Development of Novel Polysaccharide Membranes for Guided Bone Regeneration: In Vitro and In Vivo Evaluations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Cytotoxicity Assays
2.3. In Vivo Experiments
2.3.1. Biocompatibility Assessment
- -
- Surgical procedure:
- -
- Histological analysis:
2.3.2. Femoral Defect Implantation
- -
- Surgical procedure:
- -
- Radiographic analysis:
- -
- Histological analysis:
2.4. Statistical Analysis
3. Results
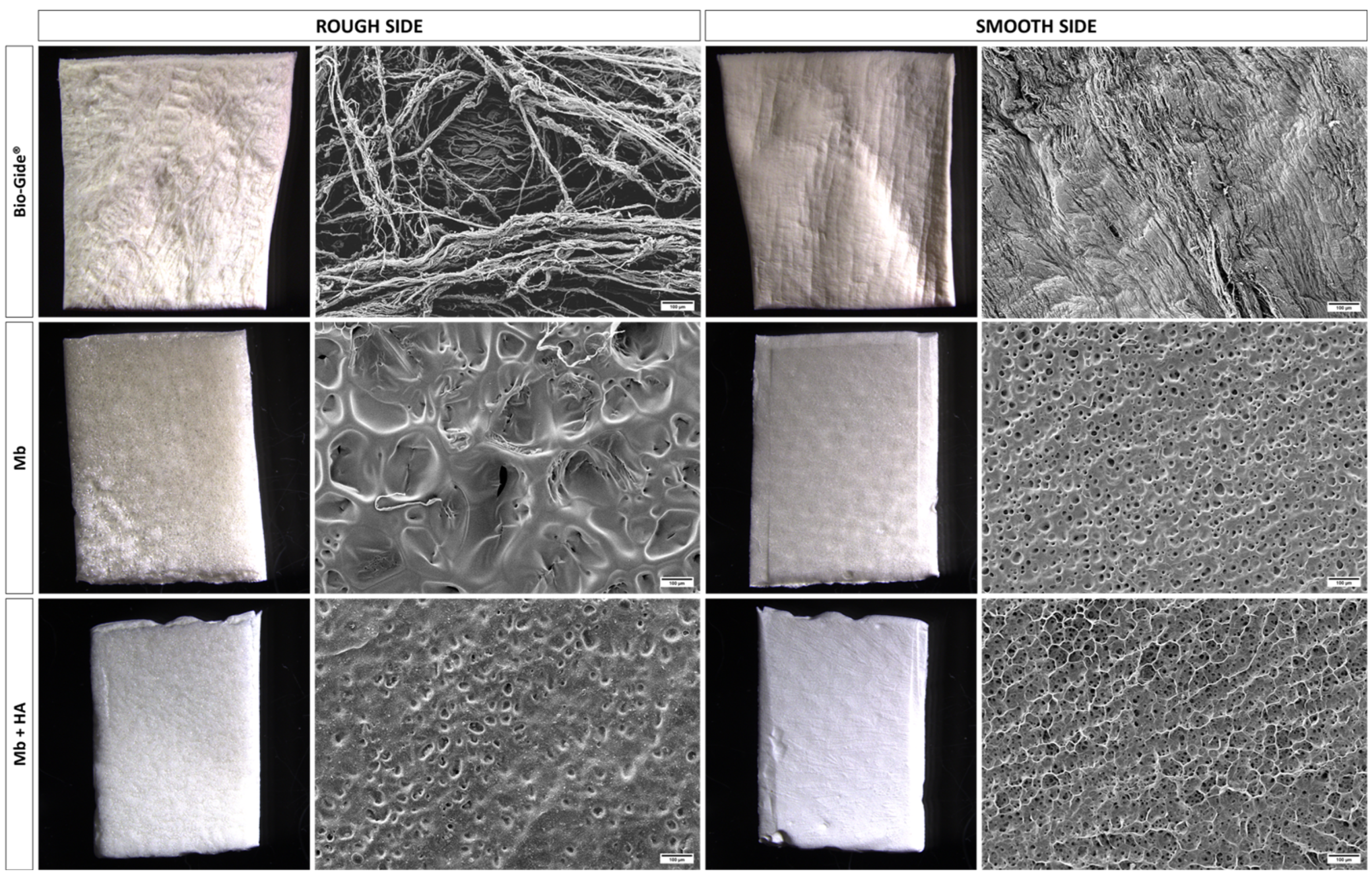
3.1. Materials
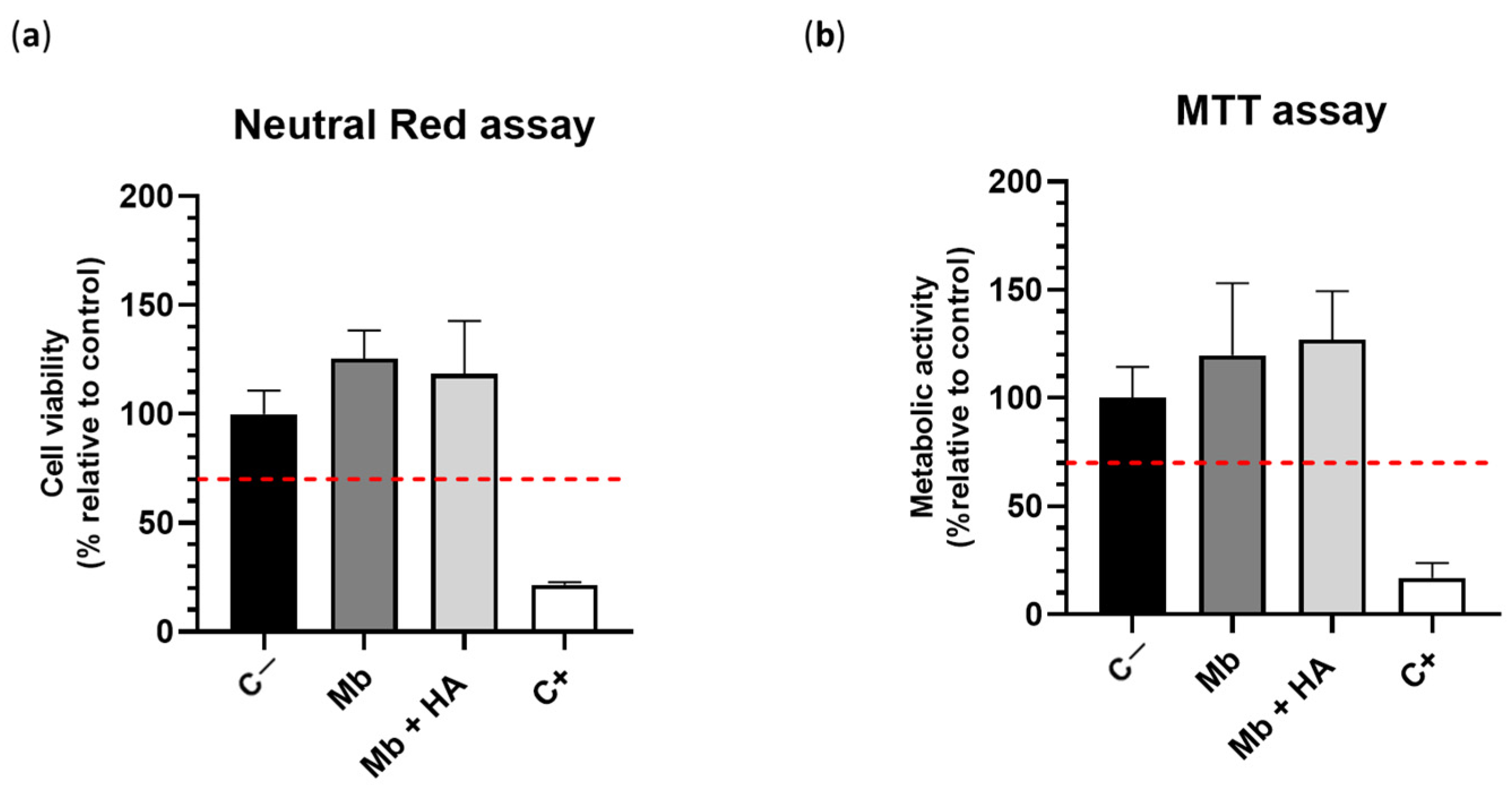
3.2. In Vitro Cytocompatibility
3.3. In Vivo Experiments
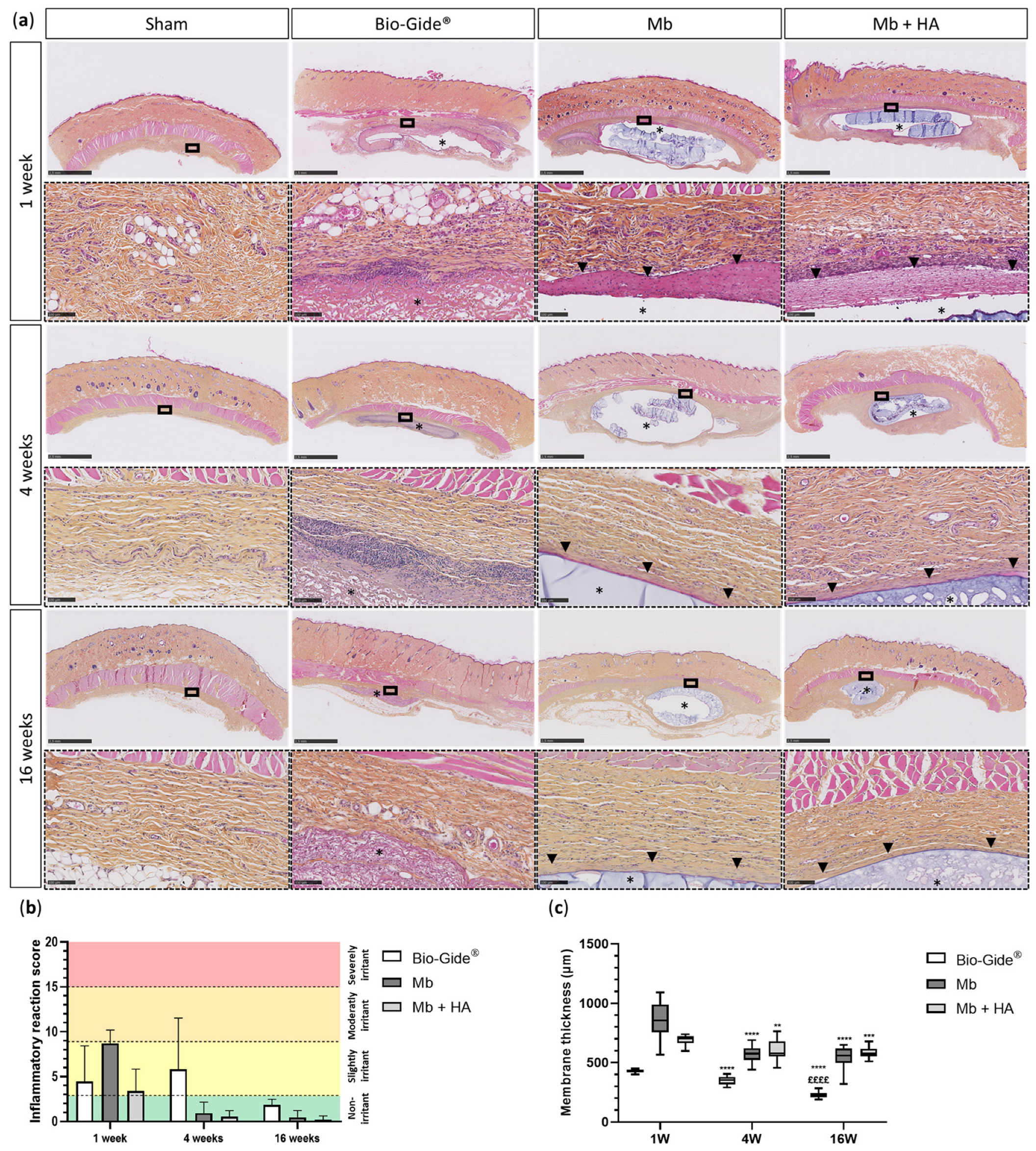
3.3.1. Biocompatibility
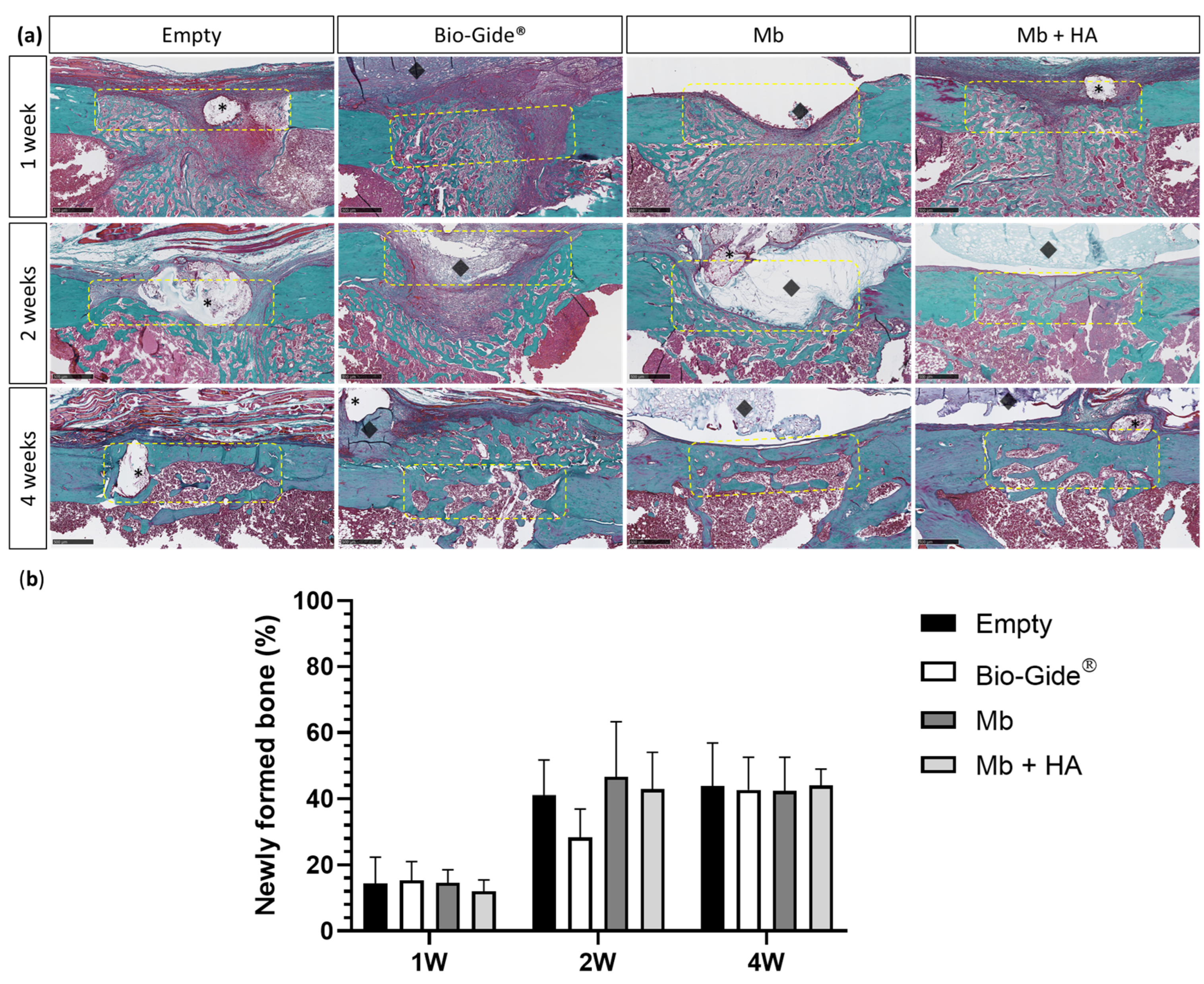
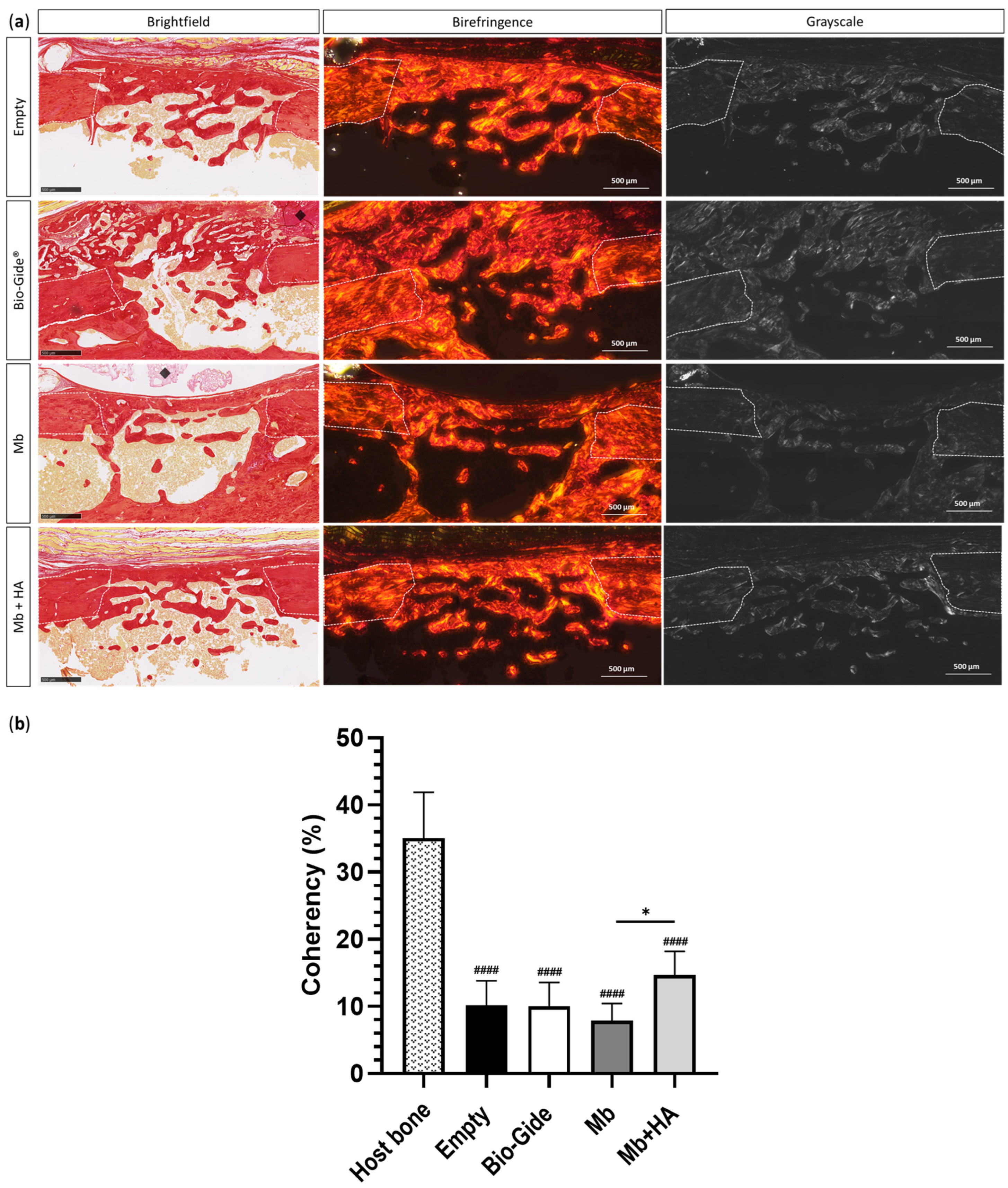
3.3.2. Bone Regeneration of Femoral Defects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Health Mater. 2020, 9, e2000707. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef]
- Solomon, S.-M.; Sufaru, I.-G.; Teslaru, S.; Ghiciuc, C.M.; Stafie, C.S. Finding the Perfect Membrane: Current Knowledge on Barrier Membranes in Regenerative Procedures: A Descriptive Review. Appl. Sci. 2022, 12, 1042. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Pellegrini, G.; Pagni, G.; Rasperini, G. Surgical Approaches Based on Biological Objectives: GTR versus GBR Techniques. Int. J. Dent. 2013, 2013, e521547. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Guillén-Carvajal, K.; Valdez-Salas, B.; Beltrán-Partida, E.; Salomón-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, X.; Chen, Y.; Yue, O.; Bai, Z.; Cui, B.; Jiang, H.; Liu, X. A Review of Recent Progress on Collagen-Based Biomaterials. Adv. Healthc. Mater. 2023, 12, 2202042. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Anderson, J.A. Refusal of animal-derived medical products in a paediatric setting: Ethical issues. Paediatr. Child Health 2020, 26, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef]
- Carvalho, L.T.; Vieira, T.A.; Zhao, Y.; Celli, A.; Medeiros, S.F.; Lacerda, T.M. Recent advances in the production of biomedical systems based on polyhydroxyalkanoates and exopolysaccharides. Int. J. Biol. Macromol. 2021, 183, 1514–1539. [Google Scholar] [CrossRef]
- Hussain, A.; Zia, K.M.; Tabasum, S.; Noreen, A.; Ali, M.; Iqbal, R.; Zuber, M. Blends and composites of exopolysaccharides; properties and applications: A review. Int. J. Biol. Macromol. 2017, 94, 10–27. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, J.F. Pullulan in pharmaceutical and cosmeceutical formulations: A review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef]
- Hayashi, C.; Hasegawa, U.; Saita, Y.; Hemmi, H.; Hayata, T.; Nakashima, K.; Ezura, Y.; Amagasa, T.; Akiyoshi, K.; Noda, M. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J. Cell Physiol. 2009, 220, 1–7. [Google Scholar] [CrossRef]
- Omar, N.A.; Amédée, J.; Letourneur, D.; Fricain, J.-C.; Fenelon, M. Recent Advances of Pullulan and/or Dextran-Based Materials for Bone Tissue Engineering Strategies in Preclinical Studies: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 889481. [Google Scholar] [CrossRef]
- Schlaubitz, S.; Derkaoui, S.M.; Marosa, L.; Miraux, S.; Renard, M.; Catros, S.; Le Visage, C.; Letourneur, D.; Amédée, J.; Fricain, J.-C. Pullulan/dextran/nHA Macroporous Composite Beads for Bone Repair in a Femoral Condyle Defect in Rats. PLoS ONE 2014, 9, e110251. [Google Scholar] [CrossRef]
- Fricain, J.; Aid, R.; Lanouar, S.; Maurel, D.; Le Nihouannen, D.; Delmond, S.; Letourneur, D.; Vilamitjana, J.A.; Catros, S. In-vitro and in-vivo design and validation of an injectable polysaccharide-hydroxyapatite composite material for sinus floor augmentation. Dent. Mater. 2018, 34, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Maurel, D.B.; Fénelon, M.; Aid-Launais, R.; Bidault, L.; Le Nir, A.; Renard, M.; Fricain, J.; Letourneur, D.; Amédée, J.; Catros, S. Bone regeneration in both small and large preclinical bone defect models using an injectable polymer-based substitute containing hydroxyapatite and reconstituted with saline or autologous blood. J. Biomed. Mater. Res. Part A 2021, 109, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Schlaubitz, S.; Le Visage, C.; Arnault, I.; Derkaoui, S.M.; Siadous, R.; Catros, S.; Lalande, C.; Bareille, R.; Renard, M.; et al. A nano-hydroxyapatite—Pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013, 34, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
- Ribot, E.J.; Tournier, C.; Aid-Launais, R.; Koonjoo, N.; Oliveira, H.; Trotier, A.J.; Rey, S.; Wecker, D.; Letourneur, D.; Vilamitjana, J.A.; et al. 3D anatomical and perfusion MRI for longitudinal evaluation of biomaterials for bone regeneration of femoral bone defect in rats. Sci. Rep. 2017, 7, 6100. [Google Scholar] [CrossRef]
- Miyahara, T.; Nyan, M.; Shimoda, A.; Yamamoto, Y.; Kuroda, S.; Shiota, M.; Akiyoshi, K.; Kasugai, S. Exploitation of a novel polysaccharide nanogel cross-linking membrane for guided bone regeneration (GBR). J. Tissue Eng. Regen. Med. 2011, 6, 666–672. [Google Scholar] [CrossRef]
- ISO 10993-5:2009. Available online: https://www.iso.org/fr/standard/36406.html (accessed on 19 October 2023).
- Vilamitjana-Amedee, J.; Bareille, R.; Rouais, F.; Caplan, A.I.; Harmand, M.-F. Human bone marrow stromal cells express an osteoblastic phenotype in culture. Vitr. Cell Dev. Biol.-Anim. 1993, 29, 699–707. [Google Scholar] [CrossRef]
- ISO 10993-12:2021. Available online: https://www.iso.org/fr/standard/75769.html (accessed on 19 October 2023).
- ISO 10993-6:2016. Available online: https://www.iso.org/fr/standard/61089.html (accessed on 19 October 2023).
- Fenelon, M.; Maurel, D.B.; Siadous, R.; Gremare, A.; Delmond, S.; Durand, M.; Brun, S.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater. Sci. Eng. C 2019, 104, 109903. [Google Scholar] [CrossRef]
- Fenelon, M.; Etchebarne, M.; Siadous, R.; Grémare, A.; Durand, M.; Sentilhes, L.; Torres, Y.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Assessment of fresh and preserved amniotic membrane for guided bone regeneration in mice. J. Biomed. Mater. Res. Part A 2020, 108, 2044–2056. [Google Scholar] [CrossRef]
- Fonck, E.; Feigl, G.G.; Fasel, J.; Sage, D.; Unser, M.; Rüfenacht, D.A.; Stergiopulos, N. Effect of Aging on Elastin Functionality in Human Cerebral Arteries. Stroke 2009, 40, 2552–2556. [Google Scholar] [CrossRef]
- Autissier, A.; Le Visage, C.; Pouzet, C.; Chaubet, F.; Letourneur, D. Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomater. 2010, 6, 3640–3648. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Duval, H.; Lv, P.; Barou, F.; Le Guilcher, C.; Aid, R.; David, B.; Letourneur, D. Interplay between crosslinking and ice nucleation controls the porous structure of freeze-dried hydrogel scaffolds. Biomater. Adv. 2022, 139, 212973. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; David, B.; Journé, C.; Cicha, I.; Letourneur, D.; Duval, H. Perfusion of MC3T3E1 Preosteoblast Spheroids within Polysaccharide-Based Hydrogel Scaffolds: An Experimental and Numerical Study at the Bioreactor Scale. Bioengineering 2023, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Labour, M.-N.; Le Guilcher, C.; Aid-Launais, R.; El Samad, N.; Lanouar, S.; Simon-Yarza, T.; Letourneur, D. Development of 3D Hepatic Constructs within Polysaccharide-Based Scaffolds with Tunable Properties. Int. J. Mol. Sci. 2020, 21, 3644. [Google Scholar] [CrossRef] [PubMed]
- Gerschenfeld, G.; Aid, R.; Simon-Yarza, T.; Lanouar, S.; Charnay, P.; Letourneur, D.; Topilko, P. Tuning Physicochemical Properties of a Macroporous Polysaccharide-Based Scaffold for 3D Neuronal Culture. Int. J. Mol. Sci. 2021, 22, 12726. [Google Scholar] [CrossRef]
- Le Guilcher, C.; Merlen, G.; Dellaquila, A.; Labour, M.-N.; Aid, R.; Tordjmann, T.; Letourneur, D.; Simon-Yarza, T. Engineered human liver based on pullulan-dextran hydrogel promotes mice survival after liver failure. Mater. Today Bio 2023, 19, 100554. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Wang, H.-L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef]
- Abed, A.; Assoul, N.; Ba, M.; Derkaoui, S.M.; Portes, P.; Louedec, L.; Flaud, P.; Bataille, I.; Letourneur, D.; Meddahi-Pellé, A. Influence of polysaccharide composition on the biocompatibility of pullulan/dextran-based hydrogels. J. Biomed. Mater. Res. Part A 2011, 96A, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zreiqat, H. Tissue Response to Biomaterials. In Encyclopedia of Biomedical Engineering, 1st ed.; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-804829-0. [Google Scholar]
- Neto, A.M.D.; Sartoretto, S.C.; Duarte, I.M.; Resende, R.F.d.B.; Alves, A.T.N.N.; Mourão, C.F.d.A.B.; Calasans-Maia, J.; Montemezzi, P.; Tristão, G.C.; Calasans-Maia, M.D. In Vivo Comparative Evaluation of Biocompatibility and Biodegradation of Bovine and Porcine Collagen Membranes. Membranes 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Lindner, C.; Alkildani, S.; Stojanovic, S.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR). Membranes 2022, 12, 378. [Google Scholar] [CrossRef]
- Zhu, M.; Duan, B.; Hou, K.; Mao, L.; Wang, X. A comparative in vitro and in vivo study of porcine- and bovine-derived non-cross-linked collagen membranes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 111, 568–578. [Google Scholar] [CrossRef]
- Piattelli, A.; Franco, M.; Ferronato, G.; Santello, M.; Martinetti, R.; Scarano, A. Resorption of composite polymer—Hydroxyapatite membranes: A time-course study in rabbit. Biomaterials 1997, 18, 629–633. [Google Scholar] [CrossRef]
- Huang, D.; Niu, L.; Li, J.; Du, J.; Wei, Y.; Hu, Y.; Lian, X.; Chen, W.; Wang, K. Reinforced chitosan membranes by microspheres for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2018, 81, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P. The Role of Collagen Organization on the Properties of Bone. Calcif. Tissue Int. 2015, 97, 229–240. [Google Scholar] [CrossRef]
- Liu, J.; Xu, M.; Wu, J.; Zhang, H.; Yang, L.; Lun, D.; Hu, Y.; Liu, B. Picrosirius-Polarization Method for Collagen Fiber Detection in Tendons: A Mini-Review. Orthop. Surg. 2021, 13, 701–707. [Google Scholar] [CrossRef]
- Rittié, L. (Ed.) Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. In Fibrosis: Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; pp. 395–407. ISBN 978-1-4939-7113-8. [Google Scholar]
- Monfoulet, L.; Malaval, L.; Aubin, J.E.; Rittling, S.R.; Gadeau, A.P.; Fricain, J.-C.; Chassande, O. Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during cortical defect healing. Bone 2010, 46, 447–452. [Google Scholar] [CrossRef]
- Hulsart-Billström, G.; Bergman, K.; Andersson, B.; Hilborn, J.; Larsson, S.; Jonsson, K.B. A uni-cortical femoral defect model in the rat: Evaluation using injectable hyaluronan hydrogel as a carrier for bone morphogenetic protein-2. J. Tissue Eng. Regen. Med. 2012, 9, 799–807. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Kormas, I.; Pedercini, A.; Alassy, H.; Wolff, L.F. The Use of Biocompatible Membranes in Oral Surgery: The Past, Present & Future Directions. A Narrative Review. Membranes 2022, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implant. Res. 2006, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 82–91. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed Omar, N.; Roque, J.; Galvez, P.; Siadous, R.; Chassande, O.; Catros, S.; Amédée, J.; Roques, S.; Durand, M.; Bergeaut, C.; et al. Development of Novel Polysaccharide Membranes for Guided Bone Regeneration: In Vitro and In Vivo Evaluations. Bioengineering 2023, 10, 1257. https://doi.org/10.3390/bioengineering10111257
Ahmed Omar N, Roque J, Galvez P, Siadous R, Chassande O, Catros S, Amédée J, Roques S, Durand M, Bergeaut C, et al. Development of Novel Polysaccharide Membranes for Guided Bone Regeneration: In Vitro and In Vivo Evaluations. Bioengineering. 2023; 10(11):1257. https://doi.org/10.3390/bioengineering10111257
Chicago/Turabian StyleAhmed Omar, Naïma, Jéssica Roque, Paul Galvez, Robin Siadous, Olivier Chassande, Sylvain Catros, Joëlle Amédée, Samantha Roques, Marlène Durand, Céline Bergeaut, and et al. 2023. "Development of Novel Polysaccharide Membranes for Guided Bone Regeneration: In Vitro and In Vivo Evaluations" Bioengineering 10, no. 11: 1257. https://doi.org/10.3390/bioengineering10111257
APA StyleAhmed Omar, N., Roque, J., Galvez, P., Siadous, R., Chassande, O., Catros, S., Amédée, J., Roques, S., Durand, M., Bergeaut, C., Bidault, L., Aprile, P., Letourneur, D., Fricain, J.-C., & Fenelon, M. (2023). Development of Novel Polysaccharide Membranes for Guided Bone Regeneration: In Vitro and In Vivo Evaluations. Bioengineering, 10(11), 1257. https://doi.org/10.3390/bioengineering10111257






