Mn-Based Methacrylated Gellan Gum Hydrogels for MRI-Guided Cell Delivery and Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Methacrylated Gellan Gum (GG-MA), MnCl2, and aCSF Solutions
2.2. Preparation of Mn-Based GG-MA Hydrogels
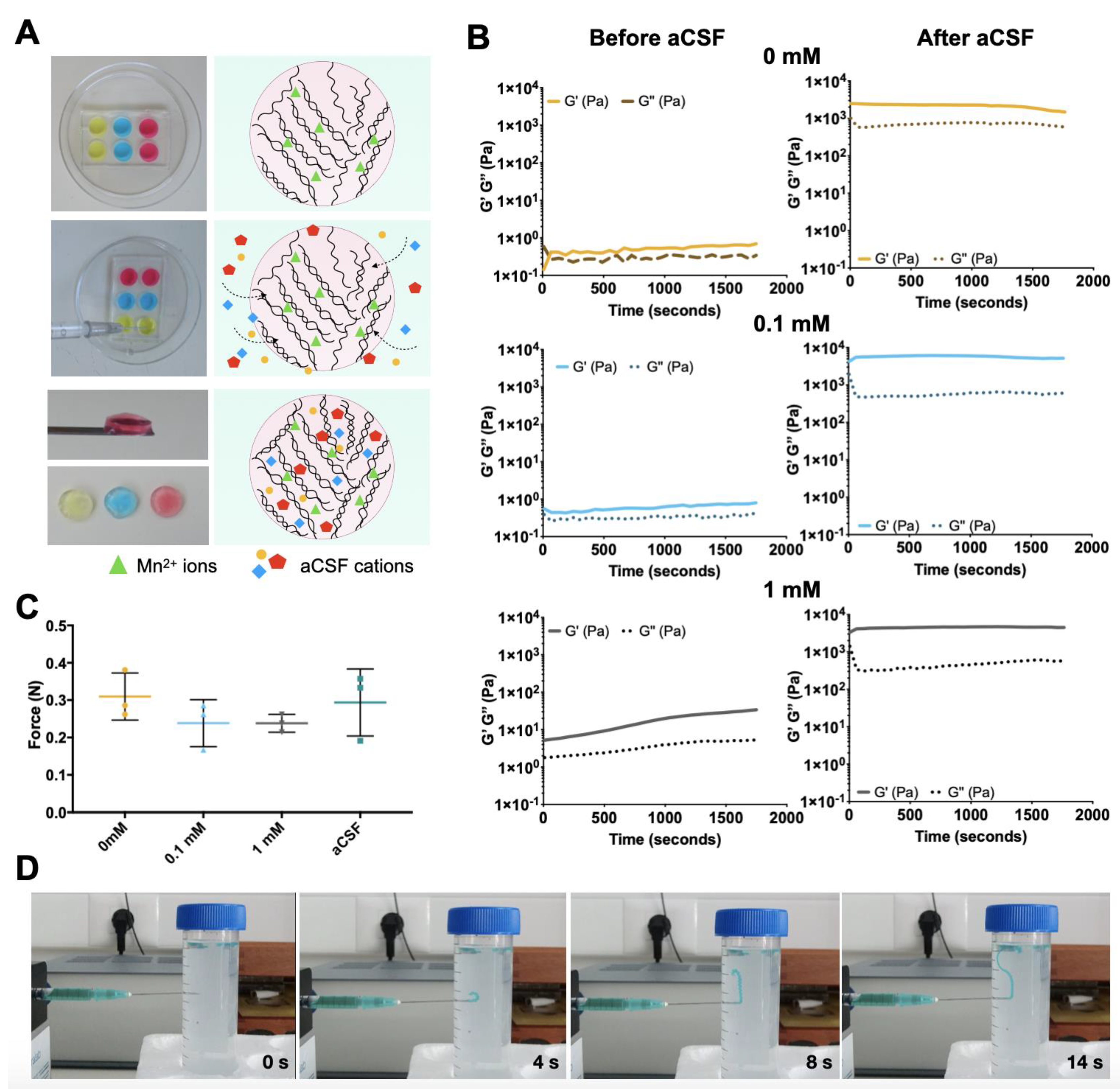
2.3. Rheological Studies
2.4. Injection Ability Test
2.5. Permeability Studies
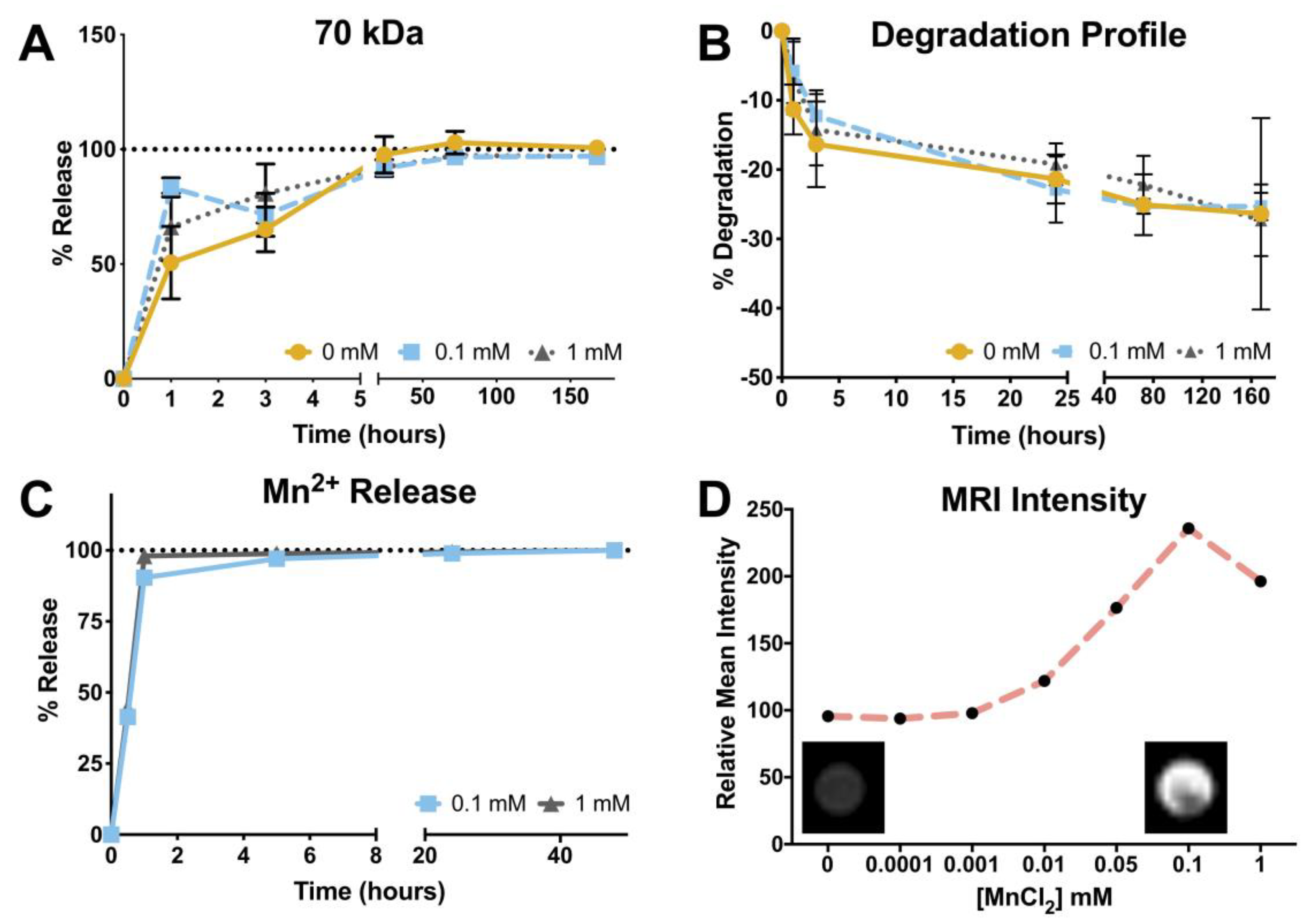
2.6. Degradation Profile
2.7. Manganese Release Profile—Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP)
2.8. Human Derived Adipose Stem Cells (hASCs) Isolation and Culture
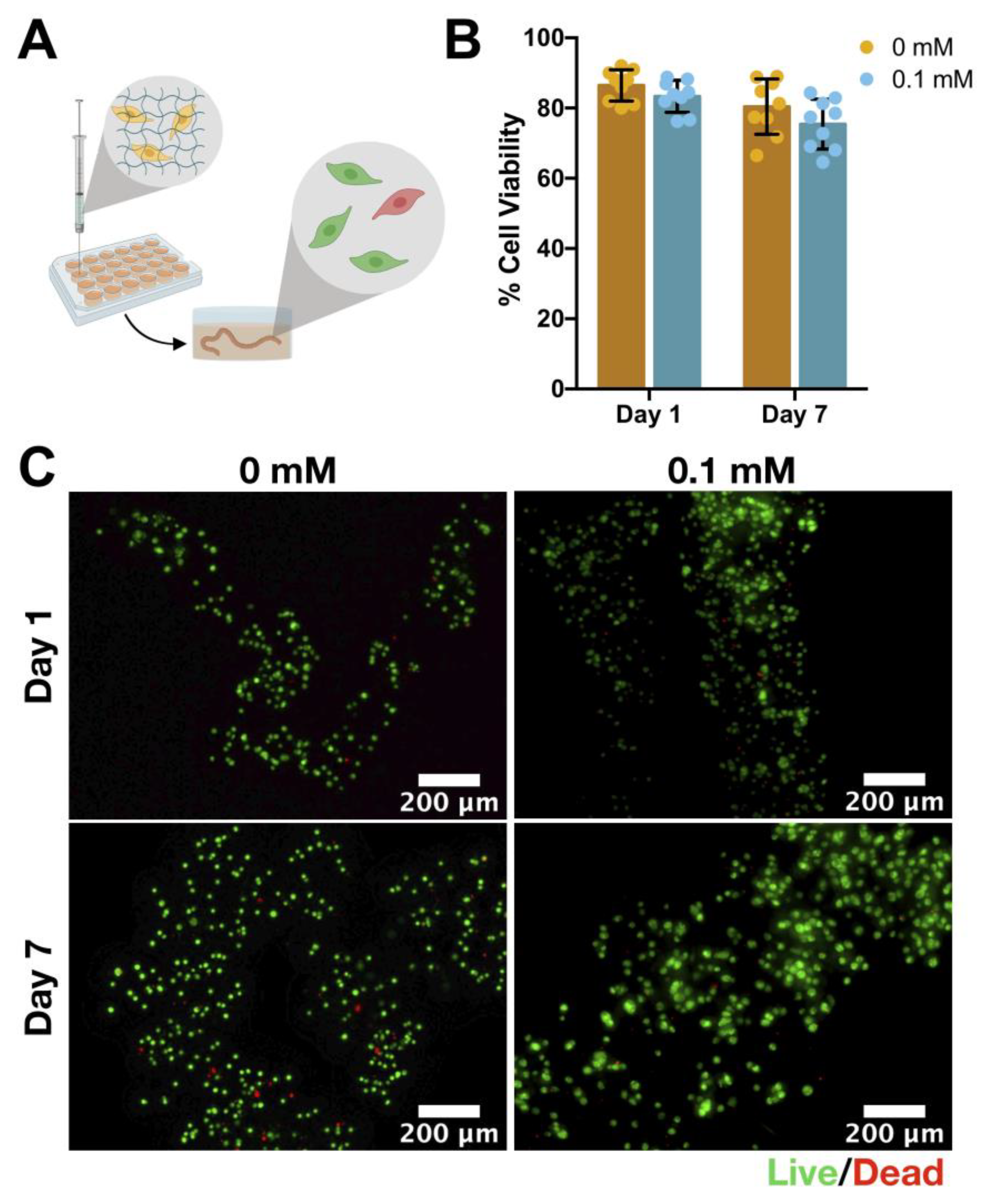
2.9. Cell Encapsulation
2.10. Live/Dead Staining
2.11. Animal Surgeries
2.12. Phantom Magnetic Resonance Imaging
2.13. In Vivo Magnetic Resonance Imaging
2.14. Statistical Analysis
3. Results and Discussion
3.1. Mn-Based GG-MA Hydrogels
3.2. Injection Ability
3.3. Hydrogel Permeability
3.4. Degradation Profile
3.5. Manganese Release Profile and Magnetic Resonance Imaging
3.6. Cell Viability: hASCs Encapsulation
3.7. In Vivo Magnetic Resonance Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyczek, A.; Arnold, A.; Zhang, J.; Campanelli, J.T.; Janowski, M.; Bulte, J.W.; Walczak, P. Transplanted human glial-restricted progenitors can rescue the survival of dysmyelinated mice independent of the production of mature, compact myelin. Exp. Neurol. 2017, 291, 74–86. [Google Scholar] [CrossRef]
- Golubczyk, D.; Malysz-Cymborska, I.; Kalkowski, L.; Janowski, M.; Coates, J.R.; Wojtkiewicz, J.; Maksymowicz, W.; Walczak, P. The Role of Glia in Canine Degenerative Myelopathy: Relevance to Human Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2019, 56, 5740–5748. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Lamanna, J.J.; Grin, N.; Hurtig, C.V.; Miller, J.H.; Riley, J.; Urquia, L.; Avalos, P.; Svendsen, C.N.; Federici, T.; et al. Preclinical Validation of Multilevel Intraparenchymal Stem Cell Therapy in the Porcine Spinal Cord. Neurosurgery 2015, 77, 604–612. [Google Scholar] [CrossRef]
- Glass, J.D.; Hertzberg, V.S.; Boulis, N.M.; Riley, J.; Federici, T.; Polak, M.; Bordeau, J.; Fournier, C.; Johe, K.; Hazel, T.; et al. Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology 2016, 87, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qian, K.; Han, X.; Li, X.; Zheng, Y.; Chen, Z.; Huang, X.; Chen, H. Intraparenchymal Neural Stem/Progenitor Cell Transplantation for Ischemic Stroke Animals: A Meta-Analysis and Systematic Review. Stem. Cells Int. 2018, 2018, 4826407. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xi, H.; Huang, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transpl. 2013, 22 (Suppl. S1), S83–S91. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Bliss, T.M.; Shah, A.K.; Sun, G.H.; Ma, M.; Foo, W.C.; Masel, J.; Yenari, M.A.; Weissman, I.L.; Uchida, N.; et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 11839–11844. [Google Scholar] [CrossRef]
- Zhong, J.; Chan, A.; Morad, L.; Kornblum, H.I.; Fan, G.; Carmichael, S.T. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil. Neural Repair 2010, 24, 636–644. [Google Scholar] [CrossRef]
- Goldman, S.A. Stem and Progenitor Cell-Based Therapy of the Central Nervous System: Hopes, Hype, and Wishful Thinking. Cell Stem Cell 2016, 18, 174–188. [Google Scholar] [CrossRef]
- Boltze, J.; Arnold, A.; Walczak, P.; Jolkkonen, J.; Cui, L.; Wagner, D.C. The Dark Side of the Force—Constraints and Complications of Cell Therapies for Stroke. Front. Neurol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Carvalho, L.; Silva-Correia, J.; Vieira, S.; Majchrzak, M.; Lukomska, B.; Stanaszek, L.; Strymecka, P.; Malysz-Cymborska, I.; Golubczyk, D.; et al. Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: Clinical needs, biomaterials, and imaging technologies. NPJ Regen. Med. 2018, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Arab, L.; Jarooghi, N.; Bolurieh, T.; Abbasi, F.; Mardpour, S.; Azimyian, V.; Moeininia, F.; Maroufizadeh, S.; Sanjari, L.; et al. Safety, Feasibility of Intravenous and Intrathecal Injection of Autologous Bone Marrow Derived Mesenchymal Stromal Cells in Patients with Amyotrophic Lateral Sclerosis: An Open Label Phase I Clinical Trial. Cell J. 2019, 20, 592–598. [Google Scholar] [PubMed]
- Barczewska, M.; Grudniak, M.; Maksymowicz, S.; Siwek, T.; Ołdak, T.; Jezierska-Woźniak, K.; Gładysz, D.; Maksymowicz, W. Safety of intrathecal injection of Wharton’s jelly-derived mesenchymal stem cells in amyotrophic lateral sclerosis therapy, Neural Regen. Res. 2019, 14, 313–318. [Google Scholar]
- Habisch, H.J.; Janowski, M.; Binder, D.; Kuzma-Kozakiewicz, M.; Widmann, A.; Habich, A.; Schwalenstocker, B.; Hermann, A.; Brenner, R.; Lukomska, B.; et al. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: Limited intraparenchymal migration and survival narrows therapeutic effects. J. Neural. Transm. 2007, 114, 1395–1406. [Google Scholar] [CrossRef]
- Chotivichit, A.; Ruangchainikom, M.; Chiewvit, P.; Wongkajornsilp, A.; Sujirattanawimol, K. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: A case report. J. Med. Case Rep. 2015, 9, 79. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Malysz-Cymborska, I.; Golubczyk, D.; Kalkowski, L.; Burczyk, A.; Janowski, M.; Holak, P.; Olbrych, K.; Sanford, J.; Stachowiak, K.; Milewska, K.; et al. MRI-guided intrathecal transplantation of hydrogel-embedded glial progenitors in large animals. Sci. Rep. 2018, 8, 16490. [Google Scholar] [CrossRef]
- Oliveira, E.P.; Malysz-Cymborska, I.; Golubczyk, D.; Kalkowski, L.; Kwiatkowska, J.; Reis, R.L.; Oliveira, J.M.; Walczak, P. Advances in bioinks and in vivo imaging of biomaterials for CNS applications. Acta Biomater. 2019, 95, 60–72. [Google Scholar] [CrossRef]
- Gale, E.M.; Wey, H.Y.; Ramsay, I.; Yen, Y.F.; Sosnovik, D.E.; Caravan, P. A Manganese-based Alternative to Gadolinium: Contrast-enhanced MR Angiography, Excretion, Pharmacokinetics, and Metabolism. Radiology 2018, 286, 865–872. [Google Scholar] [CrossRef]
- Morch, Y.A.; Sandvig, I.; Olsen, O.; Donati, I.; Thuen, M.; Skjak-Braek, G.; Haraldseth, O.; Brekken, C. Mn-alginate gels as a novel system for controlled release of Mn2+ in manganese-enhanced MRI. Contrast Media Mol. Imaging 2012, 7, 265–275. [Google Scholar] [CrossRef]
- Addisu, K.D.; Hailemeskel, B.Z.; Mekuria, S.L.; Andrgie, A.T.; Lin, Y.C.; Tsai, H.C. Bioinspired, Manganese-Chelated Alginate-Polydopamine Nanomaterials for Efficient in Vivo T1-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2018, 10, 5147–5160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Ramsay, I.A.; Erstad, D.J.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Manganese-Based Contrast Agents for Magnetic Resonance Imaging of Liver Tumors: Structure-Activity Relationships and Lead Candidate Evaluation. J. Med. Chem. 2018, 61, 8811–8824. [Google Scholar] [CrossRef] [PubMed]
- Panich, A.M.; Shames, A.I.; Aleksenskii, A.E.; Yudina, E.B.; Vul, A.Y. Manganese-grafted detonation nanodiamond, a novel potential MRI contrast agent. Diam. Relat. Mater. 2021, 119, 108590. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Aleksenskii, A.E.; Kulvelis, Y.V.; Chizhikova, A.; Vul, A.Y.; Shames, A.I. Suspensions of manganese-grafted nanodiamonds: Preparation, NMR, and MRI study. Diam. Relat. Mater. 2023, 131, 109591. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Koren, S.A.; Abisambra, J.F. Manganese-Enhanced Magnetic Resonance Imaging: Overview and Central Nervous System Applications with a Focus on Neurodegeneration. Front. Aging Neurosci. 2018, 10, 403. [Google Scholar]
- Araszkiewicz, A.M.; Oliveira, E.P.; Svendsen, T.; Drela, K.; Rogujski, P.; Malysz-Cymborska, I.; Fiedorowicz, M.; Reis, R.L.; Oliveira, J.M.; Walczak, P.; et al. Manganese-Labeled Alginate Hydrogels for Image-Guided Cell Transplantation. Int. J. Mol. Sci. 2022, 23, 2465. [Google Scholar] [CrossRef]
- Roth, J.A.; Ganapathy, B.; Ghio, A.J. Manganese-induced toxicity in normal and human B lymphocyte cell lines containing a homozygous mutation in parkin. Toxicol. Vitr. 2012, 26, 1143–1149. [Google Scholar] [CrossRef]
- Ding, D.; Roth, J.; Salvi, R. Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology 2011, 32, 233–241. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Santos, T.C.; Martins, L.; Picciochi, R.; Marques, A.P.; Castro, A.G.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum injectable hydrogels for cartilage tissue engineering applications: In vitro studies and preliminary in vivo evaluation. Tissue Eng. Part A 2010, 16, 343–353. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef]
- Vieira, S.; Vial, S.; Maia, F.R.; Carvalho, M.; Reis, R.L.; Granja, P.L.; Oliveira, J.M. Gellan gum-coated gold nanorods: An intracellular nanosystem for bone tissue engineering. RSC Adv. 2015, 5, 77996–78005. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; Panhuis, M.I.H.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; Panhuis, M.I.H. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Snodgrass, S.R.; Johanson, C.E. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp. Neurol. 2015, 273, 57–68. [Google Scholar] [CrossRef]
- Kawahara, S.; Yoshikawa, A.; Hiraoki, T.; Tsutsumi, A. Interactions of paramagnetic metal ions with gellan gum studied by ESR and NMR methods. Carbohydr. Polym. 1996, 30, 29–133. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Ya, D.; Hiraoki, T.; Mochiku, H.; Yamaguchi, R.; Takahashi, N. ESR studies of Mn(II) binding to gellan and carrageenan gels. Food Hydrocoll. 1993, 7, 427–434. [Google Scholar] [CrossRef]
- Vieira, S.; Strymecka, P.; Stanaszek, L.; Silva-Correia, J.; Drela, K.; Fiedorowicz, M.; Malysz-Cymborska, I.; Rogujski, P.; Janowski, M.; Reis, R.L.; et al. Methacrylated gellan gum and hyaluronic acid hydrogel blends for image-guided neurointerventions. J. Mater. Chem. B 2020, 8, 5928–5937. [Google Scholar] [CrossRef]
- Janowski, M.; Kuzma-Kozakiewicz, M.; Binder, D.; Habisch, H.J.; Habich, A.; Lukornska, B.; Domanska-Janik, K.; Ludolph, A.C.; Storch, A. Neurotransplantation in mice: The concorde-like position ensures minimal cell leakage and widespread distribution of cells transplanted into the cisterna magna. Neurosci. Lett. 2008, 430, 169–174. [Google Scholar] [PubMed]
- Fiedorowicz, M.; Orzel, J.; Kossowski, B.; Welniak-Kaminska, M.; Choragiewicz, T.; Swiatkiewicz, M.; Rejdak, R.; Bogorodzki, P.; Grieb, P. Anterograde Transport in Axons of the Retinal Ganglion Cells and its Relationship to the Intraocular Pressure during Aging in Mice with Hereditary Pigmentary Glaucoma. Curr. Eye Res. 2018, 43, 539–546. [Google Scholar] [CrossRef]
- Fratini, M.; Abdollahzadeh, A.; DiNuzzo, M.; Salo, R.A.; Maugeri, L.; Cedola, A.; Giove, F.; Grohn, O.; Tohka, J.; Sierra, A. Multiscale Imaging Approach for Studying the Central Nervous System: Methodology and Perspective. Front. Neurosci. 2020, 14, 72. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, and Applications; Chemtec Publishing: Scarborough, ON, Canada, 2017. [Google Scholar]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.H.; Atluri, P.; Burdick, J.A. Methods to Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Forostyak, S.; Sykova, E. Neuroprotective Potential of Cell-Based Therapies in ALS: From Bench to Bedside. Front. Neurosci. 2017, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Sakowski, S.A.; Feldman, E.L. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Ann. Neurol. 2016, 79, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Li, Z.Q.; Jiang, S.; Bratlie, K.M. Chemically Modified Gellan Gum Hydrogels with Tunable Properties for Use as Tissue Engineering Scaffolds. ACS Omega 2018, 3, 6998–7007. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Zavan, B.; Vindigni, V.; Silva, T.H.; Oliveira, J.M.; Abatangelo, G.; Reis, R.L. Biocompatibility evaluation of ionic- and photo-crosslinked methacrylated gellan gum hydrogels: In vitro and in vivo study. Adv. Healthc. Mater. 2013, 2, 568–575. [Google Scholar] [CrossRef]
- Grasdalen, H.; Smidsrød, O. Gelation of gellan gum. Carbohydr. Polym. 1987, 7, 371–393. [Google Scholar] [CrossRef]
- Turner, M.R.; Modo, M. Advances in the application of MRI to amyotrophic lateral sclerosis. Expert Opin. Med. Diagn. 2010, 4, 483–496. [Google Scholar] [CrossRef]
- Grolez, G.; Moreau, C.; Danel-Brunaud, V.; Delmaire, C.; Lopes, R.; Pradat, P.F.; El Mendili, M.M.; Defebvre, L.; Devos, D. The value of magnetic resonance imaging as a biomarker for amyotrophic lateral sclerosis: A systematic review. BMC Neurol. 2016, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, J.M.; Paiva, F.F.; Longo, B.M.; Hamani, C.; Covolan, L. Manganese-Enhanced MRI: Biological Applications in Neuroscience. Front. Neurol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal Stem Cells: A Potential Therapeutic Approach for Amyotrophic Lateral Sclerosis? Stem Cells Int. 2019, 2019, 3675627. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, H.J.; An, J.; Kim, Y.B.; Ra, J.C.; Lim, I.; Kim, S.U. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant. 2014, 23, 1585–1597. [Google Scholar] [CrossRef]
- Walker, C.L. Adipose-derived stem cell conditioned medium for the treatment of amyotrophic lateral sclerosis: Pre-clinical evidence and potential for clinical application. Neural Regen. Res. 2019, 14, 1522–1524. [Google Scholar] [CrossRef]

| Concentration (mM) | |
|---|---|
| NaCl | 125 |
| KCl | 2.5 |
| MgCl2·6H2O | 1 |
| NaH2PO4 | 1.25 |
| CaCl2·2H2O | 2 |
| NaHCO3 | 25 |
| Glucose | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, S.; Strymecka, P.; Stanaszek, L.; Silva-Correia, J.; Drela, K.; Fiedorowicz, M.; Malysz-Cymborska, I.; Janowski, M.; Reis, R.L.; Łukomska, B.; et al. Mn-Based Methacrylated Gellan Gum Hydrogels for MRI-Guided Cell Delivery and Imaging. Bioengineering 2023, 10, 427. https://doi.org/10.3390/bioengineering10040427
Vieira S, Strymecka P, Stanaszek L, Silva-Correia J, Drela K, Fiedorowicz M, Malysz-Cymborska I, Janowski M, Reis RL, Łukomska B, et al. Mn-Based Methacrylated Gellan Gum Hydrogels for MRI-Guided Cell Delivery and Imaging. Bioengineering. 2023; 10(4):427. https://doi.org/10.3390/bioengineering10040427
Chicago/Turabian StyleVieira, Sílvia, Paulina Strymecka, Luiza Stanaszek, Joana Silva-Correia, Katarzyna Drela, Michał Fiedorowicz, Izabela Malysz-Cymborska, Miroslaw Janowski, Rui Luís Reis, Barbara Łukomska, and et al. 2023. "Mn-Based Methacrylated Gellan Gum Hydrogels for MRI-Guided Cell Delivery and Imaging" Bioengineering 10, no. 4: 427. https://doi.org/10.3390/bioengineering10040427
APA StyleVieira, S., Strymecka, P., Stanaszek, L., Silva-Correia, J., Drela, K., Fiedorowicz, M., Malysz-Cymborska, I., Janowski, M., Reis, R. L., Łukomska, B., Walczak, P., & Oliveira, J. M. (2023). Mn-Based Methacrylated Gellan Gum Hydrogels for MRI-Guided Cell Delivery and Imaging. Bioengineering, 10(4), 427. https://doi.org/10.3390/bioengineering10040427












