Application of a Radiomics Machine Learning Model for Differentiating Aldosterone-Producing Adenoma from Non-Functioning Adrenal Adenoma
Abstract
:1. Introduction
2. Materials and Methods
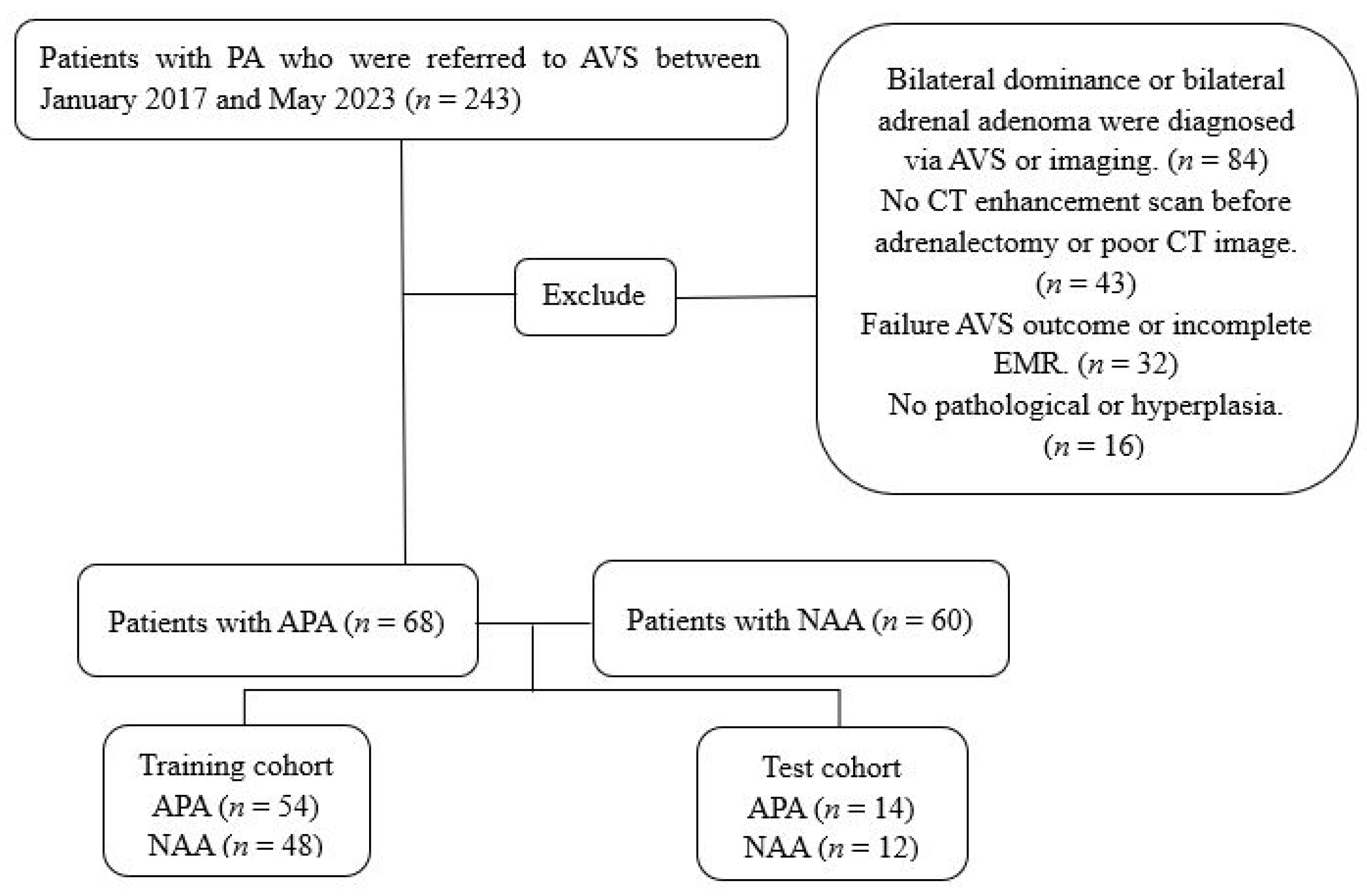
2.1. Study Design and Patients
2.2. Image Acquisition
2.3. Extraction of Radiomics Features
2.4. Selection of Radiomics Features and Model Development
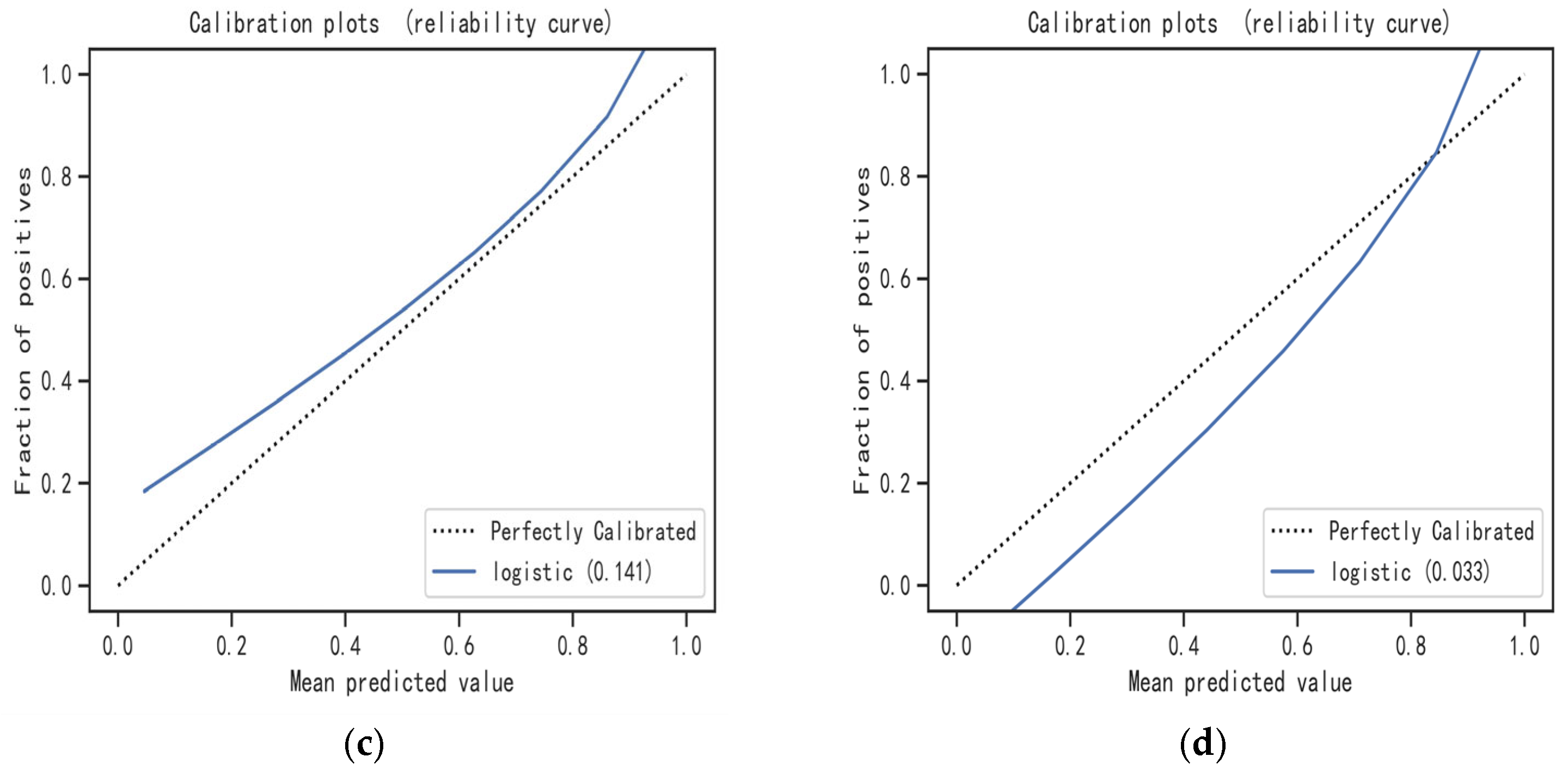
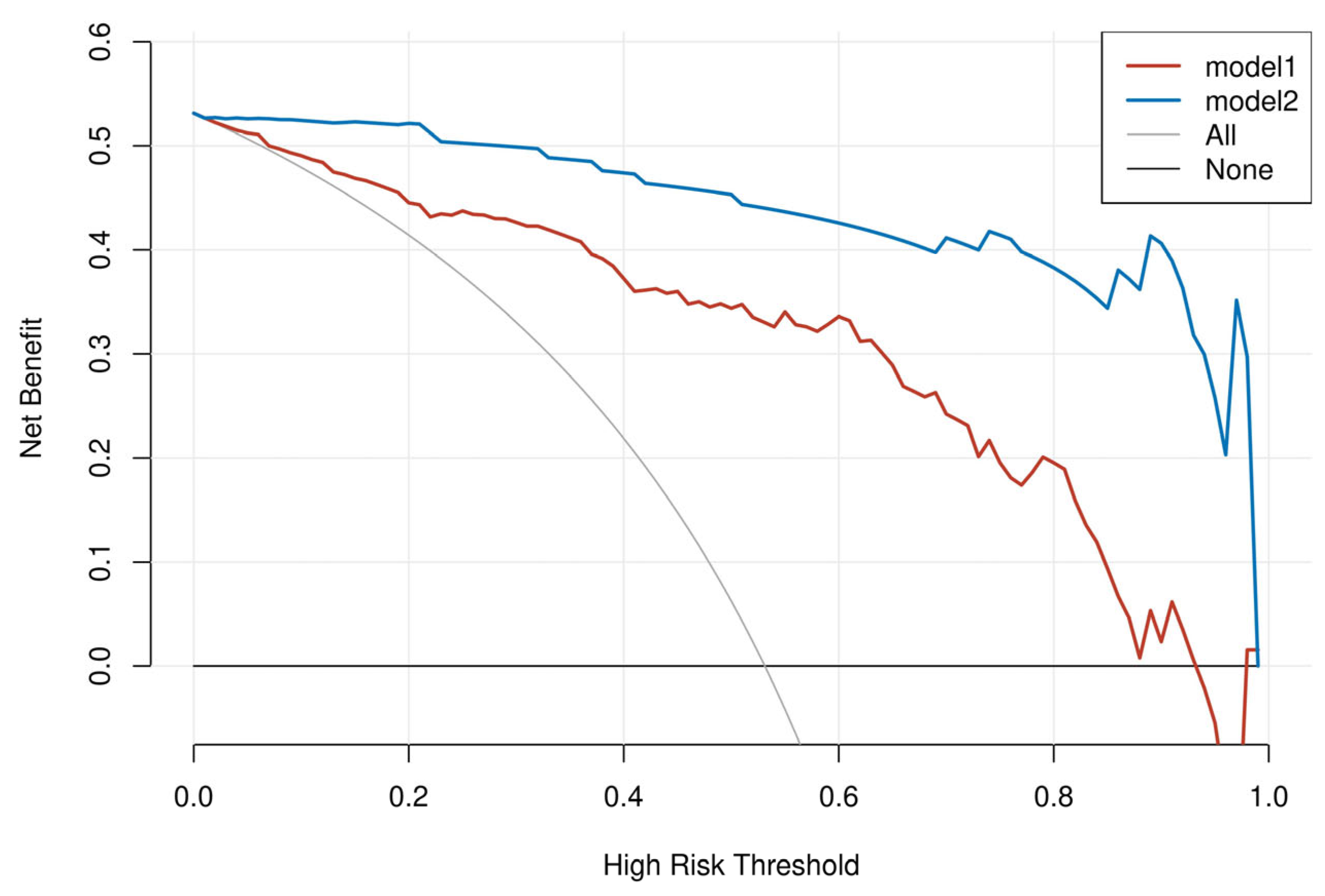
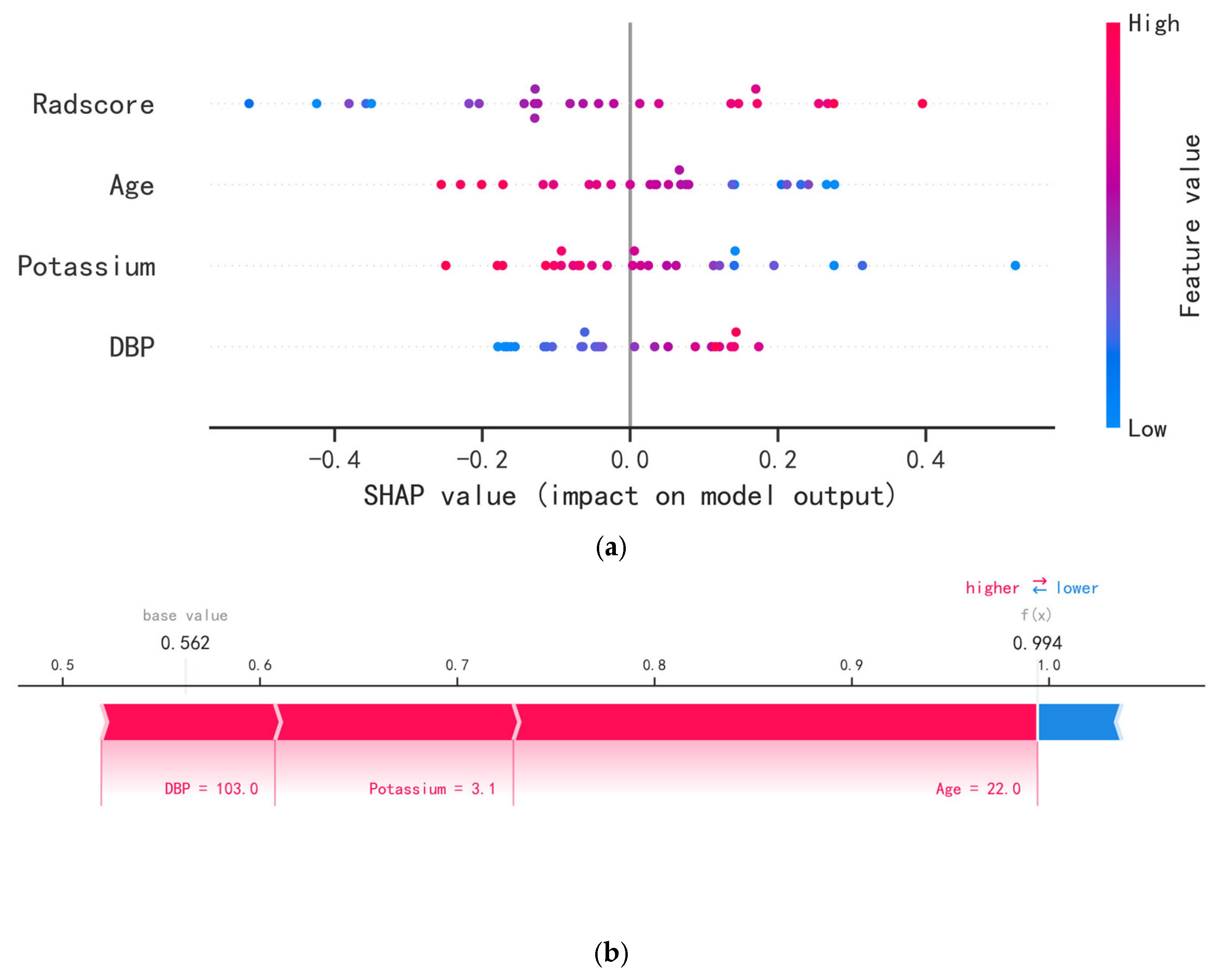
2.5. Model Evaluation and Interpretation
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
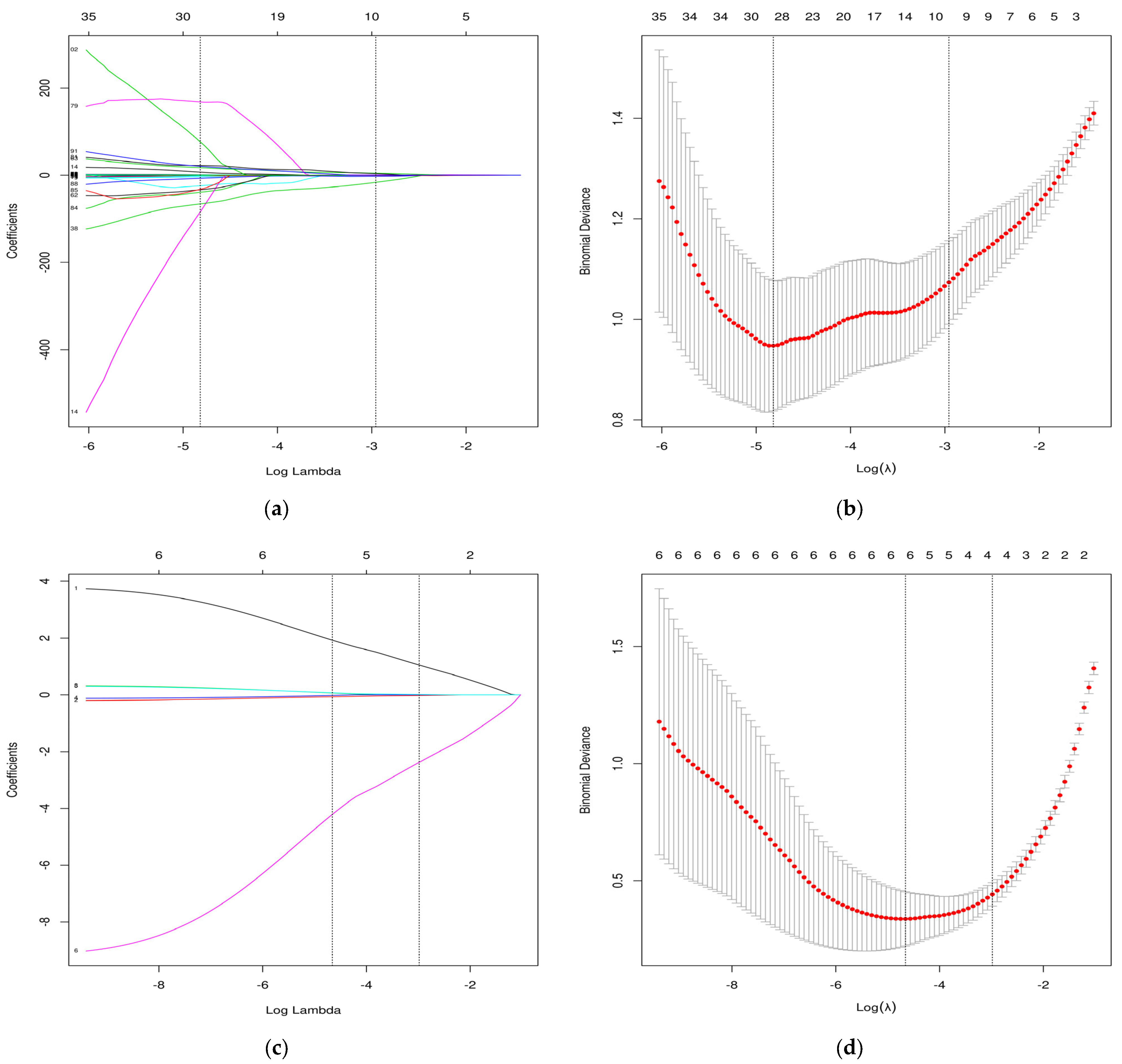
3.2. Selection of Radiomic Features and Model Development
+0.739 × (original_(shape_SurfaceVolumeRatio))
+0.007 × (wavelet-LLH_(firstorder_10Percentile))
+0.007 × (wavelet-LLH_(gldm_LargeDependenceLowGrayLevelEmphasis))
−0.557 × (wavelet-LLH_(glszm_ZoneEntropy)) + 4.712 × (wavelet-LHL_(glcm_Imc2))
−0.044 × (wavelet-LHL_(ngtdm_Busyness)) − 2.578 × (wavelet-LHH_(glrlm_RunEntropy))
−16.345 × (wavelet-HHL_(gldm_DependenceNonUniformityNormalized))
+3.628 × (wavelet-LLL_(glcm_MaximumProbability))
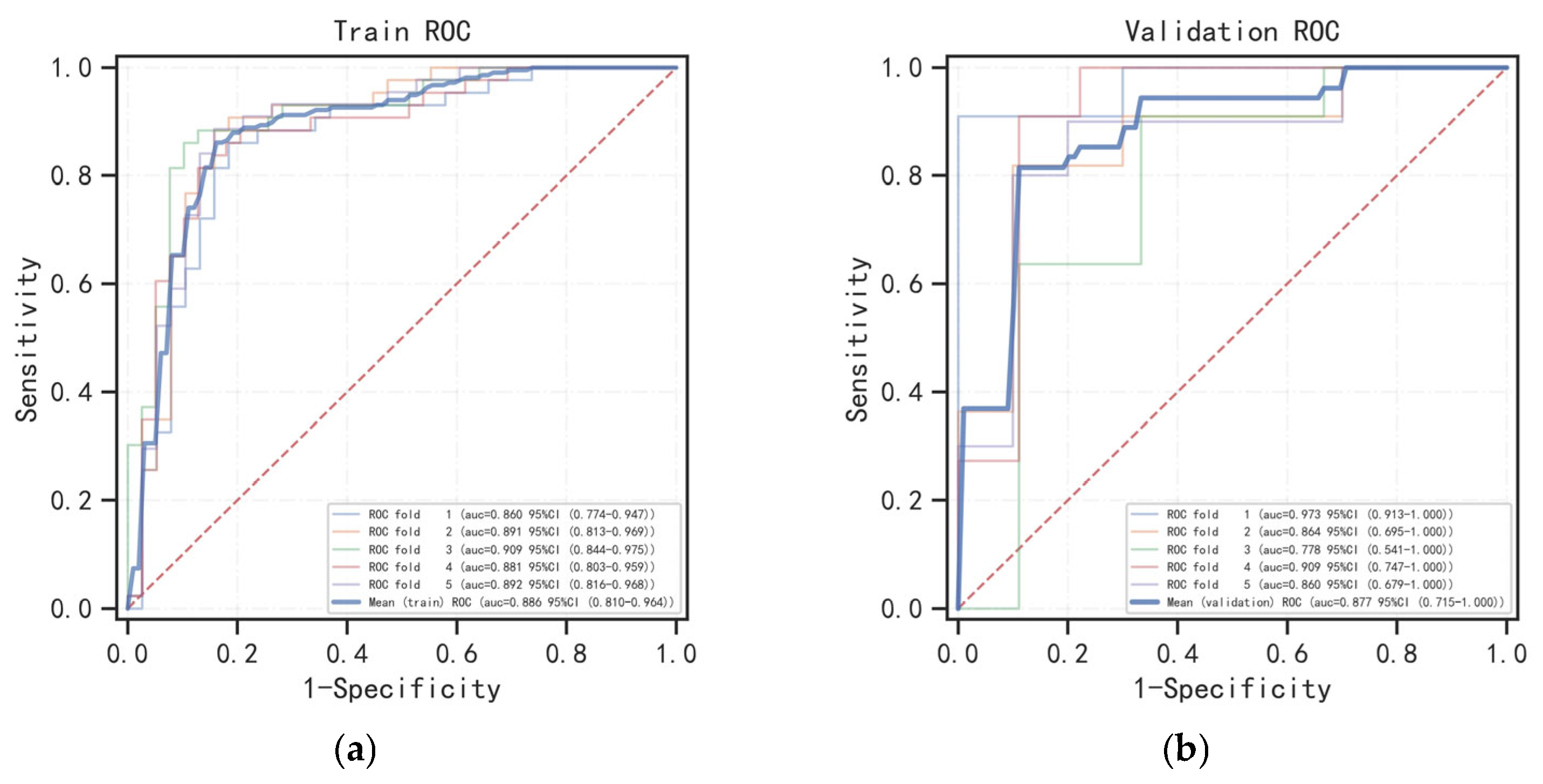
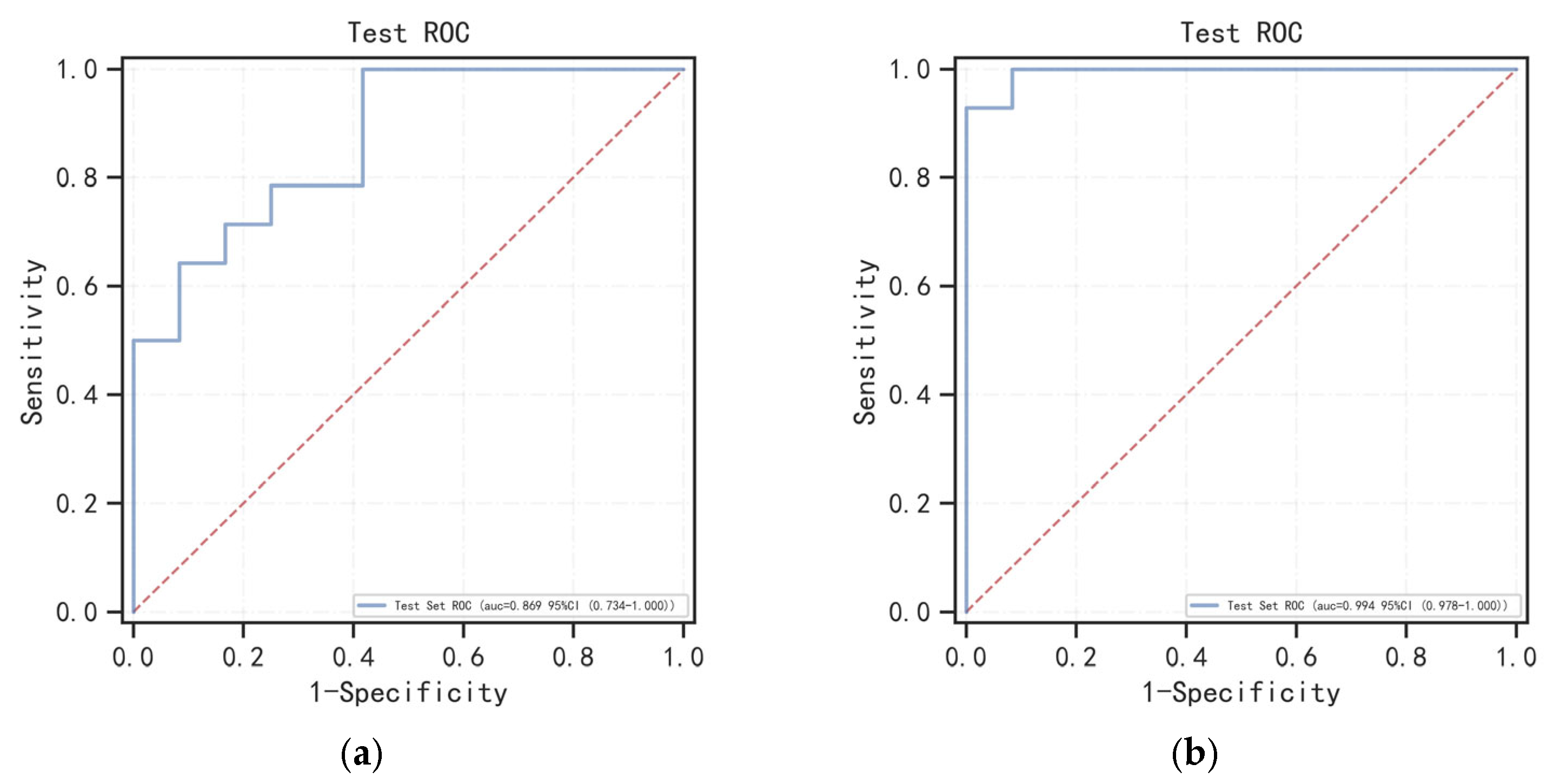
3.3. Model Evaluation and Interpretation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terzolo, M.; Stigliano, A.; Chiodini, I.; Loli, P.; Furlani, L.; Arnaldi, G.; Reimondo, G.; Pia, A.; Toscano, V.; Zini, M.; et al. AME position statement on adrenal incidentaloma. Eur. J. Endocrinol. 2011, 164, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Papanicolas, I.; Woskie, L.R.; Jha, A.K. Health Care Spending in the United States and Other High-Income Countries. JAMA 2018, 319, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Auchus, R. Approach to the Patient with Primary Aldosteronism: Utility and Limitations of Adrenal Vein Sampling. J. Clin. Endocrinol. Metab. 2021, 106, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, J.; Hu, J.; Song, Y.; He, W.; Luo, T.; Cheng, Q.; Ma, L.; Luo, R.; Fuller, P.J.; et al. Primary Aldosteronism in Patients in China with Recently Detected Hypertension. J. Am. Coll. Cardiol. 2020, 75, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M. Primary Aldosteronism: Where Are We Now? Where to from Here? Hypertension 2022, 79, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Abe, M.; Kobayashi, H. The Effect of Aldosterone on Cardiorenal and Metabolic Systems. Int. J. Mol. Sci. 2023, 24, 5370. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Wu, X.; Senanayake, R.; Goodchild, E.; Bashari, W.A.; Salsbury, J.; Cabrera, C.P.; Argentesi, G.; O’Toole, S.M.; Matson, M.; Koo, B.; et al. [(11)C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: A prospective, within-patient trial. Nat. Med. 2023, 29, 190–202. [Google Scholar] [CrossRef]
- Fuss, C.T.; Treitl, M.; Rayes, N.; Podrabsky, P.; Fenske, W.K.; Heinrich, D.A.; Reincke, M.; Petersen, T.O.; Fassnach, M.; Quinkler, M.; et al. Radiation exposure of adrenal vein sampling: A German Multicenter Study. Eur. J. Endocrinol. 2018, 179, 261–267. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.S.; Costigliola, V. Medicine in the early twenty-first century: Paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kang, B.; Cui, J.; Deng, Y.; Zhao, Y.; Ji, C.; Wang, X. Two nomograms based on radiomics models using triphasic CT for differentiation of adrenal lipid-poor benign lesions and metastases in a cancer population: An exploratory study. Eur. Radiol. 2023, 33, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Torresan, F.; Crimi, F.; Ceccato, F.; Zavan, F.; Barbot, M.; Lacognata, C.; Motta, R.; Armellin, C.; Scaroni, C.; Quaia, E.; et al. Radiomics: A new tool to differentiate adrenocortical adenoma from carcinoma. BJS Open 2021, 5, zraa061. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Guan, X.; Zhang, Y.; Liu, L.; Long, X.; Yin, H.; Wang, Z.; Li, X.; Liao, W.; Chen, B.T.; et al. Radiomics improves efficiency for differentiating subclinical pheochromocytoma from lipid-poor adenoma: A predictive, preventive and personalized medical approach in adrenal incidentalomas. EPMA J. 2018, 9, 421–429. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, Z.T.; Wang, Z.H.; Wang, Y.; Wang, Y.X.; Zhang, H.Z.; Dong, Y.F.; Xiao, X.L. A Clinical-Radiomic Nomogram Based on Unenhanced Computed Tomography for Predicting the Risk of Aldosterone-Producing Adenoma. Front. Oncol. 2021, 11, 634879. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.T.; Chang, D.; Liu, K.L.; Liao, W.C.; Wang, W.; Chang, C.C.; Wu, V.C.; Lin, Y.H. Radiomics utilization to differentiate nonfunctional adenoma in essential hypertension and functional adenoma in primary aldosteronism. Sci. Rep. 2022, 12, 8892. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou-Marketou, N.; Vaidya, A.; Dluhy, R.; Chrousos, G.P. Hyperaldosteronism. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Funder, J.W.; Carey, R.M.; Fardella, C.; Gomez-Sanchez, C.E.; Mantero, F.; Stowasser, M.; Young, W.F., Jr.; Montori, V.M.; Endocrine, S. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 3266–3281. [Google Scholar] [CrossRef]
- Rossi, G.P.; Bernini, G.; Caliumi, C.; Desideri, G.; Fabris, B.; Ferri, C.; Ganzaroli, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 2006, 48, 2293–2300. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef]
- Lundberg, S.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar] [CrossRef]
- Bioletto, F.; Lopez, C.; Bollati, M.; Arata, S.; Procopio, M.; Ponzetto, F.; Beccuti, G.; Mengozzi, G.; Ghigo, E.; Maccario, M.; et al. Predictive performance of aldosterone-to-renin ratio in the diagnosis of primary aldosteronism in patients with resistant hypertension. Front. Endocrinol. 2023, 14, 1145186. [Google Scholar] [CrossRef] [PubMed]
- Parasiliti-Caprino, M.; Lopez, C.; Prencipe, N.; Lucatello, B.; Settanni, F.; Giraudo, G.; Rossato, D.; Mengozzi, G.; Ghigo, E.; Benso, A.; et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J. Hypertens. 2020, 38, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; Nishizaka, M.K.; Zaman, M.A.; Thakkar, R.B.; Weissmann, P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002, 40, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Ekert, K.; Hinterleitner, C.; Baumgartner, K.; Fritz, J.; Horger, M. Extended Texture Analysis of Non-Enhanced Whole-Body MRI Image Data for Response Assessment in Multiple Myeloma Patients Undergoing Systemic Therapy. Cancers 2020, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Harmon, S.; Perk, T.; Li, X.; Chen, M.; Li, Y.; Jeraj, R. Using neighborhood gray tone difference matrix texture features on dual time point PET/CT images to differentiate malignant from benign FDG-avid solitary pulmonary nodules. Cancer Imaging 2019, 19, 56. [Google Scholar] [CrossRef]
- Geady, C.; Keller, H.; Siddiqui, I.; Bilkey, J.; Dhani, N.C.; Jaffray, D.A. Bridging the gap between micro- and macro-scales in medical imaging with textural analysis—A biological basis for CT radiomics classifiers? Phys. Med. 2020, 72, 142–151. [Google Scholar] [CrossRef]
- Williams, T.A.; Gomez-Sanchez, C.E.; Rainey, W.E.; Giordano, T.J.; Lam, A.K.; Marker, A.; Mete, O.; Yamazaki, Y.; Zerbini, M.C.N.; Beuschlein, F.; et al. International Histopathology Consensus for Unilateral Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2021, 106, 42–54. [Google Scholar] [CrossRef]
- Grossmann, P.; Stringfield, O.; El-Hachem, N.; Bui, M.M.; Rios Velazquez, E.; Parmar, C.; Leijenaar, R.T.; Haibe-Kains, B.; Lambin, P.; Gillies, R.J.; et al. Defining the biological basis of radiomic phenotypes in lung cancer. eLife 2017, 6, e23421. [Google Scholar] [CrossRef]
- Wu, X.L.; Xu, Q.Z.; Chen, T.; Wang, F.L.; Jiang, W.H.; Lyu, G.M.; Lu, G. Establishment and analysis of prediction model for invasive subsolid pulmonary nodules based on radiomics. Zhonghua Yi Xue Za Zhi 2022, 102, 209–215. [Google Scholar] [CrossRef]
- Ho, L.M.; Samei, E.; Mazurowski, M.A.; Zheng, Y.; Allen, B.C.; Nelson, R.C.; Marin, D. Can Texture Analysis Be Used to Distinguish Benign from Malignant Adrenal Nodules on Unenhanced CT, Contrast-Enhanced CT, or In-Phase and Opposed-Phase MRI? AJR Am. J. Roentgenol. 2019, 212, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, H.; Li, X.; Jin, S.; Yang, J.; Pan, W.; Dong, X.; Chen, J.; Ji, W. Can Radiomics Provide Additional Diagnostic Value for Identifying Adrenal Lipid-Poor Adenomas from Non-Adenomas on Unenhanced CT? Front. Oncol. 2022, 12, 888778. [Google Scholar] [CrossRef] [PubMed]
- Crimi, F.; Agostini, E.; Toniolo, A.; Torresan, F.; Iacobone, M.; Tizianel, I.; Scaroni, C.; Quaia, E.; Campi, C.; Ceccato, F. CT Texture Analysis of Adrenal Pheochromocytomas: A Pilot Study. Curr. Oncol. 2023, 30, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Ansquer, C.; Drui, D.; Mirallie, E.; Renaudin-Autain, K.; Denis, A.; Gimenez-Roqueplo, A.P.; Leux, C.; Toulgoat, F.; Kraeber-Bodere, F.; Carlier, T. Usefulness of FDG-PET/CT-Based Radiomics for the Characterization and Genetic Orientation of Pheochromocytomas Before Surgery. Cancers 2020, 12, 2424. [Google Scholar] [CrossRef]
- Akai, H.; Yasaka, K.; Kunimatsu, A.; Ohtomo, K.; Abe, O.; Kiryu, S. Application of CT texture analysis to assess the localization of primary aldosteronism. Sci. Rep. 2020, 10, 472. [Google Scholar] [CrossRef]


| Package Classification | Package Name | Version | Usage |
|---|---|---|---|
| R | glmnet | 4.1.2 | LASSO |
| Python | sklearn | 0.22.1 | ROC |
| R | rmda | 1.6 | DCA |
| Python | shap | 0.39.0 | SHAP |
| Python | sklearn | 0.22.1 | Logistic regression ML |
| Variables | Training (n = 102) | Test (n = 26) | χ2/t/z | p Value |
|---|---|---|---|---|
| Age, years | 51.00 (40.75–57.00) | 48.50 (36.50–54.25) | −1.301 | 0.193 |
| Sex | 0.927 | 0.336 | ||
| Male | 50 (49.02%) | 10 (38.46%) | ||
| Female | 52 (50.98%) | 16 (61.54%) | ||
| Time 1 | 1.00 (0.00–6.25) | 2.50 (0.13–8.50) | −1.456 | 0.145 |
| SBP, mmHg | 140.36 ± 20.05 | 139.15 ± 19.88 | 0.275 | 0.784 |
| DBP, mmHg | 88.00 (80.00–97.25) | 83 (78.25–103.25) | −0.684 | 0.494 |
| FPG, mmol/L | 5.57 (4.93–6.97) | 6.26 (5.44–7.91) | −1.963 | 0.050 |
| Potassium, mmol/L | 3.57 ± 0.68 | 3.51 ± 0.58 | 0.454 | 0.651 |
| TC, mmol/L | 4.04 (3.69–4.71) | 4.54 (3.70–4.96) | −1.108 | 0.268 |
| Scr, umol/L | 68.50 (56.00–82.25) | 63.50 (52.75–82.50) | −0.892 | 0.373 |
| eGFR, ml/min/1.73 m2 | 100.35 (87.10–107.38) | 102.10 (87.48–112.50) | −0.980 | 0.327 |
| BMI, kg/m2 | 24.19 ± 3.51 | 24.27 ± 2.93 | −0.104 | 0.917 |
| Variables | APA (n = 54) | NAA (n = 48) | χ2/t/z | p |
|---|---|---|---|---|
| Age, years | 48.00 (36.75–54.00) | 55.50 (51.00–57.75) | −3.965 | <0.001 |
| Sex | 0.961 | 0.327 | ||
| Male | 24 (44.44%) | 26 (54.17%) | ||
| Female | 30 (55.56%) | 22 (45.83%) | ||
| Time 1 | 3.00 (0.56–8.00) | 0.00 (0.00–3.00) | −4.537 | <0.001 |
| SBP, mmHg | 147 (134.00–165.50) | 127.00 (121.25–142.75) | −3.692 | <0.001 |
| DBP, mmHg | 94.56 ± 14.36 | 84.75 ± 10.39 | 3.980 | <0.001 |
| FPG, mmol/L | 5.66 (4.93–7.08) | 5.53 (4.92–6.48) | −0.674 | 0.050 |
| Potassium, mmol/L | 3.10 ± 0.54 | 4.10 ± 0.35 | −11.243 | <0.001 |
| TC, mmol/L | 3.91 (3.61–4.65) | 4.19 (3.71–4.36) | −1.438 | 0.150 |
| Scr, umol/L | 68.50 (56.00–82.50) | 68.50 (59.25–82.50) | −0.054 | 0.957 |
| eGFR, ml/min/1.73 m2 | 101.75 (89.85–108.05) | 99.50 (85.43–106.58) | −1.200 | 0.230 |
| BMI, kg/m2 | 23.68 ± 3.43 | 24.77 ± 3.55 | −1.585 | 0.116 |
| Radscore | 0.86 ± 0.76 | −0.67 ± 1.02 | 8.455 | <0.001 |
| Models | Groups | 1-Fold AUC (95% CI) | 2-Fold AUC (95% CI) | 3-Fold AUC (95% CI) | 4-Fold AUC (95% CI) | 5-Fold AUC (95% CI) |
|---|---|---|---|---|---|---|
| Radscore | Training | 0.860 (0.774–0.947) | 0.891 (0.813–0.969) | 0.909 (0.844–0.975) | 0.881 (0.803–0.959) | 0.892 (0.816–0.968) |
| Validation | 0.973 (0.913–1.000) | 0.864 (0.695–1.000) | 0.778 (0.541–1.000) | 0.909 (0.747–1.000) | 0.860 (0.679–1.000) | |
| Clinic–Radscore | Training | 0.993 (0.981–1.000) | 0.984 (0.961–1.000) | 0.999 (0.996–1.000) | 0.989 (0.973–1.000) | 0.992 (0.979–1.000) |
| Validation | 0.991 (0.966–1.000) | 1.000 (nan–nan 1) | 0.960 (0.885–1.000) | 1.000 (nan–nan) | 0.950 (0.865–1.000) |
| Models | Groups | Mean AUC (95% CI) | Accuracy | Sensitivity | Specificity | F1 |
|---|---|---|---|---|---|---|
| Radscore | Training | 0.886 (0.810–0.964) | 0.846 | 0.861 | 0.854 | 0.863 |
| Validation | 0.877 (0.715–1.000) | 0.823 | 0.869 | 0.871 | 0.854 | |
| Clinic–Radscore | Training | 0.988 (0.978–1.000) | 0.958 | 0.986 | 0.953 | 0.972 |
| Validation | 0.976 (nan-nan 1) | 0.921 | 0.964 | 0.938 | 0.946 |
| Models | AUC (95% CI) | Accuracy | Sensitivity | Specificity | F1 | z | p |
|---|---|---|---|---|---|---|---|
| Radscore | 0.869 (0.734–1.000) | 0.731 | 1.000 | 0.583 | 0.900 | 1.859 | 0.063 |
| Clinic–Radscore | 0.994 (0.978–1.000) | 0.962 | 0.929 | 1.000 | 0.931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Hao, Y.; Mu, K.; Li, J.; Tao, Z.; Ma, D.; Xu, A. Application of a Radiomics Machine Learning Model for Differentiating Aldosterone-Producing Adenoma from Non-Functioning Adrenal Adenoma. Bioengineering 2023, 10, 1423. https://doi.org/10.3390/bioengineering10121423
Yang W, Hao Y, Mu K, Li J, Tao Z, Ma D, Xu A. Application of a Radiomics Machine Learning Model for Differentiating Aldosterone-Producing Adenoma from Non-Functioning Adrenal Adenoma. Bioengineering. 2023; 10(12):1423. https://doi.org/10.3390/bioengineering10121423
Chicago/Turabian StyleYang, Wenhua, Yonghong Hao, Ketao Mu, Jianjun Li, Zihui Tao, Delin Ma, and Anhui Xu. 2023. "Application of a Radiomics Machine Learning Model for Differentiating Aldosterone-Producing Adenoma from Non-Functioning Adrenal Adenoma" Bioengineering 10, no. 12: 1423. https://doi.org/10.3390/bioengineering10121423
APA StyleYang, W., Hao, Y., Mu, K., Li, J., Tao, Z., Ma, D., & Xu, A. (2023). Application of a Radiomics Machine Learning Model for Differentiating Aldosterone-Producing Adenoma from Non-Functioning Adrenal Adenoma. Bioengineering, 10(12), 1423. https://doi.org/10.3390/bioengineering10121423







