Evaluating the Effectiveness of 2D and 3D CT Image Features for Predicting Tumor Response to Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
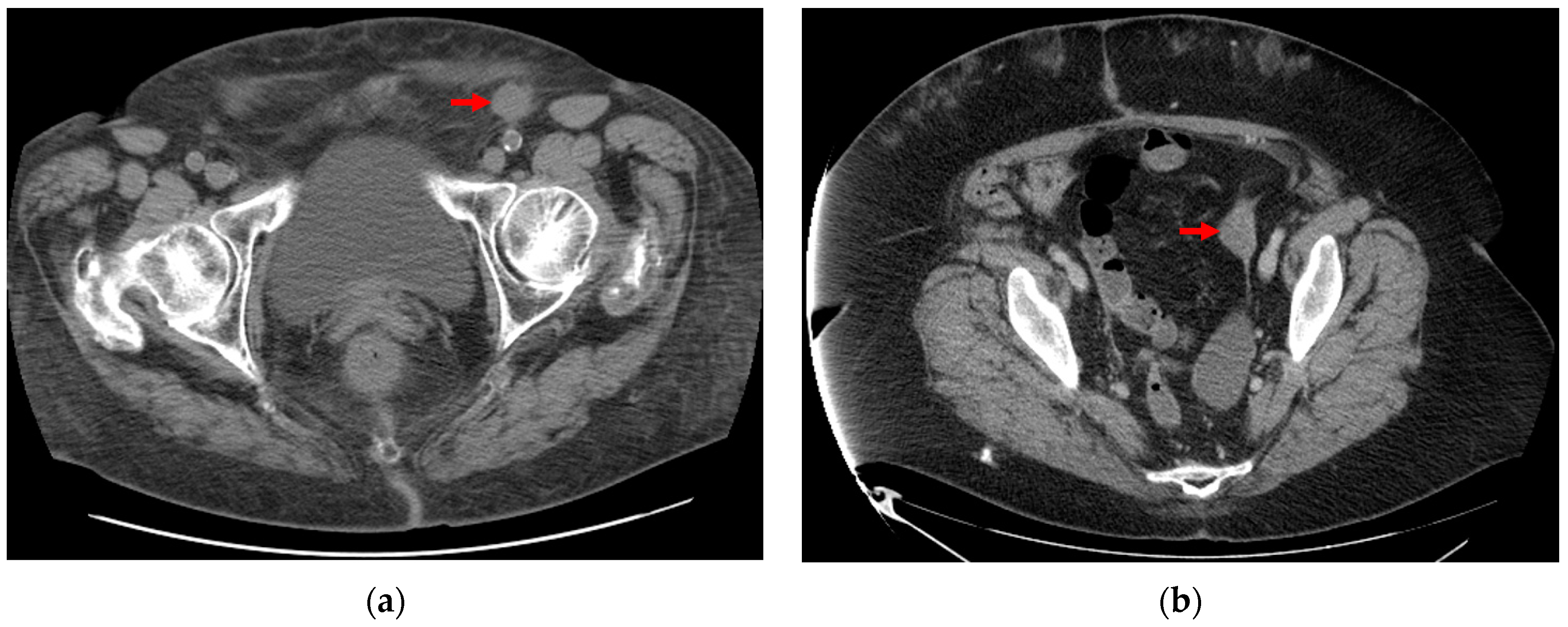
2.1. Database
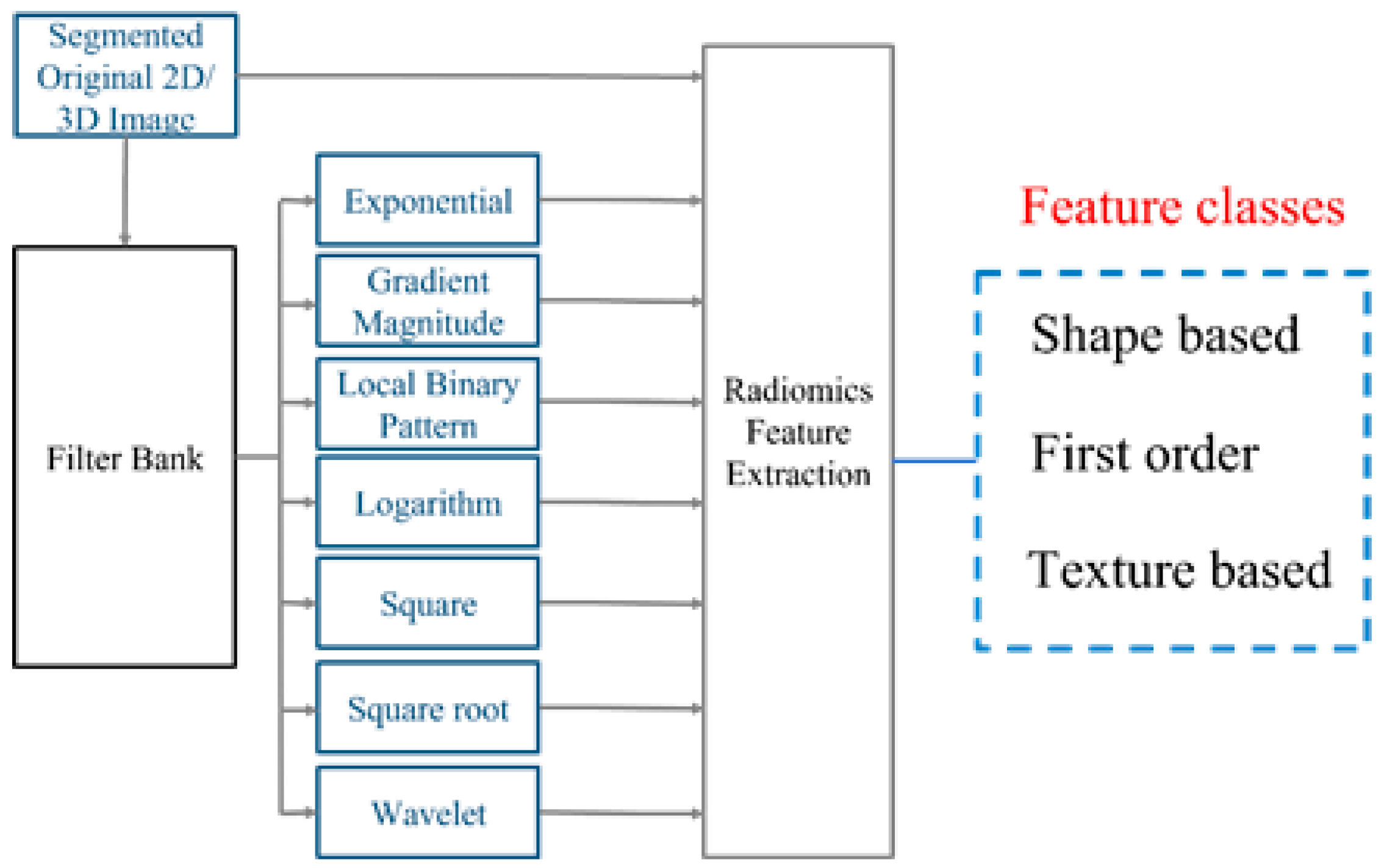
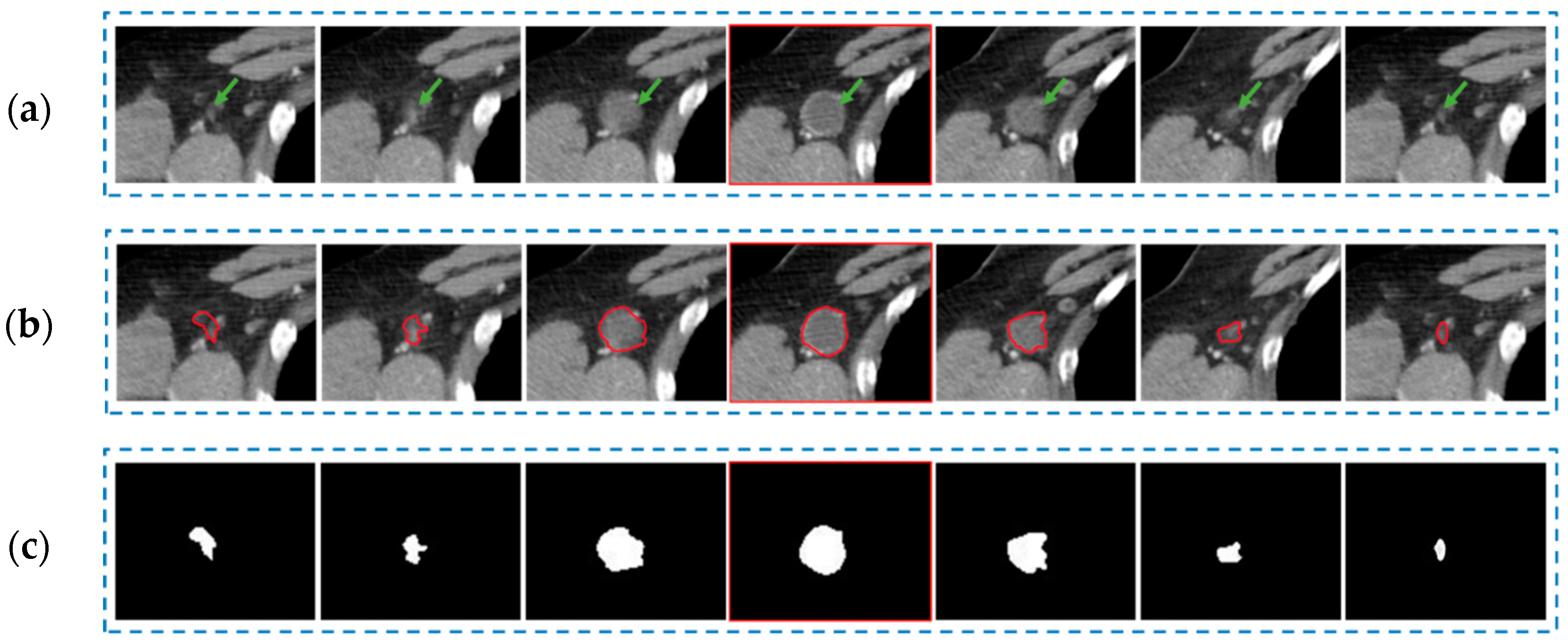
2.2. Radiomics Feature Extraction
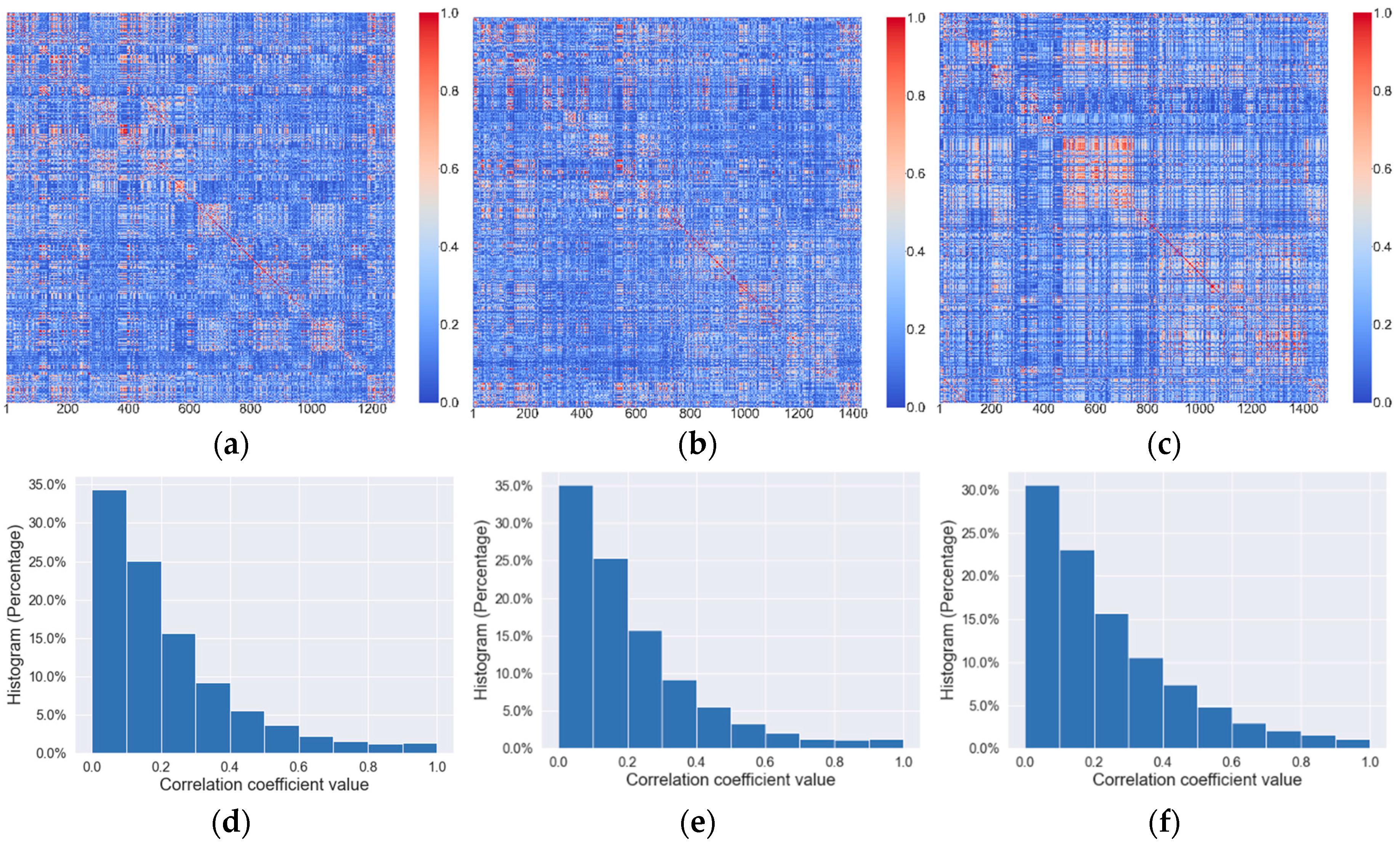
2.3. Develop Machine Learning-Based Models to Predict Tumor Response to Chemotherapy
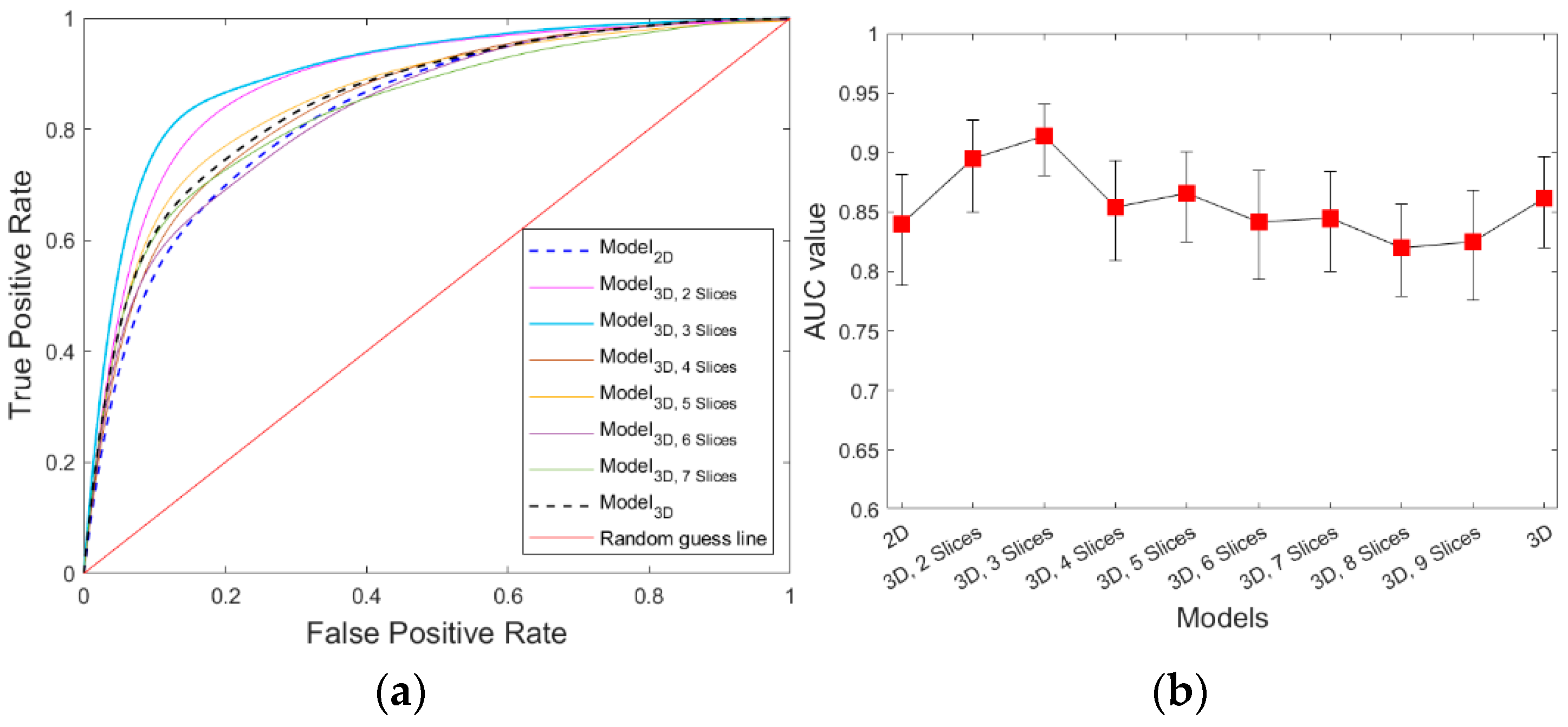
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Hamed, S.; Mansour, S.M.; Mounir, Y.; Sallam, S.A. Ovarian cancer screening—Ultrasound; impact on ovarian cancer mortality. Br. J. Radiol. 2018, 91, 20170571. (In English) [Google Scholar] [CrossRef]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. (In English) [Google Scholar] [CrossRef] [PubMed]
- Rein, B.J.D.; Gupta, S.; Dada, R.; Safi, J.; Michener, C.; Agarwal, A. Potential Markers for Detection and Monitoring of Ovarian Cancer. J. Oncol. 2011, 2011, 475983. [Google Scholar] [CrossRef] [PubMed]
- MAhAdevAPPA, A.; Krishna, S.M.; Vimala, M.G. Diagnostic and prognostic significance of Ki-67 immunohistochemical expression in surface epithelial ovarian carcinoma. J. Clin. Diagn. Res. 2017, 11, EC08. [Google Scholar] [CrossRef]
- Zorn, K.K.; Tian, C.; McGuire, W.P.; Hoskins, W.J.; Markman, M.; Muggia, F.M.; Rose, P.G.; Ozols, R.F.; Spriggs, D.; Armstrong, D.K. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma. Cancer 2009, 115, 1028–1035. [Google Scholar] [CrossRef]
- Sölétormos, G.; Duffy, M.J.; Abu Hassan, S.O.; Verheijen, R.H.; Tholander, B.; Bast, R.C.; Gaarenstroom, K.N.; Sturgeon, C.M.; Bonfrer, J.M.; Petersen, P.H.; et al. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines from the European Group on Tumor Markers. Int. J. Gynecol. Cancer 2016, 26, 43–51. [Google Scholar] [CrossRef]
- Park, B.W.; Kim, J.K.; Heo, C.; Park, K.J. Reliability of CT radiomic features reflecting tumour heterogeneity according to image quality and image processing parameters. Sci. Rep. 2020, 10, 3852. (In English) [Google Scholar] [CrossRef]
- Sharma, M.R.; Maitland, M.L.; Ratain, M.J. RECIST: No Longer the Sharpest Tool in the Oncology Clinical Trials Toolbox—Point. Cancer Res. 2012, 72, 5145–5149; discussion 5150. (In English) [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691.e13. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liu, Z.; Guan, M.; Song, J.; Lian, Y.; Wang, S.; Tang, Z.; Dong, D.; Kong, L.; Wang, M.; et al. 2D and 3D CT Radiomics Features Prognostic Performance Comparison in Non-Small Cell Lung Cancer. Transl. Oncol. 2017, 10, 886–894. [Google Scholar] [CrossRef]
- Meng, L.; Dong, D.; Chen, X.; Fang, M.; Wang, R.; Li, J.; Liu, Z.; Tian, J. 2D and 3D CT Radiomic Features Performance Comparison in Characterization of Gastric Cancer: A Multi-Center Study. IEEE J. Biomed. Health Inform. 2020, 25, 755–763. [Google Scholar] [CrossRef]
- Xu, L.; Yang, P.; Yen, E.A.; Wan, Y.; Jiang, Y.; Cao, Z.; Shen, X.; Wu, Y.; Wang, J.; Luo, C.; et al. A multi-organ cancer study of the classification performance using 2D and 3D image features in radiomics analysis. Phys. Med. Biol. 2019, 64, 215009. (In English) [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.Y.; Park, H.; Schiebler, M.L.; van Beek, E.J.; Ohno, Y.; Seo, J.B.; Leung, A. Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur. J. Radiol. 2017, 86, 297–307. [Google Scholar] [CrossRef]
- Fallowfield, L.J.; Fleissig, A. The value of progression-free survival to patients with advanced-stage cancer. Nat. Rev. Clin. Oncol. 2011, 9, 41–47. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Roy, P.; Dutta, S.; Dey, N.; Dey, G.; Chakraborty, S.; Ray, R. Adaptive thresholding: A comparative study. In Proceedings of the 2014 International Conference on Control, Instrumentation, Communication and Computational Technologies, Kanyakumari, India, 10–11 July 2014; pp. 1182–1186. [Google Scholar] [CrossRef]
- Caselles, V.; Kimmel, R.; Sapiro, G. Geodesic active contours. Int. J. Comput. Vis. 1997, 22, 61–79. [Google Scholar] [CrossRef]
- Qiu, Y.; Tan, M.; McMeekin, S.; Thai, T.; Ding, K.; Moore, K.; Liu, H.; Zheng, B. Early prediction of clinical benefit of treating ovarian cancer using quantitative CT image feature analysis. Acta Radiol. 2016, 57, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Danala, G.; Thai, T.; Gunderson, C.C.; Moxley, K.M.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Applying Quantitative CT Image Feature Analysis to Predict Response of Ovarian Cancer Patients to Chemotherapy. Acad. Radiol. 2017, 24, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Danala, G.; Wang, Y.; Thai, T.; Gunderson, C.C.; Moxley, K.M.; Moore, K.; Mannel, R.S.; Cheng, S.; Liu, H.; Zheng, B.; et al. Improving efficacy of metastatic tumor segmentation to facilitate early prediction of ovarian cancer patients’ response to chemotherapy. SPIE BiOS 2017, 10065, 47–52. [Google Scholar] [CrossRef]
- Abdoli, N.; Gilley, P.; Zhang, K.; Chen, X.; Thai, T.C.; Moore, K.; Mannel, R.S.; Qiu, Y. Comparing the effectiveness of 2D and 3D features on predicting the response to chemotherapy for ovarian cancer patients. Biophotonics Immune Responses XVIII 2023, 12380, 13–18. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef]
- Nasir, I.M.; Khan, M.A.; Yasmin, M.; Shah, J.H.; Gabryel, M.; Scherer, R.; Damaševičius, R. Pearson Correlation-Based Feature Selection for Document Classification Using Balanced Training. Sensors 2020, 20, 6793. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. Wiley R. Stat. Soc. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Piles, M.; Bergsma, R.; Gianola, D.; Gilbert, H.; Tusell, L. Feature Selection Stability and Accuracy of Prediction Models for Genomic Prediction of Residual Feed Intake in Pigs Using Machine Learning. Front. Genet. 2021, 12, 611506. (In English) [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Sun, Z.; Zurada, J.M.; Pal, N.R. Feature Selection for Neural Networks Using Group Lasso Regularization. IEEE Trans. Knowl. Data Eng. 2019, 32, 659–673. [Google Scholar] [CrossRef]
- Usai, M.G.; Goddard, M.E.; Hayes, B.J. LASSO with cross-validation for genomic selection. Genet. Res. 2009, 91, 427–436. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Orrù, G.; Pettersson-Yeo, W.; Marquand, A.F.; Sartori, G.; Mechelli, A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci. Biobehav. Rev. 2012, 36, 1140–1152. [Google Scholar] [CrossRef]
- Steardo, L.; Carbone, E.A.; de Filippis, R.; Pisanu, C.; Segura-Garcia, C.; Squassina, A.; De Fazio, P. Application of Support Vector Machine on fMRI Data as Biomarkers in Schizophrenia Diagnosis: A Systematic Review. Front. Psychiatry 2020, 11, 588. (In English) [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.S.; Anuradha, M.; Justus, J.J.; Bellam, K.; Jayasankar, T. An Efficient Coding Network Based Feature Extraction with Support Vector Machine Based Classification Model for CT Lung Images. J. Med. Imaging Health Inform. 2020, 10, 2628–2633. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, B.; Yoon, S.W.; Ko, H.S. A support vector machine-based ensemble algorithm for breast cancer diagnosis. Eur. J. Oper. Res. 2018, 267, 687–699. [Google Scholar] [CrossRef]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar] [CrossRef]
- Chang, C.; Lin, C. LIBSVM: A Library for Support Vector Machines. ACM Trans. Intell. Syst. Technol. 2013, 2, 27. (In English) [Google Scholar] [CrossRef]
- Liu, R.; Liu, E.; Yang, J.; Li, M.; Wang, F. Optimizing the Hyper-parameters for SVM by Combining Evolution Strategies with a Grid Search. In Proceedings of the Intelligent Control and Automation: International Conference on Intelligent Computing, ICIC 2006, Kunming, China, 16–19 August 2006; Huang, D.-S., Li, K., Irwin, G.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 712–721. [Google Scholar]
- Li, Q.; Doi, K. Reduction of bias and variance for evaluation of computer-aided diagnosis schemes. Med. Phys. 2006, 33, 868–875. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. (In English) [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Freixenet, J.; Martí, J.; Pérez, E.; Pont, J.; Denton, E.R.; Zwiggelaar, R. A review of automatic mass detection and segmentation in mammographic images. Med. Image Anal. 2010, 14, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. (In English) [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | 6-Month PFS | p-Value | |
|---|---|---|---|
| Responders | Non-Responders | ||
| Number of patients | 130 | 58 | |
| Age | 63 ± 10 | 61 ± 10 | 0.24 |
| Number of tumors | 272 | 133 | |
| Average tumor diameter (mm) | 31.3 ± 19.3 | 31.5 ± 16.4 | 0.94 |
| Image Type | 2D | 3D |
|---|---|---|
| Original | 104 | 110 |
| Exponential | 93 | 93 |
| Gradient Magnitude | 93 | 93 |
| Local binary pattern | 93 | 279 |
| Logarithm | 93 | 93 |
| Square | 93 | 93 |
| Square root | 93 | 93 |
| Wavelet | 741 | 741 |
| Total | 1403 | 1595 |
| Features | Radiomics Category | Filter | Total | ||||
|---|---|---|---|---|---|---|---|
| Shape | Density | Texture | Wavelet | LBP | Other | ||
| 2D | 0 | 23 | 92 | 71 | 21 | 23 | 115 |
| 3D2 Slices | 4 | 18 | 104 | 77 | 19 | 30 | 126 |
| 3D3 Slices | 0 | 16 | 75 | 51 | 9 | 31 | 91 |
| 3D4 Slices | 1 | 20 | 75 | 52 | 13 | 31 | 96 |
| 3D5 Slices | 1 | 17 | 64 | 44 | 12 | 26 | 82 |
| 3D6 Slices | 1 | 16 | 72 | 51 | 14 | 24 | 89 |
| 3D7 Slices | 1 | 15 | 61 | 37 | 16 | 24 | 77 |
| 3D8 Slices | 0 | 11 | 51 | 27 | 7 | 28 | 62 |
| 3D9 Slices | 1 | 8 | 47 | 30 | 4 | 22 | 56 |
| 3D | 1 | 8 | 64 | 34 | 13 | 26 | 73 |
| Model | STD 95% CI | STD 95% CI |
|---|---|---|
| 2D | 0.840.02 [0.78, 0.88] | 0.750.03 [0.69, 0.80] |
| 3D2 Slices | 0.890.01 [0.85, 0.92] | 0.830.02 [0.77, 0.87] |
| 3D3 Slices | 0.91 0.01 [0.88, 0.94] | 0.84 0.02 [0.78, 0.88] |
| 3D4 Slices | 0.850.02 [0.80, 0.89] | 0.760.02 [0.71, 0.82] |
| 3D5 Slices | 0.860.02 [0.82, 0.90] | 0.780.02 [0.72, 0.83] |
| 3D6 Slices | 0.840.02 [0.79, 0.88] | 0.740.03 [0.69, 0.79] |
| 3D7 Slices | 0.86 0.02 [0.80, 0.88] | 0.76 0.03 [0.70, 0.80] |
| 3D8 Slices | 0.840.02 [0.78, 0.86] | 0.750.03 [0.70, 0.80] |
| 3D9 Slices | 0.830.01 [0.78, 0.87] | 0.730.02 [0.69, 0.78] |
| 3D | 0.860.02 [0.82, 0.89] | 0.770.02 [0.72, 0.83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoli, N.; Zhang, K.; Gilley, P.; Chen, X.; Sadri, Y.; Thai, T.; Dockery, L.; Moore, K.; Mannel, R.; Qiu, Y. Evaluating the Effectiveness of 2D and 3D CT Image Features for Predicting Tumor Response to Chemotherapy. Bioengineering 2023, 10, 1334. https://doi.org/10.3390/bioengineering10111334
Abdoli N, Zhang K, Gilley P, Chen X, Sadri Y, Thai T, Dockery L, Moore K, Mannel R, Qiu Y. Evaluating the Effectiveness of 2D and 3D CT Image Features for Predicting Tumor Response to Chemotherapy. Bioengineering. 2023; 10(11):1334. https://doi.org/10.3390/bioengineering10111334
Chicago/Turabian StyleAbdoli, Neman, Ke Zhang, Patrik Gilley, Xuxin Chen, Youkabed Sadri, Theresa Thai, Lauren Dockery, Kathleen Moore, Robert Mannel, and Yuchen Qiu. 2023. "Evaluating the Effectiveness of 2D and 3D CT Image Features for Predicting Tumor Response to Chemotherapy" Bioengineering 10, no. 11: 1334. https://doi.org/10.3390/bioengineering10111334
APA StyleAbdoli, N., Zhang, K., Gilley, P., Chen, X., Sadri, Y., Thai, T., Dockery, L., Moore, K., Mannel, R., & Qiu, Y. (2023). Evaluating the Effectiveness of 2D and 3D CT Image Features for Predicting Tumor Response to Chemotherapy. Bioengineering, 10(11), 1334. https://doi.org/10.3390/bioengineering10111334






