 | rRUNX2 | TCAGGCATGTCCCTCGGTAT | TGGCAGGTAGGTATGGTAGTGG |
| Collagen type I alpha 1 chain | rCOL1A1 | GAGGTGGACACCACCCTCAA | CCAGTGTCCATGTCGCAGAA |
| Collagen type II alpha 1 chain | rCOL2A1 | CTGTCCTGTGCGACGACATA | TCCTTTCTGCCCCTTTGGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Thanh, T.; Nguyen-Tran, B.-S.; Cruciani, S.; Nguyen-Thi, T.-D.; Dang-Cong, T.; Maioli, M. Osteochondral Regeneration Ability of Uncultured Bone Marrow Mononuclear Cells and Platelet-Rich Fibrin Scaffold. Bioengineering 2023, 10, 661. https://doi.org/10.3390/bioengineering10060661
Nguyen-Thanh T, Nguyen-Tran B-S, Cruciani S, Nguyen-Thi T-D, Dang-Cong T, Maioli M. Osteochondral Regeneration Ability of Uncultured Bone Marrow Mononuclear Cells and Platelet-Rich Fibrin Scaffold. Bioengineering. 2023; 10(6):661. https://doi.org/10.3390/bioengineering10060661
Chicago/Turabian StyleNguyen-Thanh, Tung, Bao-Song Nguyen-Tran, Sara Cruciani, Thuy-Duong Nguyen-Thi, Thuan Dang-Cong, and Margherita Maioli. 2023. "Osteochondral Regeneration Ability of Uncultured Bone Marrow Mononuclear Cells and Platelet-Rich Fibrin Scaffold" Bioengineering 10, no. 6: 661. https://doi.org/10.3390/bioengineering10060661
APA StyleNguyen-Thanh, T., Nguyen-Tran, B.-S., Cruciani, S., Nguyen-Thi, T.-D., Dang-Cong, T., & Maioli, M. (2023). Osteochondral Regeneration Ability of Uncultured Bone Marrow Mononuclear Cells and Platelet-Rich Fibrin Scaffold. Bioengineering, 10(6), 661. https://doi.org/10.3390/bioengineering10060661