A Review of Conventional and Novel Treatments for Osteoporotic Hip Replacements
Abstract
1. Introduction
2. Materials and Methods
3. Results
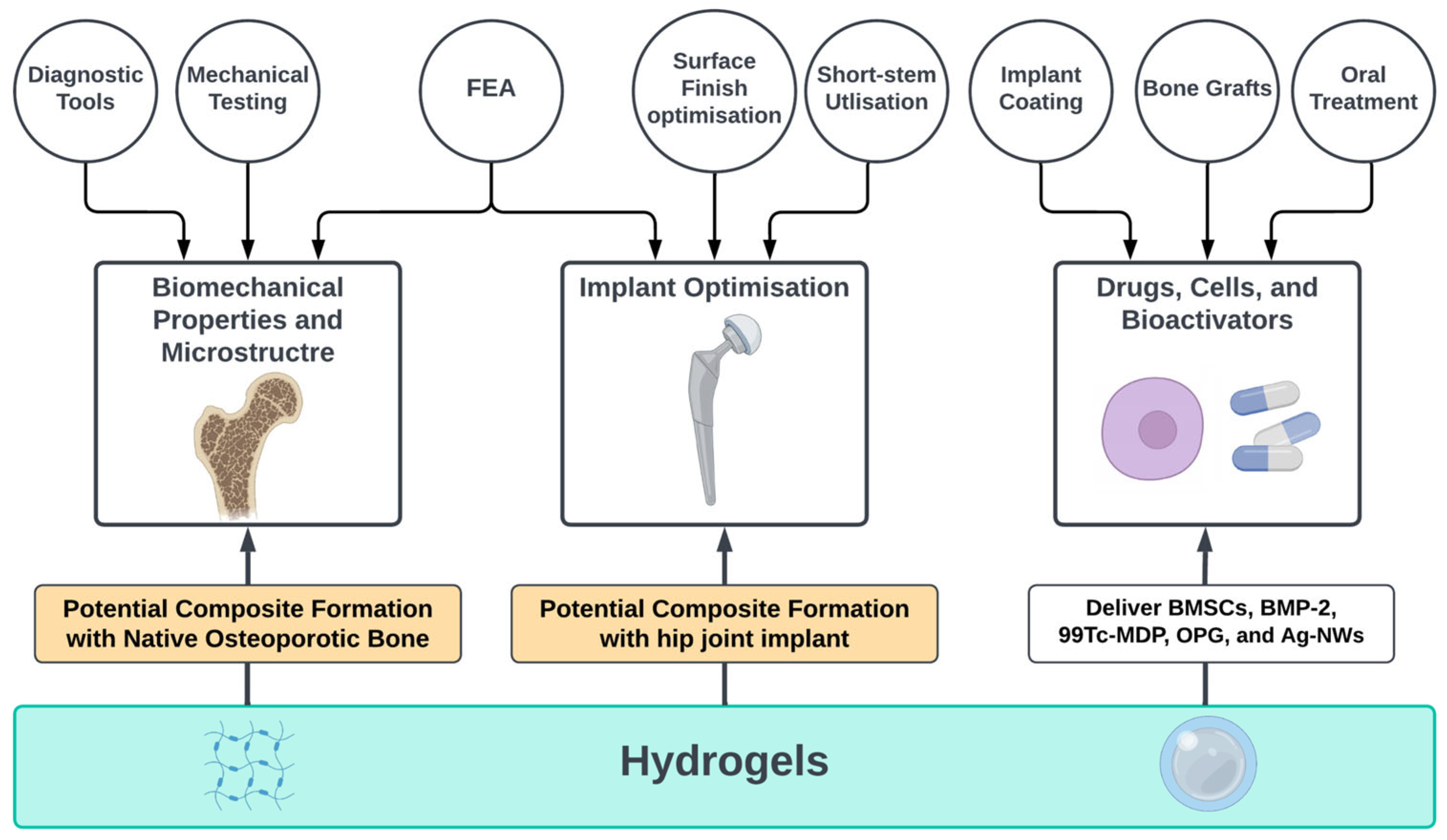
3.1. Biomechanical Properties and Microstructure
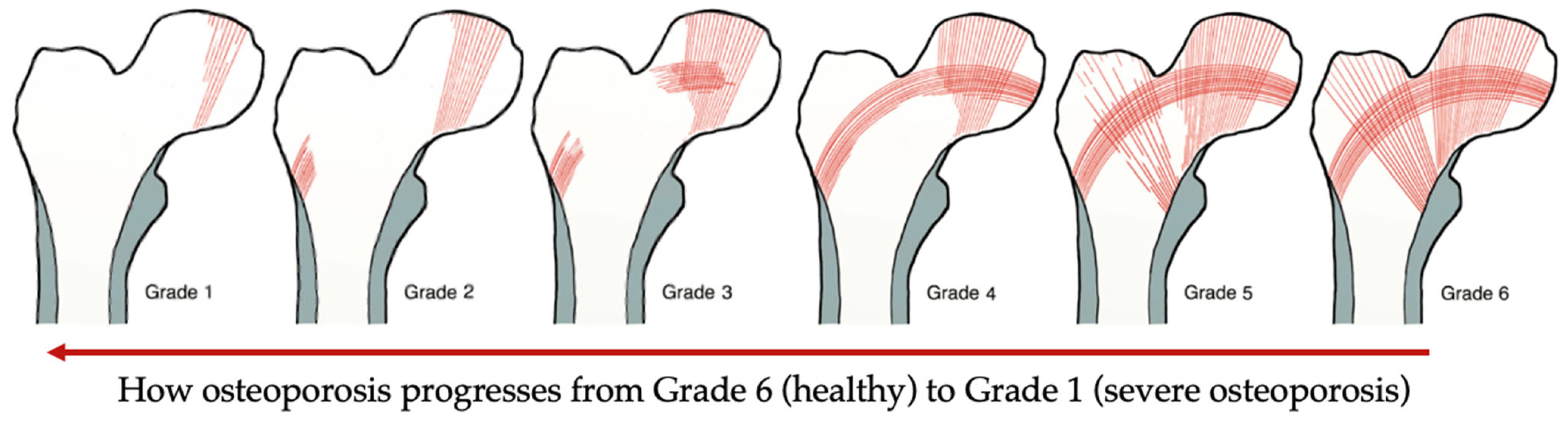
3.1.1. Diagnostic Tools
| Tool | Use | Results | Reference |
|---|---|---|---|
| Singh Index (SI) | Bone architecture assessment | Inexpensive tool, but not accurate results | [11] |
| Singh Index (SI) + Bone Mineral Density (BMD) | Mechanical competence and architecture of the bone | Acceptable estimation compared to Singh Index alone | [12] |
| Velocity Ultrasound + Bone Mineral Density (BMD) | Fracture risk assessment | Improved in comparison with Singh Index alone | [13] |
| Dual-Energy X-ray Absorptiometry (DEXA) | Evaluate (BMD) | Excellent for the assessment of (BMD) | [15] |
| Magnetic Resonance Imaging (MRI) | Evaluate (BMD) | Enhance accuracy of (DEXA) results | [14] |
| Low Field Nuclear Magnetic Resonance (LF-NMR), High Resolution Computed Tomography (HR-CT), and micro-CT (μCT) | Evaluate bone porosity and structure | Qualitative and quantitative information that can be used for Finite Elements Analysis | [16] |
3.1.2. Mechanical Testing
3.1.3. Finite Element Analysis
3.2. Implant Optimisation
3.2.1. Design Optimisation
3.2.2. Surface Finish Optimisation
3.2.3. Finite Element Analysis
3.3. Drugs, Cells, and Bioactivators
3.3.1. Implant Coating
3.3.2. Bone Grafts
3.3.3. Hydrogels within Metallic Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dickenson, R.P.; Hutton, W.C.; Stott, J.R.R. The mechanical properties of bone in osteoporosis. J. Bone Jt. Surg. Br. 1981, 63, 233–238. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporosis Foundation Epidemiology of Osteoporosis and Fragility Fractures|International Osteoporosis Foundation. Available online: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures (accessed on 19 July 2022).
- National Institute for Health and Care Excellence. NICE Impact Falls and Fragility Fractures; National Institute for Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.v.; Willers, C.; Borgström, F. SCOPE 2021: A New Scorecard for Osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Ortendahl, J.D.; Vanderpuye-Orgle, J.; Grauer, A.; Arellano, J.; Lemay, J.; Harmon, A.L.; Broder, M.S.; Singer, A.J. Healthcare Policy Changes in Osteoporosis Can Improve Outcomes and Reduce Costs in the United States. JBMR Plus 2019, 3, e10192. [Google Scholar] [CrossRef] [PubMed]
- Bukata, S.v.; Crawford, B.M.; Vallera, C. Orthopedic Aspects of Osteoporosis. In Marcus and Feldman’s Osteoporosis, 5th ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 2, pp. 1613–1625. ISBN 9780128130735. [Google Scholar]
- Kammerlander, C.; Neuerburg, C.; Verlaan, J.J.; Schmoelz, W.; Miclau, T.; Larsson, S. The Use of Augmentation Techniques in Osteoporotic Fracture Fixation. Injury 2016, 47, S36–S43. [Google Scholar] [CrossRef]
- Springer, B.D.; Fehring, T.K.; Griffin, W.L.; Odum, S.M.; Masonis, J.L. Why Revision Total Hip Arthroplasty Fails. Clin. Orthop. Relat. Res. 2009, 467, 166–173. [Google Scholar] [CrossRef]
- Barrack, R.L.; Sawhney, J.; Joe, H.; Cofield, R.H. Cost Analysis of Revision Total Hip Arthroplasty. A 5-Year Followup Study. Clin. Orthop. Relat. Res. 1999, 369, 175–178. [Google Scholar] [CrossRef]
- Singh, M.; Nagrath, A.R.; Maini, P.S. Changes in Trabecular Pattern of the Upper End of the Femur as an Index of Osteoporosis. J. Bone Jt. Surg. Am. 1970, 52, 457–467. [Google Scholar] [CrossRef]
- Wachter, N.J.; Augat, P.; Hoellen, I.P.; Krischak, G.D.; Sarkar, M.R.; Mentzel, M.; Kinzl, L.; Claes, L. Predictive Value of Singh Index and Bone Mineral Density Measured by Quantitative Computed Tomography in Determining the Local Cancellous Bone Quality of the Proximal Femur. Clin. Biomech. 2001, 16, 257–262. [Google Scholar] [CrossRef]
- D’Amelio, P.; Rossi, P.; Isaia, G.; Lollino, N.; Castoldi, F.; Girardo, M.; Dettoni, F.; Sattin, F.; Delise, M.; Bignardi, C. Bone Mineral Density and Singh Index Predict Bone Mechanical Properties of Human Femur. Connect. Tissue Res. 2008, 49, 99–104. [Google Scholar] [CrossRef]
- Njeh, C.F.; Kuo, C.W.; Langton, C.M.; Atrah, H.I.; Boivin, C.M. Prediction of Human Femoral Bone Strength Using Ultrasound Velocity and BMD: An In Vitro Study. Osteoporos. Int. 1997, 7, 471–477. [Google Scholar] [CrossRef]
- Endo, K.; Takahata, M.; Sugimori, H.; Yamada, S.; Tadano, S.; Wang, J.; Todoh, M.; Ito, Y.M.; Takahashi, D.; Kudo, K.; et al. Magnetic Resonance Imaging T1 and T2 Mapping Provide Complementary Information on the Bone Mineral Density Regarding Cancellous Bone Strength in the Femoral Head of Postmenopausal Women with Osteoarthritis. Clin. Biomech. 2019, 65, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gasbarra, E.; Iundusi, R.; Perrone, F.L.; Saturnino, L.; Tarantino, U. Densitometric Evaluation of Bone Remodelling around Trabecular Metal Primary Stem: A 24-Month Follow-Up. Aging Clin. Exp. Res. 2015, 27, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Porrelli, D.; Abrami, M.; Pelizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Grassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular Bone Porosity and Pore Size Distribution in Osteoporotic Patients—A Low Field Nuclear Magnetic Resonance and Microcomputed Tomography Investigation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104933. [Google Scholar] [CrossRef] [PubMed]
- Kanakaris, N.K.; Lasanianos, N.G. Singh Index for Osteoporosis. In Trauma and Orthopaedic Classifications: A Comprehensive Overview; Springer-Verlag London Ltd.: London, UK, 2015; pp. 405–407. ISBN 9781447165729. [Google Scholar]
- Vale, A.C.; Aleixo, I.P.; Lúcio, M.; Saraiva, A.; Caetano-Lopes, J.; Rodrigues, A.; Amaral, P.M.; Rosa, L.G.; Monteiro, J.; Fonseca, J.E.; et al. At the Moment of Occurrence of a Fragility Hip Fracture, Men Have Higher Mechanical Properties Values in Comparison with Women. BMC Musculoskelet. Disord. 2013, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.; Knox, R.; Huang, A.; Herfat, S. Acetabulum Cup Stability in an Early Weight-Bearing Cadaveric Model of Geriatric Posterior Wall Fractures. J. Orthop. Trauma 2020, 34, 55–61. [Google Scholar] [CrossRef]
- Jenkins, T.; Katsamenis, O.L.; Andriotis, O.G.; Coutts, L.v.; Carter, B.; Dunlop, D.G.; Oreffo, R.O.C.; Cooper, C.; Harvey, N.C.; Thurner, P.J.; et al. The Inferomedial Femoral Neck Is Compromised by Age but Not Disease: Fracture Toughness and the Multifactorial Mechanisms Comprising Reference Point Microindentation. J. Mech. Behav. Biomed. Mater. 2017, 75, 399–412. [Google Scholar] [CrossRef]
- Gluek, C.; Zdero, R.; Quenneville, C.E. Evaluating the Mechanical Response of Novel Synthetic Femurs for Representing Osteoporotic Bone. J. Biomech. 2020, 111, 110018. [Google Scholar] [CrossRef]
- Prendergast, P.J. Review Paper Finite Element Models in Tissue Mechanics and Orthopaedic Implant Design. Clin. Biomech. 1997, 12, 343–366. [Google Scholar] [CrossRef]
- Rieger, R.; Auregan, J.C.; Hoc, T. Micro-Finite-Element Method to Assess Elastic Properties of Trabecular Bone at Micro- and Macroscopic Level. Morphologie 2018, 102, 12–20. [Google Scholar] [CrossRef]
- He, Z.; Chu, L.; Liu, X.; Han, X.; Zhang, K.; Yan, M.; Li, X.; Yu, Z. Differences in Subchondral Trabecular Bone Microstructure and Finite Element Analysis-Based Biomechanical Properties between Osteoporosis and Osteoarthritis. J. Orthop. Translat. 2020, 24, 39–45. [Google Scholar] [CrossRef]
- Boelch, S.P.; Jordan, M.C.; Meffert, R.H.; Jansen, H. Comparison of Open Reduction and Internal Fixation and Primary Total Hip Replacement for Osteoporotic Acetabular Fractures: A Retrospective Clinical Study. Int. Orthop. 2017, 41, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Santori, N.; Falez, F.; Potestio, D.; Santori, F.S. Fourteen-Year Experience with Short Cemented Stems in Total Hip Replacement. Int. Orthop. 2019, 43, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhen, P.; Chang, Y.; Yue, H.; Chen, H.; Zhou, S.; Liu, J.; He, X. Primary Total Hip Arthroplasty Using a Short Bone-Conserving Stem in Young Adult Osteoporotic Patients with Dorr Type C Femoral Bone. J. Orthop. Surg. Res. 2021, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, G.; Cao, L.; Sun, W.; Zeng, X.; Xiong, N.; Wang, S.; Yu, W.; Liu, Q.; Lin, H. Dual-Mobility Cup Total Hip Arthroplasty for Displaced Femoral Neck Fractures: A Retrospective Study With a Median Follow-Up of 5 Years. Geriatr. Orthop. Surg. Rehabil. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Goriainov, V.; Cook, R.B.; Murray, J.W.; Walker, J.C.; Dunlop, D.G.; Clare, A.T.; Oreffo, R.O.C. Human Skeletal Stem Cell Response to Multiscale Topography Induced by Large Area Electron Beam Irradiation Surface Treatment. Front. Bioeng. Biotechnol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Kadir, A.; Kamsah, N. The Effect of Bone Properties Due to Skeletal Diseases on Stability of Cementless Hip Stems. Am. J. Appl. Sci. 1988, 6, 1988–1994. [Google Scholar]
- Peter, B.; Gauthier, O.; Laı¨b, S.L.; Bujoli, B.; Me Guicheux, J.; Janvier, P.; Harry Van Lenthe, G.; Mü, R.; Zambelli, P.-Y.; Bouler, J.-M.; et al. Local Delivery of Bisphosphonate from Coated Orthopedic Implants Increases Implants Mechanical Stability in Osteoporotic Rats. J. Biomed. Mater. Res. A 2005, 76, 133–143. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, S.; Liu, X.; Bao, C.; Hu, J. The Effect of Surface Immobilized Bisphosphonates on the Fixation of Hydroxyapatite-Coated Titanium Implants in Ovariectomized Rats. Biomaterials 2009, 30, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.; Stumpf, U.C.; Kurth, A.H.A. Simulated Osteopenia Impairs Metaphyseal Bone Ingrowth of Metal Implants in an Animal Model. Eur. J. Trauma 2005, 31, 51–56. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Y.; Xu, D.; Gong, K. The Calcium Phosphate Modified Titanium Implant Combined With Platelet-Rich Plasma Treatment Promotes Implant Stabilization in an Osteoporotic Model. J. Craniofacial Surg. 2021, 32, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Janna, S. Alendronate in Bone Cement: Fatigue Life Degraded by Liquid, Not by Powder. Clin. Orthop. Relat. Res. 2006, 445, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Brewster, N.T.; Gillespie, W.J.; Howie, C.R.; G Madabhushi, S.P.; Usmani, A.S.; Fairbairn, D.R. Mechanical Considerations in Impaction Bone Grafting. J. Bone Jt. Surg. Br. 1999, 81, 118–124. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Wang, Z.; Ren, M.; Wang, X.; Liu, H.; Lin, Q.; Wang, J. Engineering Multifunctional Hydrogel-Integrated 3D Printed Bioactive Prosthetic Interfaces for Osteoporotic Osseointegration. Adv. Healthc. Mater. 2022, 11, 2102535. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Wang, Z.; Liu, H.; Cui, Y.; Liu, Y.; Ren, M.; Zhan, H.; Li, Z.; Wu, M.; et al. Incorporation of Bone Morphogenetic Protein-2 and Osteoprotegerin in 3D-Printed Ti6Al4V Scaffolds Enhances Osseointegration Under Osteoporotic Conditions. Front. Bioeng. Biotechnol. 2021, 9, 754205. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Z.; Li, Z.; Ji, X.; Yuan, B.; Sun, Y.; Peng, C.; Leng, Y.; Dou, M.; Wang, J.; et al. Functionalized Anti-Osteoporosis Drug Delivery System Enhances Osseointegration of an Inorganic–Organic Bioactive Interface in Osteoporotic Microenvironment. Mater. Des. 2021, 206, 109753. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, Y.; Wang, C.; Wang, Z.; Wang, J.; Liu, H.; Feng, Y.; Lin, Q.; Li, Z.; Liu, H. Enhanced Osseointegration of Three-Dimensional Supramolecular Bioactive Interface through Osteoporotic Microenvironment Regulation. Theranostics 2020, 10, 4779–4794. [Google Scholar] [CrossRef]
- von Knoch, M.; Wedemeyer, C.; Pingsmann, A.; von Knoch, F.; Hilken, G.; Sprecher, C.; Henschke, F.; Barden, B.; Löer, F. The Decrease of Particle-Induced Osteolysis after a Single Dose of Bisphosphonate. Biomaterials 2005, 26, 1803–1808. [Google Scholar] [CrossRef]
- von Knoch, F.; Eckhardt, C.; Alabre, C.I.; Schneider, E.; Rubash, H.E.; Shanbhag, A.S. Anabolic Effects of Bisphosphonates on Peri-Implant Bone Stock. Biomaterials 2007, 28, 3549–3559. [Google Scholar] [CrossRef]
- Migliorati, C.A. Bisphosphanates and Oral Cavity Avascular Bone Necrosis. J. Clin. Oncol. 2003, 21, 4253–4254. [Google Scholar] [CrossRef]
- Hooten, J.P., Jr.; Engh, C.A.; Heekin, R.D.; Vinh, T.N. Structural Bulk Allografts in Acetabular Reconstruction. Analysis of Two Grafts Retrieved at Post-Mortem. J. Bone Jt. Surg. Br. 1996, 78, 270–275. [Google Scholar] [CrossRef]


| Aim | Type of Test | Results | Reference |
|---|---|---|---|
| Determine gender effect on fracture risk | Compression | Males have a bone Young’s modulus of 293.68 MPa and an ultimate stress of 8.04 MPa, whereas females have 174.26 MPa and 4.46 MPa for young’s modulus and ultimate stress, respectively. Therefore, men have lower fracture risk compared to women. | [18] |
| Evaluate the weightbearing immediately after fixation of posterior wall (PW) fractures | Cyclic loading | With assistance, immediate load bearing is allowable with 50% of PW and 25% of acetabular rim, regardless of PW fixation. | [19] |
| Investigate effect osteoporosis on bone fracture toughness | Fracture toughness | Fracture toughness decreased with ageing (7.0% each decade, r = −0.36, p = 0.029), while comparable fracture resistance properties were found in osteoporotic, osteoarthritic and control groups (10% difference for indentation and p > 0.05 for fracture properties). | [20] |
| Introduce synthetic bone that represent osteoporotic cadaveric bones. | Four-point bending, axial compression, and pullout | There was good correlation found between the cadaveric and synthetic bone samples. The p-values in all mechanical tests were acceptable, ranging between 0.1–0.9 except in pullout tests (p = 0.005). | [21] |
| Aim | Bone Model | Software | Results | Reference |
|---|---|---|---|---|
| Evaluate macroscopic mechanical properties the bone | Virtual trabecular bone biopsy from CT scan | Abaqus 6.9-2 | Osteoporotic bones have comparable elasticity to healthy ones, with young’s modulus mean (±SD) of 18.92 ± 5.43 GPa. However, the yield stress was found to be lower in osteoporotic bones with a mean (±SD) of (85.6 ± 16.7 MPa). | [23] |
| Evaluate the influence of plate and rod in osteoporotic and osteoarthritic patients | Virtual subchondral trabecular bone biopsy from CT scan | Scanco Medical Finite Element Software 1.06 | Osteoarthritic subchondral bones had higher stiffness with a mean (±SD) of 12,003.56 (±7590.42) kN/mm, while the mean stiffness of osteoporotic bones was 4964.01 (±3778.37) kN/mm. Similarly, the failure load was reported to be higher in osteoarthritic bones compared to osteoporotic ones with 477.7 (±279.56) MPa and 215.89 (±143.73), respectively. | [24] |
| Implant Design | Targeted Complication | Results | Limitations | Reference |
|---|---|---|---|---|
| Short stem implant | Aseptic loosening | The mean of the Harris Hip Score (HHS) in the two groups increased from 45.0 ± 16 (29–61) and 40.0± 11 (29–51) prior to surgery, to 93 ± 9 (84–100) and 96 ± 7 (89–100), respectively. The survival rate with stem revision for aseptic loosening was 100%. | Some cases with Vancouver B1 and Vancouver B2 fractures were reported in both groups. | [26] |
| Cementless short metaphyseal fitting stem | implant instability | The mean HHS improved from 48.0 ± 8.0(38.0–61.0) prior to surgery to 91.0 ± 8.0 (85.0–98.0). In addition, there were no postoperative complications such as infection, deep vein thrombosis, loosening, or peri-prosthetic fracture. | Low number of patients, and short follow-up duration. | [27] |
| Dual-mobility cups in total hip arthroplasty (DM-THA) | Femoral Neck Fractures (FNFs) | The mean HHS increased from 58.62 (+15.79) preopratively to 86.13 (+9.92). | Cases of loosening, revision DM-THA, intra-prosthetic dislocation, migration, tilting, and severe wear were reported in the study. | [28] |
| Treatment | Targeted Complication | Results | Limitation | Reference |
|---|---|---|---|---|
| Zoledronate | Instability and poor bone formation in the bone–implant interface | Bone formation was enhanced by the elimination of osteoclastic activity by Zolendronate. Thus, in comparison to the implant not coated with Zoledronate, coated implants showed significantly higher maximal pullout force (p < 0.05) and (p < 0.01). | Used with hydroxyapatite coating, which is reported to impair osteoporotic bone ingrowth, consequently long-term survival. | [31,32] |
| Hydroxyapatite (HA) coated implants | Poor bone–implant ingrowth | The mean osseointegrated implant surface (OIS) in implants coated with HA and uncoated ones were 23.7 and 23.5 in ovariectomised rats, respectively. HA have no effect on osteoporotic bones while it enhances the OIS in healthy bones. | The results of the study indicate that HA-coated implants deteriorate bone ingrowth in the long term. | [33] |
| Calcium Phosphates coating (CaP) with platelet-rich plasma (PRP) | Implant instability | Enhanced stability, evident by the increase in the maximal push-out force in the group treated with CaP and PRP compared to the control group (p < 0.05). | No limitations mentioned in the study | [34] |
| Surface Large Area Electron Beam melting (LAEB) | Implant surface nanotopography | When compared to the untreated group, the group treated with a cathode voltage of 35 kV and 25 shots showed a significant increase in osteogenic activity (two- to three-fold). This peak was observed to correlate with a surface roughness () of 44 nm. | The technique has not been investigated in vivo for mechanical interface strength | [29] |
| Material | Fabrication Process | Impregnated Drugs | Reference |
|---|---|---|---|
| NCECS-PVA and AGPVA | Chemical crosslinking | Autophagy-regulated rapamycin | [37] |
| Poloxamer 407 | Thermosensitive mixture | Bone morphogenetic protein-2 (BMP-2) | [38] |
| Poloxamer 407 | Thermosensitive mixture | Technetium methylenediphosphonate (-MDP) | [39] |
| N-carboxyethyl chitosan (N-chitosan) | In situ crosslinking | Bone marrow stem cells (BMSCs) + (BMP-2) | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdah, F.; Alshammari, A.; Hidalgo-Bastida, A.; Cooper, G. A Review of Conventional and Novel Treatments for Osteoporotic Hip Replacements. Bioengineering 2023, 10, 161. https://doi.org/10.3390/bioengineering10020161
Alabdah F, Alshammari A, Hidalgo-Bastida A, Cooper G. A Review of Conventional and Novel Treatments for Osteoporotic Hip Replacements. Bioengineering. 2023; 10(2):161. https://doi.org/10.3390/bioengineering10020161
Chicago/Turabian StyleAlabdah, Fahad, Adel Alshammari, Araida Hidalgo-Bastida, and Glen Cooper. 2023. "A Review of Conventional and Novel Treatments for Osteoporotic Hip Replacements" Bioengineering 10, no. 2: 161. https://doi.org/10.3390/bioengineering10020161
APA StyleAlabdah, F., Alshammari, A., Hidalgo-Bastida, A., & Cooper, G. (2023). A Review of Conventional and Novel Treatments for Osteoporotic Hip Replacements. Bioengineering, 10(2), 161. https://doi.org/10.3390/bioengineering10020161








