Improvement of Metal-Doped β-TCP Scaffolds for Active Bone Substitutes via Ultra-Short Laser Structuring
Abstract
:1. Introduction
2. Materials and Methods
2.1. β-TCP Disk Production
2.2. Laser Ablation
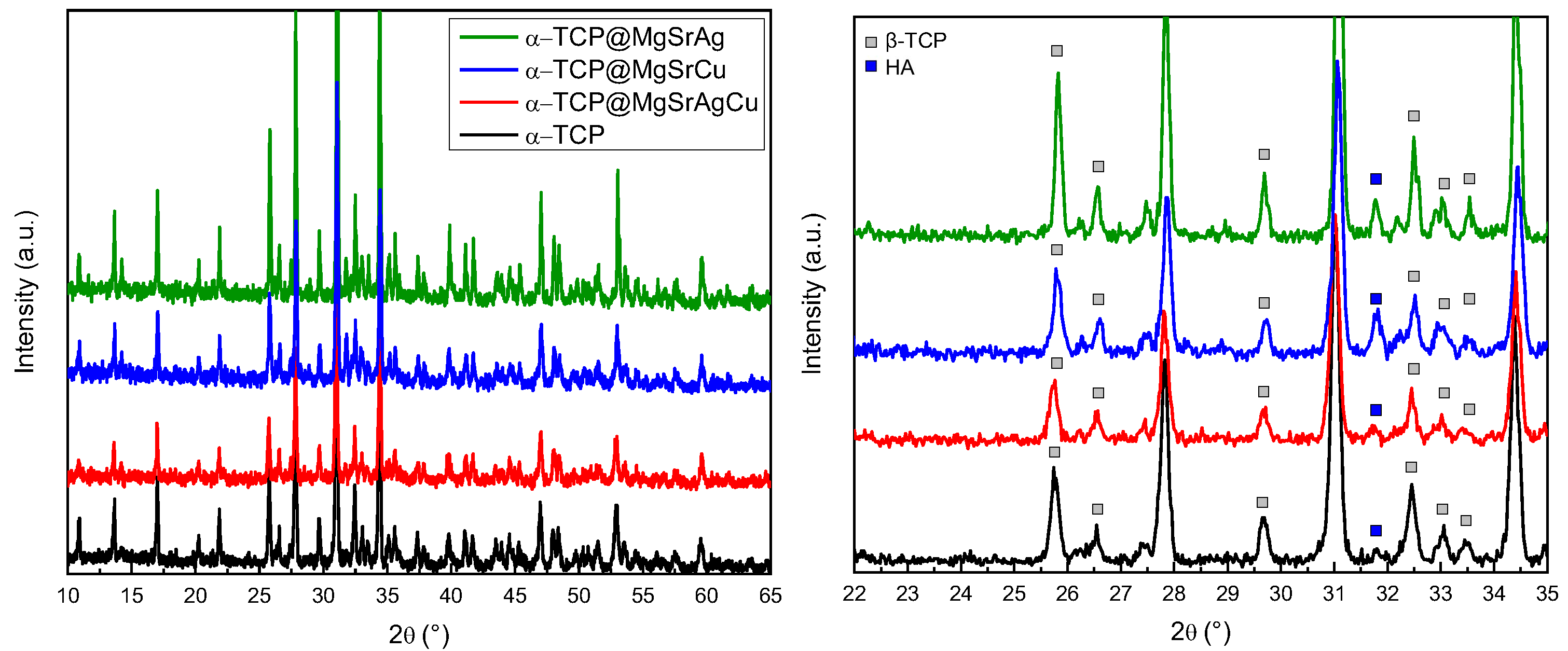
2.3. X-ray Diffraction (XRD)
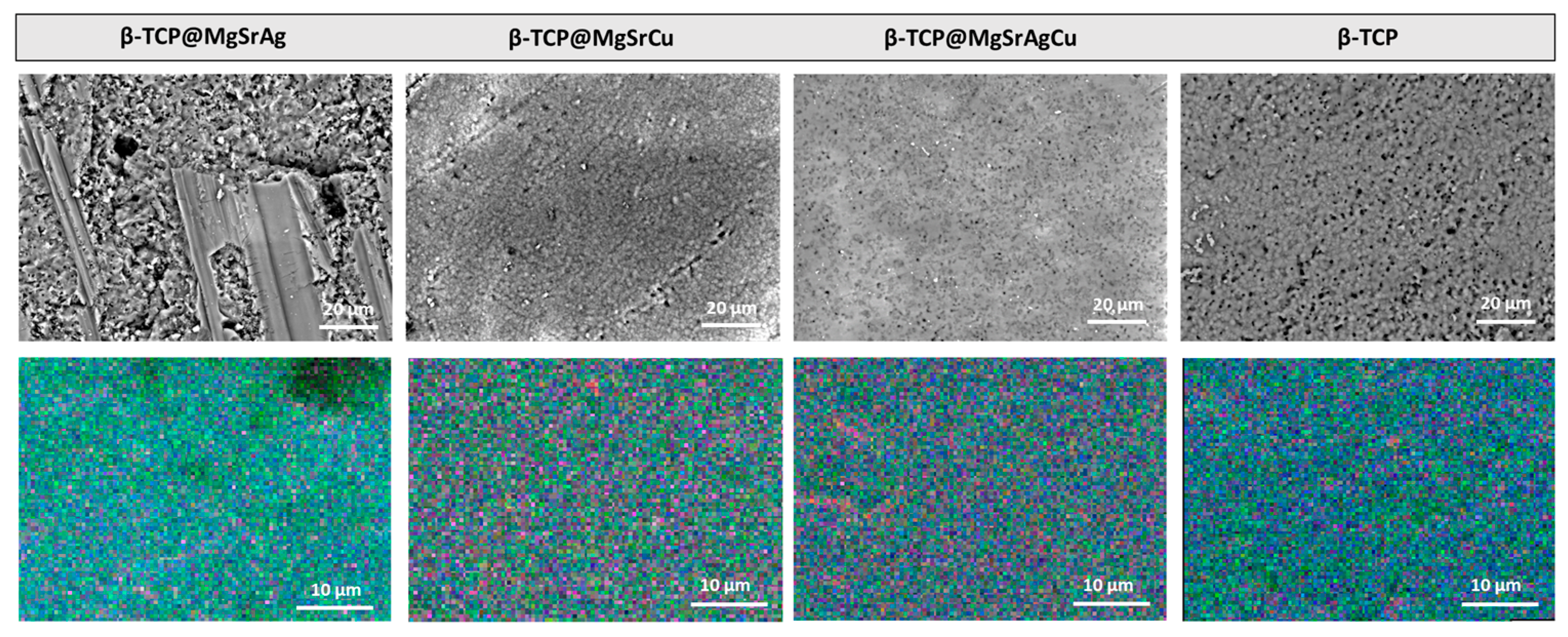
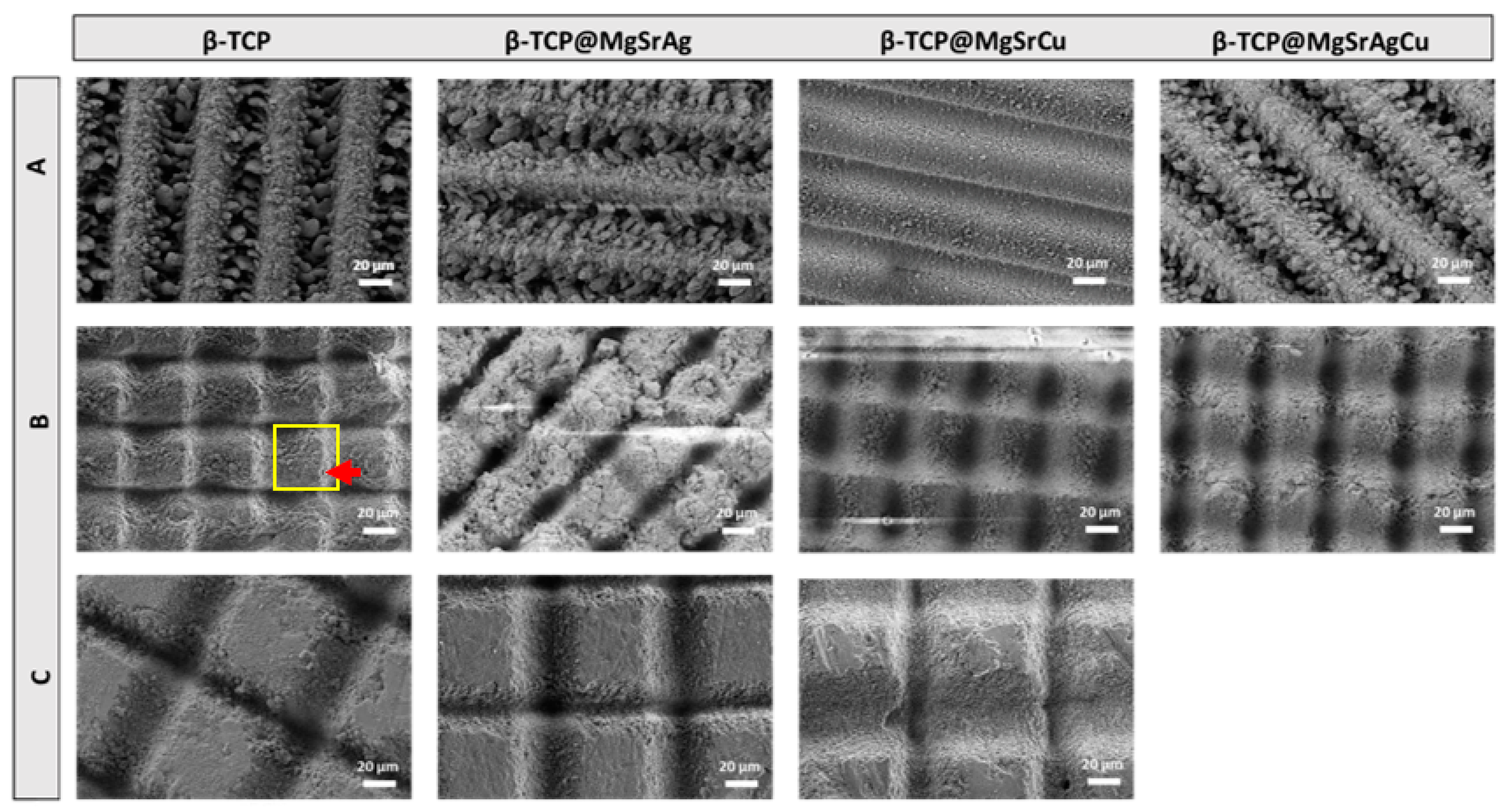
2.4. Scanning Electron Microscopy–Energy Dispersive Spectroscopy (SEM-EDS)
2.5. Optical Profilometer
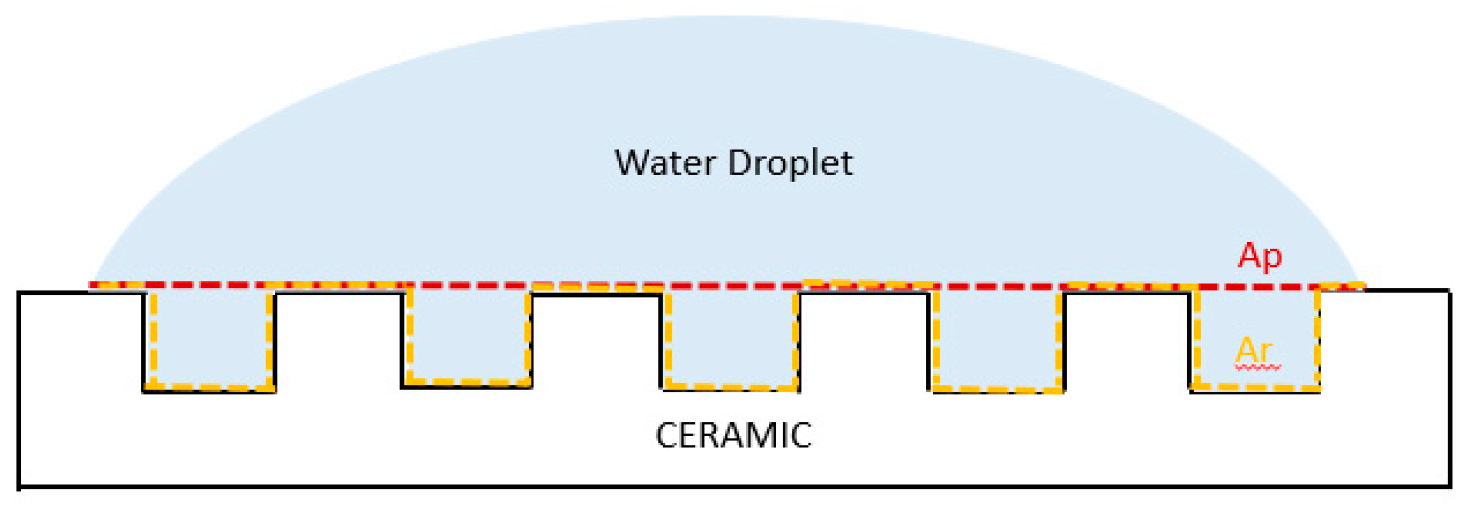
2.6. Water Contact Angle (WCA) and Surface-Free Energy (SFE)
2.7. Raman Spectroscopy
2.8. In Vitro Stability Studies
2.9. Confocal Microscopy
3. Results and Discussion
3.1. X-ray Diffraction
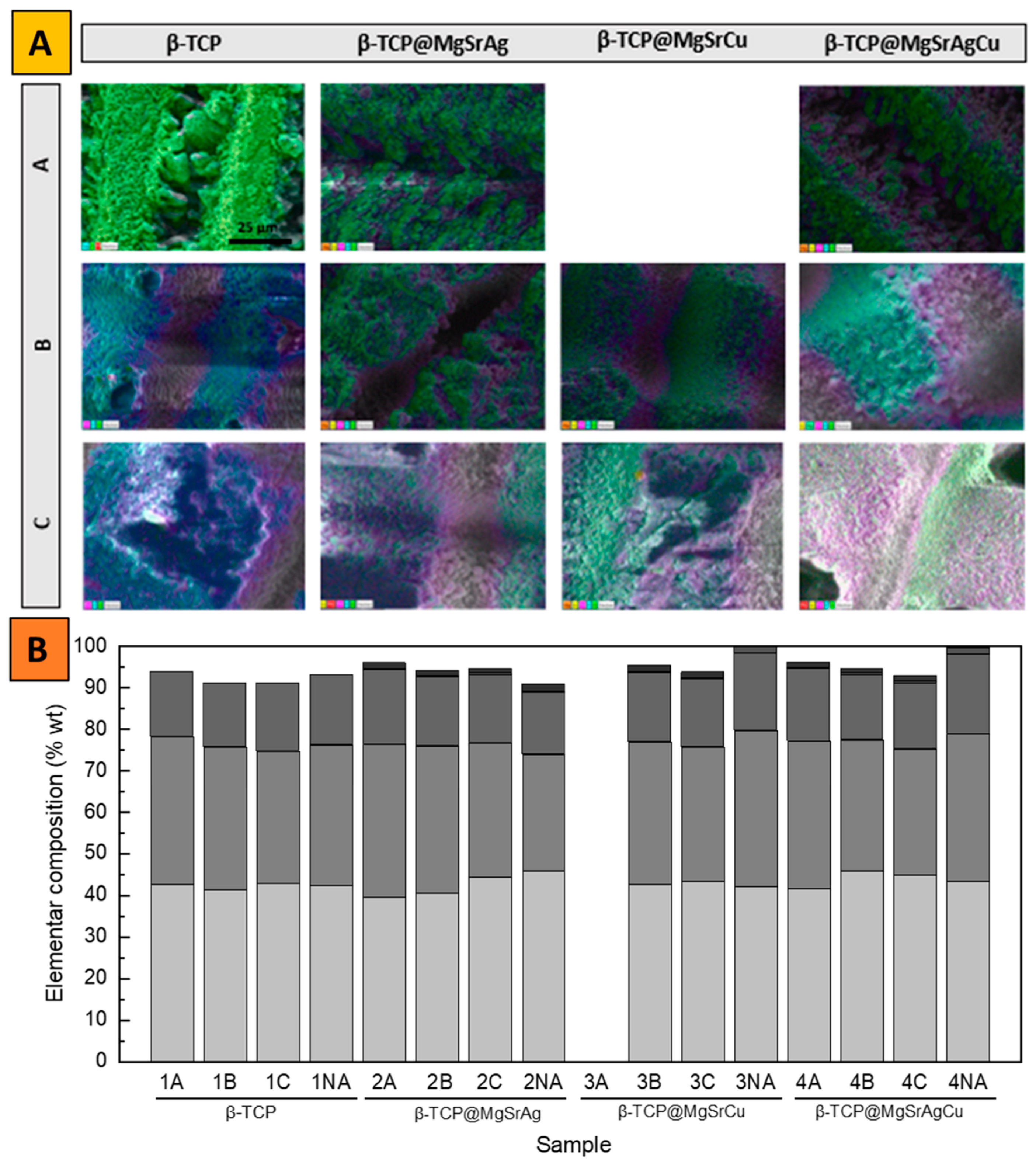
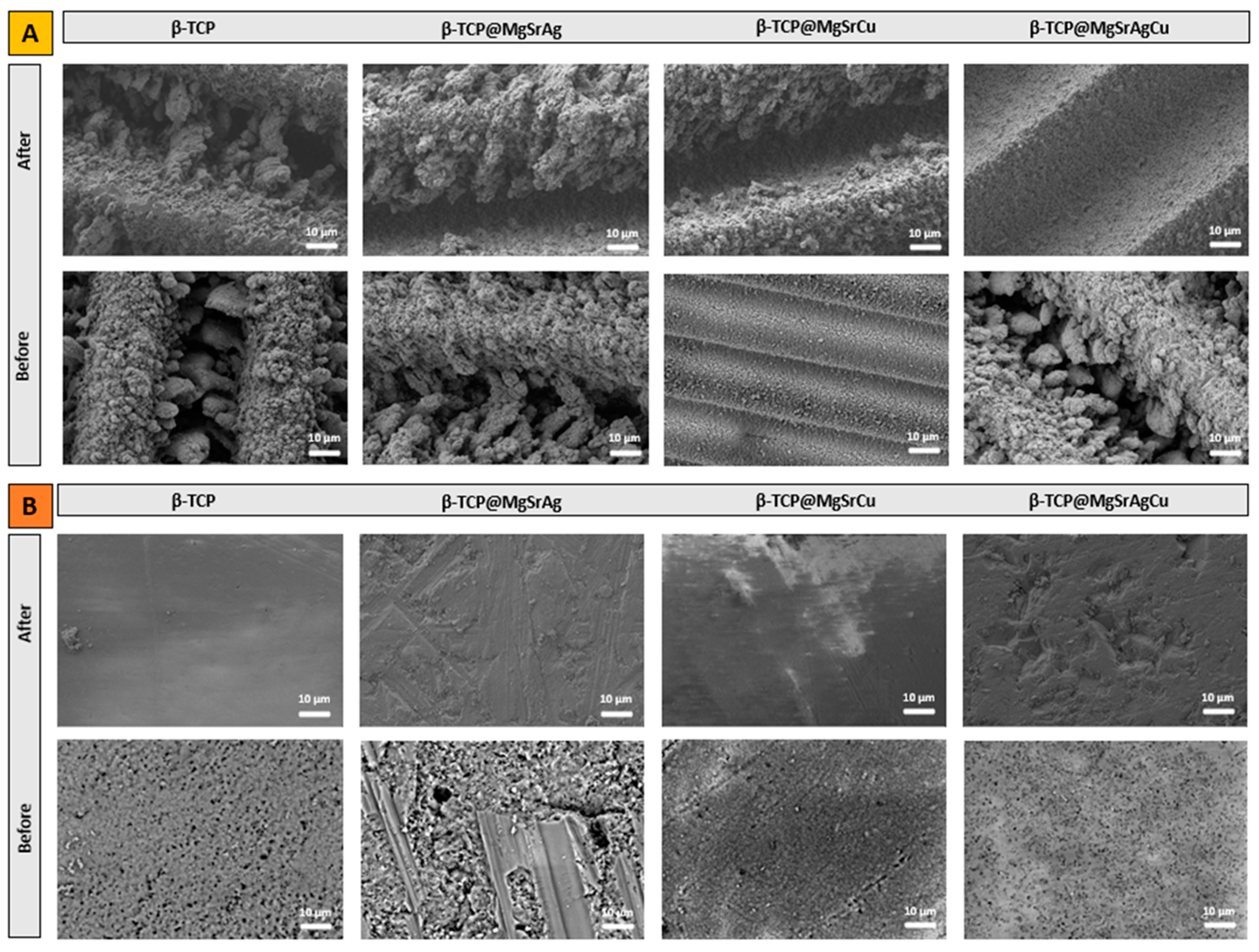
3.2. SEM-EDS
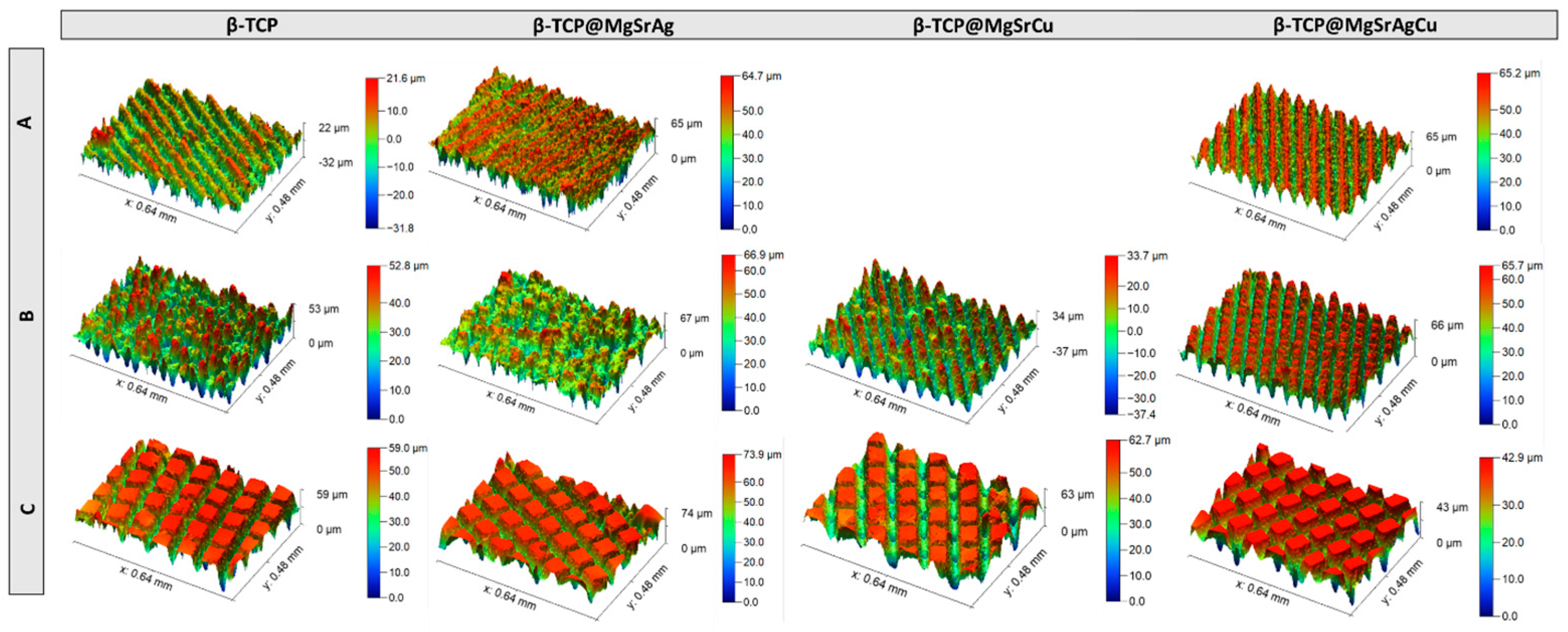
3.3. Optical Profilometer of Ablated TCP Disks
3.4. Wettability and Surface-Free Energy (SFE)
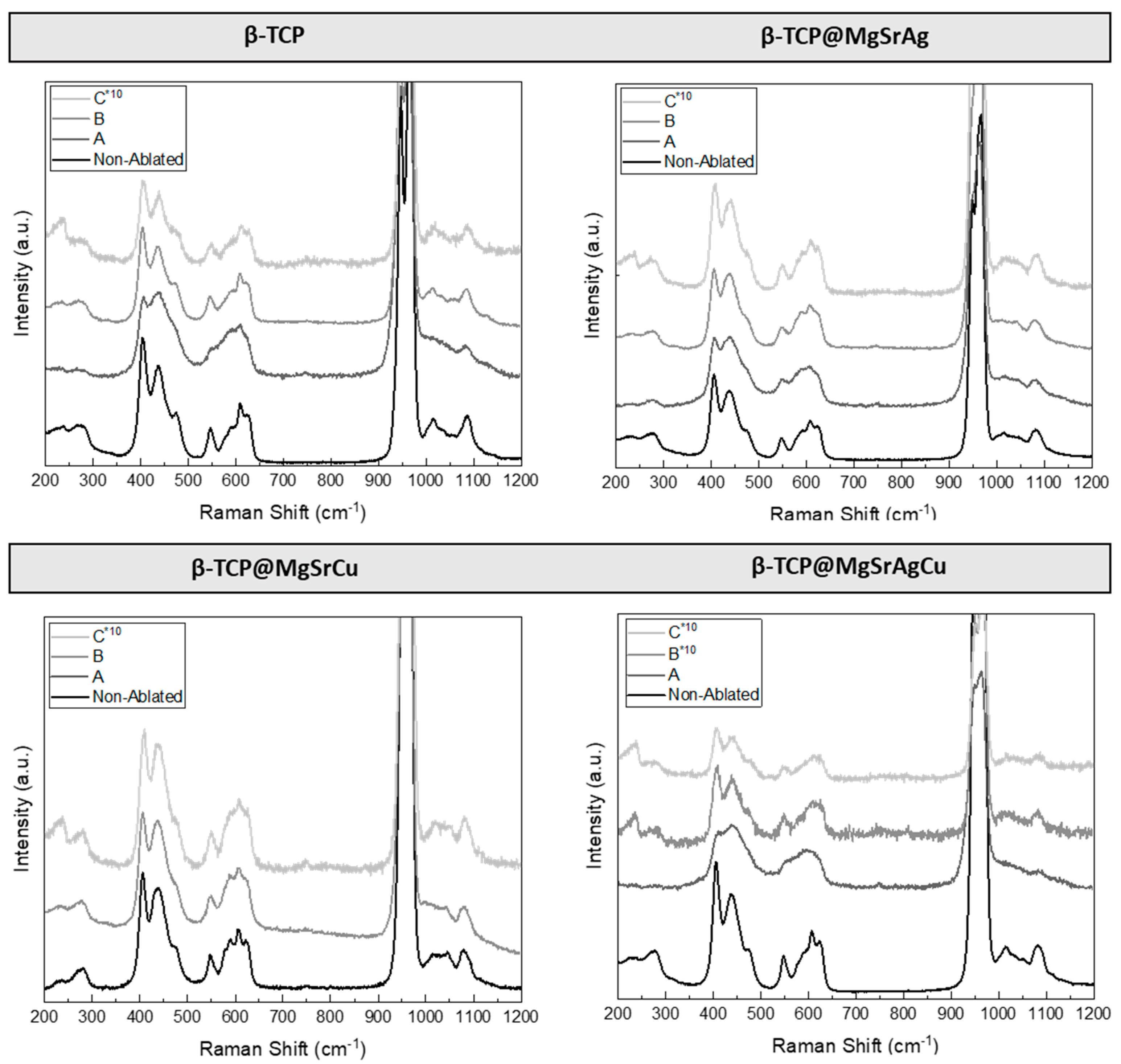
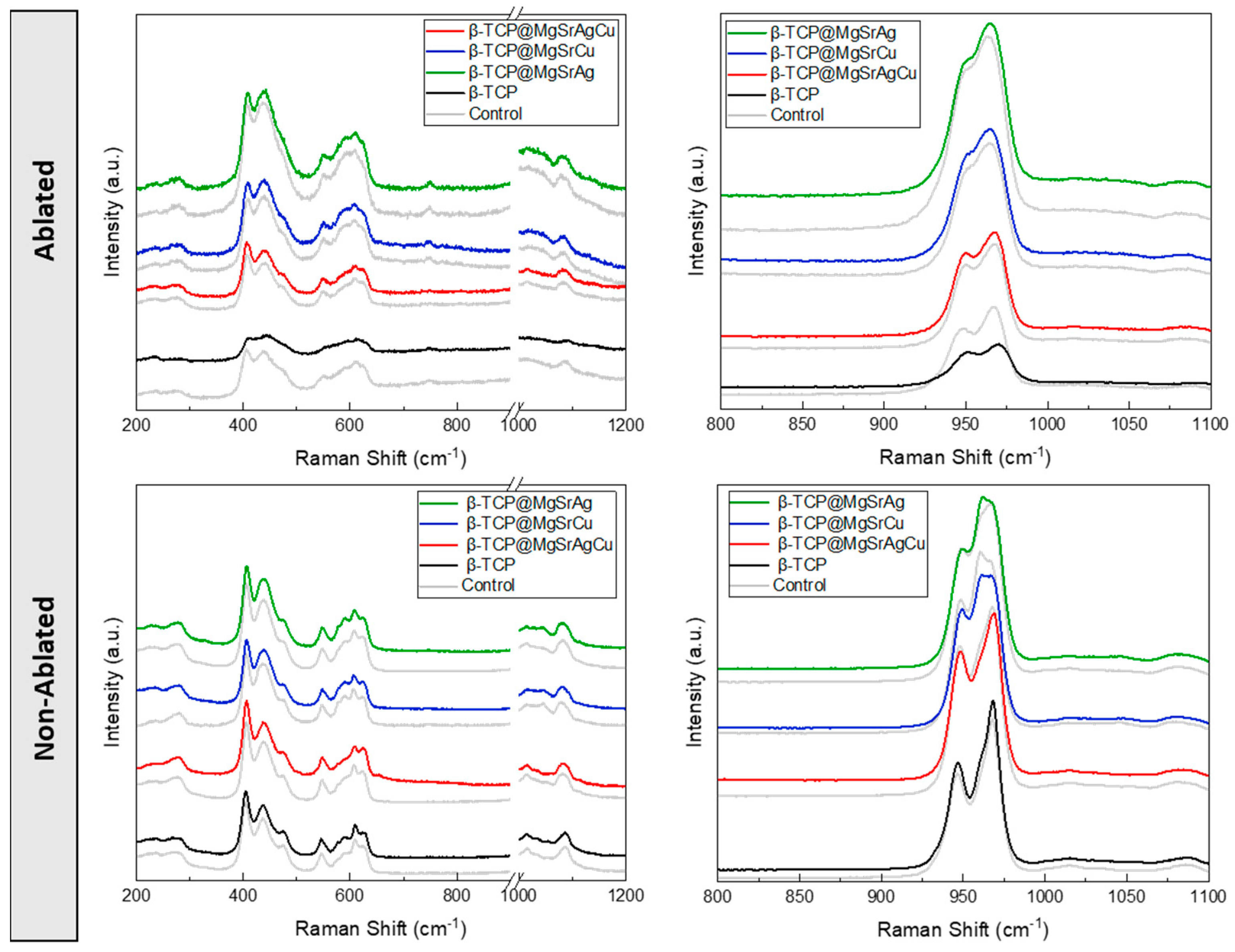
3.5. Raman Spectroscopy
3.6. In Vitro Bioactivity
3.7. Confocal Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36 (Suppl. S3), 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ross, N.; Tacconi, L.; Miles, J.B. Heterotopic Bone Formation Causing Recurrent Donor Site Pain Following Iliac Crest Bone Harvesting. Br. J. Neurosurg. 2000, 14, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Gusić, N.; Ivković, A.; VaFaye, J.; Vukasović, A.; Ivković, J.; Hudetz, D.; Janković, S. Nanobiotechnology and Bone Regeneration: A Mini-Review. Int. Orthop. 2014, 38, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Sola, A.; Cacciotti, I.; Bartoli, C.; Gazzarri, M.; Bianco, A.; Chiellini, F.; Cannillo, V. Mg and/or Sr-Doped Tricalcium Phosphate/Bioactive Glass Composites: Synthesis, Microstructure and Biological Responsiveness. Mater. Sci. Eng. C 2014, 42, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.M.C.; Vieira, S.I.; Cerqueira, A.R.; Pina, S.; Da Cruz Silva, O.A.B.; Abrantes, J.C.C.; Ferreira, J.M.F. Effects of Mn-Doping on the Structure and Biological Properties of β-Tricalcium Phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Vichery, C.; Descamps, S.; Martinez, H.; Kaur, A.; Jacobs, A.; Nedelec, J.M.; Renaudin, G. Cu-Doping of Calcium Phosphate Bioceramics: From Mechanism to the Control of Cytotoxicity. Acta Biomater. 2018, 65, 462–474. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Fadeeva, I.V.; Borovikova, E.Y.; Dzhevakov, P.B.; Slukin, P.V.; Zheng, Y.; Xia, D.; Lazoryak, B.I.; Rau, J.V. Antimicrobial Properties of Co-Doped Tricalcium Phosphates Ca3-2x(M′M′′)x(PO4)2 (M = Zn2+, Cu2+, Mn2+ and Sr2+). Ceram. Int. 2022, 48, 29770–29781. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C.; Li, H.; Yuen, J.; Chang, J.; Xiao, Y. Preparation, Characterization and in Vitro Angiogenic Capacity of Cobalt Substituted β-Tricalcium Phosphate Ceramics. J. Mater. Chem. 2012, 22, 21686–21694. [Google Scholar] [CrossRef]
- Dongxu, K.; Dernell, W.; Bandyopadhyay, A.; Bose, S. Doped Tricalcium Phosphate Scaffolds by Thermal Decomposition of Naphthalene: Mechanical Properties and In Vivo Osteogenesis in a Rabbit Femur Model. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 176, 139–148. [Google Scholar]
- Xue, W.; Dahlquist, K.; Banerjee, A.; Bandyopadhyay, A.; Bose, S. Synthesis and Characterization of Tricalcium Phosphate with Zn and Mg Based Dopants. J. Mater. Sci. Mater. Med. 2008, 19, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Obadia, L.; Deniard, P.; Alonso, B.; Rouillon, T.; Jobic, S.; Guicheux, J.; Julien, M.; Massiot, D.; Bujoli, B.; Bouler, J.M. Effect of Sodium Doping in β-Tricalcium Phosphate on Its Structure and Properties. Chem. Mater. 2006, 18, 1425–1433. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Zheng, Y.; Barbaro, K.; Lebedev, V.N.; Aksenov, S.M.; Borovikova, E.Y.; Gafurov, M.R.; Fadeeva, I.V.; Lazoryak, B.I.; Di Giacomo, G.; et al. Dependence of Antimicrobial Properties on Site-Selective Arrangement and Concentration of Bioactive Cu2+ Ions in Tricalcium Phosphate. Ceram. Int. 2023, 49, 21308–21323. [Google Scholar] [CrossRef]
- Hurle, K.; Maia, F.R.; Ribeiro, V.P.; Pina, S.; Oliveira, J.M.; Goetz-Neunhoeffer, F.; Reis, R.L. Osteogenic Lithium-Doped Brushite Cements for Bone Regeneration. Bioact. Mater. 2022, 16, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Vieira, S.; Rego, P.; Torres, P.; da Cruz e Silva, O.; da Cruz e Silva, E.; Ferreira, J. Biological Responses of Brushite-Forming Zn- and ZnSr- Substituted Beta-Tricalcium Phosphate Bone Cements. Eur. Cells Mater. 2010, 20, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Tarafder, S.; Vahabzadeh, S.; Bose, S. Effects of MgO, ZnO, SrO, and SiO2 in Tricalcium Phosphate Scaffolds on in Vitro Gene Expression and in Vivo Osteogenesis. Mater. Sci. Eng. C 2019, 96, 10–19. [Google Scholar] [CrossRef]
- Somers, N.; Jean, F.; Lasgorceix, M.; Preux, N.; Delmotte, C.; Boilet, L.; Petit, F.; Leriche, A. Fabrication of Doped β-Tricalcium Phosphate Bioceramics by Direct Ink Writing for Bone Repair Applications. J. Eur. Ceram. Soc. 2023, 43, 629–638. [Google Scholar] [CrossRef]
- Lu, X.; Leng, Y. Comparison of the Osteoblast and Myoblast Behavior on Hydroxyapatite Microgrooves. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2009, 90B, 438–445. [Google Scholar] [CrossRef]
- Sirdeshmukh, N.; Dongre, G. Laser Micro & Nano Surface Texturing for Enhancing Osseointegration and Antimicrobial Effect of Biomaterials: A Review. Mater. Today Proc. 2021, 44, 2348–2355. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium Phosphate: Synthesis, Properties and Biomedical Applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Lasgorceix, M.; Ott, C.; Boilet, L.; Hocquet, S.; Leriche, A.; Asadian, M.; De Geyter, N.; Declercq, H.; Lardot, V.; Cambier, F. Micropatterning of Beta Tricalcium Phosphate Bioceramic Surfaces, by Femtosecond Laser, for Bone Marrow Stem Cells Behavior Assessment. Mater. Sci. Eng. C 2019, 95, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast Laser Processing of Materials: A Review. Adv. Opt. Photonics 2015, 7, 684. [Google Scholar] [CrossRef]
- Aceti, D.M.; Filipov, E.; Angelova, L.; Sotelo, L.; Fontanot, T.; Yousefi, P.; Christiansen, S.; Leuchs, G.; Stanimirov, S.; Trifonov, A.; et al. Single-Step Process for Titanium Surface Micro-and Nano-Structuring and In Situ Silver Nanoparticles Formation by Ultra-Short Laser Patterning. Materials 2022, 15, 4670. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Angelova, L.; Filipov, E.; Aceti, D.; Mincheva, R.; Carrete, X.; Kerdjoudj, H.; Dubus, M.; Chevrier, J.; Trifonov, A.; et al. Biomimetic Hierarchical Structuring of Pla by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior. Polymers 2021, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Rihakova, L.; Chmelickova, H. Laser Micromachining of Glass, Silicon, and Ceramics. Adv. Mater. Sci. Eng. 2015, 2015, 584952. [Google Scholar] [CrossRef]
- Götz, H.E.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of Surface Finish on the Osseointegration of Laser-Treated Titanium Alloy Implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Sotelo, L.; Fontanot, T.; Vig, S.; Fernandes, M.H.; Sarau, G.; Leuchs, G.; Christiansen, S. Initial Surface Roughness Influence on the Generation of LIPSS on Titanium and Stainless Steel and Their Effect on Cell/Bacteria Viability. In Proceedings of the Volume 12409, Laser-based Micro- and Nanoprocessing XVII, San Francisco, CA, USA, 28 January–3 February 2023; p. 34. [Google Scholar] [CrossRef]
- Carvalho, A.; Cangueiro, L.; Oliveira, V.; Vilar, R.; Fernandes, M.H.; Monteiro, F.J. Femtosecond Laser Microstructured Alumina Toughened Zirconia: A New Strategy to Improve Osteogenic Differentiation of HMSCs. Appl. Surf. Sci. 2018, 435, 1237–1245. [Google Scholar] [CrossRef]
- Filipov, E.; Angelova, L.; Vig, S.; Fernandes, M.H.; Moreau, G.; Lasgorceix, M.; Buchvarov, I.; Daskalova, A. Investigating Potential Effects of Ultra-Short Laser-Textured Porous Poly-ε-Caprolactone Scaffolds on Bacterial Adhesion and Bone Cell Metabolism. Polymers 2022, 14, 2382. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Trifonov, A.; Lasgorceix, M.; Hocquet, S.; Minne, M.; Declercq, H.; Leriche, A.; Aceti, D.; Buchvarov, I. Development of Femtosecond Laser-engineered Β-tricalcium Phosphate (Β-tcp) Biomimetic Templates for Orthopaedic Tissue Engineering. Appl. Sci. 2021, 11, 2565. [Google Scholar] [CrossRef]
- Madhukumar, K.; Varma, H.K.; Komath, M.; Elias, T.S.; Padmanabhan, V.; Nair, C.M.K. Photoluminescence and Thermoluminescence Properties of Tricalcium Phosphate Phosphors Doped with Dysprosium and Europium. Bull. Mater. Sci. 2007, 30, 527–534. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR Crystallinity Indices in Sound Human Tooth Enamel and Synthetic Hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, J.; Poinsot, D.; Krespi, L.; Nénon, J.P.; Cortesero, A.M. Costs of Secondary Parasitism in the Facultative Hyperparasitoid Pachycrepoideus Dubius: Does Host Size Matter? Entomol. Exp. Appl. 2002, 103, 239–248. [Google Scholar] [CrossRef]
- Samanta, A.; Wang, Q.; Shaw, S.K.; Ding, H. Roles of Chemistry Modification for Laser Textured Metal Alloys to Achieve Extreme Surface Wetting Behaviors. Mater. Des. 2020, 192, 108744. [Google Scholar] [CrossRef]
- Kliuev, M.; Wiessner, M.; Büttner, H.; Maradia, U.; Wegener, K. Super-Hydrophobic and Super-Hydrophilic Effect by Means of EDM Surface Structuring of γ-TiAl. Procedia CIRP 2020, 95, 393–398. [Google Scholar] [CrossRef]
- Chaudhuri, B. Biopolymers-Graphene Oxide Nanoplatelets Composites with Enhanced Conductivity and Biocompatibility Suitable for Tissue Engineering Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128136911. [Google Scholar]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion Release from Hydroxyapatite and Substituted Hydroxyapatites in Different Immersion Liquids: In Vitro Experiments and Theoretical Modelling Study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Naseri, E.; Van Beek, A.; Ahmadi, A. Application of Electrowetting on Dielectric (EWOD) in Drug Release Control and Release-on-Demand. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 654, 130131. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface Characteristics Influencing Bacterial Adhesion to Polymeric Substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic Surfaces for the Reduction of Bacterial Adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Quillard, S.; Mellier, C.; Gildenhaar, R.; Hervelin, J.; Deniard, P.; Berger, G.; Bouler, J.M. Raman and Infrared Studies of Substituted β-TCP. Key Eng. Mater. 2012, 493–494, 225–230. [Google Scholar] [CrossRef]
- Cuscó, R.; Guitián, F.; De Aza, A.; Artús, L. Differentiation between Hydroxyapatite and β-Tricalcium Phosphate by Means of μ-Raman Spectroscopy. J. Eur. Ceram. Soc. 1998, 18, 1301–1305. [Google Scholar] [CrossRef]
- Mayer, S.T.; Muller, R.H. An In Situ Raman Spectroscopy Study of the Anodic Oxidation of Copper in Alkaline Media. J. Electrochem. Soc. 1992, 139, 426–434. [Google Scholar] [CrossRef]
- Bakan, F. A Systematic Study of the Effect of PH on the Initialization of Ca-Deficient Hydroxyapatite to β- TCP Nanoparticles. Materials 2019, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Zwahr, C.; Helbig, R.; Werner, C.; Lasagni, A.F. Fabrication of Multifunctional Titanium Surfaces by Producing Hierarchical Surface Patterns Using Laser Based Ablation Methods. Sci. Rep. 2019, 9, 6721. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating Superhydrophobic and Antibacterial Surfaces on Gold by Femtosecond Laser Pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef]
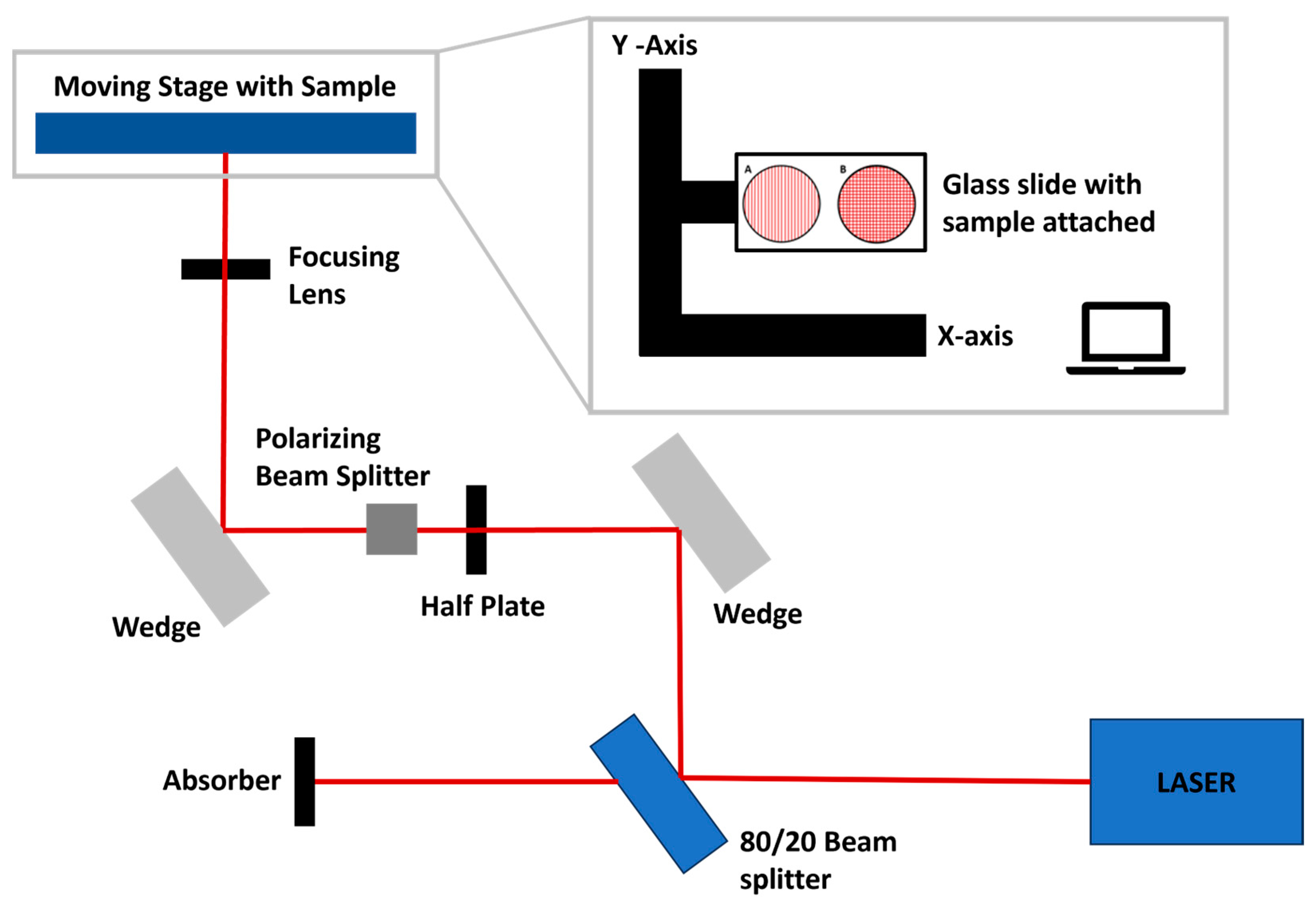



| Designation | V (mm/s) | F (J/cm2) | H (μm) | Pattern |
|---|---|---|---|---|
| Parameter A | 0.75 | 8.15 | 50 | Lines |
| Parameter B | 1.5 | 6.11 | 50 | Grid |
| Parameter C | 1.5 | 6.11 | 100 | Grid |
| Sample | Sr (%) | Mg (%) | Cu (%) | Ag (%) | Ca/P |
|---|---|---|---|---|---|
| Theoretical value | 2 | 2 | 0.1 | 0.1 | 1.5 |
| Non-Doped | - | - | - | - | 1.60 |
| β-TCP@MgSrAgCu | 1.95 (97.5) | 1.85 (92.5) | 0.14 (140) | - | 1.50 |
| β-TCP@MgSrCu | 1.61 (80.5) | 0.65 (32.5) | 0.17 (170) | - | 1.19 |
| β-TCP@MgSrAg | 1.91 (95.5) | 1.00 (50.0) | - | - | 1.56 |
| Solvent Contact Angle (°) | SFE (mJ/m2) | |||||
|---|---|---|---|---|---|---|
| Sample | MI | MEG | H2O | Total | Dis | Pol |
| β-TCP@MgSrAgCu | 51.5 ± 1.00 | 51.2 ± 8.25 | 92.8 ± 11.4 | 34.31 | 33.43 | 0.080 |
| β-TCP@MgSrCu | 60.3 ± 3.19 | 85.7 ± 2.00 | 70.6 ± 5.02 | 27.8 | 27.12 | 0.65 |
| β-TCP@MgSrAg | 54.1 ± 6.04 | 65.6 ± 14.6 | 69.2 ± 0.18 | 30.86 | 30.78 | 0.08 |
| β-TCP | 52.1 ± 0.82 | 61.8 ± 2.33 | 82.1 ± 10.1 | 73.45 | 16.8 | 56.64 |
| Parameter | Sample | SA (mm2) | SSA (m−1) |
|---|---|---|---|
| A | β-TCP | 0.46 ± 0.01 | 1.51 |
| β-TCP@MgSrAg | 0.56 ± 0.03 | 1.85 | |
| β-TCP@MgSrCu | |||
| β-TCP@MgSrAgCu | 0.55 ± 0.00 | 1.80 | |
| B | β-TCP | 0.55 ± 0.01 | 1.80 |
| β-TCP@MgSrAg | 0.47 ± 0.01 | 1.54 | |
| β-TCP@MgSrCu | 0.59 ± 0.00 | 1.95 | |
| β-TCP@MgSrAgCu | 0.65 ± 0.00 | 2.13 | |
| C | β-TCP | 0.48 ± 0.01 | 1.57 |
| β-TCP@MgSrAg | 0.48 ± 0.01 | 1.59 | |
| β-TCP@MgSrCu | 0.47 ± 0.00 | 1.54 | |
| β-TCP@MgSrAgCu | 0.45 ± 0.00 | 1.48 |
| TSa ± TSq (μm) | GSa ± GSq (μm) | CSa ± cSq (μm) | |||||
|---|---|---|---|---|---|---|---|
| Sample | A | B | C | A | B | C | NA |
| β-TCP | 5.7 ± 7.0 | 8.4 ± 11 | 16 ± 19 | 1.7 ± 2.1 | 2.9 ± 3.4 | 1.3 ± 1.9 | 1.8 ± 2.4 |
| β-TCP@MgSrAg | 16 ± 19 | 6.8 ± 8.6 | 10 ± 12 | 2.7 ± 3.3 | 0.8 ± 1.1 | 0.99 ± 1.3 | 1.1 ± 1.6 |
| β-TCP@MgSrCu | 15 ± 18 | 14 ± 17 | 1.6 ± 1.8 | 0.17 ± 0.24 | 0.5 ± 1.1 | ||
| β-TCP@MgSrAgCu | 7.2 ± 8.4 | 1.0 ± 1.2 | 0.13 ± 0.18 | 0.47 ± 6.0 | 16 ± 19 | 8.5 ± 9.9 | 0.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, Í.; Sotelo, L.; Erceg, I.; Jean, F.; Lasgorceix, M.; Leriche, A.; Sikirić, M.D.; Marušić, K.; Christiansen, S.; Daskalova, A. Improvement of Metal-Doped β-TCP Scaffolds for Active Bone Substitutes via Ultra-Short Laser Structuring. Bioengineering 2023, 10, 1392. https://doi.org/10.3390/bioengineering10121392
Soares Í, Sotelo L, Erceg I, Jean F, Lasgorceix M, Leriche A, Sikirić MD, Marušić K, Christiansen S, Daskalova A. Improvement of Metal-Doped β-TCP Scaffolds for Active Bone Substitutes via Ultra-Short Laser Structuring. Bioengineering. 2023; 10(12):1392. https://doi.org/10.3390/bioengineering10121392
Chicago/Turabian StyleSoares, Íris, Lamborghini Sotelo, Ina Erceg, Florian Jean, Marie Lasgorceix, Anne Leriche, Maja Dutour Sikirić, Katarina Marušić, Silke Christiansen, and Albena Daskalova. 2023. "Improvement of Metal-Doped β-TCP Scaffolds for Active Bone Substitutes via Ultra-Short Laser Structuring" Bioengineering 10, no. 12: 1392. https://doi.org/10.3390/bioengineering10121392
APA StyleSoares, Í., Sotelo, L., Erceg, I., Jean, F., Lasgorceix, M., Leriche, A., Sikirić, M. D., Marušić, K., Christiansen, S., & Daskalova, A. (2023). Improvement of Metal-Doped β-TCP Scaffolds for Active Bone Substitutes via Ultra-Short Laser Structuring. Bioengineering, 10(12), 1392. https://doi.org/10.3390/bioengineering10121392







